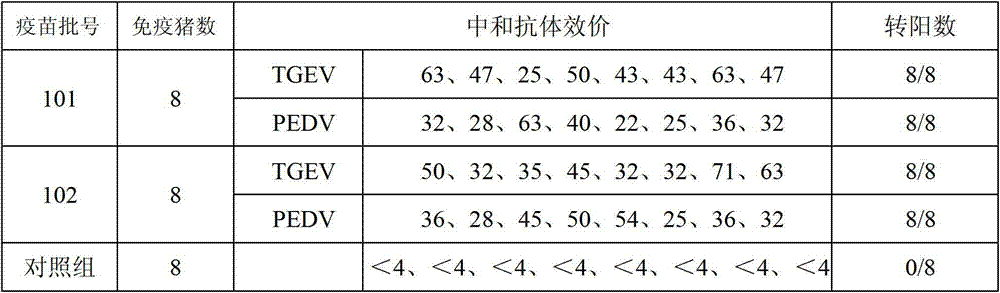
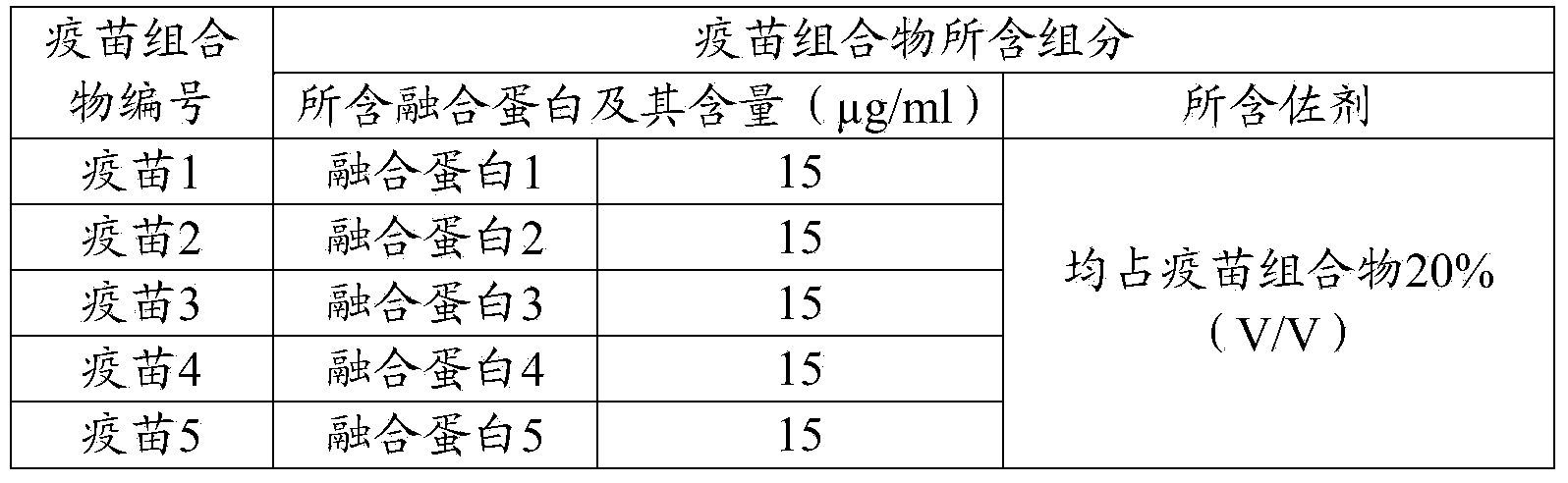
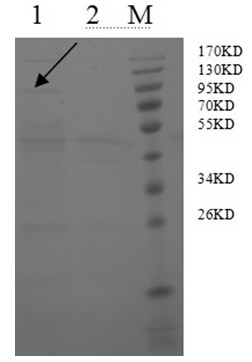
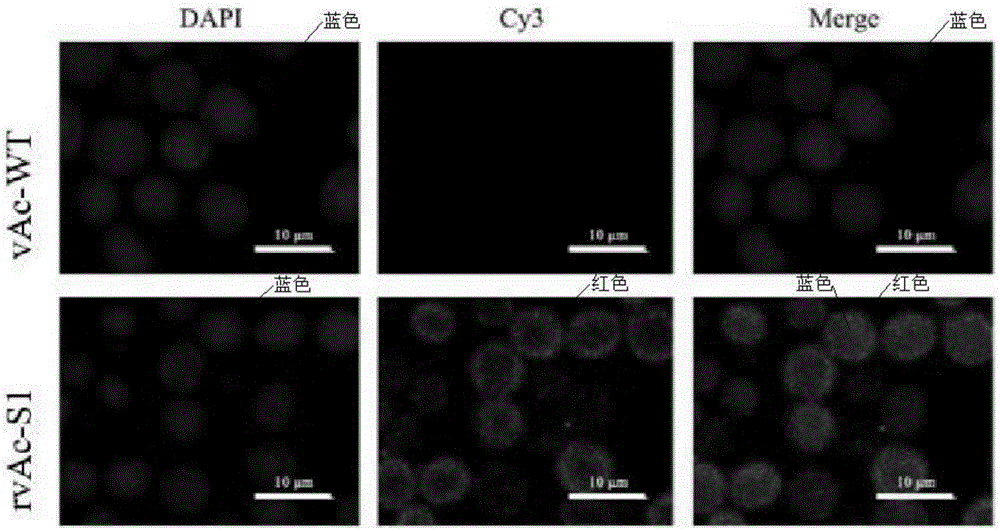
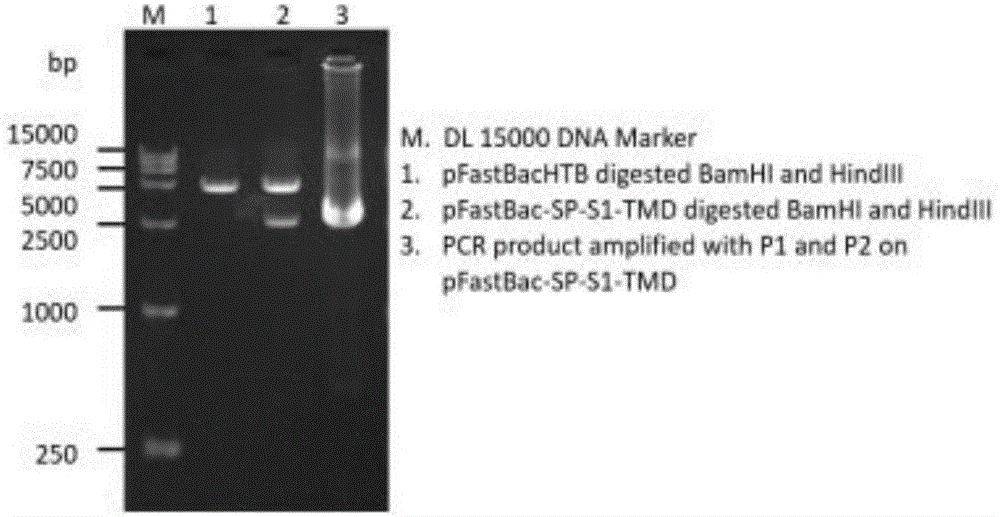
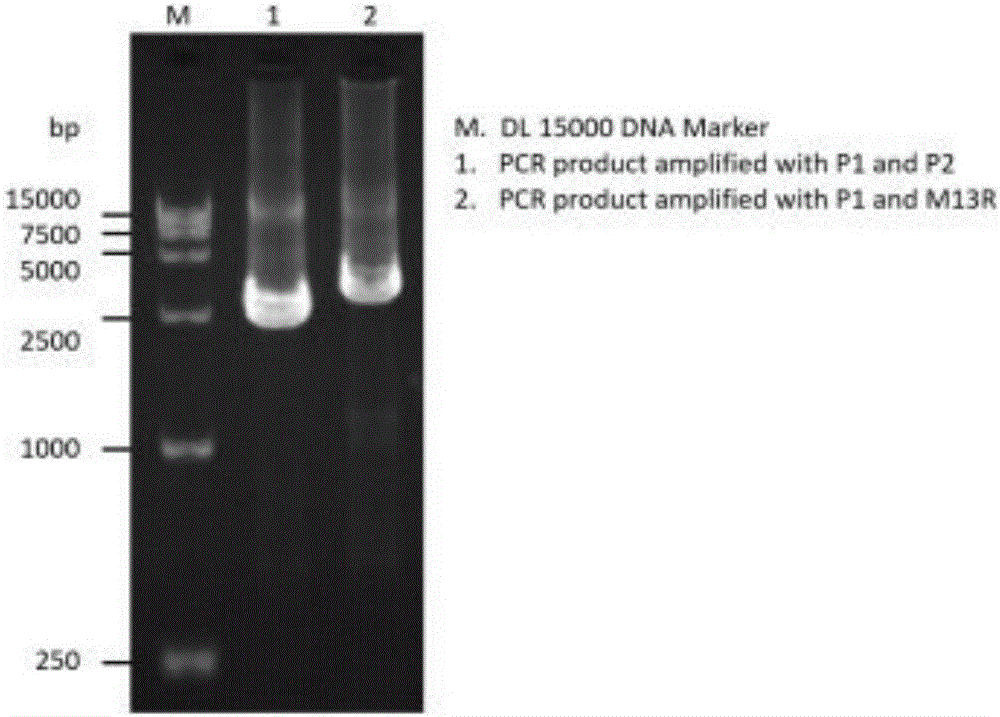
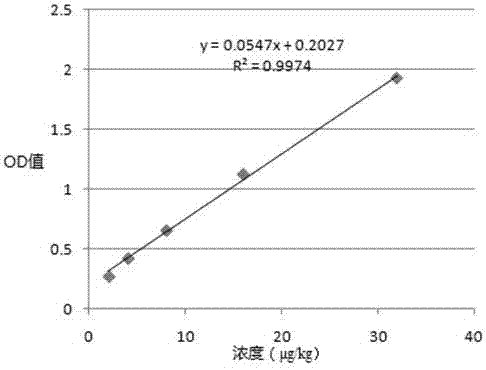
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
269 results about "Porcine epidemic diarrhoea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Porcine epidemic diarrhea is a condition caused by the porcine epidemic diarrhea virus that leads to severe gastrointestinal disease in pigs. It is closely related to the agent responsible for transmissible gastroenteritis in pigs. Piglets are most susceptible to the disease, as are young adults during periods of stress. Transmission is via the fecal-oral route.
