Porcine epidemic diarrhea vaccine composition, and preparation method and application thereof
A porcine epidemic diarrhea and vaccine composition technology, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as side effects, insufficient antigen content, and increased miscellaneous protein content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation, Identification and Content Determination of Porcine Epidemic Diarrhea Virus S1 Protein
[0070] 1.1 Preparation, identification and content determination of porcine epidemic diarrhea virus S1a protein
[0071] 1.1.1 Construction of recombinant vector Bacmid-S1a
[0072] According to the nucleotide sequence of porcine epidemic diarrhea virus S1 protein (see the sequence table SEQ ID No.1), it is synthesized by Shanghai Bioengineering Co., Ltd. by artificial synthesis, and the synthesized gene fragment has a full length of 2367bp. The template of S1 protein was prepared on the basis of the artificially synthesized S1 gene fragment.
[0073] The S1 full-length gene fragment synthesized in the previous step was used as a template for the PCR reaction, a pair of specific primers were designed, and XhoI and PstI restriction sites were added in the upstream and downstream respectively. The primer sequences are as follows:
[0074] S1a-P1: 5'GCTCTCGAGAT...
Embodiment 3
[0102] Example 3 Preparation of Porcine Epidemic Diarrhea Virus Vaccine Composition
[0103] 3.1 Preparation of porcine epidemic diarrhea virus subunit antigen
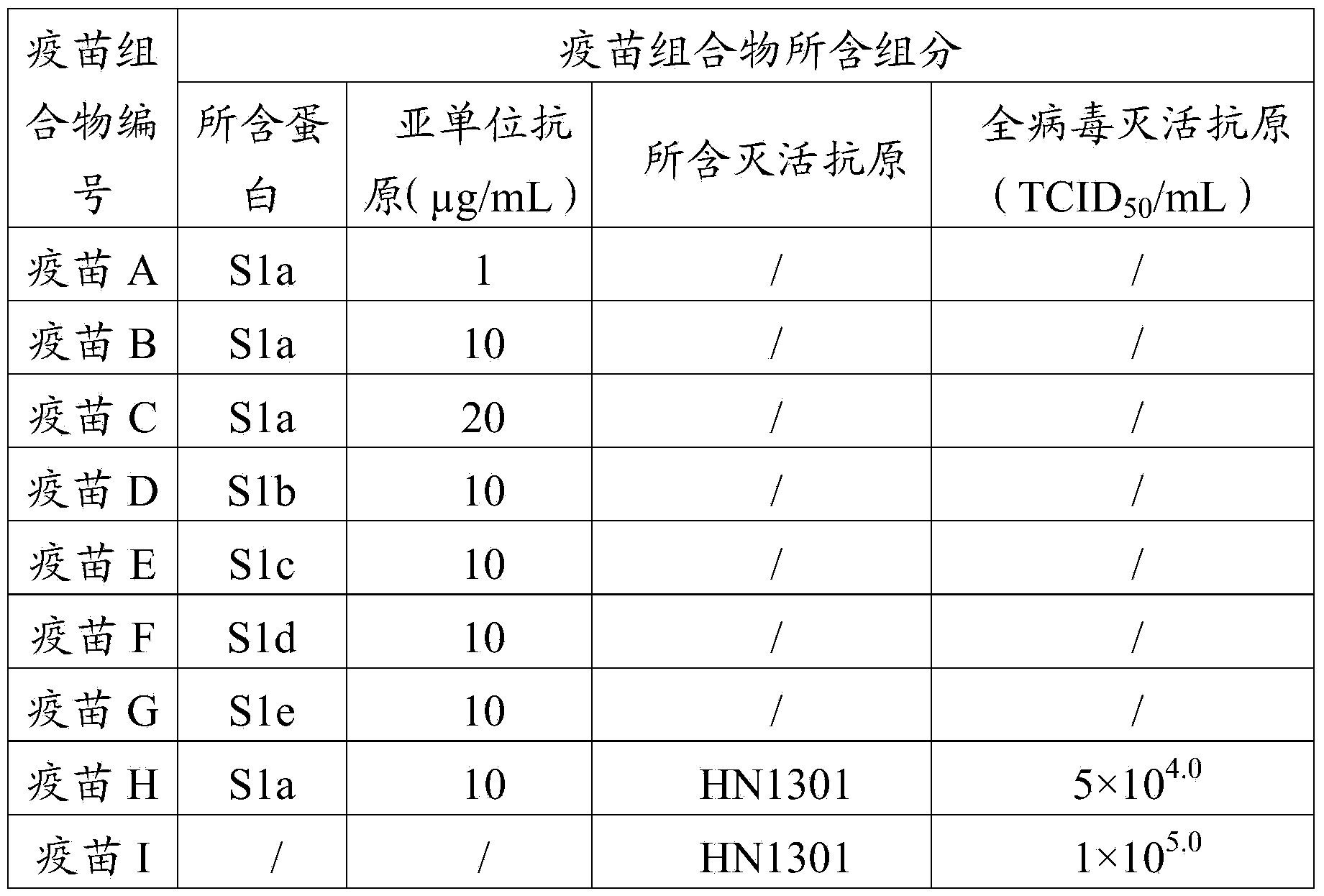
[0104] Dilute the recombinant proteins S1a, S1b, S1c, S1d, and S1e prepared in Example 1 using PBS at pH 7.2, and add aluminum gel adjuvant to mix well, so that the recombinant proteins S1a, S1b, S1c, and S1d in the vaccine composition The contents of S1e and S1e are shown in Table 1, while ensuring that the volume ratio of the aluminum gel adjuvant to the vaccine composition is 1:5. The prepared vaccine composition was used as an immunogen and stored at 4°C for future use.
