Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
405 results about "Biopharmaceutical" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A biopharmaceutical, also known as a biologic(al) medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living cells used in cell therapy. Biologics can be composed of sugars, proteins, or nucleic acids or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial.
Preservation by Vaporization
ActiveUS20080229609A1Easy to controlImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
Hydrogel particle formulation
InactiveUS7022313B2Avoid lostEasy to customizePowder deliveryPeptide/protein ingredientsDiagnostic agentParticle injection
New compositions formed from the combination of an active substance with a hydrogel carrier moiety are provided. The compositions are suitable for use in high-velocity transdermal particle injection techniques. Methods of providing the new compositions are also provided. In addition, methods for administering pharmacologically active agent to a subject are provided. These methods are useful for delivering drugs, biopharmaceuticals, vaccines and diagnostics agents.
Owner:POWDERJECT RES LTD OXFORD (GB)
Interferon tau mutants and methods for making them
InactiveUS6833256B1High activityLow cytotoxicityPeptide/protein ingredientsDepsipeptidesADAMTS ProteinsAutoimmune disease
The present invention is directed to the field of animal and human health, and more particularly to pharmacological uses of analogs or mutants of interferon-tau (IFN-tau) that differ from native IFN-tau because of substitutions of amino acids near the amino terminus of the IFN-tau molecule that impart improved biological activity. The IFN-tau mutants described in this disclosure have low toxicity, retain the same or slightly reduced antiviral activity compared with highly effective IFN-alpha, and have enhanced antiproliferative activity compared to native IFN-tau, making them useful in treating viral infections, cancer, and immune system diseases including autoimmune diseases. The present invention is also directed to a method for making novel recombinant proteins, especially interferons, interleukins, and cytokines, polypeptide hormones and other biopharmaceuticals that have improved biological activity over known proteins and / or lower toxicity and / or increased stability.
Owner:UNIV OF MARYLAND
Preservation by vaporization
ActiveUS9469835B2Maximize potencyMaximize viabilityBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
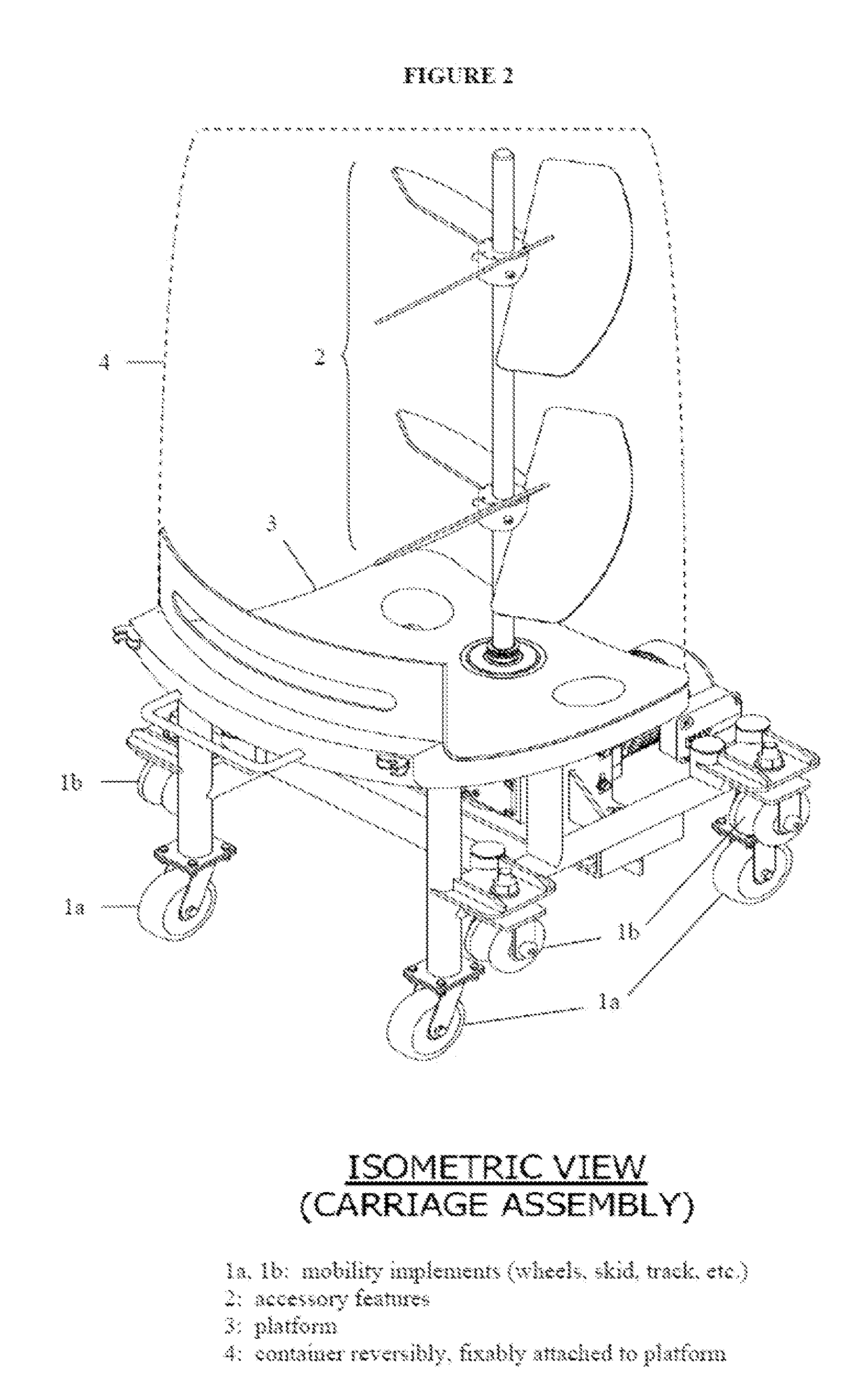
Reactor systems
ActiveUS20120282688A1Sequential/parallel process reactionsPeptide/protein ingredientsReactor systemCarriage