Multi-fluorescence immunity analysis method for quickly distinguishing PEDV, TGEV and PoRV
ActiveCN105154589AAvoid crossbreedingGuaranteed temperatureMicrobiological testing/measurementDNA/RNA fragmentationImmune profilingSwine Transmissible Gastroenteritis
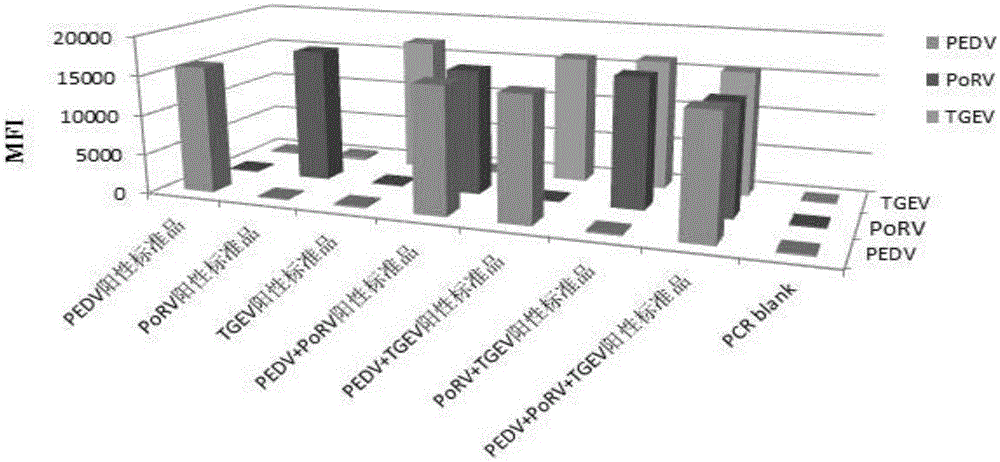
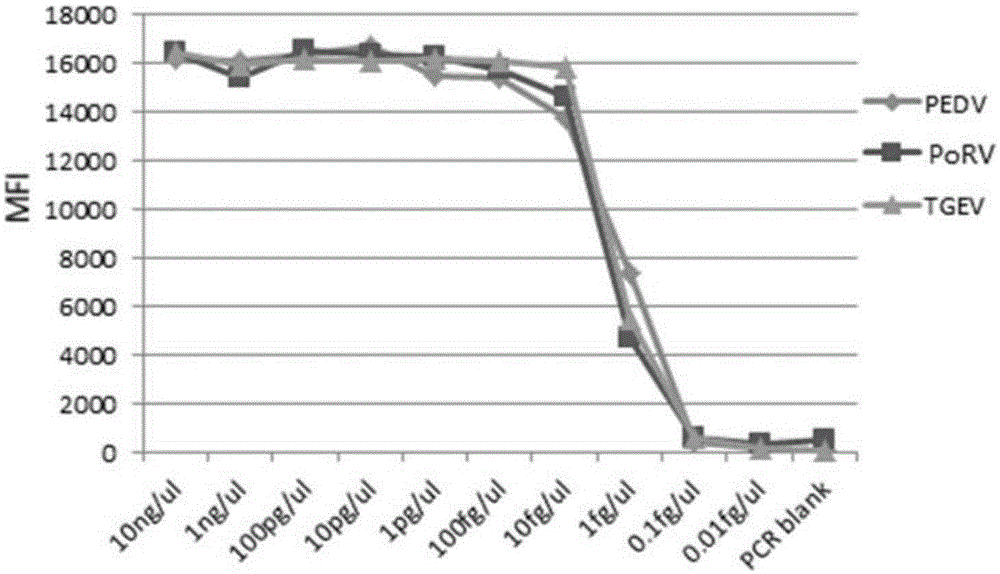
The invention discloses a multi-fluorescence immunity analysis method for quickly distinguishing PEDV, TGEV and PoRV. The method is easy to operate, a target amplified fragment is obtained through PCR, then hybridization is conducted on an amplified product, fluorescence coded microspheres and streptavidin-phycoerythrin, the MFi value is read through a detection instrument, and different types of viruses are distinguished. By means of the method, porcine epizootic diarrhea, swine transmissible gastroenteritis and pig group A rotavirus can be accurately detected at the same time, the specificity is high, the sensitivity is high, and the repeatability is good. Compared with a traditional detection method, various molecules of different purposes in the same sample are detected at the same time, the sample consumption is little, operation is simple and fast, and the detection cost can be greatly lowered.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
Preparation and application of fusion protein and vaccine composition thereof
ActiveCN104262488AShort timeEase of mass productionAntiviralsPharmaceutical non-active ingredientsDiseaseImmunoglobulin Fc Fragments
The invention provides a fusion protein, a porcine epidemic diarrhea vaccine composition containing the fusion protein and application thereof. The fusion protein contains porcine epidemic diarrhea virus antigenic protein and immunoglobulin Fc segment, wherein the porcine epidemic diarrhea virus antigenic protein contains a protein formed by series combination of porcine epidemic diarrhea virus S protein segments. The invention also provides a porcine epidemic virus vaccine composition which contains the fusion protein and a carrier. The invention also provides a preparation method of the vaccine composition and application of the vaccine composition in preparing drugs for preventing and / or treating diseases initiated by porcine epidemic diarrhea virus. The vaccine composition prepared from the fusion protein avoids the technical problem that the porcine epidemic diarrhea virus totivirus can not be easily separated and cultured in the traditional vaccine inactivation process. The fusion protein can utilize the gene engineering technique to perform abundant recombinant expressions, has the advantage of short time consumption, and is convenient for large-scale production.
