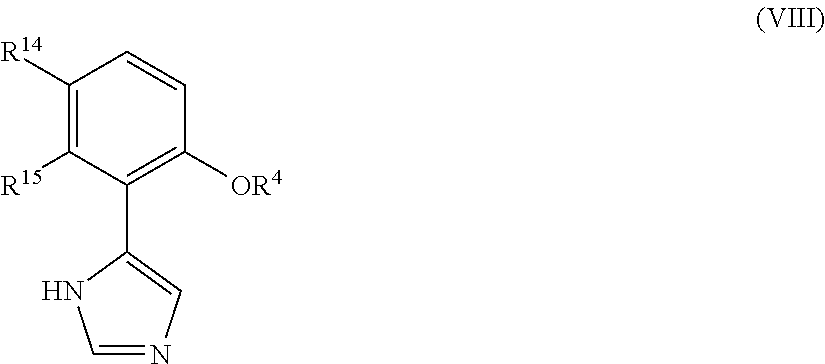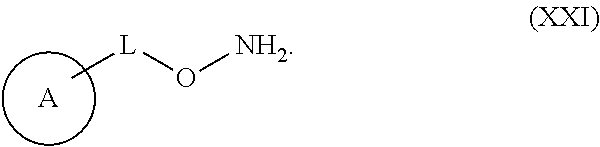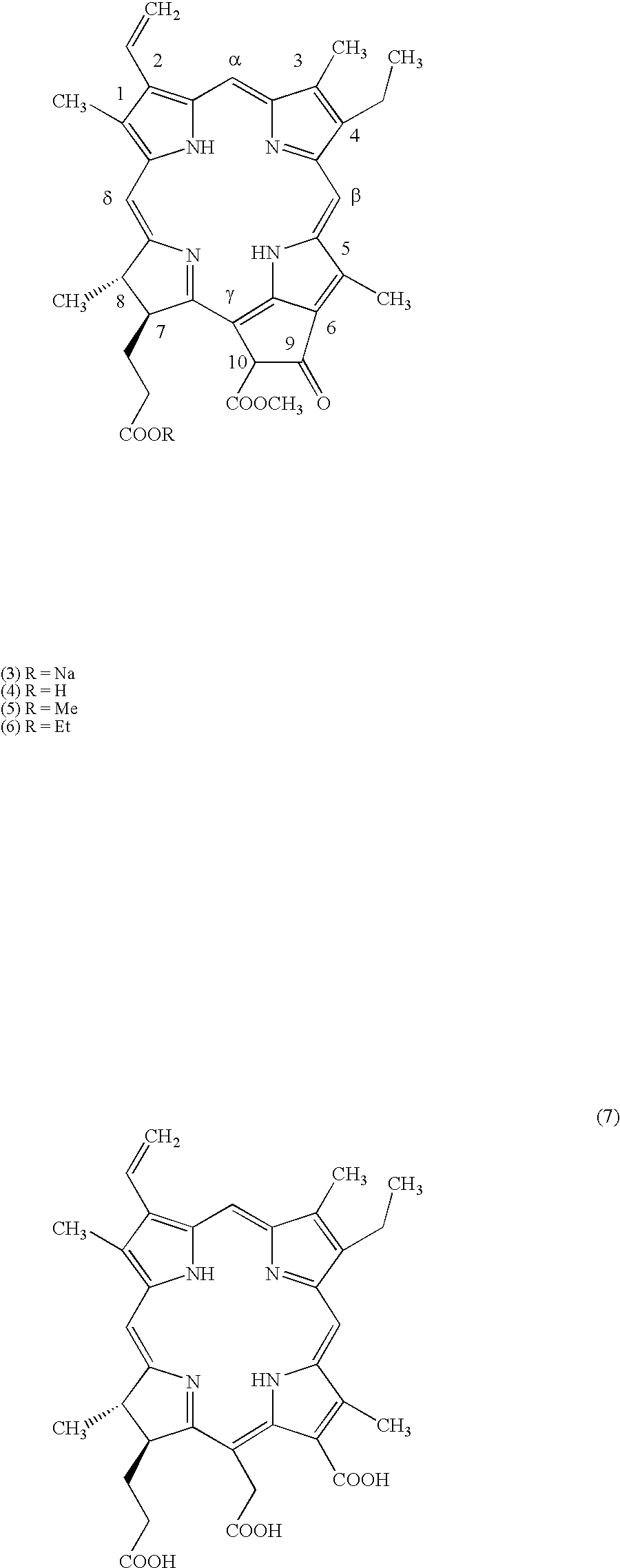Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
277 results about "Transmissible disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A transmissible disease may be transmitted through direct contact (e.g. blood exchange with an infected host) or through a vector (e.g. contaminated surfaces, fomite, air, foods, animals, etc.). Examples of transmissible disease are AIDS, herpes, etc.
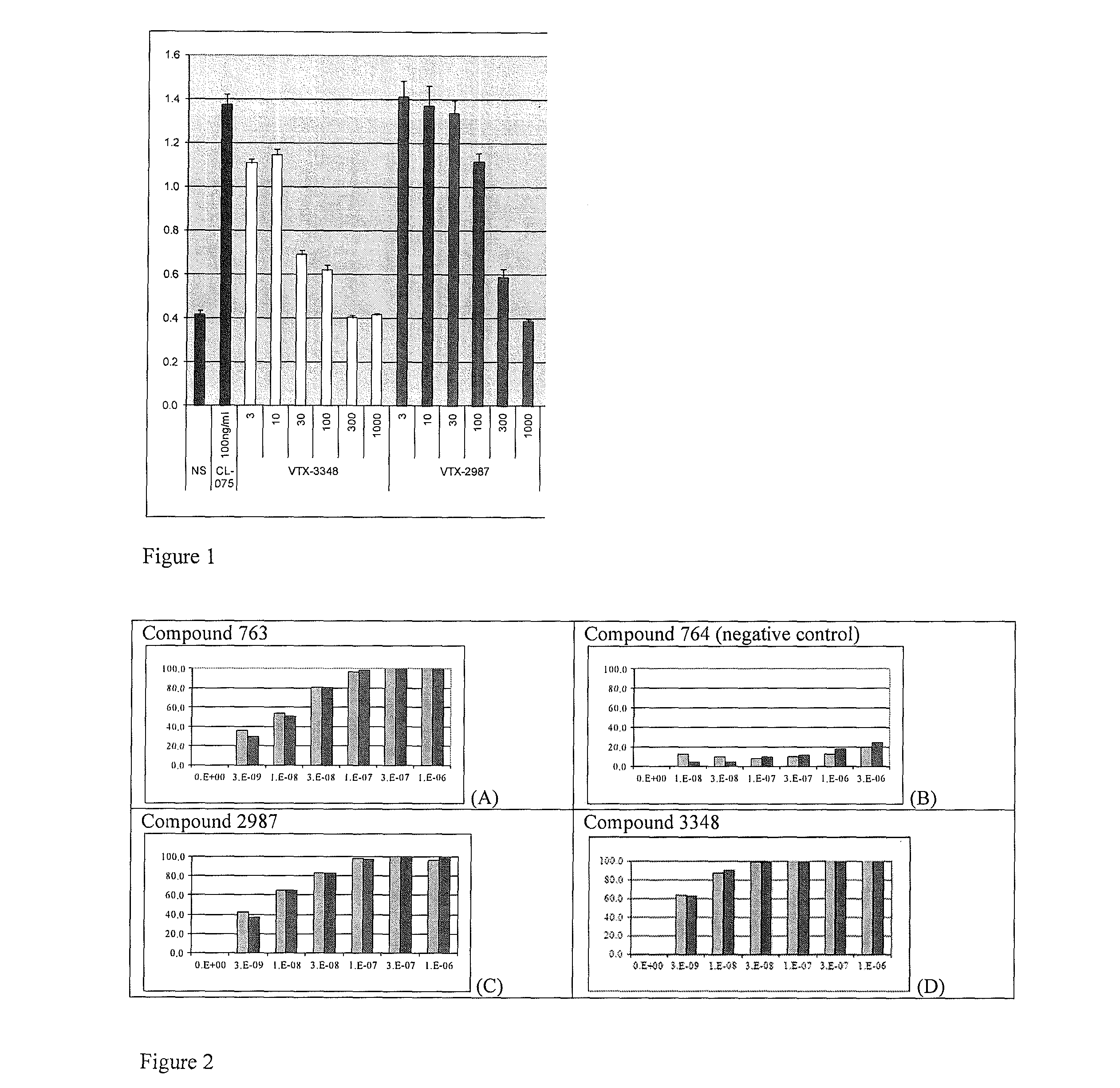
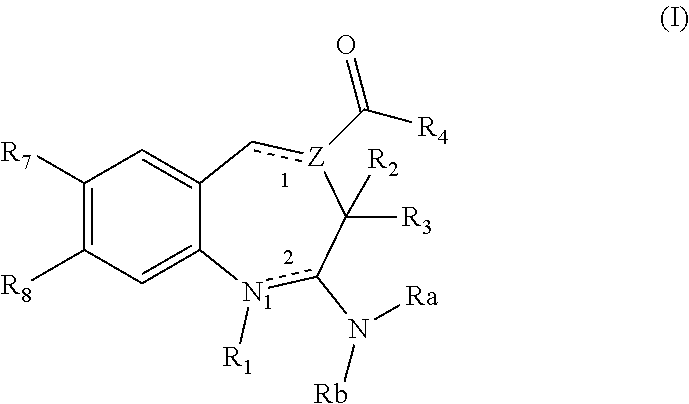
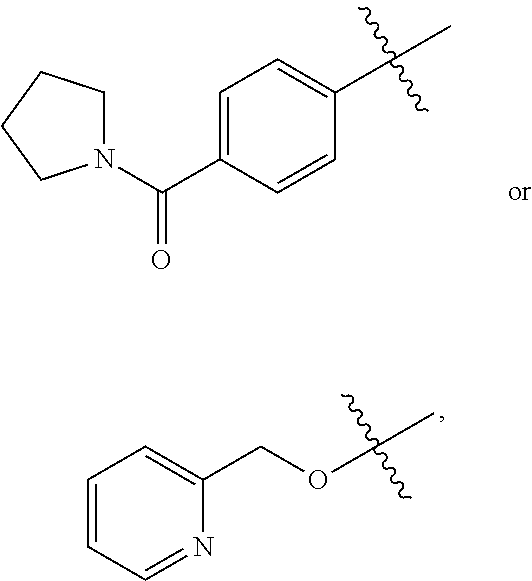
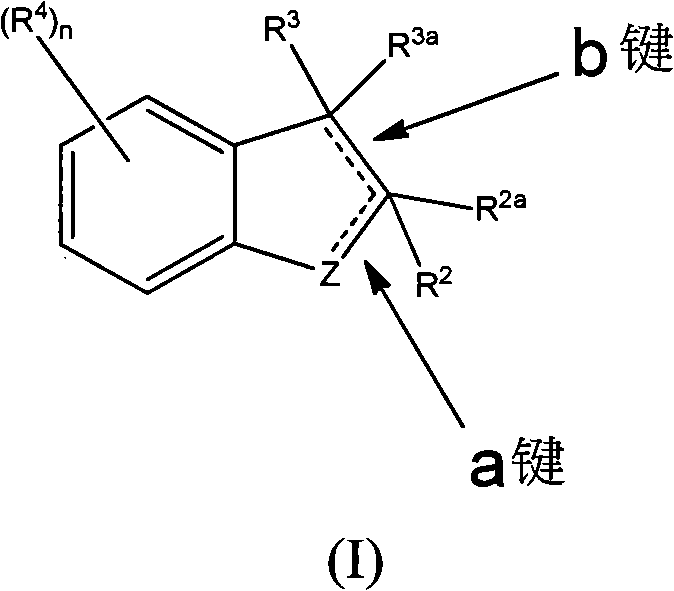
Substituted Benzoazepines As Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in treating or preventing disease, including cancer, autoimmune disease, fibrotic disease, cardiovascular disease, infectious disease, inflammatory disorder, graft rejection, or graft-versus-host disease.