[0105] 3.2 Preparation of porcine epidemic diarrhea whole virus inactivated antigen
[0106] The PEDV HN1301 strain virus solution was diluted with pH 7.2 PBS, and the diluted virus solution was inoculated with African green monkey kidney cells (Vero cells, purchased from Shanghai Institute of Biochemistry) covered with a dense monolayer by 3% ...
Embodiment 4
[0113] Example 4 Evaluation of Immunity Efficacy of Porcine Epidemic Diarrhea Vaccine Composition
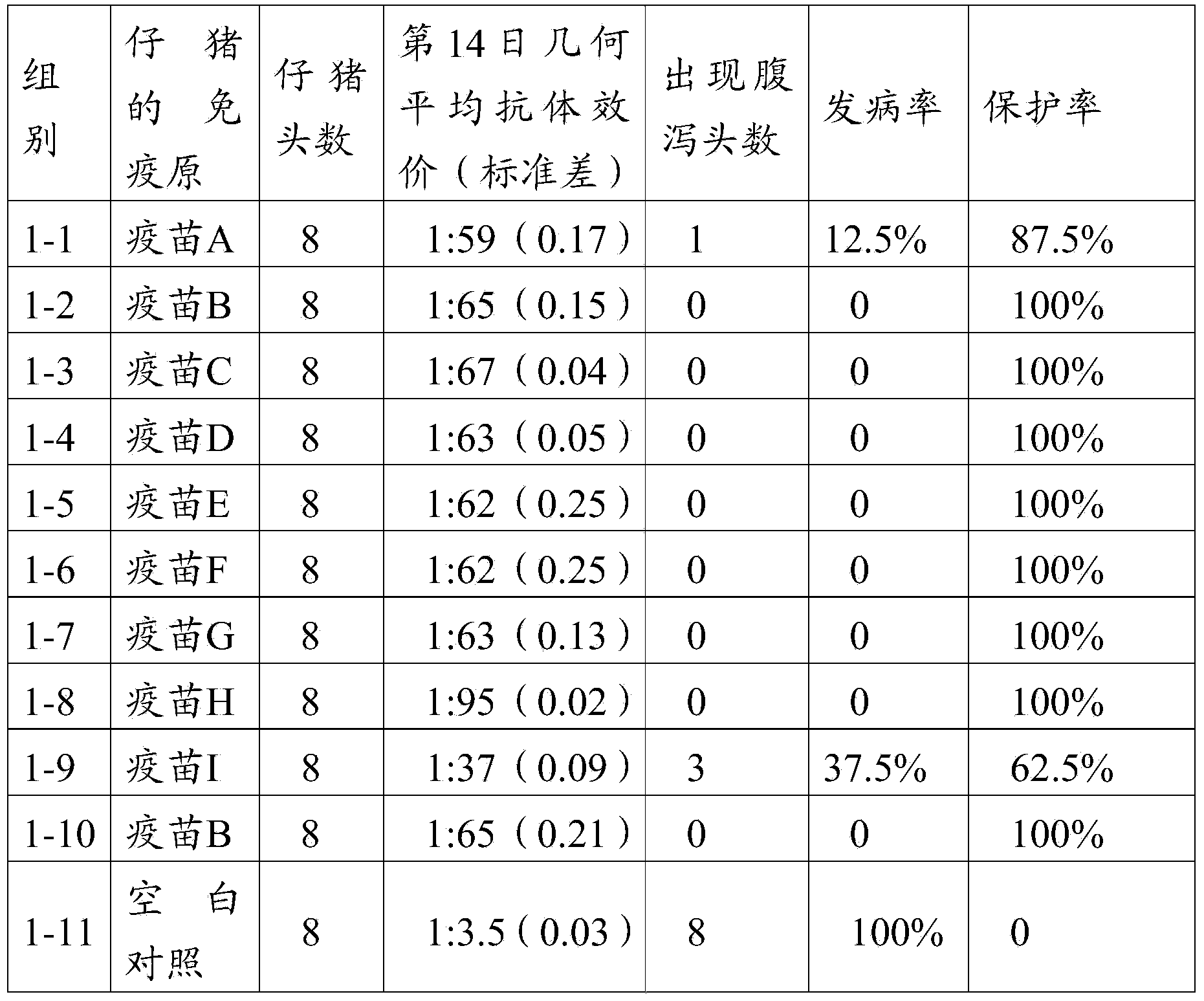
[0114] 4.1 Active immunization test
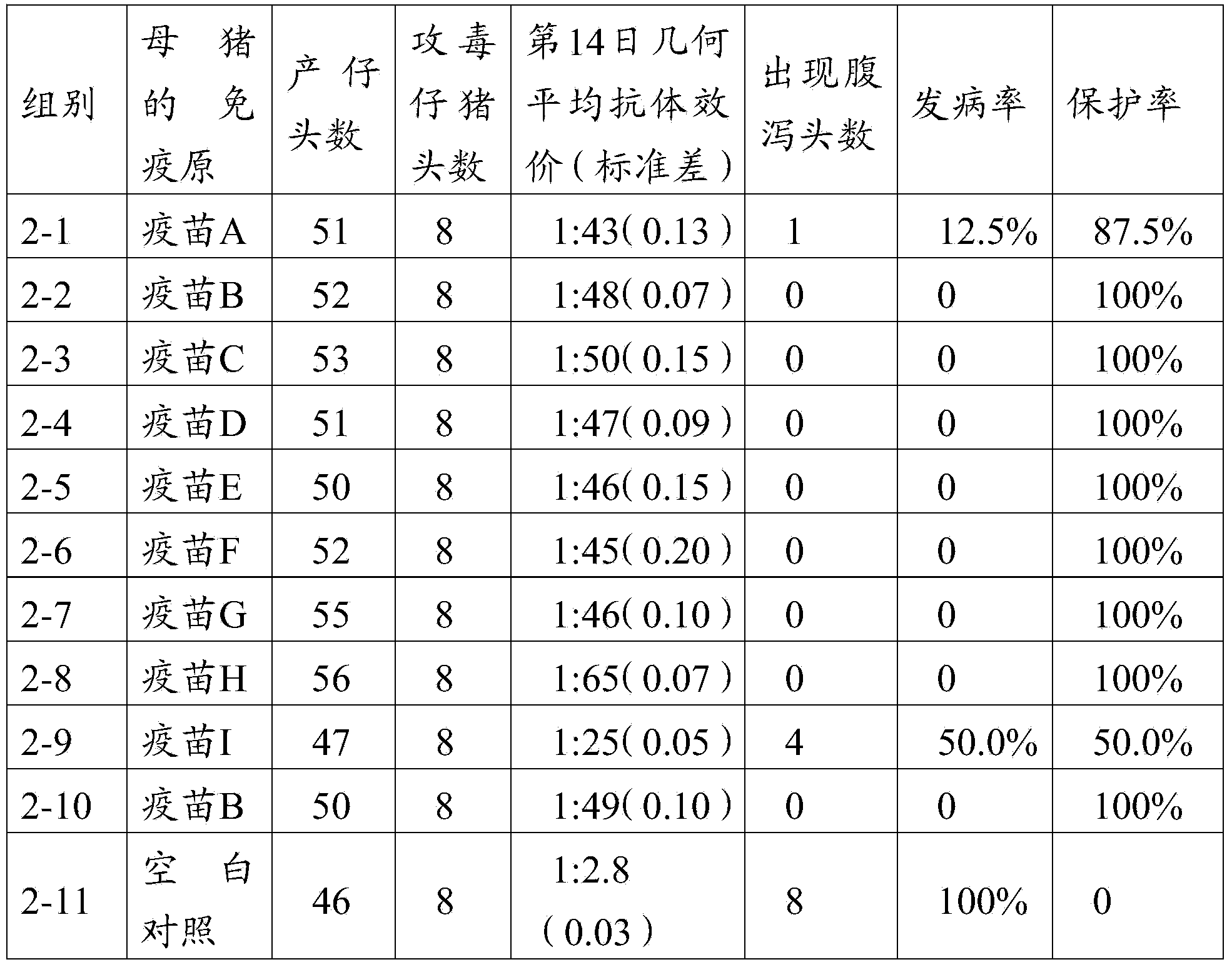
[0115] Select 88 PEDV antibody-negative piglets at the age of 3 days, and randomly divide them into 11 groups (see Table 2 for details), 8 piglets / group, and the vaccine composition prepared in Example 3 of the first 1-1 to 1-9 groups are immunized successively through Houhai Point A-I, 1mL / head; groups 1-10 were immunized with the vaccine composition B prepared in Example 3 in Houhai Point; groups 1-11 were set as blank control groups, and were injected with the same dose of PBS solution at pH 7.2. On the 14th day after immunization, the sera of pigs in the immunized group and the control group were collected, and the titers of neutralizing antibodies in the sera of each group were detected. Statistical analysis was carried out on the average antibody titer of each piglet by using SPSS15.0 software. Simultaneously, the immune group (the 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com