Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
A technology for porcine epidemic diarrhea and porcine rotavirus, which is applied in the field of triple live vaccines, can solve problems such as reinvigoration and complex virus components, and achieve the effects of avoiding trouble, good immune efficacy, and improving safety and immune efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
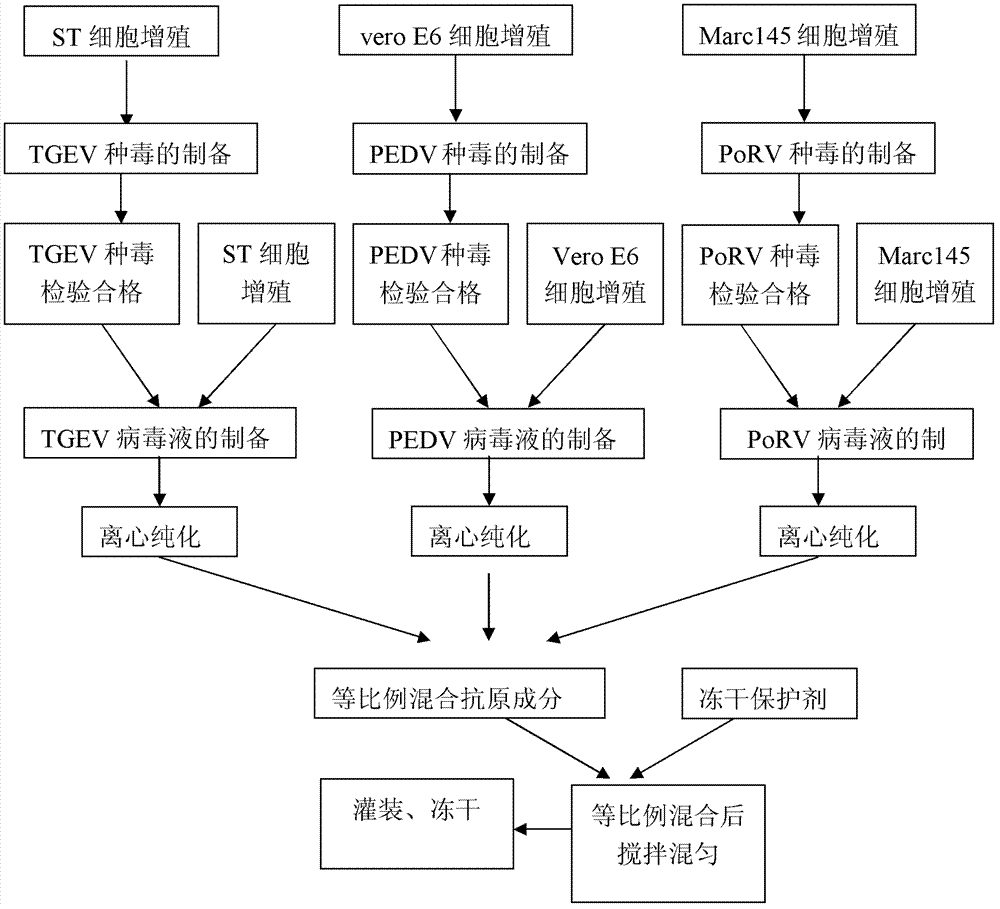
[0045] Embodiment 1, the screening of suitable cell for propagating virus
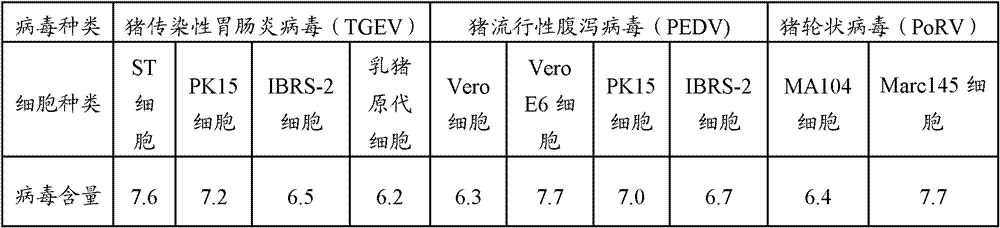
[0046] Porcine TGEV was inoculated into ST cells (ATCC), PK15 cells (China Animal Disease Control Center), IBRS-2 cells (Wuhan Type Culture Collection Center) and suckling pig kidney primary cells, and porcine epidemic diarrhea virus was inoculated in Vero cells (Shanghai Institute of Biochemistry), VeroE6 cells (Shanghai Institute of Biochemistry), PK15 cells (China Animal Disease Control Center), IBRS-2 (Wuhan Type Culture Collection Center), porcine rotavirus was inoculated into Marc145 cells (China Animal Center for Disease Control), MA104 cells (Wuhan Typical Culture Collection Center), in their respective maintenance solutions, control the pH value before filtration to 7.3-7.4, and the temperature is 35 ° C to 37 ° C, continuous subculture for 8 generations, so that the virus For suitable cells, the virus proliferation titer is measured, and the cell with the highest virus proliferation titer is ...
Embodiment 2
[0059] Embodiment 2, the preparation and inspection of the triple live vaccine of porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus
[0060] 1. Materials and methods
[0061] 1.1 cells
[0062] ST cells were purchased from American type culture collection (ATCC);
[0063] Vero E6 cells were purchased from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences;
[0064] Marc145 cells were purchased from China Animal Disease Control Center;
[0065] 1.2 Preparation of poisonous seeds for production
[0066] Preparation of transmissible gastroenteritis virus virus seeds: Inject the ST cells that have grown dense monolayers into the qualified TGEV seed virus at 0.001 M.O.I (infection dose per unit cell), absorb at 37°C for 1 hour, and add a final concentration of 2.2 g / L NaHCO 3 , 10 mg / L trypsin, 1% DMSO (v / v), 100 U / ml penicillin, 100 μg / ml streptomycin, MEM maintenance solution without fetal...
Embodiment 3
[0130] Embodiment 3, the triple live vaccine of porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus and self-made rotavirus attenuated vaccine and commercially available infectious gastroenteritis and epidemic diarrhea dual live vaccine immunization Efficacy comparison test.
[0131] 1 material
[0132] The triple live vaccine of porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus, the vaccine product (lot number: V20100303) in the implementation example 2;
[0133] Porcine rotavirus attenuated vaccine, laboratory trial product;
[0134] Porcine transmissible gastroenteritis, porcine epidemic diarrhea dual live vaccine, Jilin Zhengye Biological Products Co., Ltd. (batch number 20100102).
[0135] 2 methods
[0136] 2.1 The preparation method of rotavirus attenuated vaccine
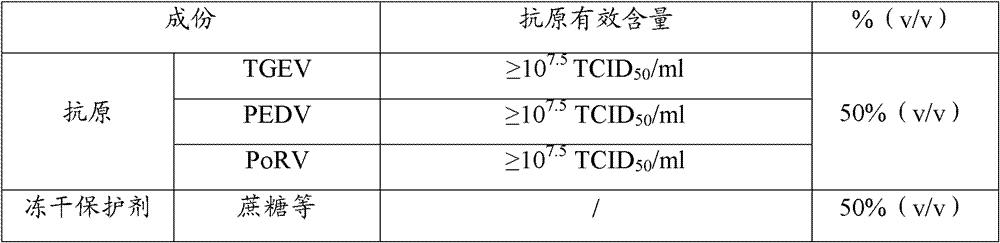
[0137] The preparation of the attenuated rotavirus vaccine is 10% of the prepared virus content in item 1.4 of Example 2 8 TCID 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com