Owner:ARRAY BIOPHARMA +1
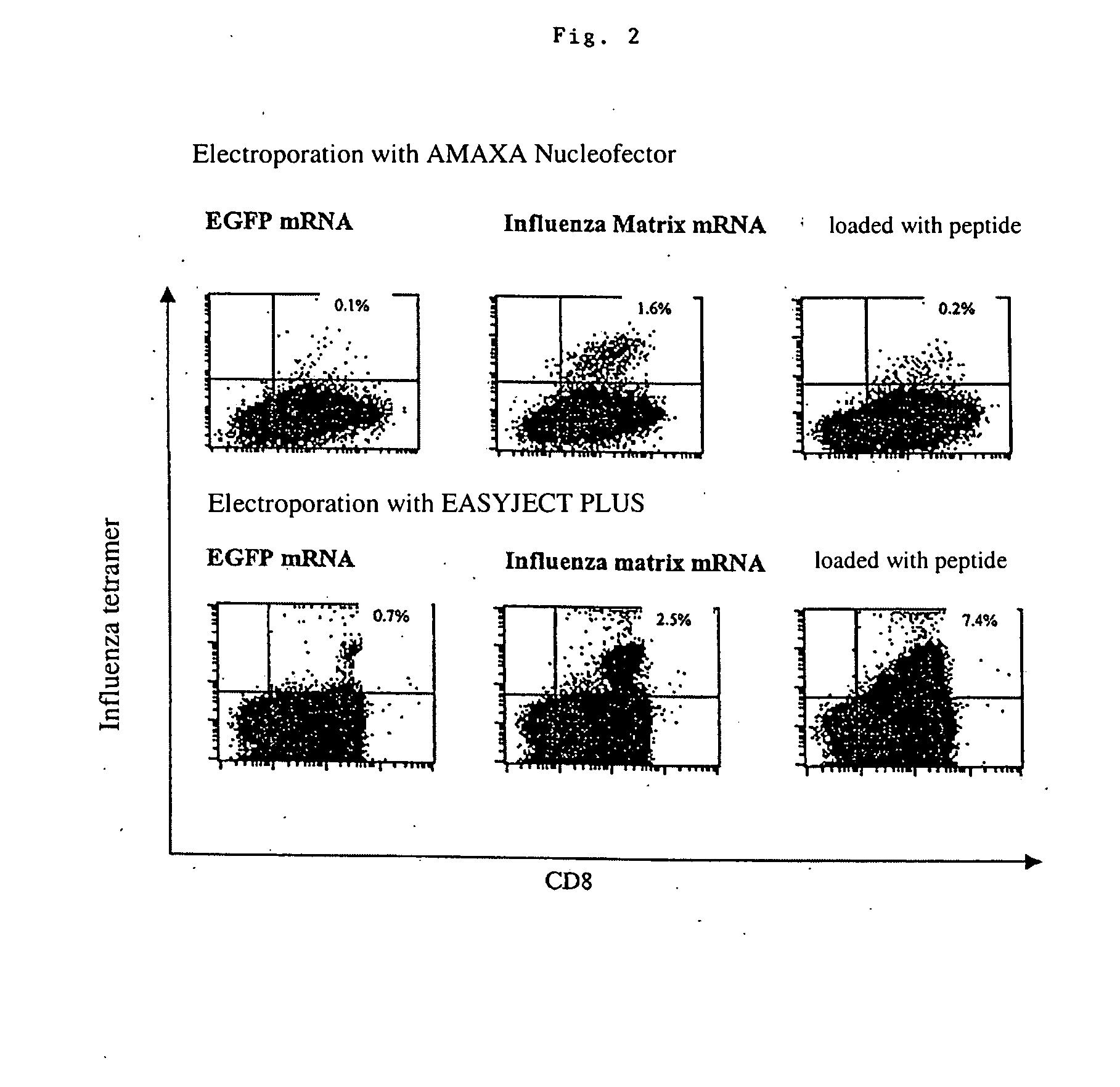
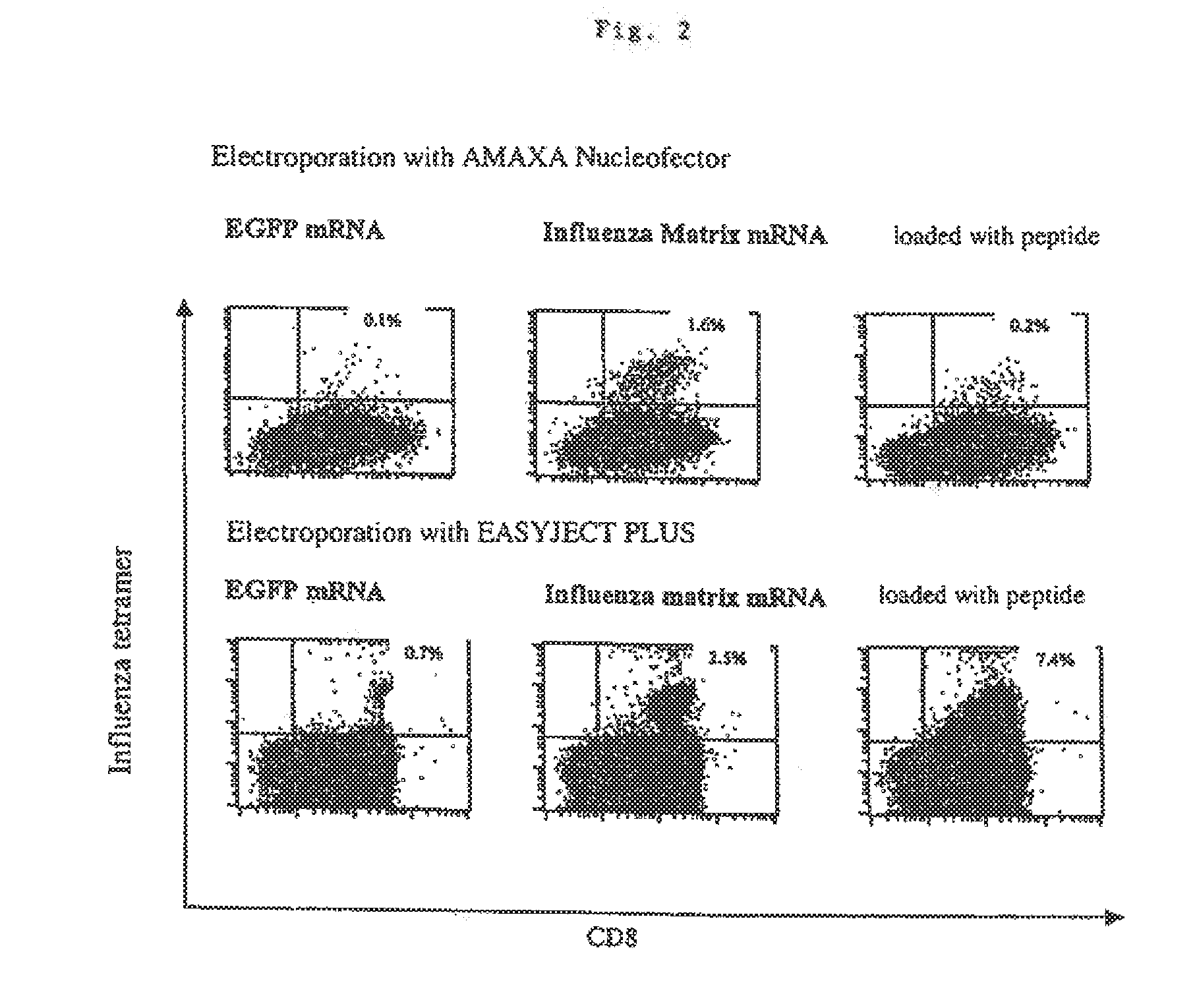
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20120009221A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseViral antigen ingredientsAntigenPharmaceutical medicine
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
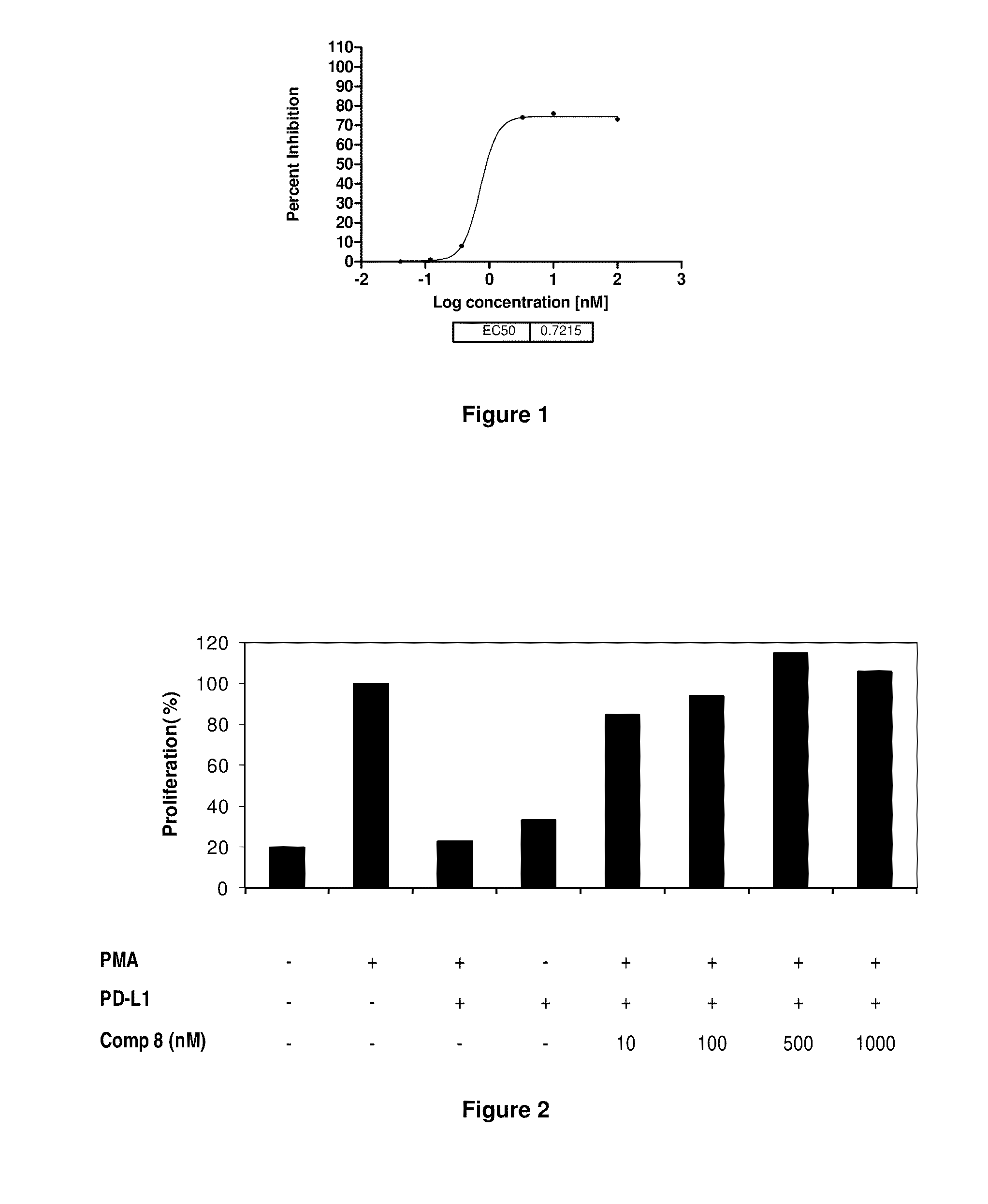
Immunosuppression modulating compounds
InactiveUS20110318373A1Reduce the binding forceAntibacterial agentsBiocideDiseaseSignalling pathways
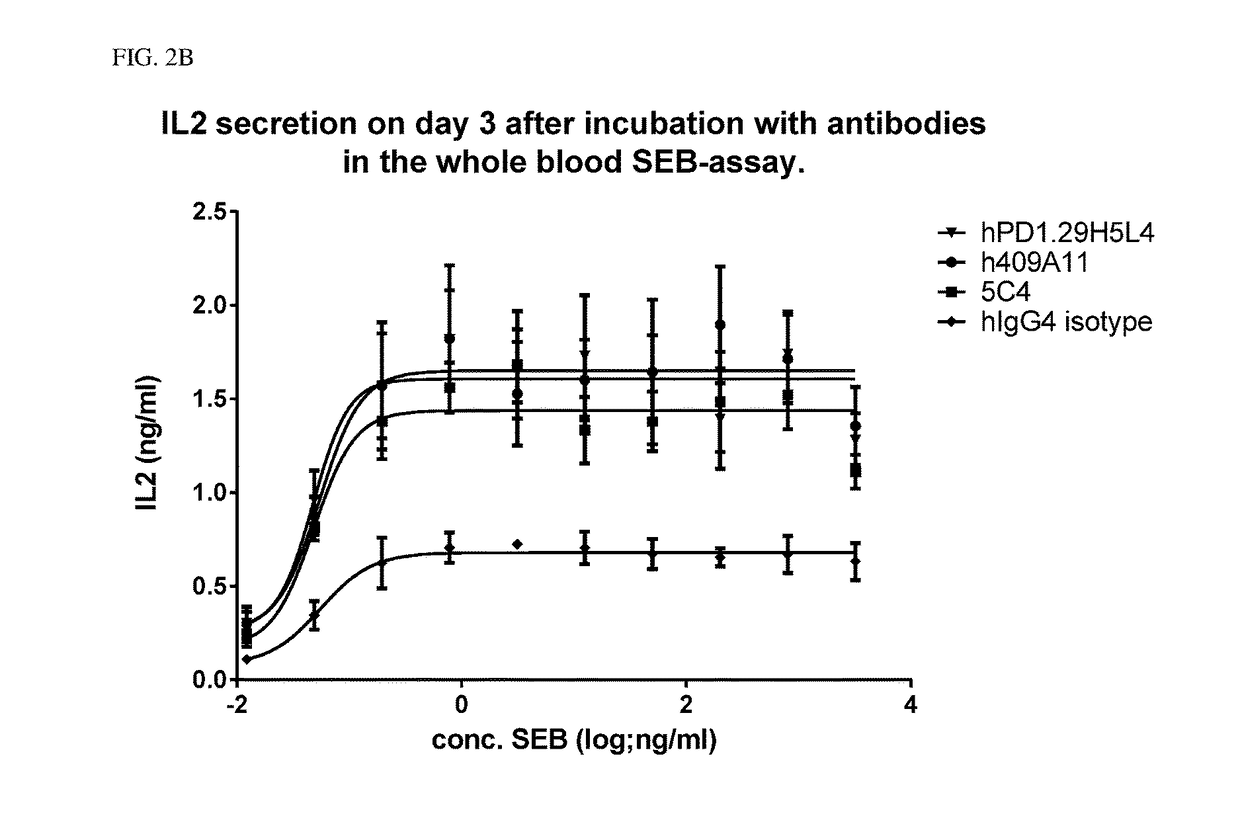
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
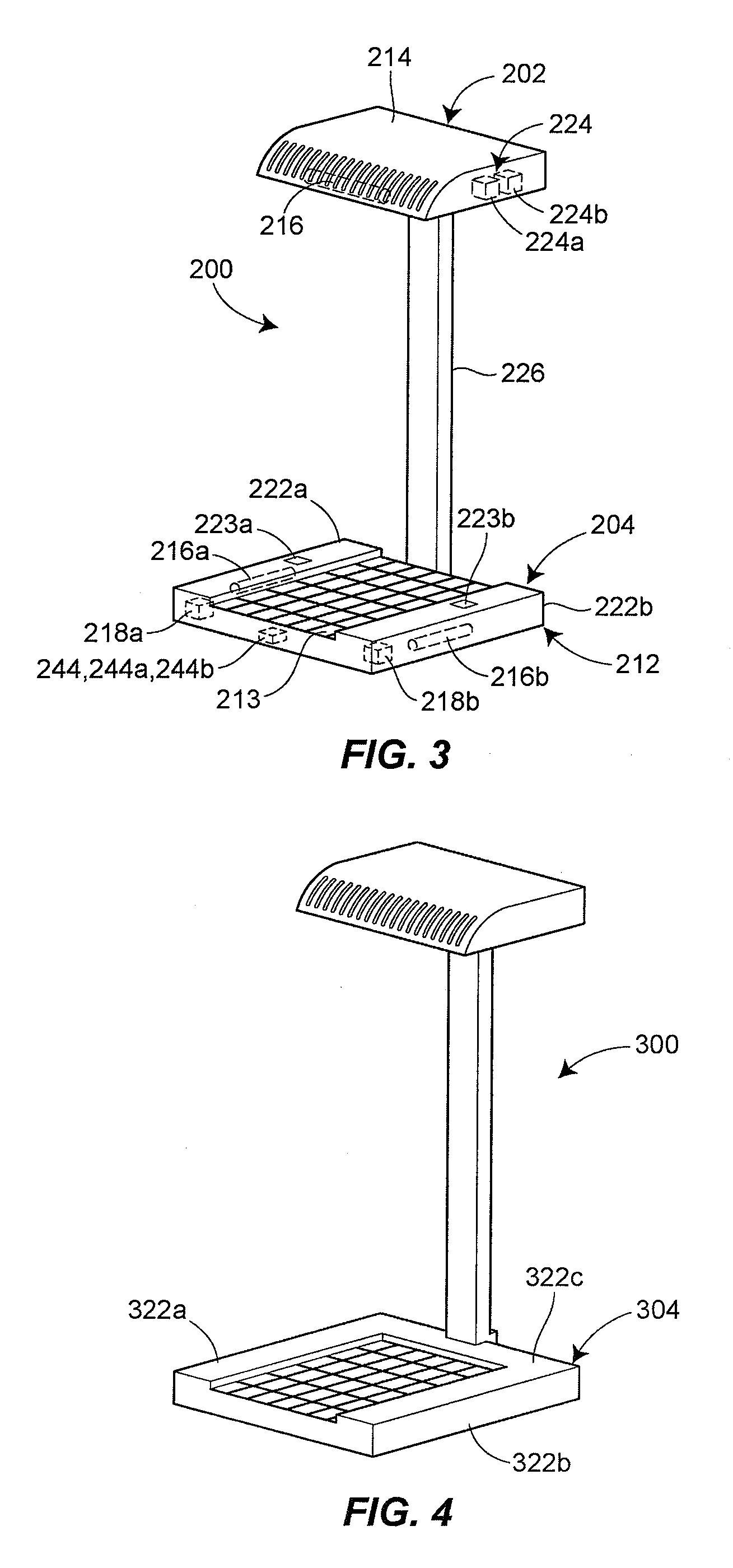
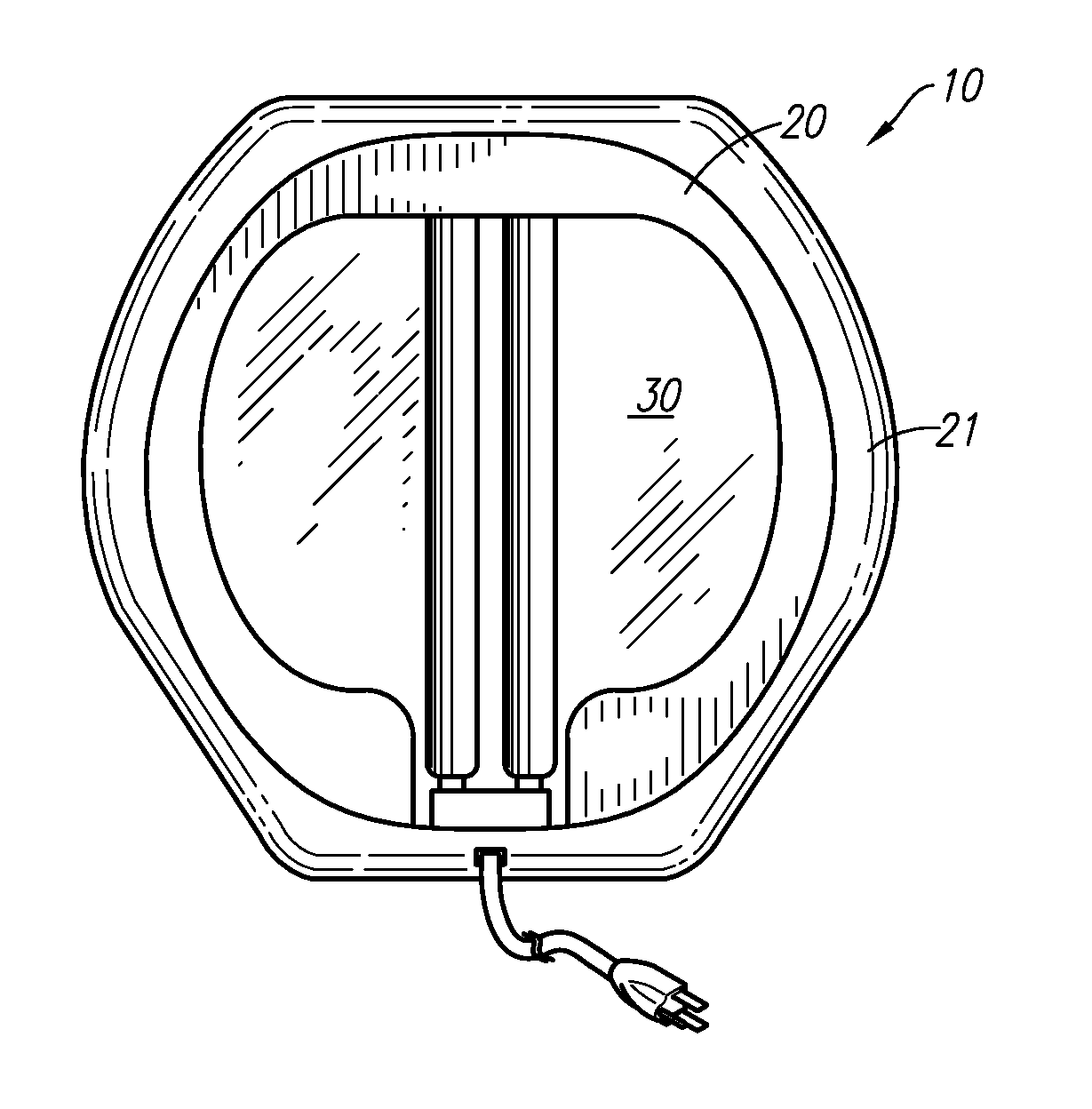
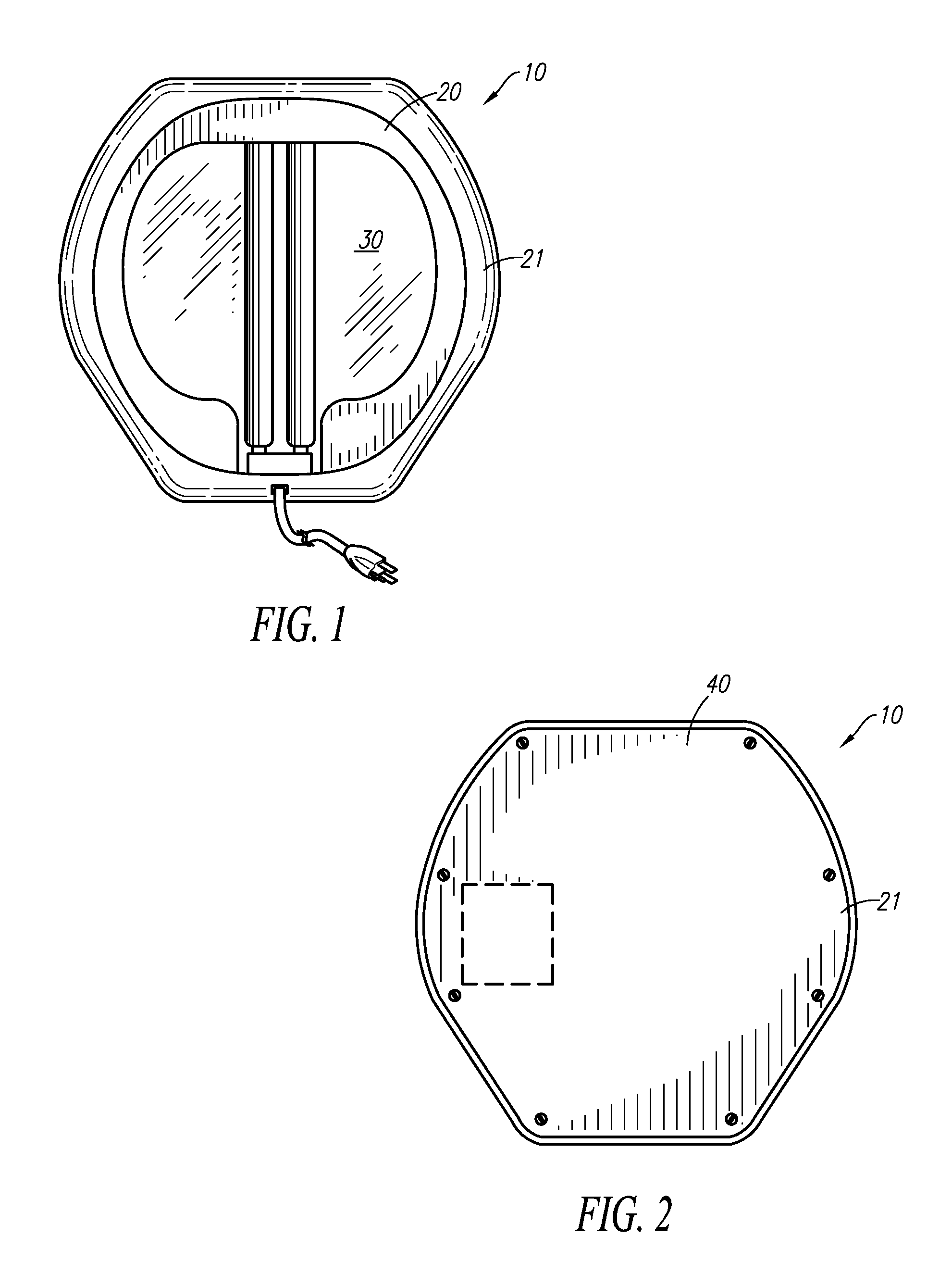
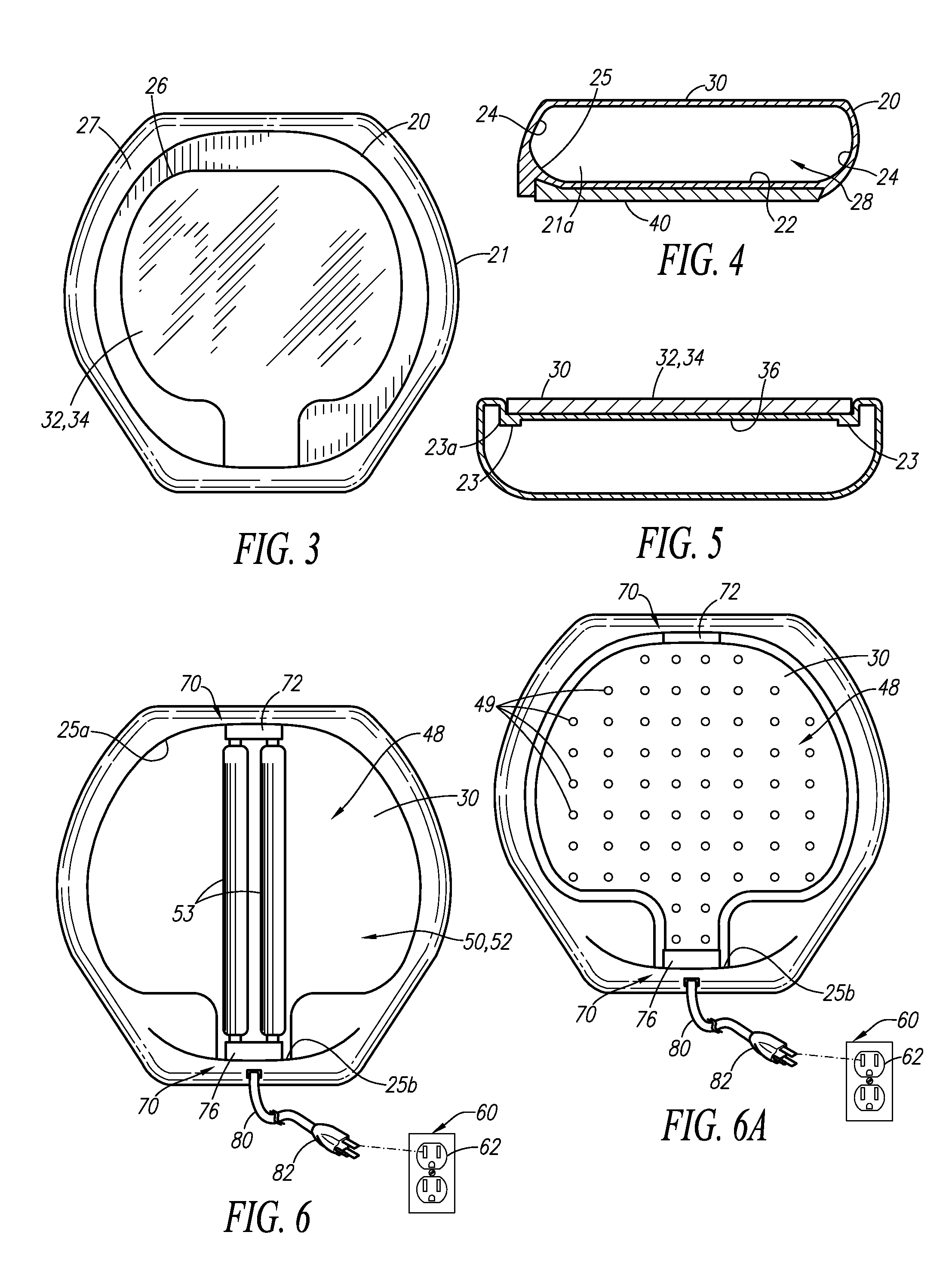
Germicidal Floor, Germicidal Foot, And Hand Cleaning System
InactiveUS20090314308A1Easy to identifyElectrostatic cleaningLight therapyPhysicsTransmissible disease
A device for simultaneously cleaning hands and feet comprises a first housing and a second housing connected by a pole. An ultraviolet light source and a light reflector is disposed within each of the first and second housings. The ultraviolet light sources and light reflectors are adapted to direct ultraviolet light waves onto the hands and feet of an individual user of