This disclosure relates to equipment utilized to manufacture chemical agents, particularly biopharmaceuticals. In some embodiments, reactor systems comprising a mobile carriage assembly; a disposable reaction container removably attached to the carriage assembly; and, a carriage holder into which the mobile carriage assembly may be removably inserted are provided.
Owner:ABEC INC
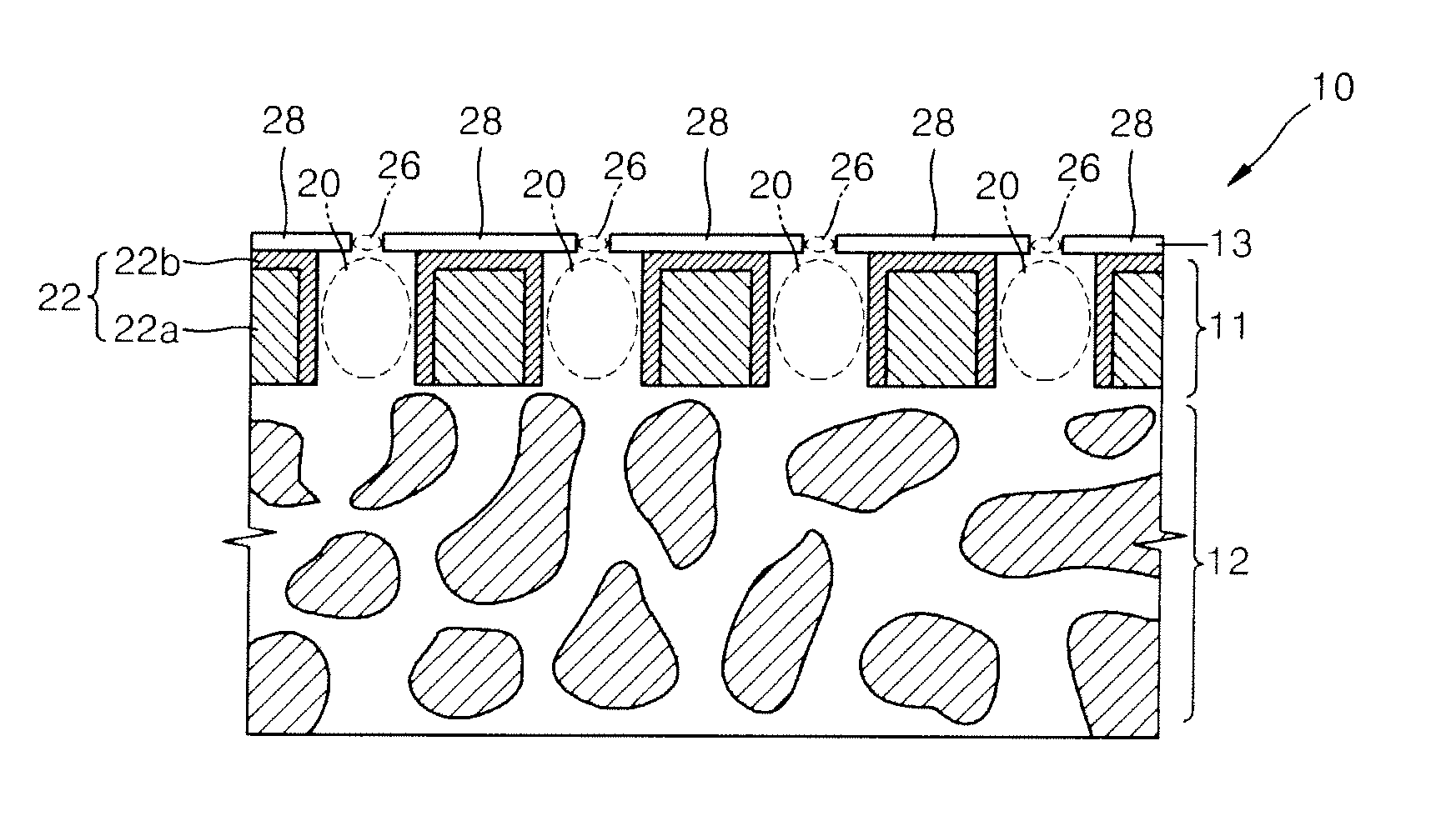
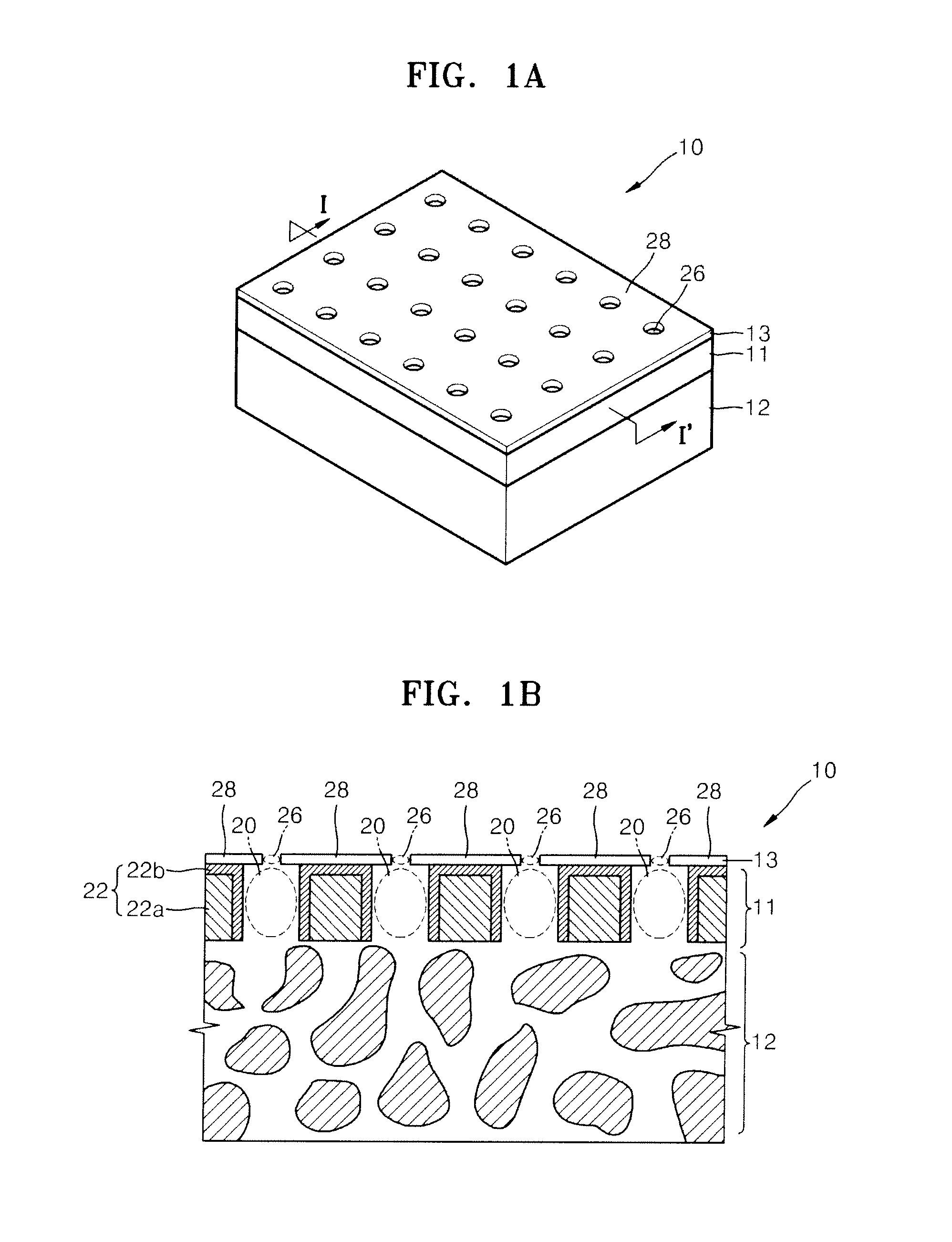
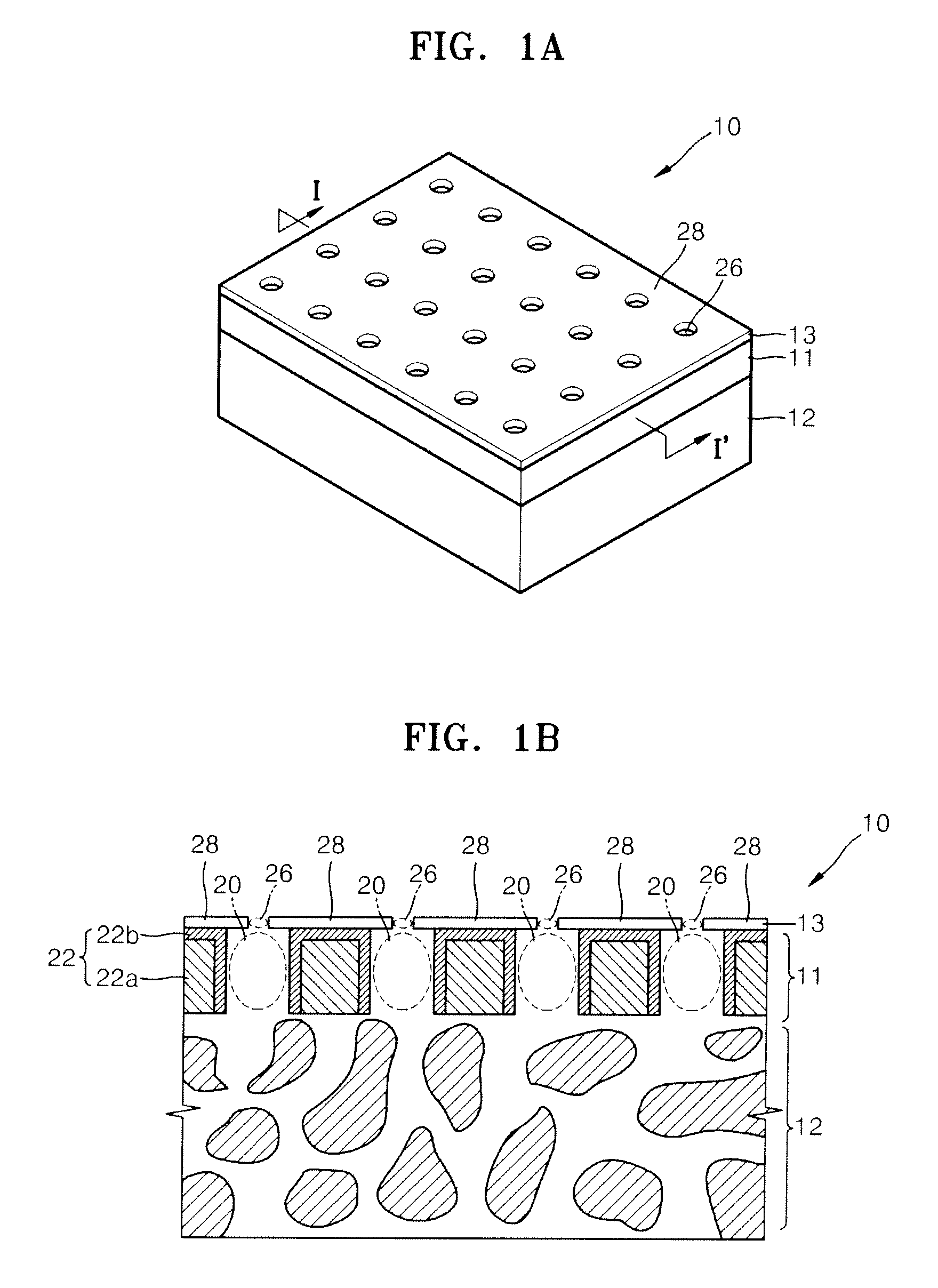
Nanoporous membrane, process of fabricating the same and device for controlled release of biopharmaceuticals comprising the same
InactiveUS7935416B2High hole densityUniform pore sizeSemi-permeable membranesMembranesControl releaseNanoporous membrane
Provided are a nanoporous membrane including a support; a first separation layer with a plurality of first nano-sized pores and a first matrix; and a second separation layer having a plurality of second pores respectively corresponding to the plurality of first pores of the first separation layer and a second matrix, and formed on the first separation layer, wherein a density of the plurality of the first pores and the second pores is equal to or greater than 1010 / cm2, and a diameter of each of the second pores is less than that of the corresponding first pore, a process of fabricating the same, and a device for a controlled release of biopharmaceuticals including the nanoporous membrane. The device for a controlled release of biopharmaceuticals including the nanoporous membrane can release biopharmaceuticals at a constant rate for a long period of time regardless of the concentration of the biopharmaceuticals including in pharmaceuticals, and high flex and selectivity.
Owner:POSTECH ACAD IND FOUND
Bulk freezing of biopharmaceuticals
A system and method for bulk freezing is provided. In one embodiment, the system and method for bulk freezing includes a bulk freezing container adapted to hold at least one and preferably a plurality of bags holding a biopharmaceutical liquid. The bulk freezing container includes at least a first and second shelf having corrugations, wherein the second shelf is vertically arranged above the first shelf with the bags disposed between the shelves. The bags and the corrugations of the first and second shelves define a plurality of substantially parallel flow channels through which a cryogenic cold fluid or a warming fluid is passed to freeze and / or thaw the biopharmaceutical fluid. In another embodiment, the bulk freezing system and method includes a bulk freezing container with a plurality of adjacent elongated chambers adapted for holding the biopharmaceutical fluid.