Owner:PU LIKE BIO ENG
Porcine epidemic diarrhea virus stain and application thereof
ActiveCN103725651AImprove securityGood immune protectionMicroorganism based processesAntiviralsEpidemic diarrheaMicroorganism
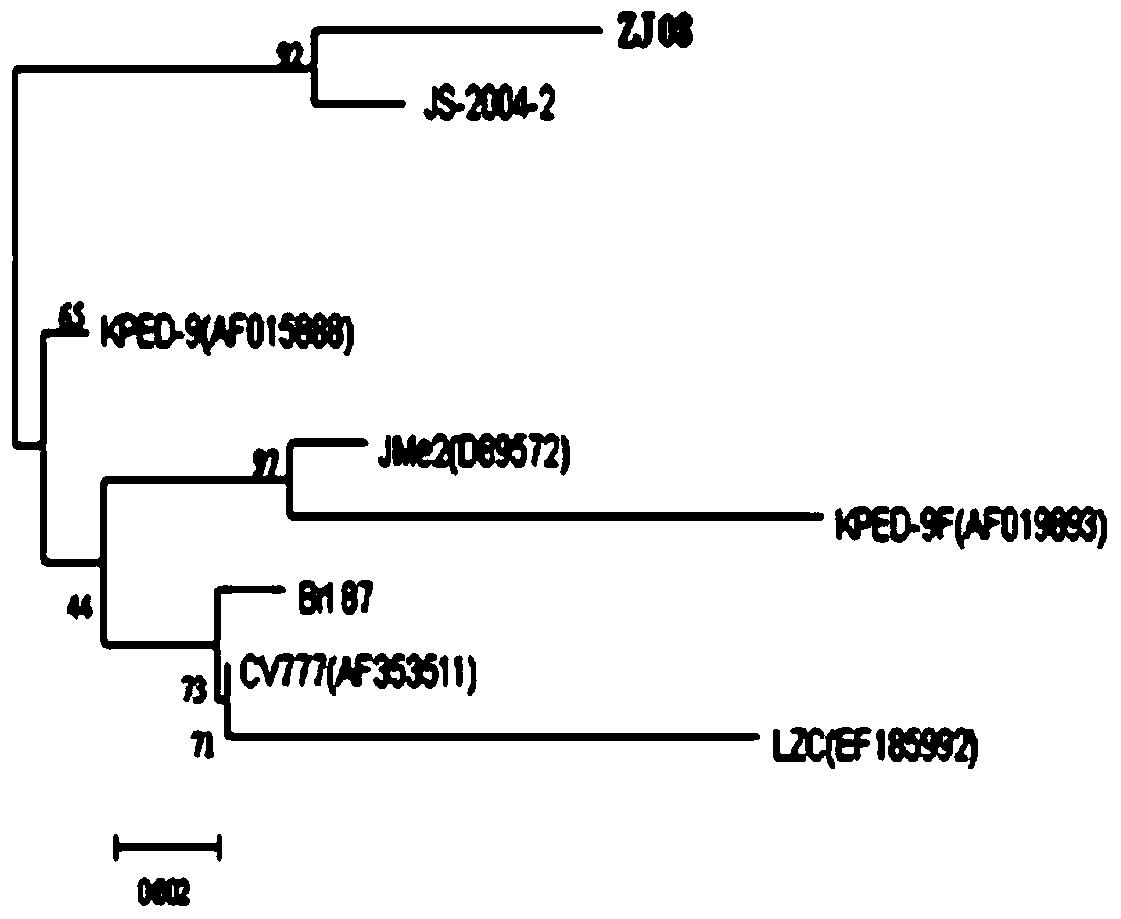
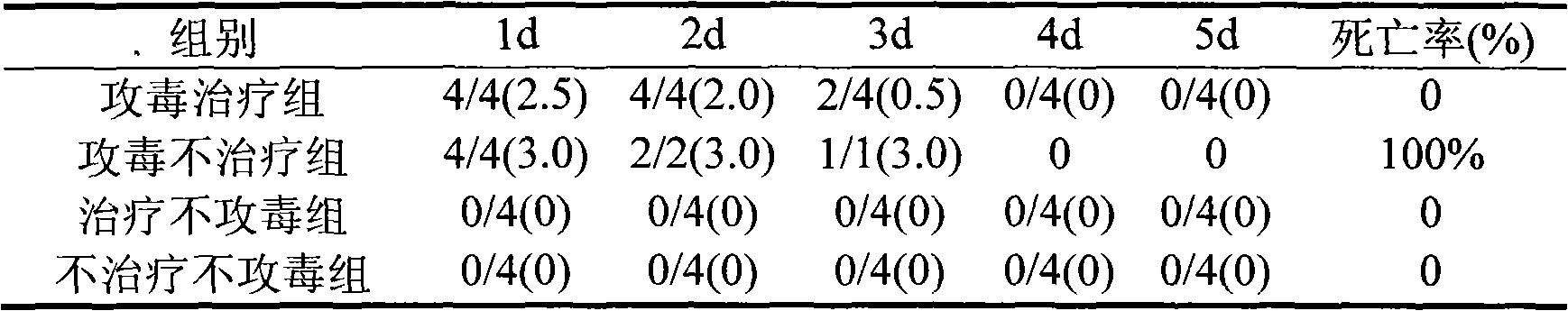
The invention discloses a porcine epidemic diarrhea virus low-virulent stain ZJ08 and application thereof. The microbial collection number of the low-virulent stain is CGMCC No.7806. The PEDV low-virulent stain has high safety, and is safe for pregnant sows, baby pigs and pigs in all ages. The active protection ratio of the low-virulent stain for 3-day baby pigs reaches 100%, and the passive protective ratio reaches higher than 94.7%, which indicates that the low-virulent stain has favorable immunoprotection effect on porcine epidemic diarrhea.
Owner:兆丰华生物科技(南京)有限公司 +3
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
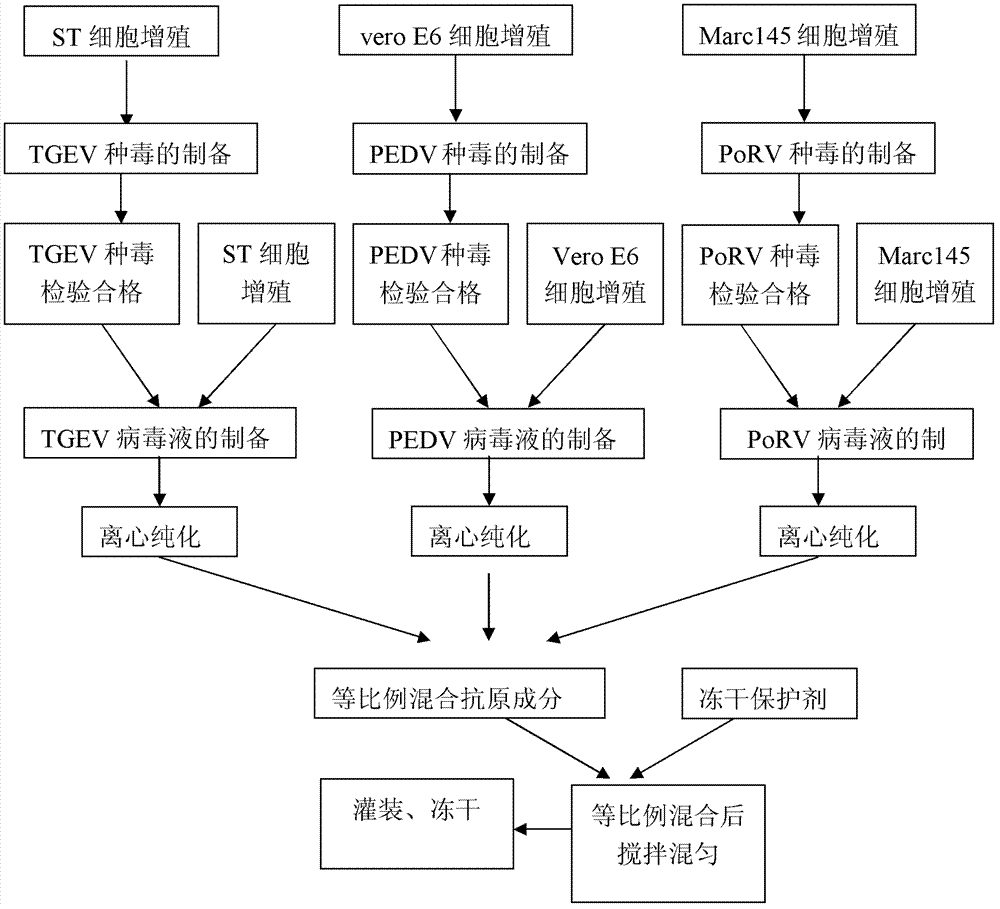
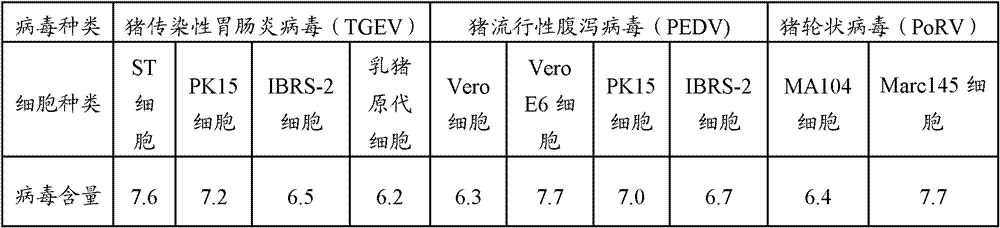
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Porcine epizootic diarrhea virus strain and vaccine composition, preparation method and application thereof
ActiveCN106148287AImprove immune efficiencyImprove securityDigestive systemAntiviralsBiologyDiarrhea
The invention discloses a porcine epizootic diarrhea virus strain with good immunogenicity and an inactivated vaccine prepared through the porcine epizootic diarrhea virus strain. The porcine epizootic diarrhea virus strain is a current epidemic strain, and good immunogenicity and stability are achieved; compared with a commercially available vaccine strain, the vaccine prepared through the strain has the advantages of being good in safety, high in immune protective capability, high in immune efficacy and the like, and porcine epizootic diarrhea can be comprehensively and effectively prevented and treated.
Owner:PU LIKE BIO ENG
Porcine epidemic diarrhea vaccine composition, and preparation method and application thereof
The invention provides a porcine epidemic diarrhea vaccine composition. The vaccine composition contains a porcine epidemic diarrhea virus subunit antigen and a vector. The invention also provides a porcine epidemic diarrhea vaccine composition, and the vaccine composition comprises a porcine epidemic diarrhea virus subunit antigen, a porcine epidemic diarrhea totivirus inactivated antigen and a vector. The invention also discloses a preparation method of the vaccine composition and application thereof to preparation of drugs for preventing and / or treating porcine epidemic diarrhea. An S1 protein in the vaccine composition can conduct large amount of recombinant expression of components in the vaccine composition by means of genetic engineering, so as to realize short time consumption as well as facilitate large-scale production. When the vaccine composition contains the S1 protein and porcine epidemic diarrhea totivirus inactivated antigen, the vaccine composition has better immune effect than single usage of totivirus inactivated antigen, and can effectively prevent diseases caused by variants of porcine epidemic diarrhea and common strains.