the device. The light waves kill or deactivate harmful germs or transmissible diseases disposed on the hands and / or carried by the feet or the socks of the individual, thereby preventing the harmful germs or transmissible diseases from spreading and causing harm to other individuals or animals.
Owner:KIM DARRICK S H L +1
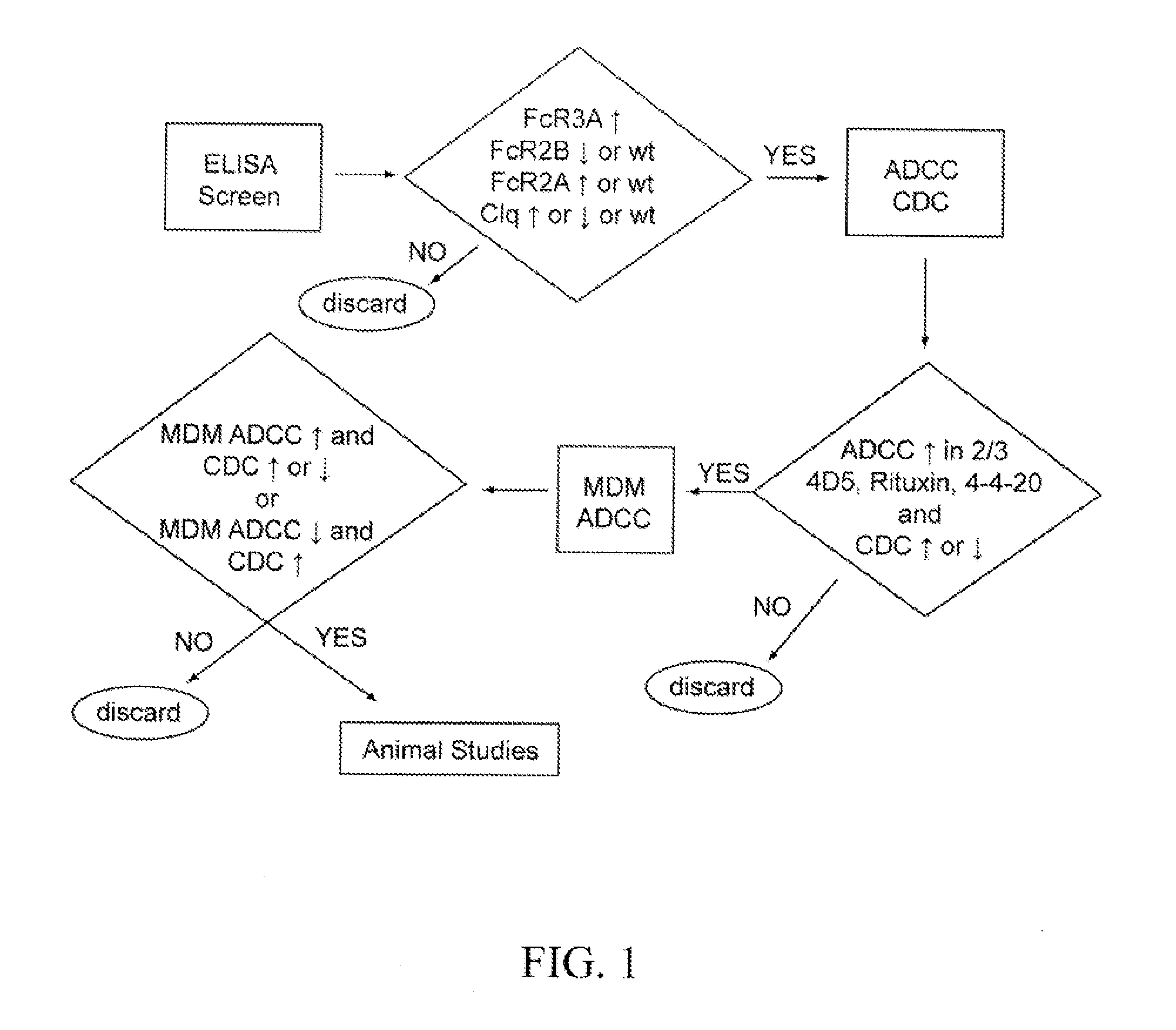
Fc Region-Containing Polypeptides That Exhibit Improved Effector Function Due To Alterations Of The Extent Of Fucosylation, And Methods For Their Use
ActiveUS20120219551A1Enhanced antibody effector functionGood curative effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsEffector cellEffector functions
The present invention relates to Fc region-containing polypeptides that exhibit improved effector function due to alterations of the extent of fucosylation, and to methods of using such polypeptides for treating or preventing cancer and other diseases. The Fc region-containing polypeptides of the present invention are preferably immunoglobulins (e.g., antibodies), in which the Fc region comprises at least one amino acid substitution relative to the corresponding amino acid sequence of a wild type Fc region, and which is sufficient to attenuate post-translational fucosylation and mediate improved binding to an activating Fc receptor and reduced binding to an inhibitory Fc receptor. The methods of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection where either an enhanced efficacy of effector cell function mediated by FcγR is desired (e.g., cancer, infectious disease) or an inhibited effector cell response mediated by FcγR is desired (e.g., inflammation, autoimmune disease).
Owner:MACROGENICS INC
Chimeric t cell receptors and related materials and methods of use
InactiveUS20090304657A1Good biological propertiesGreat T cell responseBiocideAntibody mimetics/scaffoldsDiseaseDrug biological activity
The invention provides a chimeric T cell receptor (TCR) comprising a variable region of a human TCR and a constant region comprising at least an extracellular domain of a constant region of a non-human TCR, as well as functional variants thereof. The invention also provides polypeptides and proteins related to the inventive TCRs, as well as nucleic acids encoding the TCRs, polypeptides, or proteins, recombinant expression vectors, and host cells. Further provided are pharmaceutical compositions related to the inventive TCRs and methods of preventing or treating a disease, e.g., an infectious disease, cancer, in a host, methods of detecting a diseased cell in a host, and methods of improving the biological activity of a TCR.