Owner:PRAXAIR TECH INC
Immunity enhancing agent, inactivated vaccine, and preparation method thereof
InactiveCN103083663AImprove immunityEnhance immune responseViral antigen ingredientsAntiviralsDipeptideOil phase
The invention provides an immunity enhancing agent, an inactivated vaccine, and a preparation method thereof. The invention relates to the field of biopharmaceutical. The immunity enhancing agent comprises 0.1-21mg / mL of monophosphoryl lipid A, 1.5-125mg / mL of muramyl dipeptide, and 0.7-4.5mg / mL of beta-glucan. The invention also provides the inactivated vaccine comprising the immunity enhancing agent, and a preparation method of the inactivated vaccine. According to the invention, the immunity enhancing agent is mixed with an inactivated antigen solution, such that a water-phase solution is obtained; and the water-phase solution is mixed with an oil-phase solution, such that the inactivated vaccine is obtained. According to the immunity enhancing agent provided by the invention, with a synergetic effect of the components, body immunity level can be improved, and immune response to antigen can be improved, such that antibody level after immunization can be increased, immune window period can be shortened, and vaccine immunization effect can be enhanced. According to the inactivated vaccine comprising the immunity enhancing agent, antibody level after immunization is high, a protection period is long, and immunization window period is short.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES +1
Hydrogel particle formation
InactiveUS20050191361A1Improve performanceAvoid lostPowder deliveryGenetic material ingredientsDiagnostic agentActive agent
New compositions formed from the combination of an active substance with a hydrogel carrier moiety are provided. The compositions are suitable for use in high-velocity transdermal particle injection techniques. Methods of providing the new compositions are also provided. In addition, methods for administering pharmacologically active agent to a subject are provided. These methods are useful for delivering drugs, biopharmaceuticals, vaccines and diagnostics agents.
Owner:POWEDERJECT RES
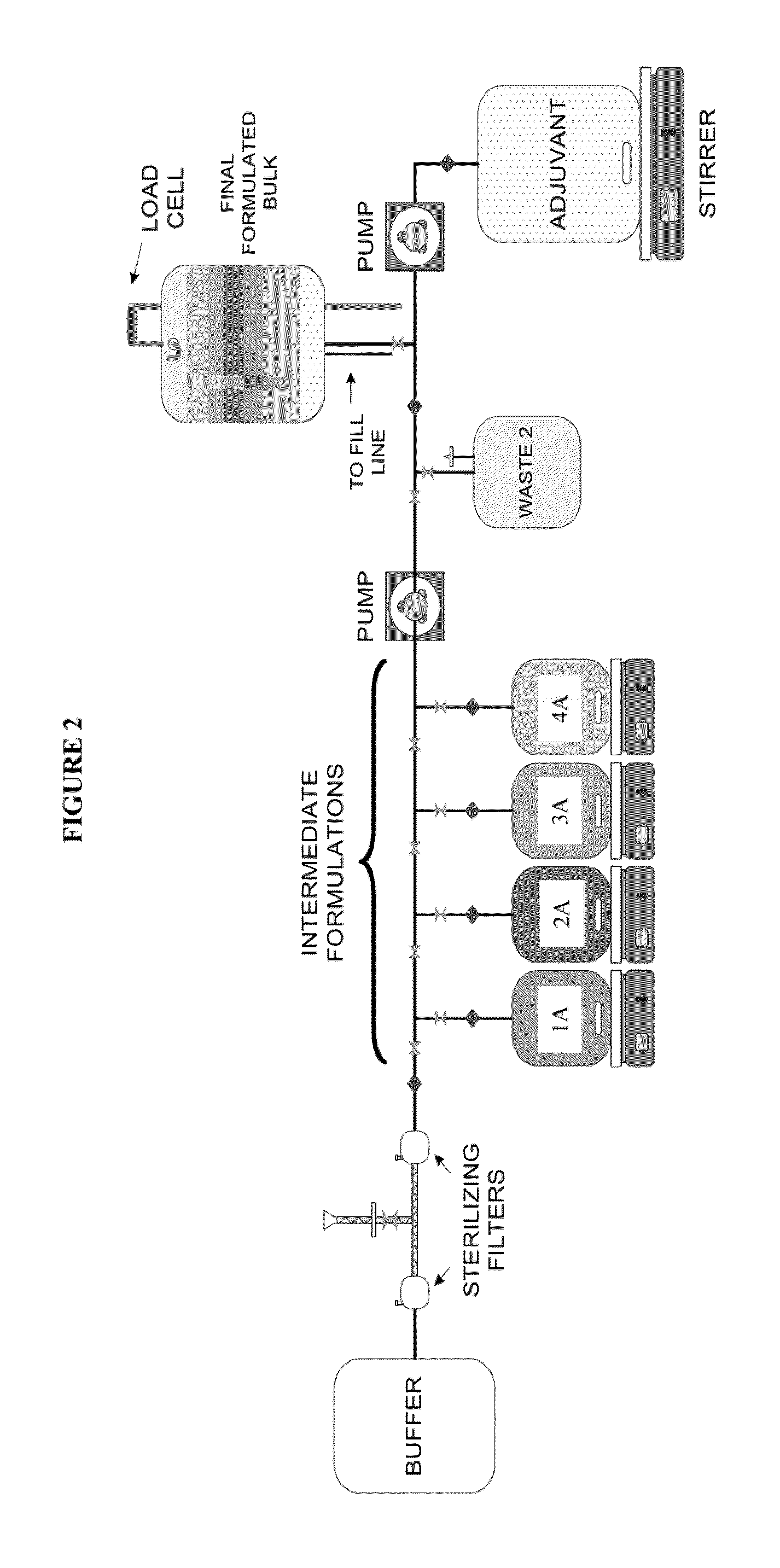
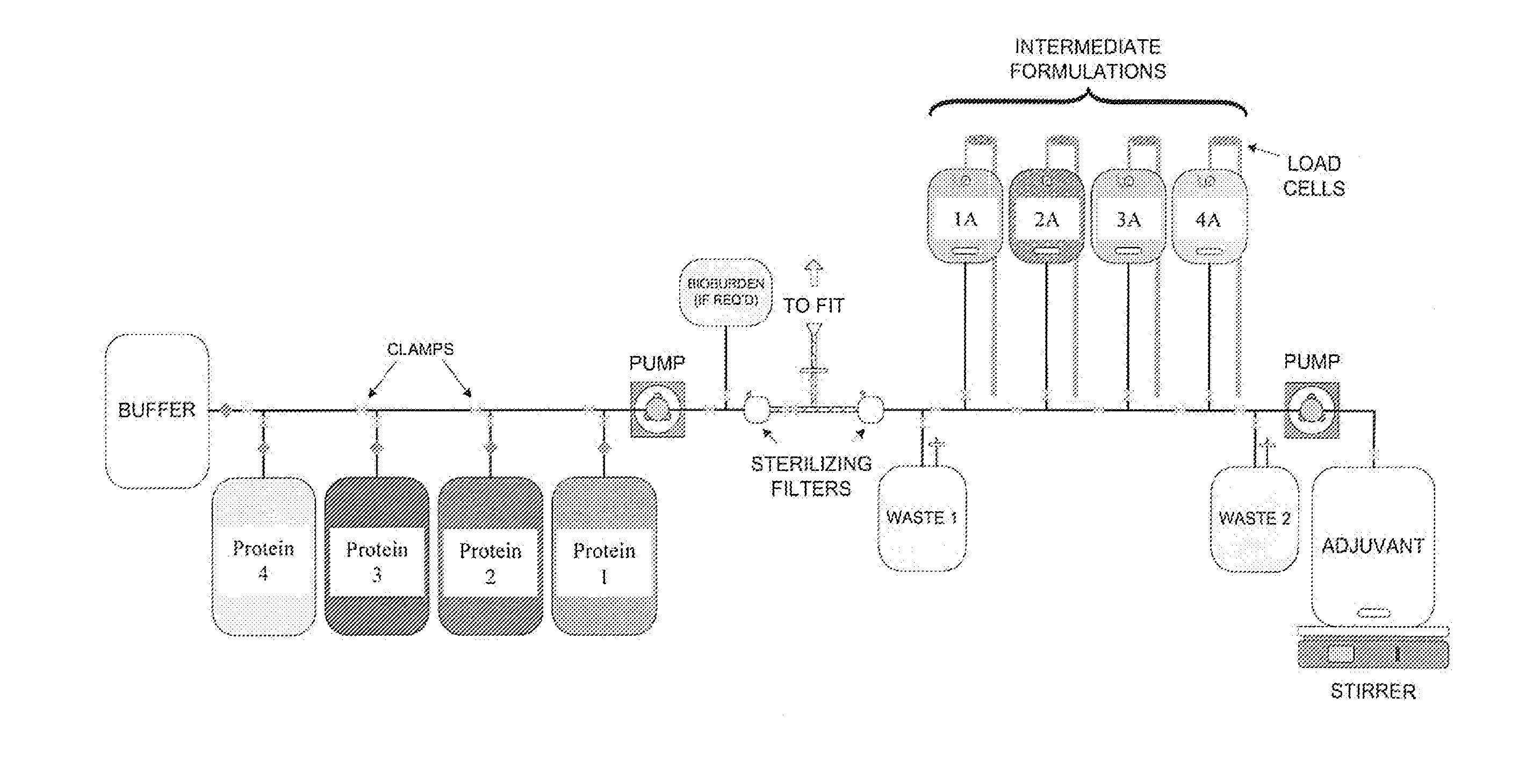
System and process for producing multi-component biopharmaceuticals
A sterile, closed, disposable system for formulating biopharmaceutical compositions containing multiple active agents is described herein.