Owner:PU LIKE BIO ENG
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
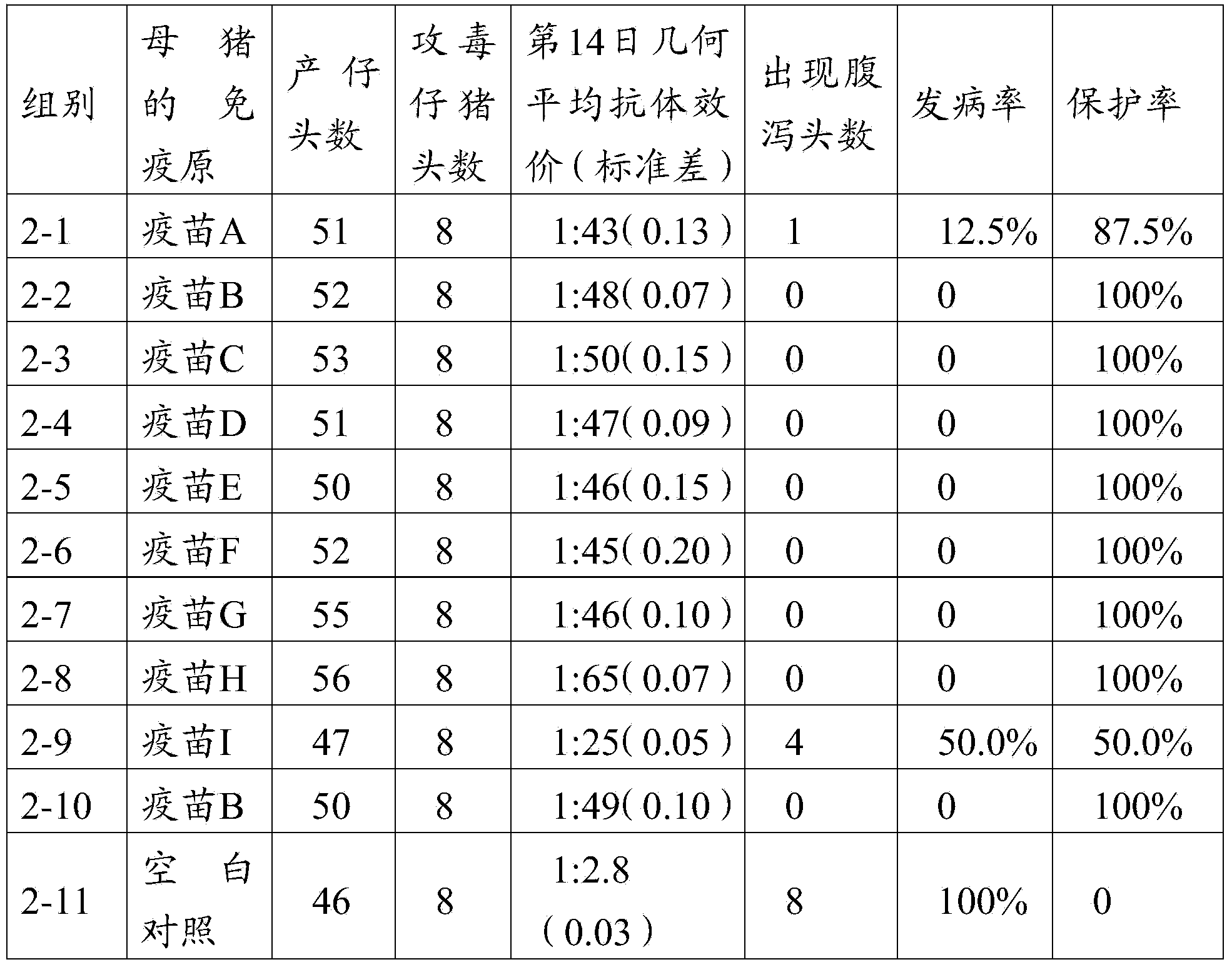
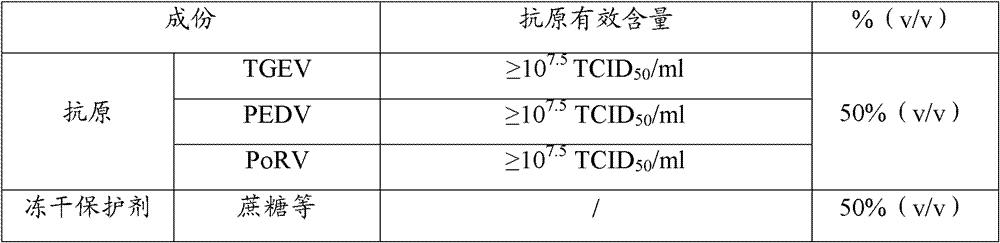
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Feed and traditional Chinese medicine for treating pigling epidemic diarrhea and preparation method thereof
InactiveCN102613412AImprove the immunityGood curative effectAnimal feeding stuffAccessory food factorsTherapeutic effectAristolochia debilis
The invention provides feed which comprises a puffing feed carrier, a micro-organism bacterial agent, a trace element additive, an amino acid additive and a traditional Chinese medicine additive, wherein the traditional Chinese medicine additive further comprises galangal, cumin, root of chinese barberry, herba lagotis, partrinia, phellodendron, astragalus, atractylodes, myrobalan, limonitum, dendrobium, liriope, hairy euphorbia, aristolochia debilis, coriolus versicolor, Kapok and schisandra chinensis. According to the invention, the traditional Chinese medicine composition has the efficacies that the treatment of epidemic diarrhea of pigling pays an attention to treating both principal and secondary aspect of epidemic diarrhea; the treatment effect is good; drug residue does not exist, functions of an organism can be aroused, the resistance of the organism is improved.
Owner:HAIMEN XINGWANG MEAT PROD
Anti-swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus egg-yolk antibody and preparation method thereof
ActiveCN104788561ANo growthGood physical propertiesEgg immunoglobulinsImmunoglobulins against virusesSwine Transmissible GastroenteritisArtificial infection
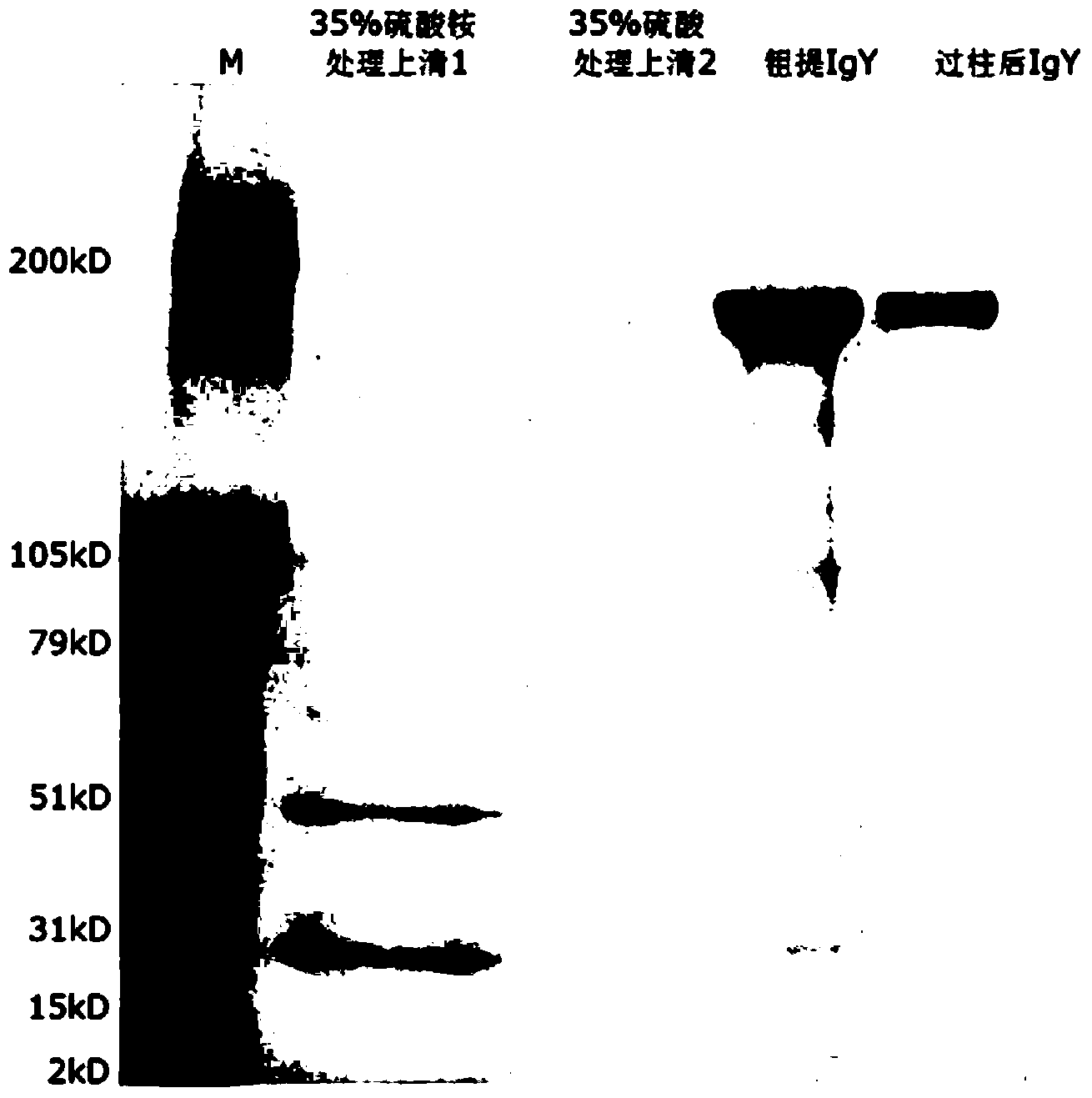
The invention discloses an anti-swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus egg-yolk antibody. Swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus combined inactivated vaccine is used as an immunogen to immune laying hens, and an egg-yolk antibody is purified from egg yolk, wherein the swine transmissible gastroenteritis virus is swine transmissible gastroenteritis virus HB08 with the collection number being CGMCC No.7807; and the porcine epidemic diarrhea virus is porcine epidemic diarrhea virus ZJ08 with the collection number being CGMCC No.7806. The prepared egg-yolk antibody has good and safe traits. Artificial infection cure rate of the prepared egg-yolk antibody reaches 100% and is obviously higher than cure rate of an egg-yolk antibody prepared from classical IBDV. Clinical case cure rate of the prepared egg-yolk antibody reaches 93.0%. In clinical preventive tests, incidence of diarrhea can be reduced by 17-24% for tested pigs.