Owner:UNITED STATES OF AMERICA
Methods and compositions for immunotherapy and detection of inflammatory and immune-dysregulatory disease, infectious disease, pathologic angiogenesis and cancer
InactiveUS20060140936A1Antibacterial agentsOrganic active ingredientsDendritic cellAutoimmune condition
Methods and compositions for immunotherapy of inflammatory and immune-dysregulatory diseases, using multispecific antagonists that target at least two different markers are disclosed. The different targets include (i) proinflammatory effectors of the innate immune system, (ii) coagulation factors, and (iii) targets specifically associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, wherein the targets included in group (iii) are neither a proinflammatory effector of the immune system nor a coagulation factor. When the multispecific antagonist reacts specifically with a target associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, it also binds specifically with at least one proinflammatory effector of the immune system or at least one coagulation factor. Thus, the multispecific antagonist contains at least one binding specificity related to the diseased cell or condition being treated and at least one specificity to a component of the immune system, such as a receptor or antigen of B cells, T cells, neutrophils, monocytes and macrophages, and dendritic cells, a modulator of coagulation, or a proinflammatory cytokine. The multispecific antagonists are used in the treatment of various diseases that are generated or exacerbated by, or otherwise involve, proinflammatory effectors of the innate immune system or coagulation factors. Such diseases more particularly include acute and chronic inflammatory disorders, autoimmune diseases, giant cell arteritis, septicemia and septic shock, coagulopathies (including diffuse intravascular coagulation), neuropathies, graft versus host disease, infectious diseases, acute respiratory distress syndrome, granulomatous diseases, transplant rejection, asthma, cachexia, myocardial ischemia, and atherosclerosis. Other diseases also responsive to these therapies include cancers and conditions with pathological angiogenesis.
Owner:IMMUNOMEDICS INC
Methods for the treatment of disease using immunoglobulins having Fc regions with altered affinities for FcgammaRactivating and FcgammaRinhibiting
ActiveUS8652466B2Enhanced antibody effector functionGood curative effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunologic disordersCancer prevention
The present invention relates to methods of treating or preventing cancer and other diseases using molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds an FcγR that activates a cellular effector (“FcγRActivating,” such as FcγRIIA or FcγRIIIA) and an FcγR that inhibits a cellular effector (“FcγRInhibiting,” such as FcγRIIA) with an altered Ratio of Affinities relative to the respective binding affinities of such FcγR for the Fc region of the wild-type immunoglobulin. The methods of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection where either an enhanced efficacy of effector cell function mediated by FcγR is desired (e.g., cancer, infectious disease) or an inhibited effector cell response mediated by FcγR is desired (e.g., inflammation, autoimmune disease).
Owner:MACROGENICS INC
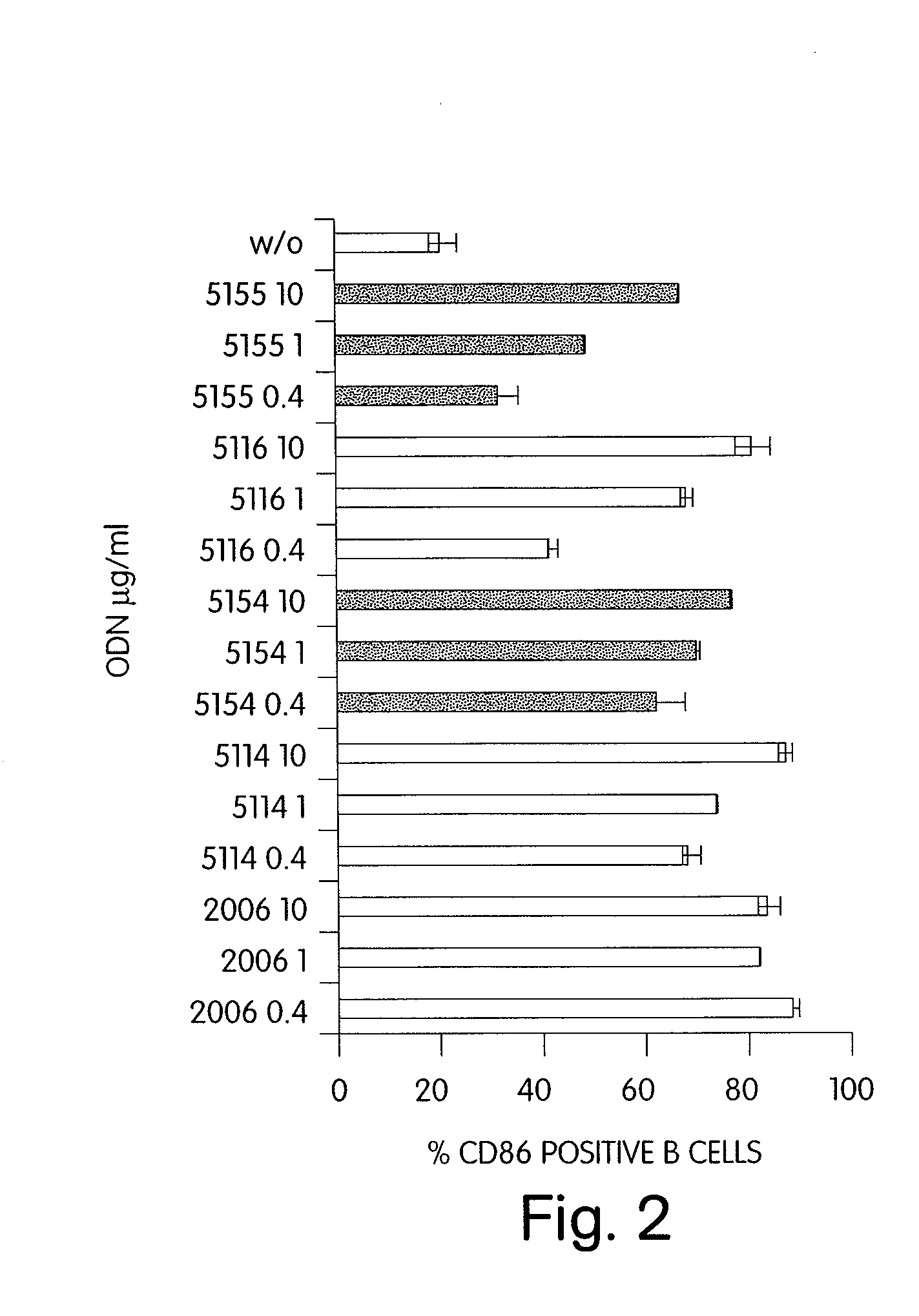
CPG-like nucleic acids and methods of use thereof
InactiveUS20080226649A1Enhancing neutrophil proliferationOrganic active ingredientsSugar derivativesAllergyPurine
Immunostimulatory compositions described as CpG-like nucleic acids are provided, including nucleic acids having immunostimulatory characteristics of CpG nucleic acid, despite certain substitutions of C, G, or C and G of the CpG dinucleotide. The substitutions can include, among others, exchange of methylated C for C, inosine for G, and ZpY for CpG, where Z is cytosine or dSpacer and Y is inosine, 2-aminopurine, nebularine, or dSpacer. Also provided are methods for inducing an immune response in a subject using the CpG-like nucleic acids. The methods are useful in the treatment of a subject that has or is at risk of developing an infectious disease, allergy, asthma, cancer, anemia, thrombocytopenia, or neutropenia.
Owner:COLEY PHARMA GMBH
Combination Scale And Germicidal Sterilization Apparatus
InactiveUS20130048876A1Avoid spreadingMilk preparationMaterial analysis using wave/particle radiationUltraviolet lightsTransmissible disease
A germicidal sterilization apparatus which includes a housing, a plate disposed atop the housing, and at least one ultraviolet light source supported by the housing. The at least one UV light source is adapted and configured to generate UV light waves which kill or deactivate harmful germs, microorganisms and spores thereof, pathogens, and / or transmissible diseases. The apparatus is integrated with a weight-measuring device for determining and indicating the weight of an individual.