Owner:SANOFI PASTEUR LTD
New particle stabilized emulsions and foams
InactiveUS20150125498A1Novel and useful emulsifying propertyFlexibility of systemBiocideCosmetic preparationsSolid particlePharmaceutical formulation
The present invention relates to a particle stabilized emulsion or foam comprising at least two phases and solid particles, wherein said solid particles are starch granules and said starch granules or a portion thereof are situated at the interface between the two phases providing the particle stabilized emulsion or foam. The invention further relates to the use of said particle stabilized emulsion or foam for encapsulation of substances chosen from biopharmaceuticals, proteins, probiotics, living cells, enzymes and antibodies, sensitive food ingredients, vitamins, and lipids in food products, cosmetic products, skin creams, and pharmaceutical formulations.
Owner:SPEXIMO
Glucagon analog for treatment of metabolic diseases
ActiveCN109836488AGood enzyme resistance and stabilityLong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDipeptidyl peptidase
The present invention relates to the field of biopharmaceuticals, and in particular to a glucagon analog for treating metabolic diseases. The structural formula is: H-X2-X3-GTFTSD-X10-SKYLD-X16-X17-AAQ-DFVQWLMN-X29-X<z> or H-S-Q-GTFTSD-Y-SKYLD-X16-X17-AAQ-DFVQWLMN-X29-Xz-NH2. The described glucagon analogue has a GLP-1 / GCG / GIP triple receptor agonist activity and better enzyme resistant stabilityfor neutral endopeptidase (NEP) and dipeptidyl peptidase-4(DPP-4), and has a longer half-life in vivo and duration of action compared with natural glucagon, GLP-1 and GIP.
Owner:ZHEJIANG DOER BIOLOGICS CO LTD
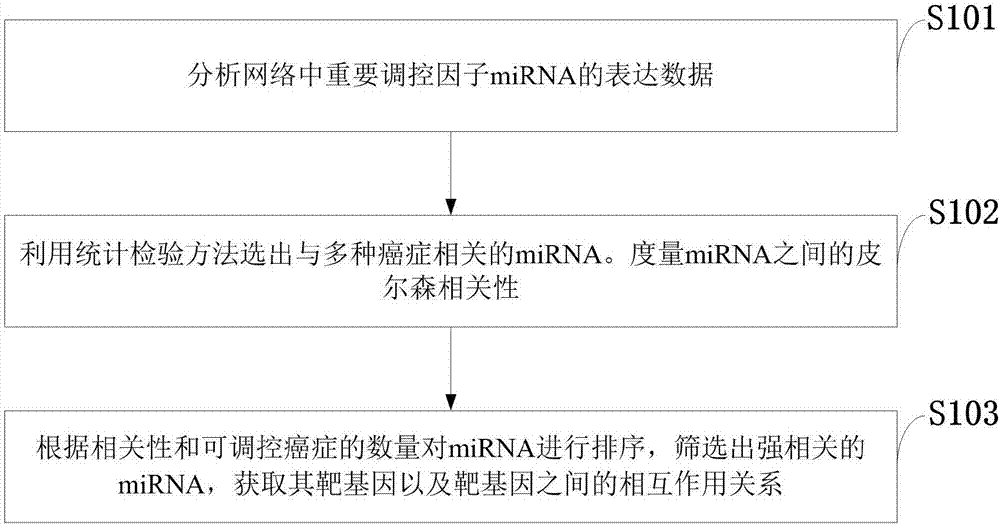
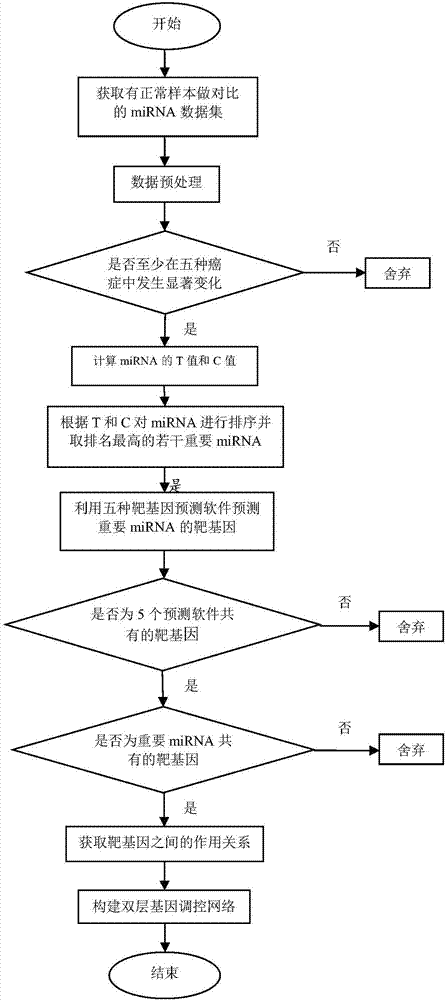
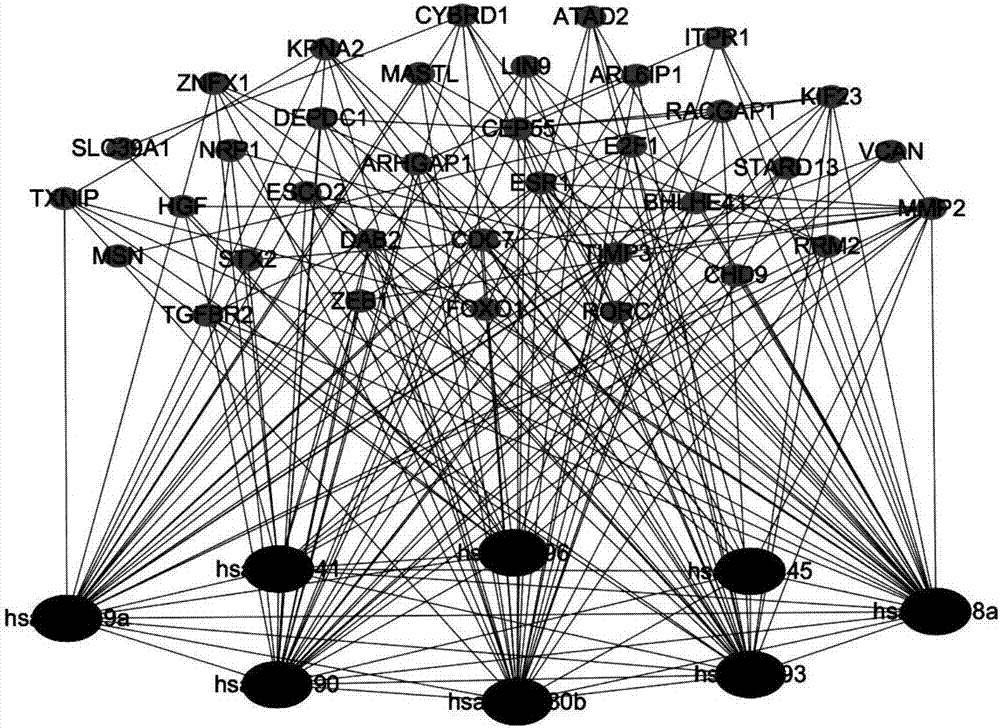
Method for constructing double-layer gene regulation and control network
ActiveCN107358062AUnderstanding PathogenesisUnderstand the processProteomicsGenomicsKey factorsTargeted therapy
The invention belongs to the technical field of data processing and discloses a method for constructing a double-layer gene regulation and control network. The method comprises following steps: analyzing expression data of an important regulation and control factor miRNA in a network; selecting miRNA relevant to multiple types of cancer by means of the statistics examination method; measuring Pearson correlation among the miRNA; ordering the miRNA according to the correlation and the number of controllable cancer, screening out strongly correlated miRNA and obtaining target genes thereof and interacting relations among the target genes. The present invention discloses the mechanism of gene and miRNA involved in the regulation of biological processes such as cancer and helps to understand the relationship between genes and cancer and other complex diseases and provides reference for the development of biopharmaceuticals and targeted therapy for pan-cancer. Identifying cancer-associated miRNAs and genes can be used to elucidate the mechanisms of cooperation that are involved in the regulation of key factors that influence a variety of cancer processes, cancer risk prediction, and the development of bio-targeted drugs.
Owner:XIDIAN UNIV
Reactor systems
ActiveUS9228165B2Sequential/parallel process reactionsPeptide/protein ingredientsReactor systemEngineering
This disclosure relates to equipment utilized to manufacture chemical agents, particularly biopharmaceuticals. In some embodiments, reactor systems comprising a mobile carriage assembly; a disposable reaction container removably attached to the carriage assembly; and, a carriage holder into which the mobile carriage assembly may be removably inserted are provided.
Owner:ABEC INC
Nanoporous membrane, process of fabricating the same and device for controlled release of biopharmaceuticals comprising the same
InactiveUS20090208726A1High hole densityUniform pore sizeSemi-permeable membranesMembranesControl releaseNanoporous membrane
Provided are a nanoporous membrane including a support; a first separation layer with a plurality of first nano-sized pores and a first matrix; and a second separation layer having a plurality of second pores respectively corresponding to the plurality of first pores of the first separation layer and a second matrix, and formed on the first separation layer, wherein a density of the plurality of the first pores and the second pores is equal to or greater than 1010 / cm2, and a diameter of each of the second pores is less than that of the corresponding first pore, a process of fabricating the same, and a device for a controlled release of biopharmaceuticals including the nanoporous membrane. The device for a controlled release of biopharmaceuticals including the nanoporous membrane can release biopharmaceuticals at a constant rate for a long period of time regardless of the concentration of the biopharmaceuticals including in pharmaceuticals, and high flex and selectivity.