Owner:兆丰华生物科技(南京)有限公司 +3
Traditional Chinese medical composition for treating porcine epizootic diarrhea and application thereof
ActiveCN103230574AAlleviate the fiercenessCoordinated liftDigestive systemAntiviralsSide effectOfficinalis
The invention discloses a traditional Chinese medical composition for treating porcine epizootic diarrhea. The composition comprises the following components in parts by weight as follows; 80+ / -5 parts of honeysuckle, 150+ / -10 parts of Agastache rugosus, 80+ / -5 parts of Codonopsis pilosula, 80+ / -5 parts of Rhizoma Atractylodis Macrocephalae, 10+ / -5 parts of Mangnolia officinalis, 100+ / -5 parts of Pinellia ternate, 80+ / -5 parts of Poria cocos, 50+ / -5 parts of Poria cocos and 80+ / -5 parts of liquorice. The traditional Chinese medical composition disclosed by the invention has the advantages of high integral curative effect, difficulty in generation of tolerance, low residue, small toxic and side effects and the like, has the remarkable inhibiting effect to growth and propagation of many virus pathogenies, even has the pharmacological effect of killing pathogenies, and has very important economic and social meanings for promoting healthy development of pig industry.
Owner:四川省羌山生物科技股份有限公司
ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for porcine epidemic diarrhea viruses and preparation method thereof
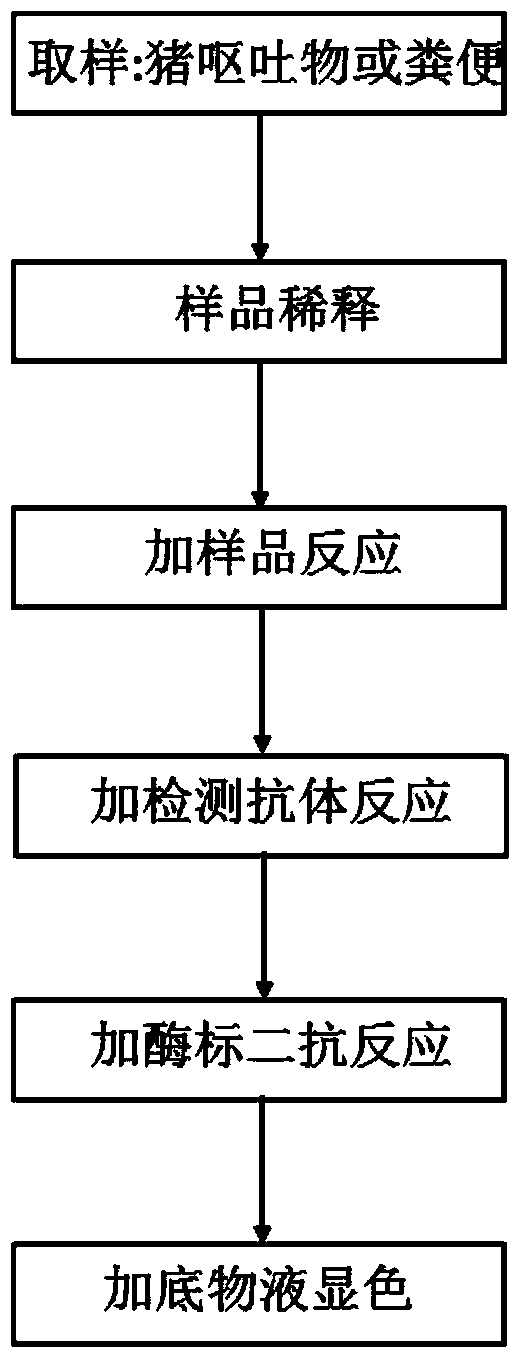
The invention relates to an ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for a porcine epidemic diarrhea virus (PEDV) and a preparation method of the ELISA detection kit. The ELISA detection kit for the porcine epidemic diarrhea virus comprises an ELISA plate, a detection antibody and an ELISA second antibody, wherein the ELISA plate is coated with a specific egg yolk antibody of the porcine epidemic diarrhea virus; the detection antibody is a polyclonal antibody of the porcine epidemic diarrhea virus; the ELISA second antibody is an antibody which is labeled by an enzyme and resists the detection antibody. The ELISA detection kit for the porcine epidemic diarrhea virus takes the PEDV of vomitus and excrement of diarrhea pigs as a detection object and can find PEDV infected pigs as soon as possible; meanwhile, the operation difficulty of the detection is reduced.
Owner:SHENZHEN BROSTIGER BIO PHARMA
Recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, vaccine and application
InactiveCN107619819AEase of mass productionGood antigenicityAntiviralsAntibody medical ingredientsEpidemic diarrheaAdjuvant
The invention discloses a recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, a vaccine and an application. The recombinant cell line is constructed by transfecting HEK-293T cells by virtue of recombinant plasmid which is constructed by carrying a target gene on a lentiviral vector, and then transfecting the HEK-293T cells by virtue of generated high-titer virus particles; and the recombinant cell line, which can achieve stable expression, can still keep an excellent protein expression level after several passages. The recombinant cell line for stable expression of the porcine epidemic diarrhea virus S1 protein provided by the invention has the characteristics of being easy for culture, rapid in proliferation, unlimited in expansion, stable in property and high in protein expression amount; and when the vaccine, which is prepared from the expression protein and adjuvants, is used for immunizing pigs, the generation of a high-titer porcine epidemicdiarrhea virus neutralizing antibody can be induced from animal bodies, and the piglets (the pigs) can resist strong attack of porcine epidemic diarrhea viruses.
Owner:GUANGZHOU BONIZZI BIOTECH CO LTD
PED (Porcine Epedemic Diarrhea) inactivated vaccine and preparation method thereof
ActiveCN104383528AEnhance immune responseImprove the level ofOrganic active ingredientsDipeptide ingredientsAntiendomysial antibodiesDipeptide
The invention provides a PED (Porcine Epedemic Diarrhea) inactivated vaccine and a preparation method thereof and relates to the field of biopharmacy. The PED inactivated vaccine comprises inactivated PEDV (Porcine Epedemic Diarrhea Virus), and is characterized in that the PED inactivated vaccine comprises 0.05-10 mg / mL Beta-glucosylceramide, 0.1-21 mg / mL monophosphoryl lipid A, 1.5-125 mg / mL muramyl dipeptide and 0.7-4.5 mg / mL Beta-glucan. According to the ingredients of the PED inactivated vaccine, Beta-glucosylceramide, monophosphoryl phosphoryl lipid A, muramyl dipeptide and Beta-glucan have the synergistic effect, the immune response of animals to antigens in the vaccine is significantly improved, the immune window phase is shortened, the antibody production duration of the animal body is obviously prolonged, the serum antibody level is improved, and the level of total intestinal mucosa secretory antibodies (the total SIgA) is improved.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
TGEV and PEDV combined live vaccine and preparation method thereof
The invention discloses a combined live vaccine of transmissible gastroenteritis virus of swine (TGEV) and porcine epidemic diarrhea virus (PEDV) and a preparation method thereof. An attenuated swine transmissible gastroenteritis virus HB08 and an attenuated porcine epidemic diarrhea virus ZJ08 which are self-separated, attenuated and stored respectively undergo viral multiplication on ST cells and VeroE6 cells, and seedling and freeze drying are then carried out by adding a freeze-drying protective additive into a virus solution which is qualified after inspected. The two diseases, transmissible gastroenteritis of swine and porcine epidemic diarrhea virus, which are epidemic in clinic at present, can be effectively prevented by the use of the combined live vaccine.