Owner:CRAWFORD DEREK G
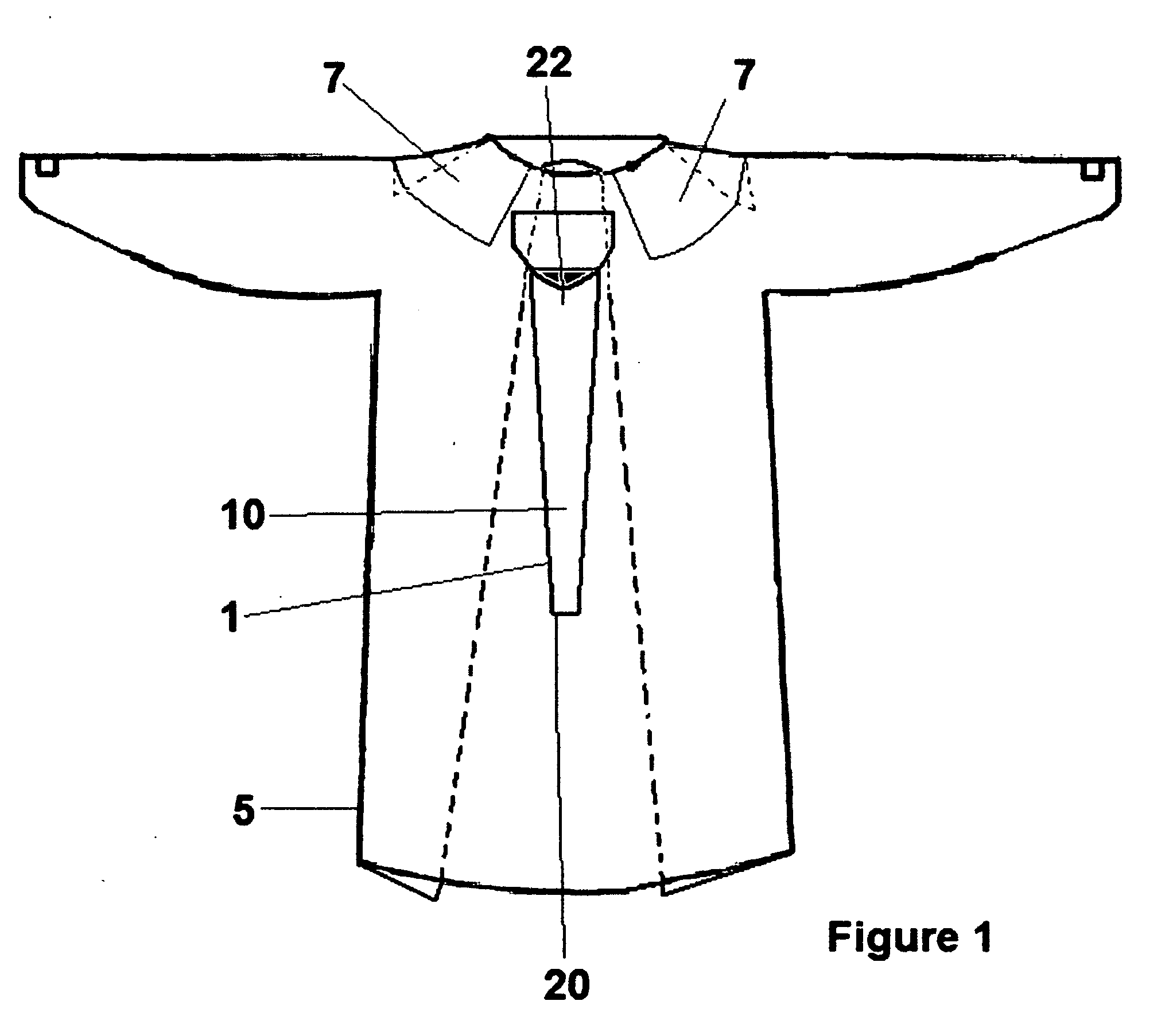
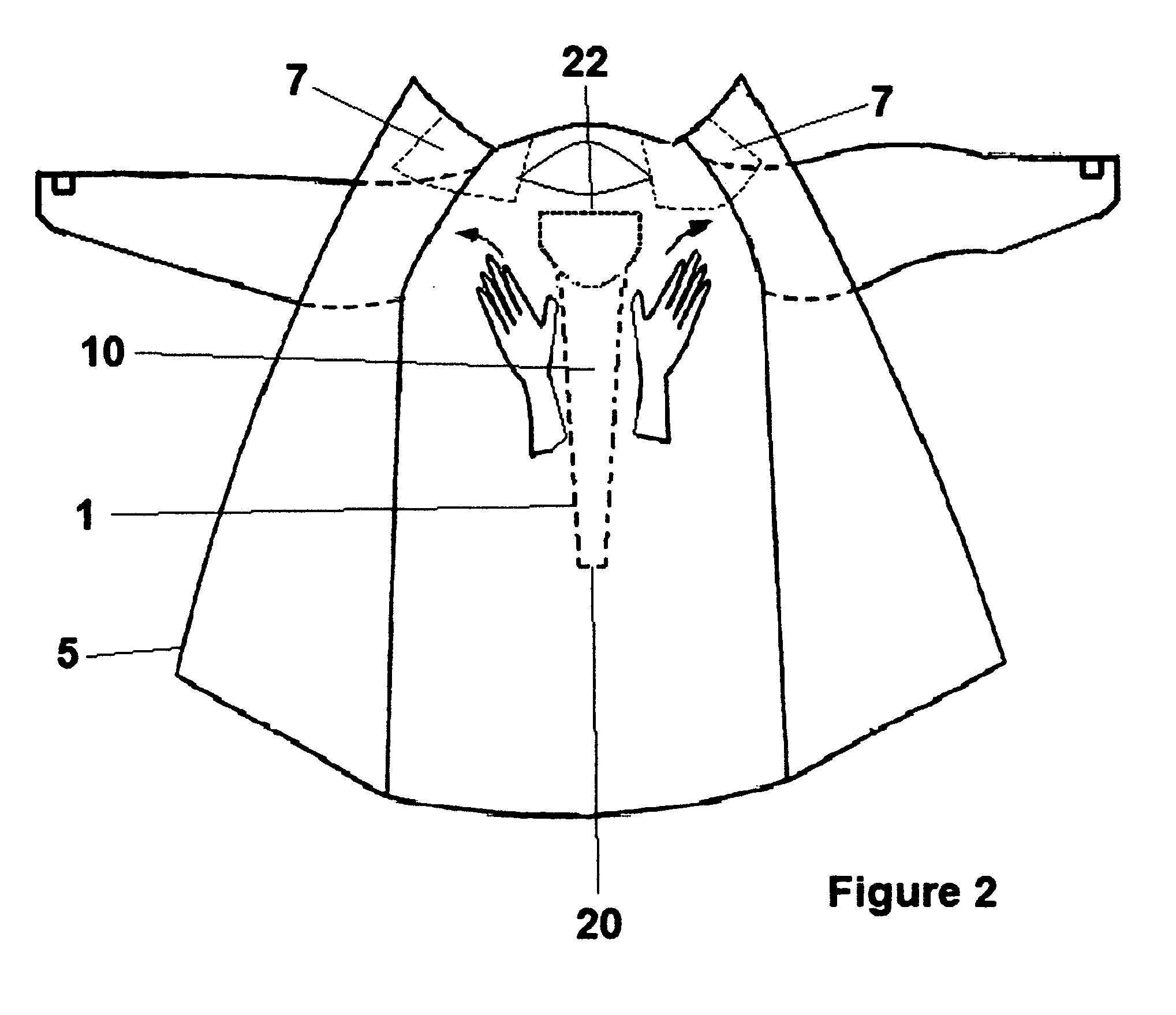
Disposable hospital gown with stethoscope protector/cover
InactiveUS20090165186A1Reduce transmissionImprove hygieneStethoscopePyjamasIntensive care medicineHospital patients
A disposable hospital patient's gown with a stethoscope protector or cover for preventing the stethoscope of healthcare provider from being contaminated, so as to prevent transmission of infectious diseases to the patients.
Owner:MIJARES DANIEL +1
Methods for the Treatment of Disease Using Immunoglobulins Having Fc Regions with Altered Affinities for FcgammaRactivating and FcgammaRinhibiting
ActiveUS20140134162A1Additive and synergistic and novel propertyImproved phenotypeSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsImmunglobulin eWild type
The present invention relates to methods of treating or preventing cancer and other diseases using molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds an FcγR that activates a cellular effector (“FcγRActivating,” such as FcγRIIA or FcγRIIIA) and an FcγR that inhibits a cellular effector (“FcγRInhibiting,” such as FcγRIIA) with an altered Ratio of Affinities relative to the respective binding affinities of such FcγR for the Fc region of the wild-type immunoglobulin. The methods of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection where either an enhanced efficacy of effector cell function mediated by FcγR is desired (e.g., cancer, infectious disease) or an inhibited effector cell response mediated by FcγR is desired (e.g., inflammation, autoimmunde disease).
Owner:MACROGENICS INC
Ido inhibitors
Presently provided are methods for (a) modulating an activity of indoleamine 2,3-dioxygenase comprising contacting an indoleamine 2,3-dioxygenase with a modulation effective amount of a compound as described in one of the aspects described herein; (b) treating indoleamine 2,3-dioxygenase (IDO) mediated immunosuppression in a subject in need thereof, comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount of a compound as described in one of the aspects described herein; (c) treating a medical conditions that benefit from the inhibition of enzymatic activity of indoleamine-2,3-dioxygenase comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount of a compound as described in one of the aspects described herein; (d) enhancing the effectiveness of an anti-cancer treatment comprising administering an anti-cancer agent and a compound as described in one of the aspects described herein; (e) treating tumor-specific immunosuppression associated with cancer comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount of a compound as described in one of the aspects described herein; and (f) treating immunosuppression associated with an infectious disease, e.g., HIV-I infection, comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount a compound as described in one of the aspects described herein.
Owner:NEWLINK GENETICS
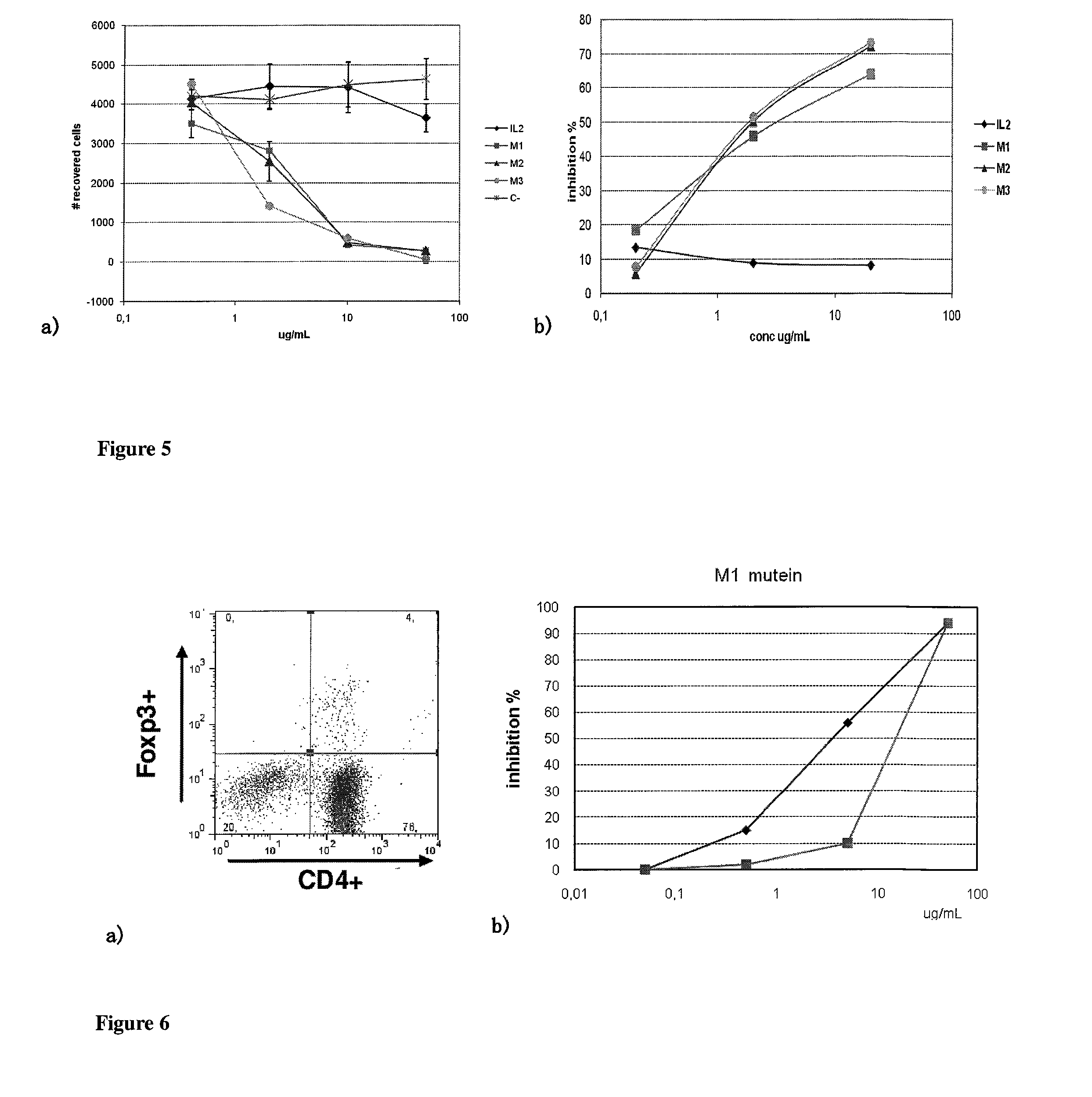
Immunomodulatory interleukin-2 polypeptides and methods of treating melanoma
The present invention relates generally to polypeptides whose primary sequence has high sequence homology with human interleukin 2 (IL-2) with some punctual mutations in the sequence of native IL-2. The polypeptides of the present invention have an immunomodulatory effect on the immune system, which is selective / preferential on regulatory T cells. The present invention also relates to specific polypeptides whose amino acid sequence is disclosed herein. In another aspect the present invention relates to pharmaceutical compositions comprising as active ingredient the polypeptides disclosed. Finally, the present invention relates to the therapeutic use of the polypeptides and pharmaceutical compositions disclosed due to their immune modulating effect on diseases such as cancer and chronic infectious diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Imidazole Derivatives as IDO Inhibitors
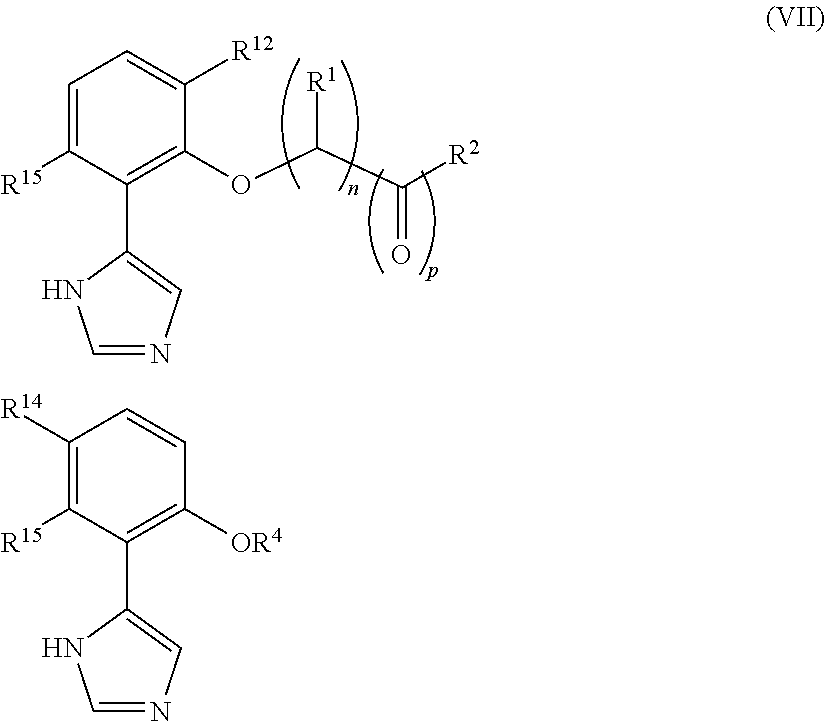
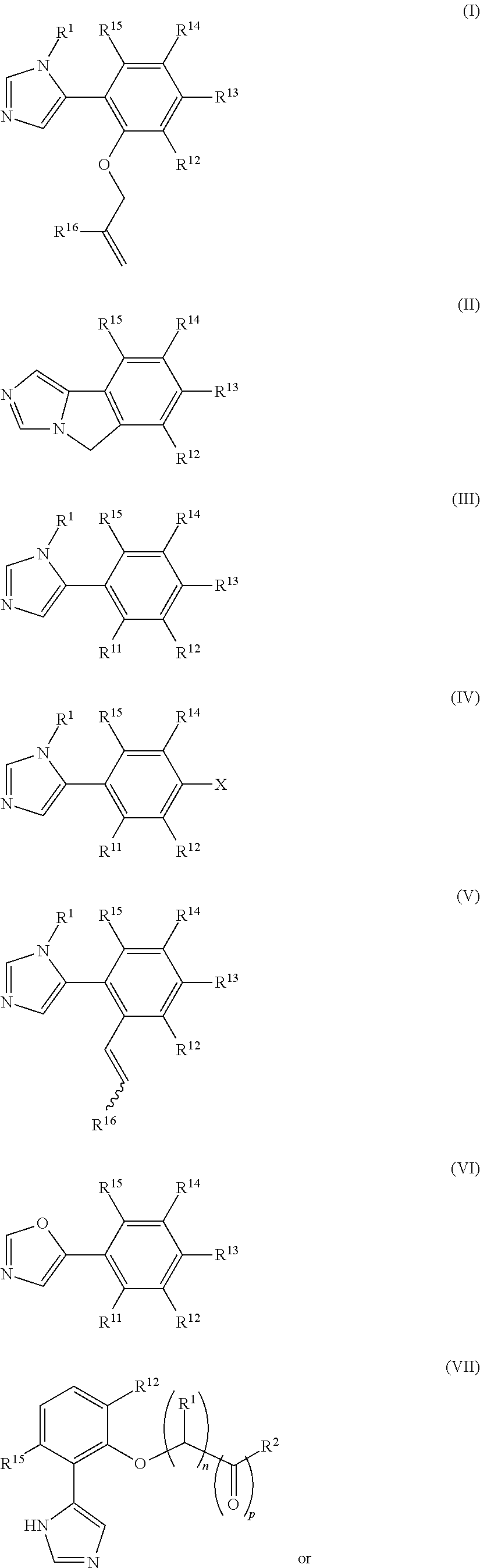
Presently provided are IDO inhibitors of general formulae (VII), (VIII) as shown below and pharmaceutical compositions thereof, useful for modulating an activity of indoleamine 2,3-dioxygenase; treating indoleamine 2,3-dioxygenase (IDO) mediated immunosuppression; treating a medical conditions that benefit from the inhibition of enzymatic activity of indoleamine-2,3-dioxygenase; enhancing the effectiveness of an anti-cancer treatment comprising administering an anti-cancer agent; treating tumor-specific immunosuppression associated with cancer; and treating immunosupression associated with an infectious disease.