Owner:POSTECH ACAD IND FOUND
Scaled down freezing and thawing system for biopharmaceuticals and biologics
ActiveUS7228688B2Domestic refrigeratorsStationary refrigeration devicesFreeze and thawBiopharmaceutical
Owner:SARTORIUS STEDIM NORTH AMERICA INC
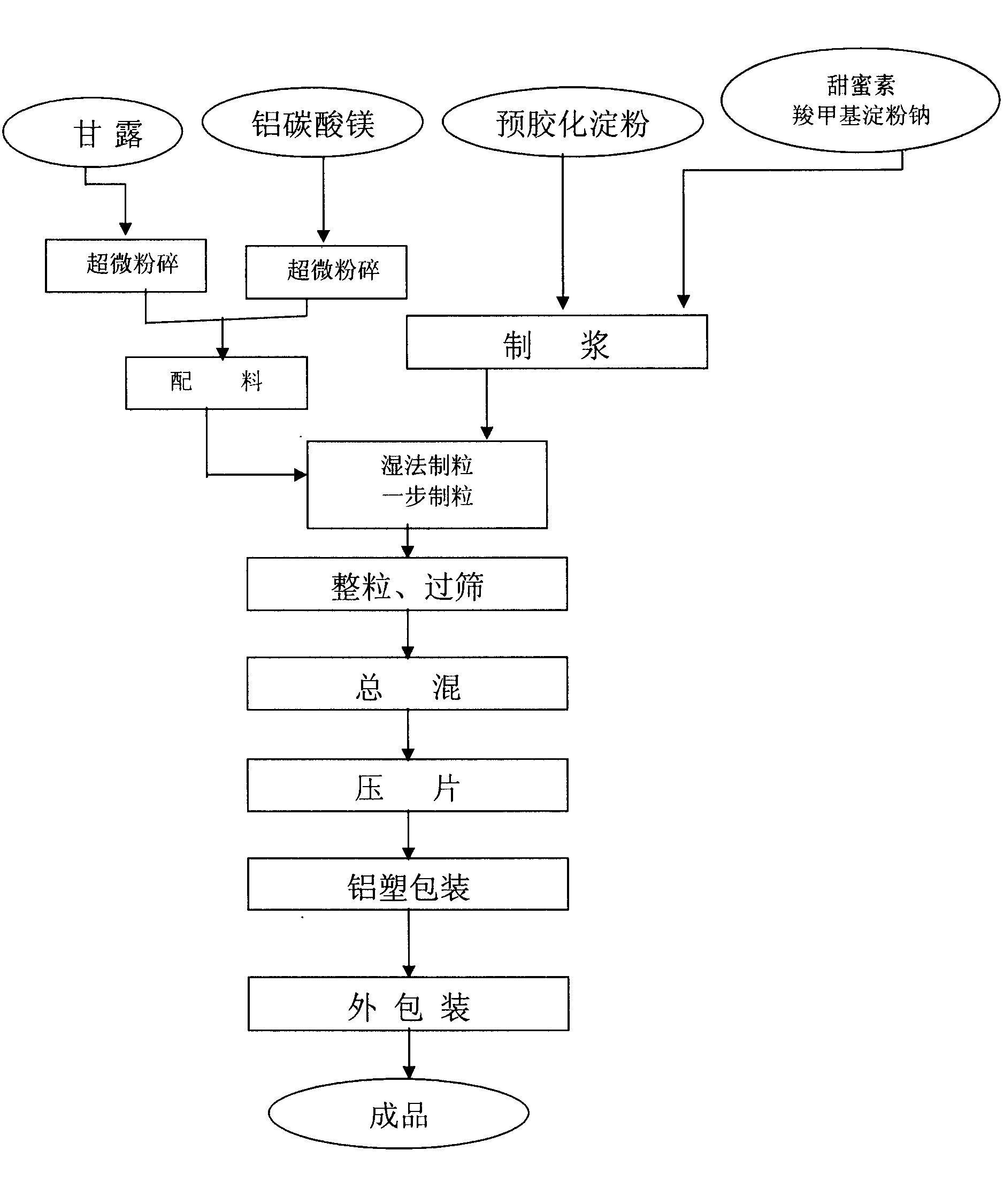
Rapidly effectual aluminum magnesium carbonate preparation and technique of preparing the same
InactiveCN101219150ANo side effectsReactivity NoneDigestive systemPill deliveryMagnesium stearateStearic acid
The invention relates to hydrotalcite preparation with rapid efficiency and a preparation process thereof, which belong to the biopharmaceuticals field. Every 1000 hydrotalcite units of the invention comprise the following constituents: 0.45-0.55kg of hydrotalcite, 0.18-0.25kg of mannitol, 12-18g of carboxymethyl starch sodium, 8.0-15.0g of magnesium stearate, 0.5-1.0g of sodium cyclamate, 0.3-0.9ml of peppermint flavor, 0.378kg-0.462kg of pre-gelatinized starch paste (12 percent by mass specific concentration) and 0.504kg-0.616kg of pre-gelatinized starch paste (5 percent by mass specific concentration). The preparation process of the invention comprises the following steps: hydrotalcite and mannitol are prepared into super-fine powder with grain diameter of 5-15um, which are blended with carboxymethyl starch sodium, sodium cyclamate and pre-gelatinized starch paste, wet granulated, sieved by a sieve with 12-16 meshes, totally mixed, packaged or tabletted to prepare finished product of hydrotalcite preparation; the hydrotalcite preparation prepared by adopting the method of the invention has fast effectiveness, good effect with low toxicity, small side-effects, pleasant taste and high suffer compliance, which is an ideal clinical antiacid.
Owner:游洪涛
Single domain antibody for recognizing human serum albumin
InactiveCN107674122AExtended half-lifeConvenient treatmentFungiBacteriaSingle-domain antibodyNucleic acid sequence
The invention discloses a single domain antibody for recognizing human serum albumin, CDR1, CDR2 and CDR3 sequences with specific recognition effects, a fusion protein prepared from the amino acid sequences and polypeptide molecules or fragments of which at least one has a treatment function, a multispecific or multifunctional molecule containing the single domain antibody, a nucleic acid moleculefor coding the above amino acid sequences, a carrier containing the amino acid sequences, a protein or polypeptide, wherein the carrier is expressed by the expression system, and a host cell for expressing the any single domain antibody, fusion protein, multispecific or multifunctional molecule, nucleic acid sequences and carrier or protein or polypeptide. Through screening, the single domain antibody with high affinity is obtained, identifies specific human serum albumin, prolongs the half-life of biopharmaceuticals and is conducive to tumor treatment.
Owner:SHENZHEN BEIKE BIOTECH
System and Process for Producing Mulit-Component Biopharmaceuticals
A sterile, closed, disposable system for formulating biopharmaceutical compositions containing multiple active agents is described herein.
Owner:SANOFI PASTEUR LTD
Method for recloning production cells
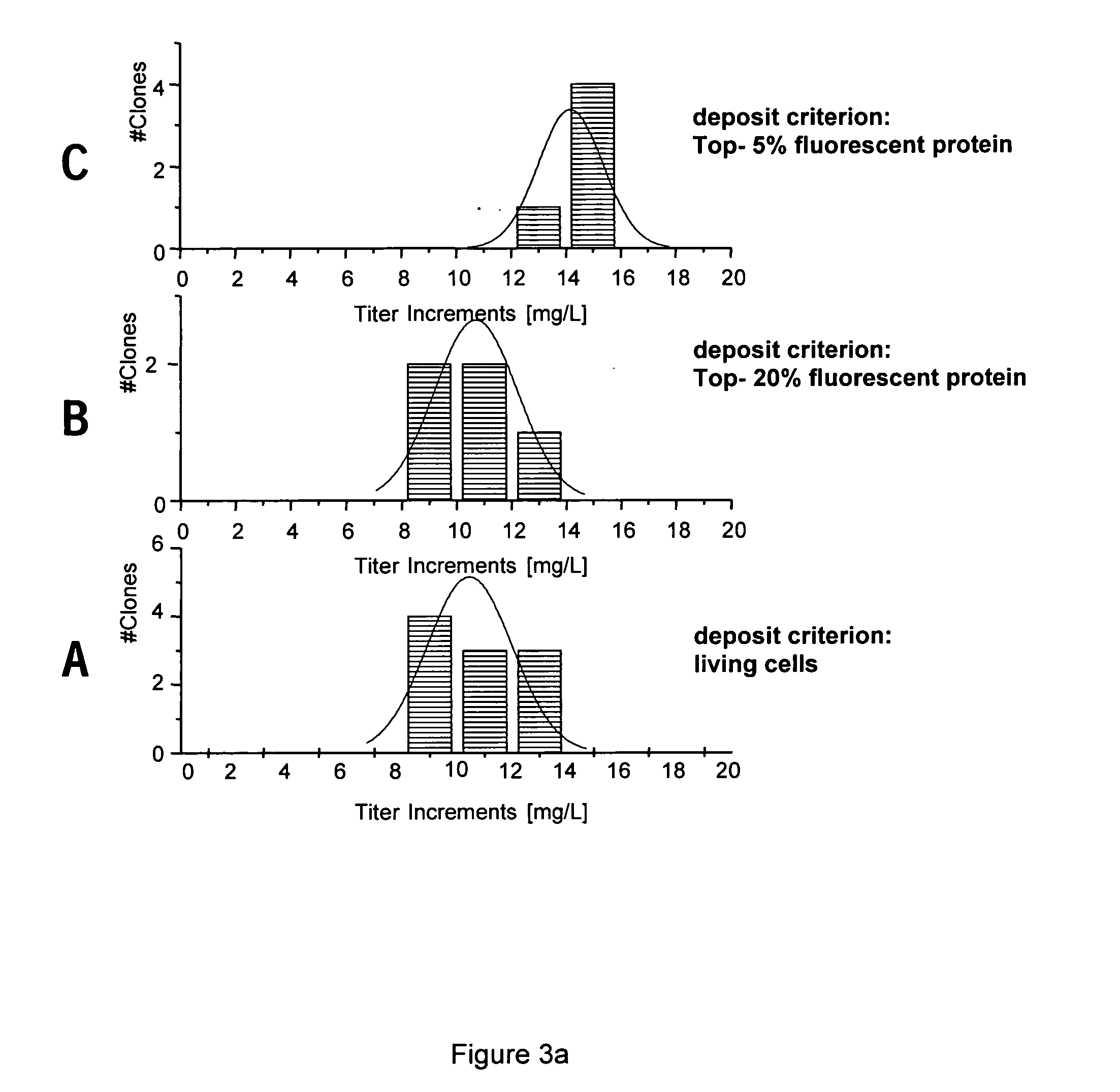
ActiveUS20050059146A1Shorten development timeReduce riskGenetically modified cellsCulture processBiopharmaceuticalBiological drugs
A new method for selecting clones and recloning mammalian cells which are of importance for the production of biopharmaceuticals, preferably hamster or mouse myeloma cells, with a high degree of automation and throughput. The invention relates to methods of depositing and replicating single cell clones of the cells in question. The invention also relates to methods of preparing proteins using cells which have been obtained and replicated by single cell deposition as well as compositions which allow the replication of single cells.