Owner:兆丰华生物科技(南京)有限公司 +3
Porcine epidemic diarrhea virus resistant hyper-immune serum and preparation method thereof
ActiveCN103705918AResist attackStable virulenceAntiviralsAntibody medical ingredientsSerum igeCure rate
The invention discloses hyper-immune serum prepared by immunizing a hybridized pig with the age of 50-60 days by taking an inactivated vaccine of porcine epidemic diarrhea virus ZJ08 strain CGMCC No.7806 as an immunogen due to immunization for three times. The prepared hyper-immune serum is high in properties, high in specificity and high in safety and is not polluted by bacteria, moulds, mycoplasma and exogenous viruses, and the artificial infection cure rate is 100 percent. The neutralization titer of the serum is over 26, the serum is separately filled at the temperature of minus 70 DEG C, and the retention period is 12 months.
Owner:兆丰华生物科技(南京)有限公司 +3
Chinese herb extract feed additive capable of effectively preventing and treating porcine epizootic diarrhea
ActiveCN103141702AImprove immunityPromote growthAntibacterial agentsBacteria material medical ingredientsFood additiveBiotechnology
The invention discloses a Chinese herb extract feed additive capable of effectively preventing and treating porcine epizootic diarrhea, and belongs to the field of feed additives. The feed additive is prepared from the main materials of snow grass meal, curcuma powder, semen cassiae powder, burdock powder, shallot white powder, Chinese herbal medicine preparation and curcuma extract, and toadstool and bacillus subtilis are scientifically compounded, so that the application amount of antibiotics medicine in cultivation of pigs is effectively reduced, the use amount of the antibiotic is reduced by above 60%, the drug property of the Chinese herbal medicine is improved, the porcine epizootic diarrhea caused by porcine epidemic diarrhea virus can be effectively prevented and treated, and the Chinese herb extract feed additive is safe and non-toxic. Besides, the amount of the additive in the feed is low, the quality is stable, an acting effect is improved by combining different Chinese herb extracts, the porcine epizootic diarrhea is reduced by 60-80%, and the taste and quality of animal products are obviously improved. A sense experimental evaluation proves that the evaluation score on the taste and quality of the animal products using the additive is improved by above 65%.
Owner:兴安双胞胎饲料有限公司
Porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application
InactiveCN102399806AReduce colonizationPromote accumulationBacteriaGenetic material ingredientsBacillus megateriumCell wall
The invention discloses a porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application. An antigen fusion gene shown as SEQ ID No. 1. is obtained by connecting an antigen locus of porcine epidemic diarrhea virus (PEDV) S glycoprotein and a cell wall anchoring sequence. The invention further constructs a secretion expression vector containing the antigen fusion gene, and secretion expression vector is converted into the bacillus megaterium to express recombinant protein on the cell wall or surface of the bacillus megaterium. Immunoblotting experiments indicate that the expressed recombinant protein can react with PEDV immune serum and has the same antigenicity as PEDV natural antigens. Immunofluorescent tests of live bacteria which are subjected to induced expression indicate that the expressed recombinant protein is positioned to the surfaces of the bacteria. Experiment results indicate that the recombinant protein can be prepared into safe and effective mucous immune live vaccines for preventing and treating porcine epidemic diarrhea.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Porcine epidemic diarrhea virus attenuated strain, vaccine composition prepared therefrom, and application
The invention relates to a porcine epidemic diarrhea virus attenuated strain. The porcine epidemic diarrhea virus attenuated strain is a nucleotide fragment or termination translator in a porcine epidemic diarrhea virus S gene encoding sequence, for encoding last nine amino acids EAFEKVHVQ or a homologous fragment thereof. The porcine epidemic diarrhea virus attenuated strain is a porcine epidemic diarrhea virus epidemic strain attenuated strain, and has the advantages of substantially reduced pig pathogenicity, no return after pig immunization, good immunogenicity and realization of effective resistance of immunized pigs to virulent attack. The invention also relates to a vaccine composition obtained by adopting the porcine epidemic diarrhea virus attenuated strain as a live virus antigen, a porcine epidemic diarrhea virus attenuated strain mutation S protein, and a preparation method of the porcine epidemic diarrhea virus attenuated strain.
Owner:PU LIKE BIO ENG
Preparation method of porcine epidemic diarrhea recombinant adenovirus vaccine
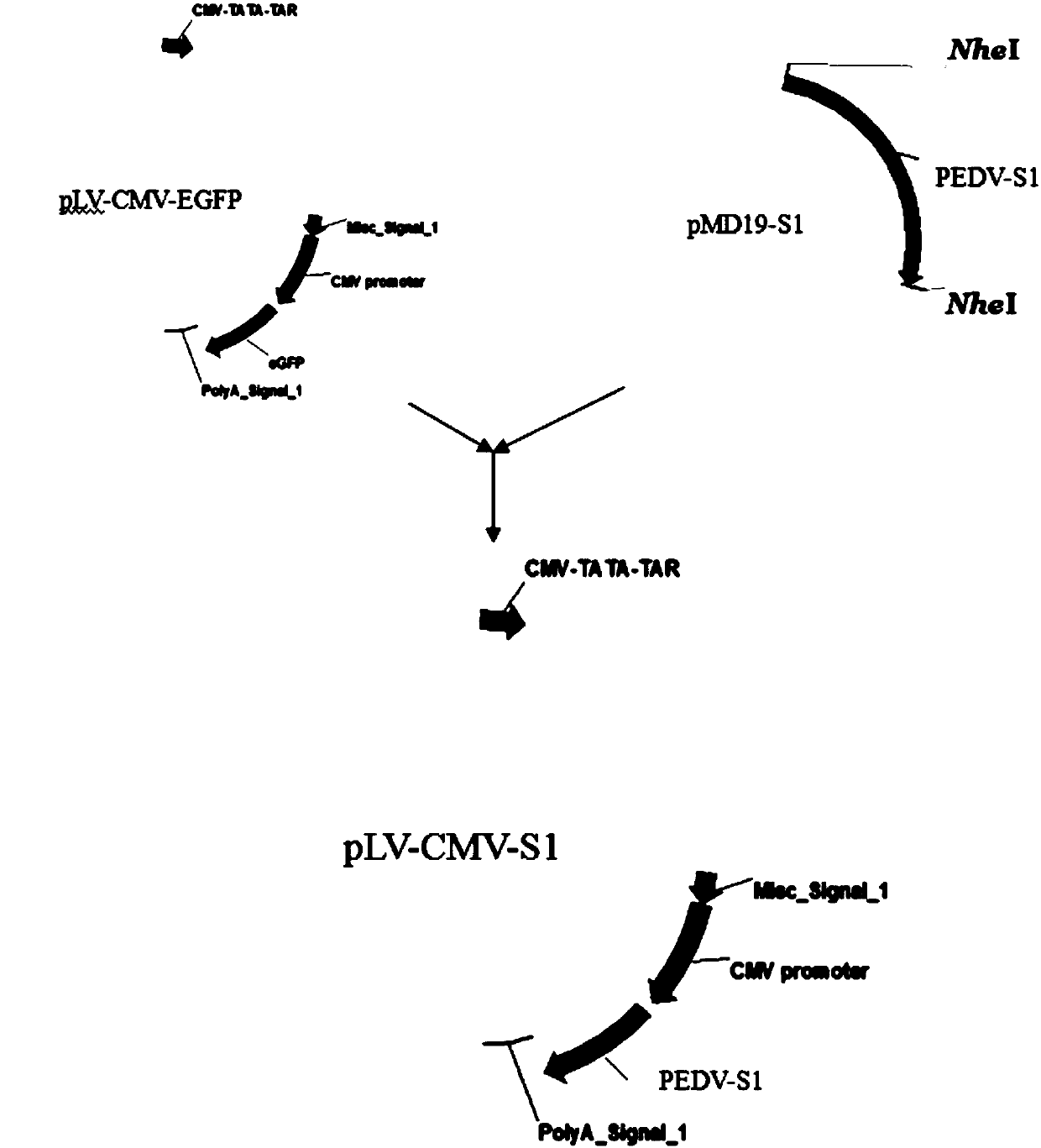
InactiveCN102512693AImprove abilitiesThe production is effectiveGenetic material ingredientsAntiviralsEnzyme digestionA-DNA
The invention discloses a preparation method of a porcine epidemic diarrhea recombinant adenovirus vaccine. The preparation method provided by the invention comprises the following steps of inserting a DNA sequence of a zone S1 of a porcine epidemic diarrhea virus (PEDV) into an adenovirus shuttle plasmid pShuttle-CMV to obtain pShuttle-CMV-S1, carrying out linearization of the pShuttle-CMV-S1, transforming the linear pShuttle-CMV-S1 into a BJ5183 competent cell containing pAdEasy-1, carrying out homologous recombination, carrying out enzyme digestion, carrying out AD-293 cell transfection, carrying out packaging to obtain a recombinant adenovirus rAd-S1, and carrying out purification, amplification and sub-packaging. After oral immunization, the porcine epidemic diarrhea recombinant adenovirus vaccine can induce generation of mucosal immunity thereby preventing porcine epidemic diarrhea (PED) well.