Owner:NEWLINK GENETICS
IDO Inhibitors
Presently provided are methods for (a) modulating an activity of indoleamine 2,3-dioxygenase comprising contacting an indoleamine 2,3-dioxygenase with a modulation effective amount of a compound as described in one of the aspects described herein; (b) treating indoleamine 2,3-dioxygenase (IDO) mediated immunosuppression in a subject in need thereof, comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount of a compound as described in one of the aspects described herein; (c) treating a medical conditions that benefit from the inhibition of enzymatic activity of indoleamine-2,3-dioxygenase comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount of a compound as described in one of the aspects described herein; (d) enhancing the effectiveness of an anti-cancer treatment comprising administering an anti-cancer agent and a compound as described in one of the aspects described herein; (e) treating tumor-specific immunosuppression associated with cancer comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount of a compound as described in one of the aspects described herein; and (f) treating immunsupression associated with an infectious disease, e.g., HIV-1 infection, comprising administering an effective indoleamine 2,3-dioxygenase inhibiting amount a compound as described in one of the aspects described herein.
Owner:NEWLINK GENETICS
Receptors
InactiveUS20060135418A1Structure miniaturizationImprove bindingImmunoglobulin superfamilyIn-vivo radioactive preparationsDisulfide bondingAutoimmune condition
A multivalent T cell receptor (TCR) complex comprising at least two TCRs, linked by a non-peptidic polymer chain or a peptidic linker sequence. Preferably the TCR complex comprises TCR heterodimers having a non-native disulfide bond between constant domain residues, said TCRs being linked via an optionally substituted, polyalkylene glycol linker. Therapeutic agents such as cytotoxic drugs may be attached to such complexes for targeted cell delivery. Such TCR complexes may be used in the diagnosis or treatment of cancer, infectious disease, or autoimmune disease.
Owner:IMMUNOCORE LTD
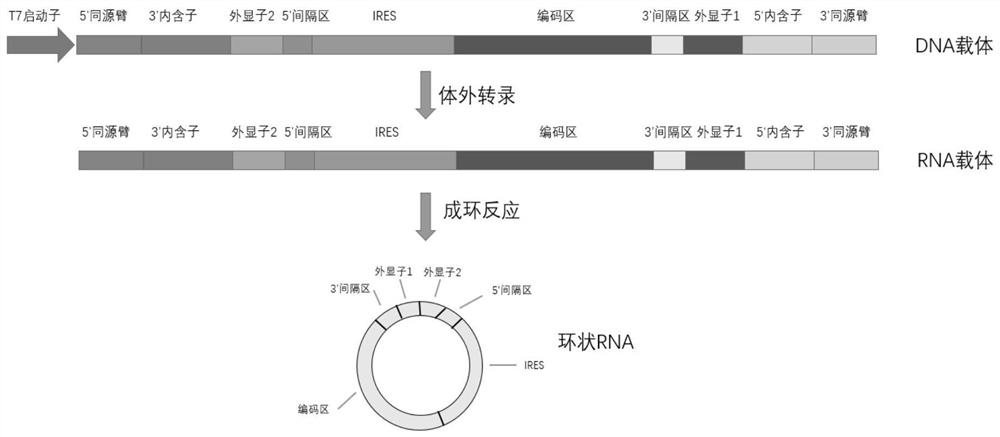
Recombinant nucleic acid molecule for transcribing circular RNA and application of recombinant nucleic acid molecule in protein expression
ActiveCN112481289AImprove expression levelImprove expression efficiencyTumor rejection antigen precursorsSsRNA viruses positive-senseDendritic cellTGE VACCINE
The invention relates to a recombinant nucleic acid molecule for transcribing circular RNA and application of the recombinant nucleic acid molecule in protein expression. Specifically, the invention relates to the recombinant nucleic acid molecule for transcribing circular RNA, a recombinant expression vector, linear RNA, circular RNA, a recombinant host cell, a pharmaceutical composition and a method for preparing protein. The recombinant nucleic acid molecule is transcribed to form circular RNA containing a specific IRES element, the IRES element can improve the protein expression level of the circular RNA in eukaryotic cells, efficient and durable expression of protein is achieved, and the recombinant nucleic acid molecule has important application values for preparing mRNA infectious disease vaccines, therapeutic mRNA tumor vaccines and mRNA-based dendritic cell tumor vaccines, and in the fields of gene therapy based on mRNA, chimeric antigen receptor T cell therapy based on mRNA,protein supplement therapy and the like.
Owner:SUZHOU CUREMED BIOMEDICAL TECH CO LTD
Multimer of extracellular domain of cell surface functional molecule
ActiveUS20090305950A1Facilitated DiffusionEnhances their cellular cytotoxicityAntibacterial agentsAntimycoticsExtracellularEfficacy
As a substance which pharmacologically regulates the function of a cell surface functional molecule, a substance which has specificity and an activity or efficacy equal or superior to an antibody and does not require an advanced production technique and facility for application thereof to a pharmaceutical product has been demanded. The invention relates to a multimer of an extracellular domain of a cell surface functional molecule, particularly a tetramer of an extracellular domain of PD-1 or PD-L1. Further, the invention relates to an application of such a tetramer as a preventive and / or therapeutic agent for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of PD-1 or PD-L1 as a testing or diagnostic agent or a research agent for such a disease.