Owner:BOEHRINGER INGELHEIM PHARM KG
Processing method for intelligent hydrogel using starch nano-particles as framework
ActiveCN108676177ADegradableOvercome the weakness of weakened strengthNanotechnologyControlled releaseSmart hydrogels
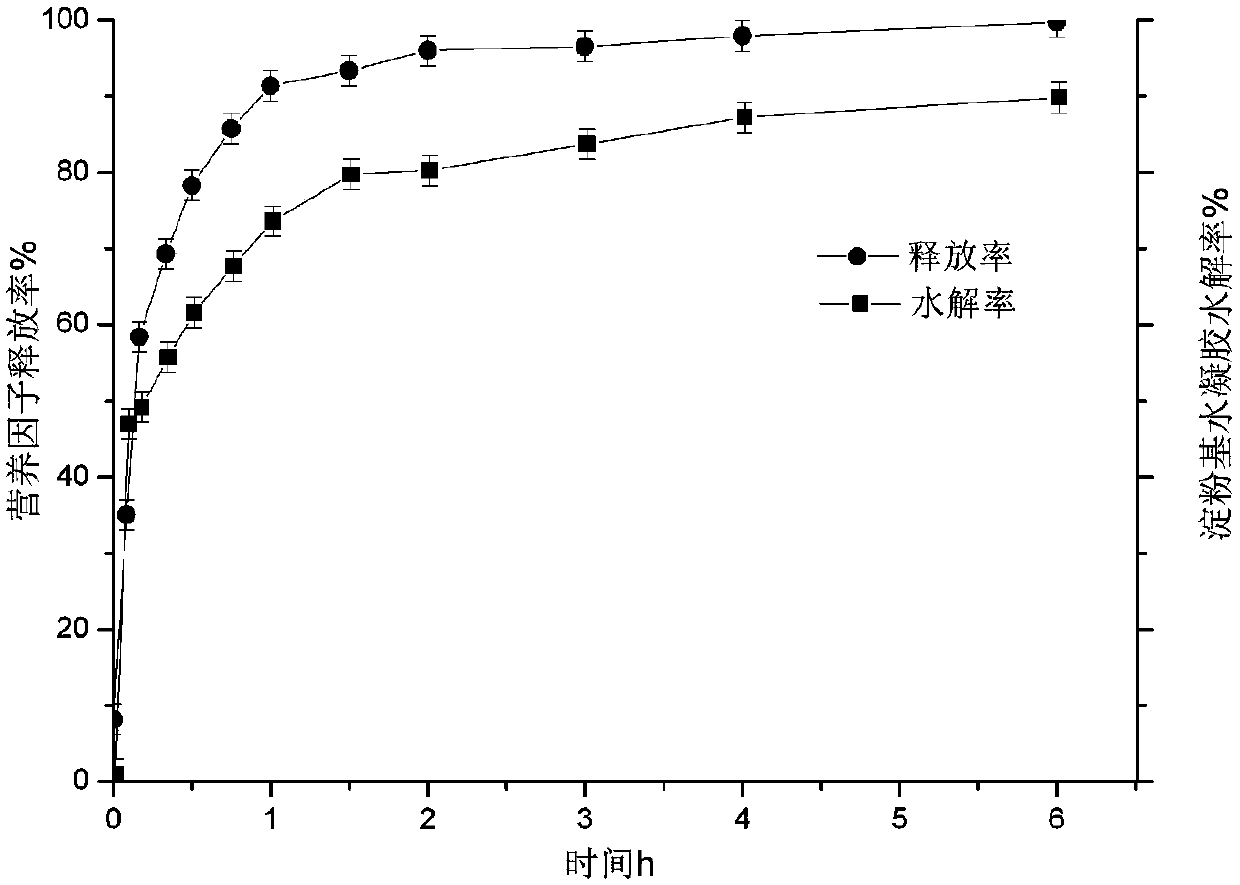
The invention discloses a processing method for intelligent hydrogel using starch nano-particles as a framework, and belongs to the technical field of nutritional and healthy foods. The intelligent hydrogel having a spatial network structure is obtained by using the transnucleosidation chain extension-sugar chain entanglement reaction of skeleton water-soluble starch nano-particles as the framework. The product is the intelligent starch-based hydrogel having the advantages of good rehydration, good biocompatibility, strong gel strength, irreversible enzymatic response, reversible pH response and carrying of multi-nutrient factors. The hydrogel can realize the protection and controlled release of food function factors, and can be applied to fields of foods, biopharmaceuticals, functional materials and the like.
Owner:JIANGNAN UNIV
Polypeptide and composition for restraining SARS-COV-2 infection, and purposes of polypeptide and composition
ActiveCN111471088AAvoid infectionPrevent intrusionOrganic active ingredientsPeptide/protein ingredientsPharmaceutical medicineBiopharmaceutical
The invention provides polypeptide for restraining SARS-COV-2 infection, and belongs to the field of biopharmaceuticals. The polypeptide is VDP-4, and has an amino acid sequence as shown in SEQID NO:1; and the polypeptide can also be a VDP-4 modifying product, or one of pharmaceutically acceptable salts, esters and prodrugs. The polypeptide can be specifically bound with an RBD region of an S protein of SARS-COV-2 to block combination of viruses and cell receptors and further effectively restrain virus infection from the source. The invention also provides a composition for restraining the SARS-COV-2, and a purpose of the composition.
Owner:北京中科微盾生物科技有限责任公司
Detection and removal of misfolded proteins/peptides
The invention concerns the field of detecting and quantifying misfolded proteins / peptides. In particular the detection and quantification of misfolded proteins / peptides in body fluids, on cell surfaces of humans and mammals, the detection of misfolded proteins / peptides in reagents to be tested for scientific research and / or diagnostic use and in pharmaceutical medication or their additives and it concerns as well the removal of misfolded proteins / peptides from reagents to be tested for scientific research and / or for diagnostic purposes and from pharmaceutical medication or their additives. Furthermore the invention includes substances to identify and methods to detect bio-films, a method to examine hemocompatibility of materials and a method to optimize therapeutical products, and to provide reagents microorganisms to charge with for more reliable diagnostics and quality control of biopharmaceuticals and identification substances for the screening for preliminary stages of amyloids that can be used for technical purposes.