Owner:GENIFARM LAB INC
Recombinant baculovirus with surface displaying porcine epidemic diarrhea virus S protein
InactiveCN106085969AImprove Surface DisplayHigh expressionSsRNA viruses positive-senseViral antigen ingredientsSurface displayViral Vaccine
The invention provides a recombinant baculovirus with the surface displaying a porcine epidemic diarrhea virus S protein, and a preparation method thereof. The virus adopts PEDV spike protein S1 gene as antigen gene, the recombinant virus is constructed by using an insect baculovirus vector expression system, and an S1 protein is successfully expressed and displayed on the surface of the virus. The recombinant virus is used to immunize and inoculate mice as a PEDV pseudo-virus vaccine, and serum neutralization test and lymphocyte propagation experiment analysis shows that the recombinant virus can arouse an effective immune protection effect.
Owner:杭州洪晟生物技术股份有限公司
Method for preparing double yolk antibody of porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus
InactiveCN101845095AStrong specificityStrong targetingEgg immunoglobulinsImmunoglobulins against virusesPig farmsYolk
The invention discloses a method for preparing a double yolk antibody of a porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The method comprises the following steps of: performing porcine transmissible gastroenteritis virus multiplication on porcine kidney cells (PK15); performing porcine epidemic diarrhea virus multiplication on African green monkey kidney cells (Vero); emulsifying the two cell cultures used as antigen with an oil emulsion adjuvant to prepare immunogen, namely, mixing the two kinds of viruses in a ratio of (1-3):(1-3) to prepare the immunogen; immunizing non-immunologic laying hens; and obtaining the double yolk antibody which can prevent and treat porcine transmissible gastroenteritis and porcine epidemic diarrhea based on the collection and purification of the yolk. When the double yolk antibody is used for curing experimental pigs, the clinical symptoms in the experiment are obviously reduced compared with a control group, and the death rate of the experimental group is obviously lower than that of the control group. The double yolk antibody has obvious preventing and treating functions when applied in a pig farm with high incidence rate of the porcine transmissible gastroenteritis and the porcine epidemic diarrhea.
Owner:PU LIKE BIO ENG
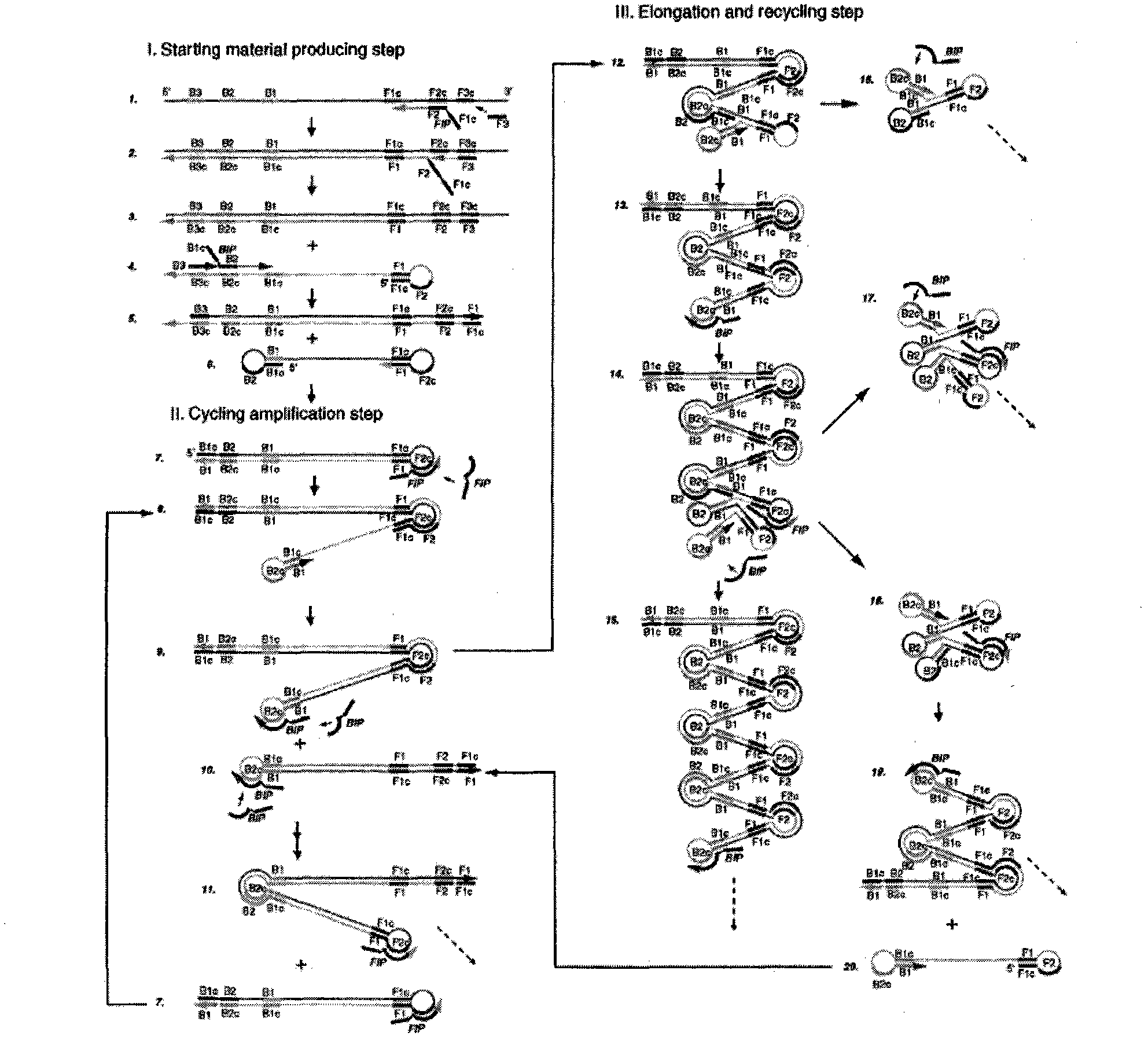
Method for detecting swine epidemic diarrhea by reverse transcription-loop-mediated isothermal amplification
InactiveCN102021249AQuick checkSensitive detectionMicrobiological testing/measurementReverse transcriptaseBiology
The invention provides a method for detecting swine epidemic diarrhea by reverse transcription-loop-mediated isothermal amplification. According to the conserved domain gene of PEDV (Porcine Epidemic Diarrhea Virus) N protein, three pairs of primers aiming at six areas on a target gene are designed, two RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primers are combined, and PED (Porcine Epidemic Diarrhea) viruses are identified by the RT-LAMP (Reverse Transcription-Loop-Mediated Isothermal Amplification) technology; RT-LAMP reacts; reverse transcription is performed on obtained RNA(Ribose Nucleic Acid); and the obtained cDNA is used for PCR reaction. The method provided by the invention ensures amplification specificity, has high amplification speed and can obtain a result within 30-60 minutes, and people can observe amplification effect with eyes without electrophoresis. Reverse transcription and nucleic acid amplification can be realized in 1 hour at the constant temperature of 65 DEG C by using enzyme mixture; RT-LAMP detection is carried out on the basis of LAMD amplification DNA; a reverse transcription enzyme is added to realize amplified detection of RNA; and reverse transcription and amplification are finished in one step so as to omit the reverse transcription step which is firstly carried out by the traditional RT-PCR.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Porcine epidemic diarrhea virus strain MY01 and application thereof
InactiveCN105462936AEpidemic preventionAvoid spreadingSsRNA viruses positive-senseViral antigen ingredientsDiseaseMicroorganism
The invention relates to a porcine epidemic diarrhea virus strain MY01 and application thereof and belongs to the field of porcine epidemic diarrhea virus vaccine reagents. The porcine epidemic diarrhea virus strain MY01 is collected in China General Microbiological Culture Collection Center under CGMCC No. 11495. The porcine epidemic diarrhea virus strain MY01 is higher in pathogenicity for pigs and good in immunogenicity, a deactivated oil emulsion vaccine prepared using the strain is safe and reliable, homologous attacking protection can be provided, protection is also provided for PEDV (porcine epidemic diarrhea virus) epidemic strains, higher immunity can be generated after immunization, inoculated pigs are significantly lower in morbidity and mortality, the immunization effect is superior than that of existing commercial vaccines in the market, and the strain has the advantage of competing with like products home and abroad, can effectively prevent the prevalence and spread of porcine epidemic diarrhea virus and reduce economic cost caused by the disease and has a promising application prospect.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Fusion protein and application thereof
ActiveCN107098974AAvoid separabilityAvoid technical difficulties of cultivationSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpidemic diarrheaNanoparticle
The invention provides fusion protein, which comprises monomeric ferritin subunit protein connected to porcine epidemic diarrhea virus antigenic protein, which includes porcine epidemic diarrhea virus S protein and / or fragments thereof, and further includes S1 protein, M protein, N protein and / or fragments thereof. The invention further provides nanoparticles containing the fusion protein. The invention further provides a vaccine composition containing the nanoparticles and / or a carrier. The invention further discloses a preparation method of the vaccine composition and an application of the vaccine composition to prepare a medicine for preventing and / or treating porcine epidemic diarrhea virus. The vaccine composition made from the nanoparticles overcomes the technical problem that whole viruses are hard to separate and culture in a process of manufacturing a conventional inactivation vaccine from porcine epidemic diarrhea whole viruses. The nanoparticles can be subjected to massive recombinant expression via genetic engineering technology. The time consumption is short, and the fusion protein is convenient for massive production.
Owner:PU LIKE BIO ENG
Double-antibody sandwich ELISA quantitative determination kit of porcine epidemic diarrhea virus and application
ActiveCN107099506AStrong responsivenessStrong specificityBiological material analysisMicroorganism based processesSerum igeProtein.monoclonal
The invention provides a double-antibody sandwich ELISA quantitative determination kit of a porcine epidemic diarrhea virus and an application and relates to the field of biodetection. A preservation number of a hybridoma cell strain PEDV of an anti-PEDV N protein monoclonal antibody is CCTCC NO: C201410. The double-antibody sandwich ELISA quantitative determination kit of the porcine epidemic diarrhea virus comprises a pre-coated elisa plate and an enzyme labeled antibody. The pre-coated elisa plate is the elisa plate coated by the anti-PEDV N protein monoclonal antibody, and the enzyme labeled antibody is a horse radish peroxidase labeled anti PEDV N protein polyclonal antibody; the anti PEDV N protein polyclonal antibody is obtained by immunizing a rabbit through the protein N of the porcine epidemic diarrhea virus to obtain serum of the rabbit. The kit provided by the invention is good in specificity, high in sensitivity and good in stability, can quickly, simply and efficiently detect the porcine epidemic diarrhea virus quantitatively, and is low in cost and suitable for high throughput detection.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Porcine epidemic diarrhea virus attenuated vaccine strain and its culture method and use
InactiveCN106591245AEfficient ProliferationNo pollution in the processSsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaDisease
The invention discloses a porcine epidemic diarrhea virus attenuated vaccine strain. The porcine epidemic diarrhea virus attenuated vaccine strain is an attenuated vaccine obtained by PEDV / CH / NB / 2014 passage attenuation and has an accession number of CGMCC No. 13299. The invention also discloses a use of the attenuated vaccine strain NB120 in prevention of porcine epidemic diarrhea diseases and the use belongs to the field of biotechnology. The attenuated vaccine strain NB120 can be processed to form a single vaccine or a multi-vaccine and the vaccine can effectively prevent porcine epidemic diarrhea diseases. The attenuated vaccine strain NB120 has the advantages of good safety and good immunogenicity. After applying to a sow or piglet, the vaccine strain can produce protective immune response to a prevalent strain of porcine epidemic diarrhea virus and thus the vaccine strain can effectively prevent infection caused by the prevalent strain of porcine epidemic diarrhea virus, can reduce a disease loss and has a good application prospect.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD
Genetic engineering live vaccine of recombinant Salmonella choleraesuis and Porcine epidemic diarrhea virus, preparation and application
ActiveCN103013895APreserve immune efficiencyHigh biosecurityAntibacterial agentsBacteriaBacteroidesBacterial genetics
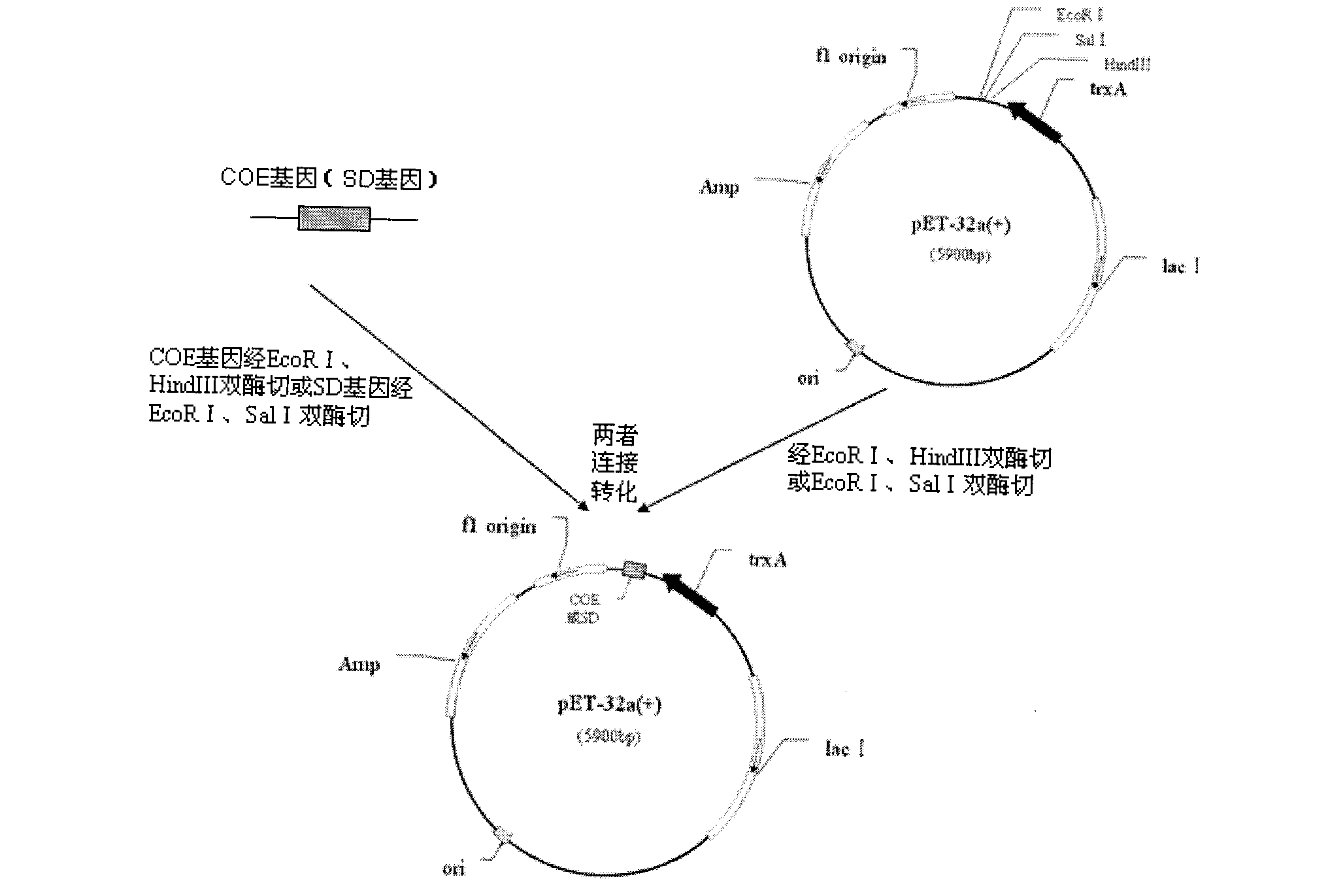
The invention belongs to the technical field of animal bacterial genetic engineering, and concretely relates to a construction of recombinant Salmonella choleraesuis strains C501-Coe and C501-SD with no resistance marker and expressing main antigenic sites of porcine epidemic diarrhea virus, a preparation of a vaccine and an application. The invention obtains the recombinant Salmonella choleraesuis strains C501-Coe and C501-SD with no resistance marker and expressing the main antigenic sites of the porcine epidemic diarrhea virus, and the strains have the following accession number respectively: CCTCC NO: M2011296 and CCTCC NO: M2011297. The two recombinant strains are deleted with an asd gene necessary for growth of the S. choleraesuis, and contain plasmids which can express the asd gene, as well as a COE gene fragment and a SD gene fragment of the Porcine epidemic diarrhea virus in the strains. <{EN3}>The invention further discloses a method and an application by using the recombinant strain to prepare the vaccine of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. <{EN4}>The vaccine provided by the invention can stimulate swine to generate a protective immunization reaction for resisting the Salmonella choleraesuis and the Porcine epidemic diarrhea virus, and can effectively prevent infection of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. The invention further discloses a method and an application by using the recombinant strain to prepare the vaccine of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus. The vaccine provided by the invention can stimulate swine to generate a protective immunization reaction for resisting the Salmonella choleraesuis and the Porcine epidemic diarrhea virus, and can effectively prevent infection of the Salmonella choleraesuis and the Porcine epidemic diarrhea virus.
Owner:HUAZHONG AGRI UNIV +1
Egg yolk antibody for preventing and treating porcine epidemic diarrhea and preparation method of egg yolk antibody
ActiveCN107177001AResist attackImproving immunogenicityEgg immunoglobulinsImmunoglobulins against virusesEpidemic diarrheaAnimal science
The invention discloses an egg yolk antibody for preventing and treating porcine epidemic diarrhea and a preparation method of the egg yolk antibody. The preparation method comprises the following steps: using an inactivated vaccine of a porcine epidemic diarrhea virus as immunogen for immunizing a laying hen; harvesting egg yolk liquid and purifying from the egg yolk liquid to obtain the egg yolk antibody, wherein a porcine epidemic diarrhea virus strain is PEDV / CH / 2014, with a preservation number of CGMCC (China General Microbiological Culture Collection Center) No. 10111. The egg yolk antibody prepared by the preparation method disclosed by the invention has the advantages of good characters, easiness in storage, high safety and good preventing and treating effects; by an in vitro neutralization test, the neutralizing titer of the egg yolk antibody is measured to reach 1:128; by means of a clinical prevention test, the diarrhea incidence of tested pigs can be reduced by 20 percent, and the cure rate of artificial infection reaches 100 percent.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com