Owner:OXPROTECT
Total-flavone extract of lindera root leaves and preparation method and use thereof
InactiveCN101450107AContent advantageAdvantages of simplicityDigestive systemAntiviralsMedicinal herbsPolyamide
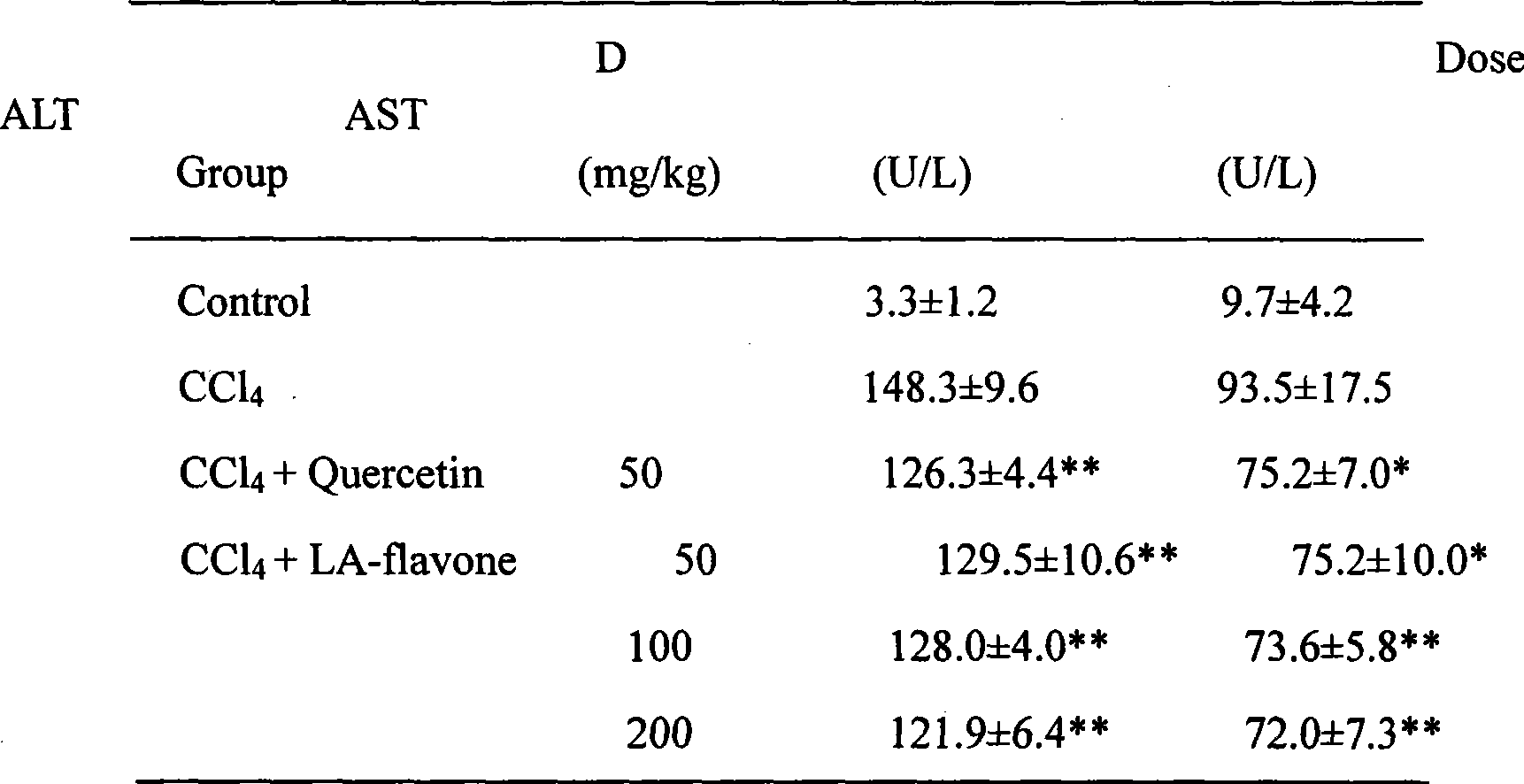
The invention relates to a lindera aggregate leaf total flavonoids extract preparing method and application thereof belonging to the biopharmaceutical technology field. The lindera aggregate leaf total flavonoids extracting method comprises the steps: smashing the medicinal materials, boiling by water and extracting, filtering out the sediments and taking the liquid, passing the extract liquid through macroporous resin or polyamide column, eluting by ethanol water solution, concentrating the eluent, and getting extract containing the total flavonoids. The method achieves the advantages of simple, low cost, high total flavonoids content in extract, and suitable for industrial production. The lindera aggregate leaf total flavonoids exact achieves remarkable oxidation resistance and liver protection activity in vivo or in vitro experiments, can be applied in preparation of health food, health medicine and medicines for treating acute / chronic viral hepatitis, chemical hepatitis, drug hepatitis and autoimmunity hepatitis.
Owner:ZHEJIANG HONGSHILIANG GRP TIANTAISHAN SPICEBUSHROOT
Humanized cytokine animal model, preparation method and application
ActiveCN111304246ACompounds screening/testingAntibody mimetics/scaffoldsAnimal scienceBiopharmaceutical
The invention relates to a humanized gene modification inhuman animal, in particular to an animal model expressing humanized IL-6R and / or IL-6 protein. In some examples, an inhuman animal expressing the humanized IL-6R and / or IL-6 and subjected to genetic modification also has an immunodeficiency phenotype, and / or contains more humanized cytokine of IL3, GM-CSF and the like. The invention also provides a construction method of the humanized inhuman animal containing IL-6R and / or IL-6 gene modification, and an application of the construction method to the field of biopharmaceutical.
Owner:BIOCYTOGEN JIANGSU CO LTD +1
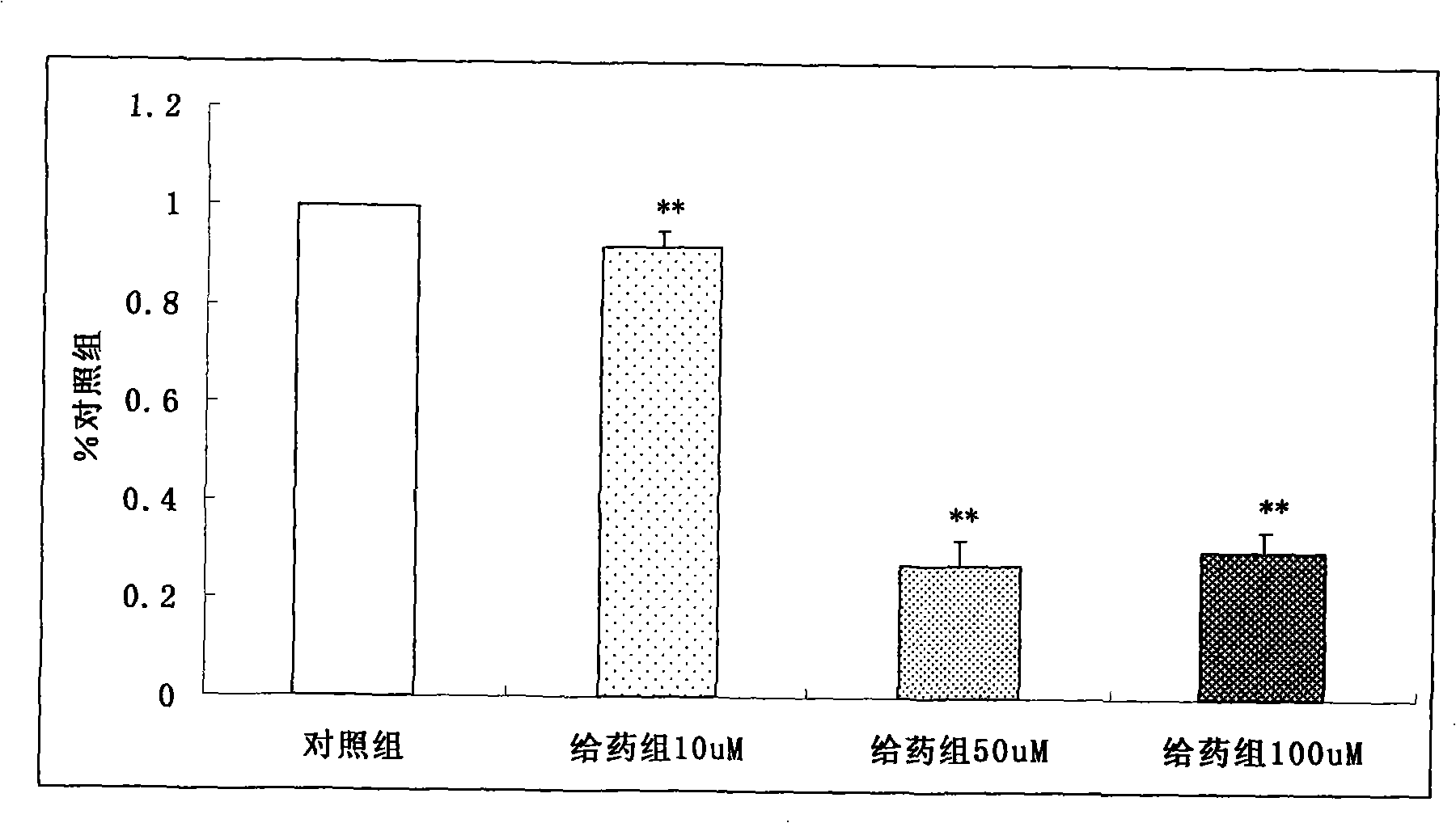
Method for improving bioactivity of exendin fusion protein
ActiveCN101875700AExtended half-lifeImprove biological activityFungiBacteriaInsulin dependent diabetesDrug biological activity
Owner:无锡和邦生物科技有限公司 +1
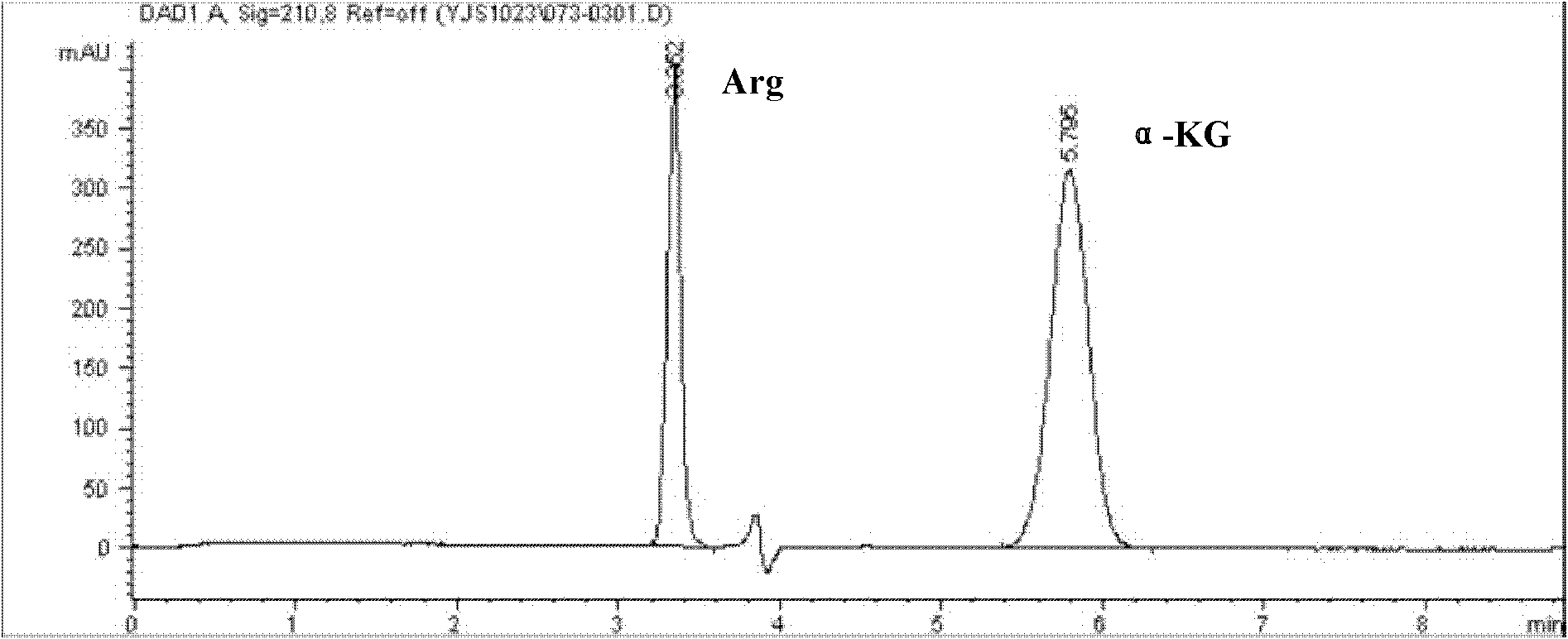
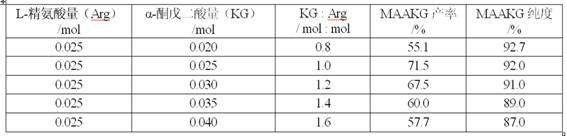
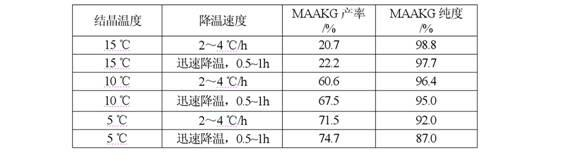
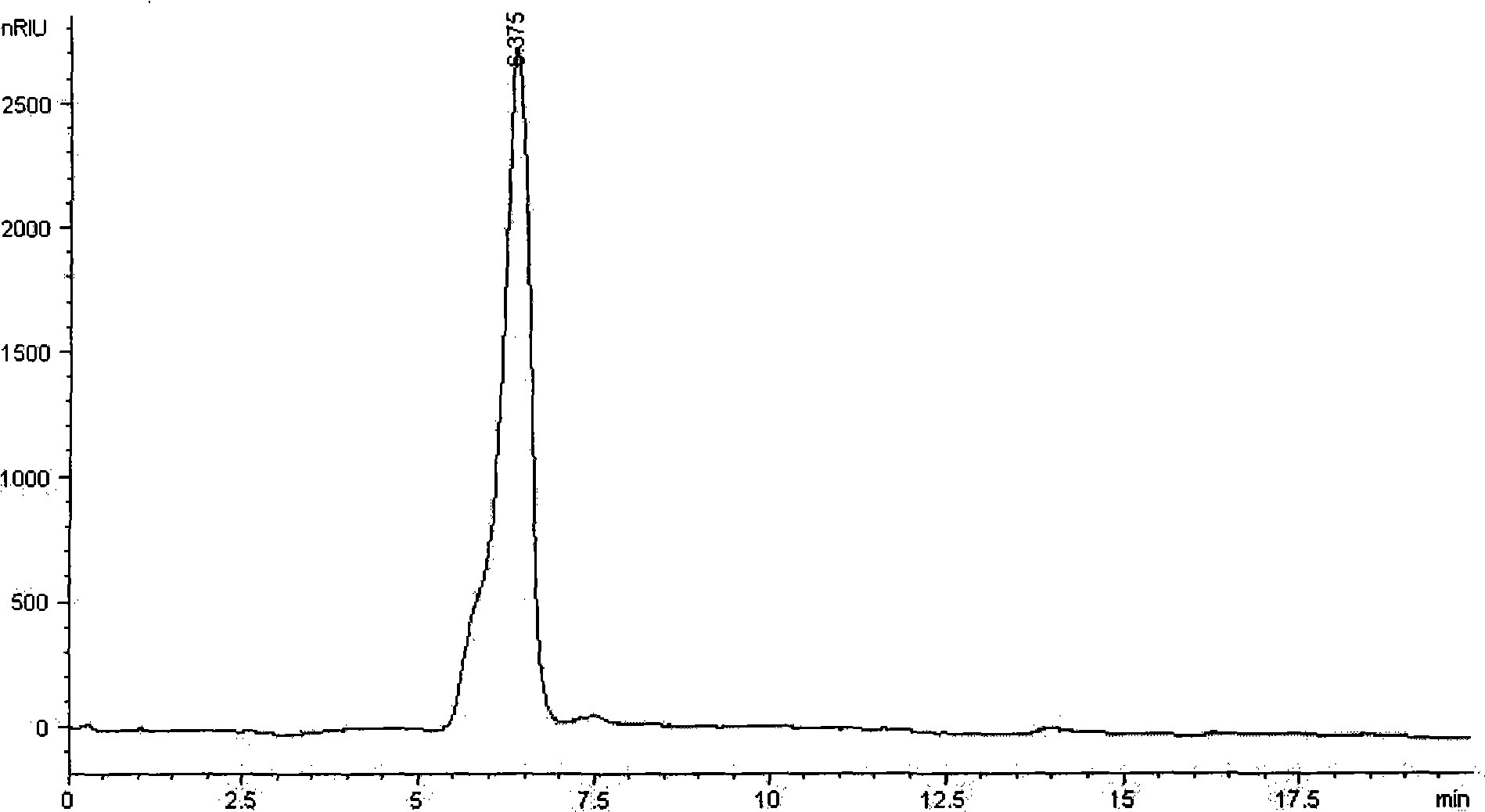
Process for preparing L-arginine-alpha-ketoglutarate (AAKG) from fermentation liquor through direct crystallization
ActiveCN102020593AHigh purityQuality improvementOrganic compound preparationCarboxylic acid salt preparationLiver functionsArginine
The invention belongs to the technical field of biopharmaceuticals and relates to a method for preparing L-arginine-alpha-ketoglutarate (AAKG) from raw materials comprising L-arginine and alpha-ketoglutarate obtained by fermentation. In the method, the L-arginine and alpha-ketoglutarate in the fermentation liquor are directly used as the raw materials for AAKG rather than being used for extracting the refined crystalline products. The process mainly comprises the following technical steps: fermentation liquor pretreatment, chelation, concentration, crystallization, dissolution for decolorization, recrystallization, drying and the like. The process is characterized by comprising the following steps: pretreating the fermentation liquor with L-arginine, adding a certain amount of fermentation filtrate with alpha-ketoglutarate to the pretreated fermentation liquor to be chelated, carrying out vacuum evaporation concentration and then cooling the crystal to obtain the coarse product; and recrystallizing the coarse product by utilizing an organic solvent to obtain the high-purity product. The process has the advantages of high product purity, short flow, obvious emission reduction effect and the like, and is easy for quality control. The AAKG is mainly used as a physique enhancer and has the functions of promoting the muscles to grow and recover rapidly, promoting liver cells to absorb the nutrients and energy, maintaining normal liver functions and the like.
Owner:FUJIAN GUTIAN PHARMA
Application of mangiferin in treating type II diabetes and vitro trial model thereof
The invention relates to an application of norathyriol in treating type II diabetes, belonging to the technology field of biopharmaceuticals and molecular biology. After co-incubation of 293A cells and norathyriol for 24 hours, the expression level of uncoupling protein 2 (UCP2) coding gene in 293A cells is remarkably down-regulated. The invention discloses that the norathyriol can be used for inhibiting expression UCP2 gene in 293A cells and can be used for preparing drugs for preventing and treating type II diabetes; and proposes an in vitro test model for treating type II diabetes.
Owner:NANJING UNIV
Gene synthesis of wild boar alpha-interferon, vector construction and method for producing outcome
InactiveCN101392256AOptimize free energySimple structurePeptide/protein ingredientsAntiviralsEscherichia coliInclusion bodies
The invention discloses a gene synthesis of Alpha-interferon of wild boars and vector construction thereof as well as a production method of a product. A number of problems of low expression capacity, products expressed in a fusion state, products with purification tags or no fermentation technology with high density exist in the domestic recombinant strains. The invention includes a method that a codon and codon pairs, preferred by Escherichia coli, are used for synthesizing an Alpha-interferon gene of the wild boar, establishing a high-efficiency expression vector and transforming a high-efficiency expression strain as well as methods of high-density fermentation of engineering bacteria, separation and purification of inclusion bodies, the modification, the renaturation and the purification of target protein and the determination of biological activity of the expressed product. The gene synthesis, the vector construction and the production method pertain to the technical field of the production of polypeptide drugs by genetic engineering in biopharmaceuticals.
Owner:黑龙江省农业科学院畜牧研究中心 +1
Duchesnea polysaccharide, as well as preparation and use thereof
InactiveCN101486771ARich sourcesBreeding is easyOrganic active ingredientsAntiviralsMonomer compositionBiopharmaceutical
The invention relates to a preparation method of polysaccharide which is extracted from plants and usage thereof, belonging to the field of biopharmaceuticals. The preparation of polysaccharide comprises extracting total polysaccharide DIP which is extracted and separated from Duchesnea indica and has clear varicella-zoster virus activity resistance property, neutral polysaccharide neutral polysaccharide DIP1 and acidic polysaccharide DIP2 in total polysaccharide, and two major active monomer compositions polysaccharide DIP30 and polysaccharide DIP 60 in acidic polysaccharide DIP2. The raw material of Duchesnea indica has wide source range, easy regeneration, easily-operated preparation method, and high reproducibility. The polysaccharide shows significant inhibition on the varicella-zoster virus, is safe, non-toxic and stable, and is a high-quality anti-viral drug candidate.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com