Owner:ONO PHARMA CO LTD
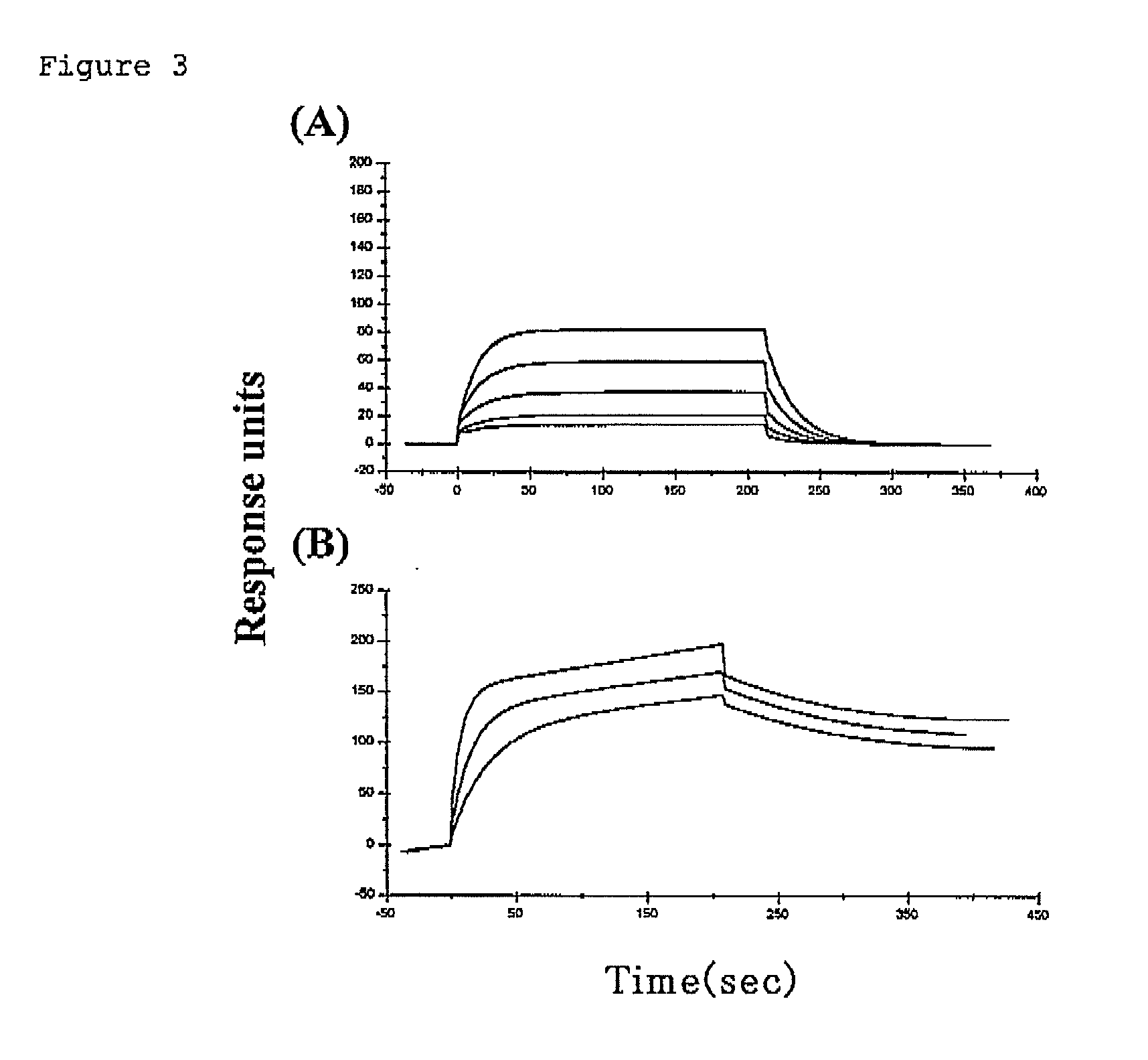
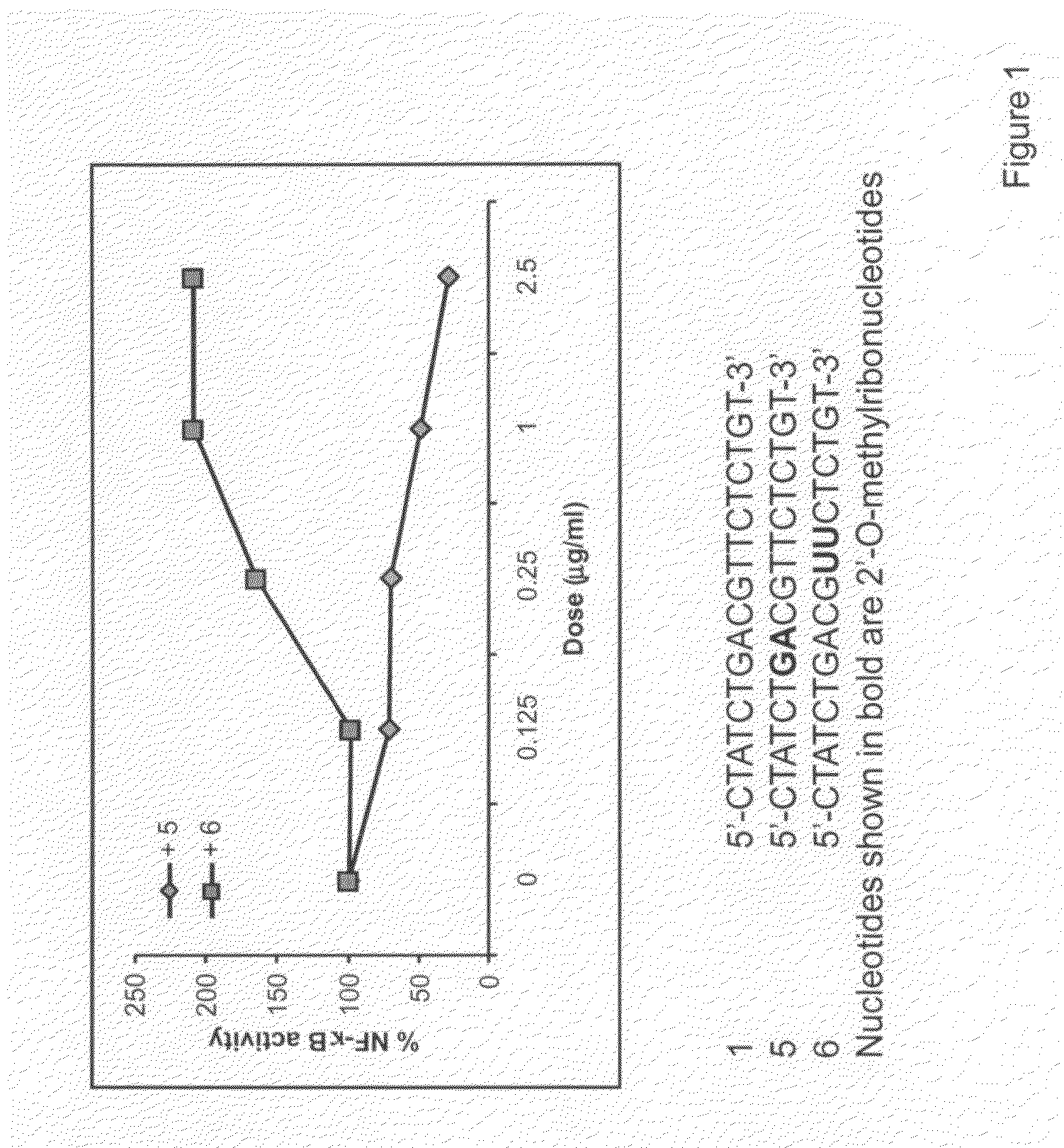
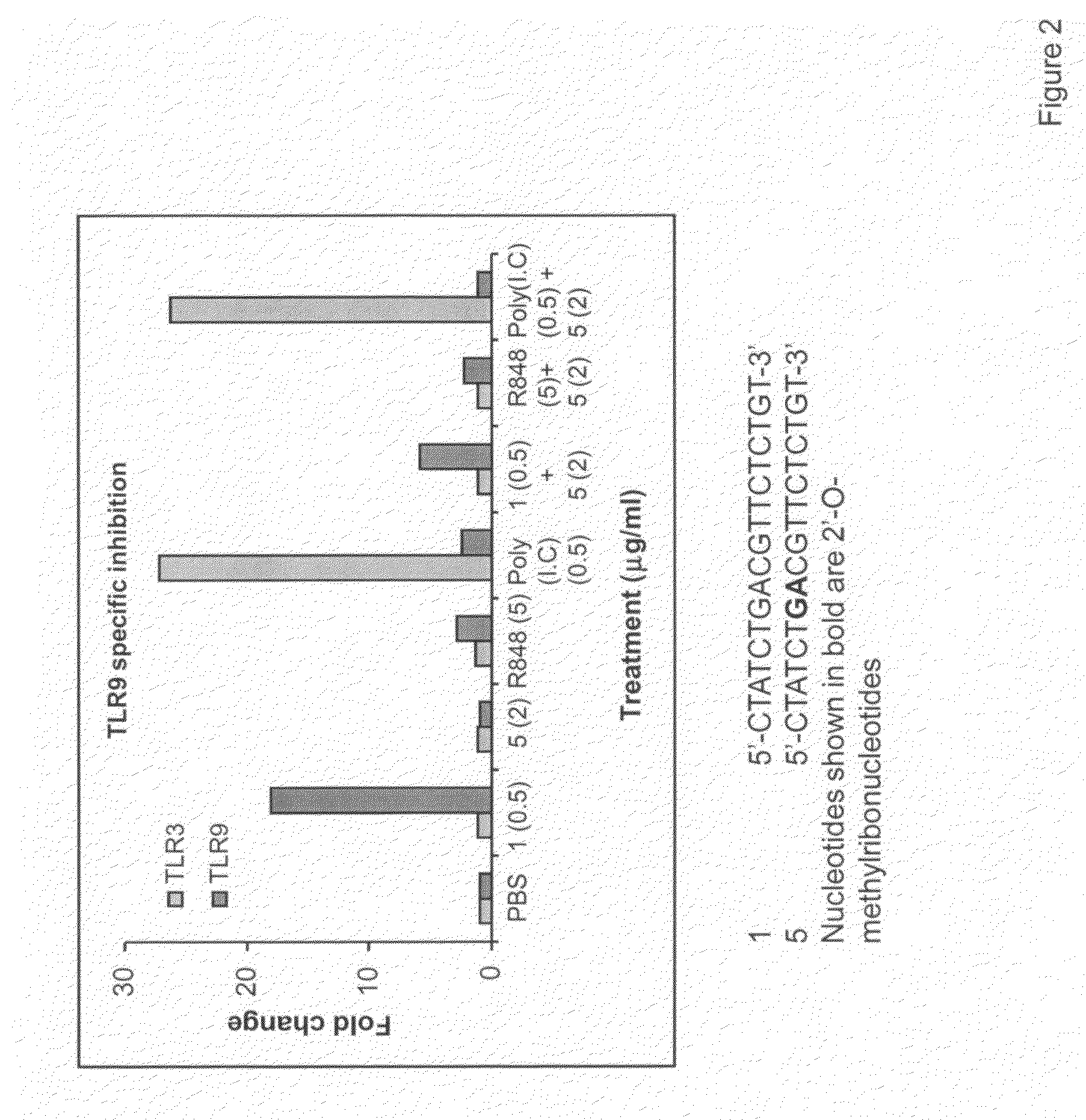
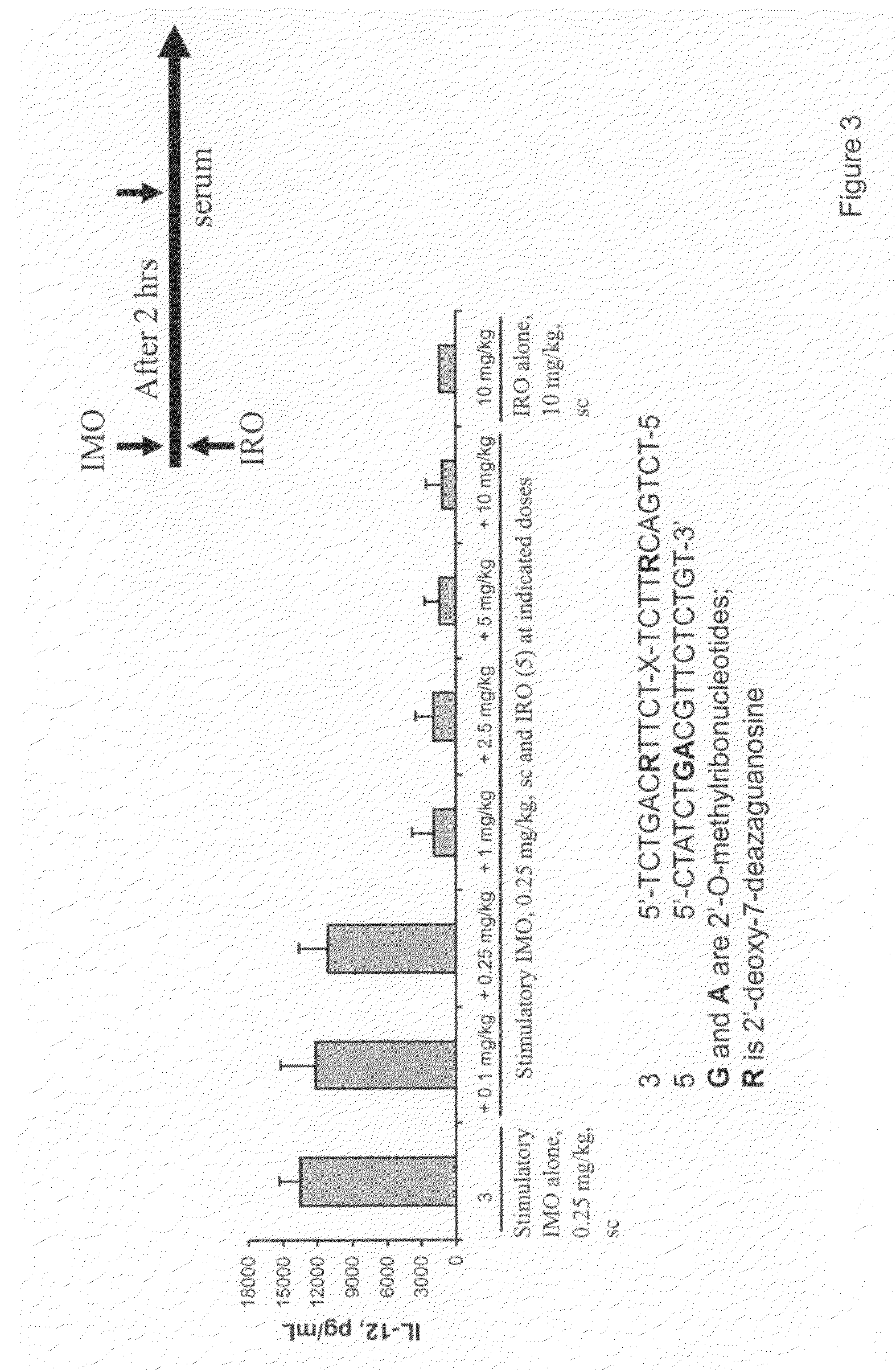
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
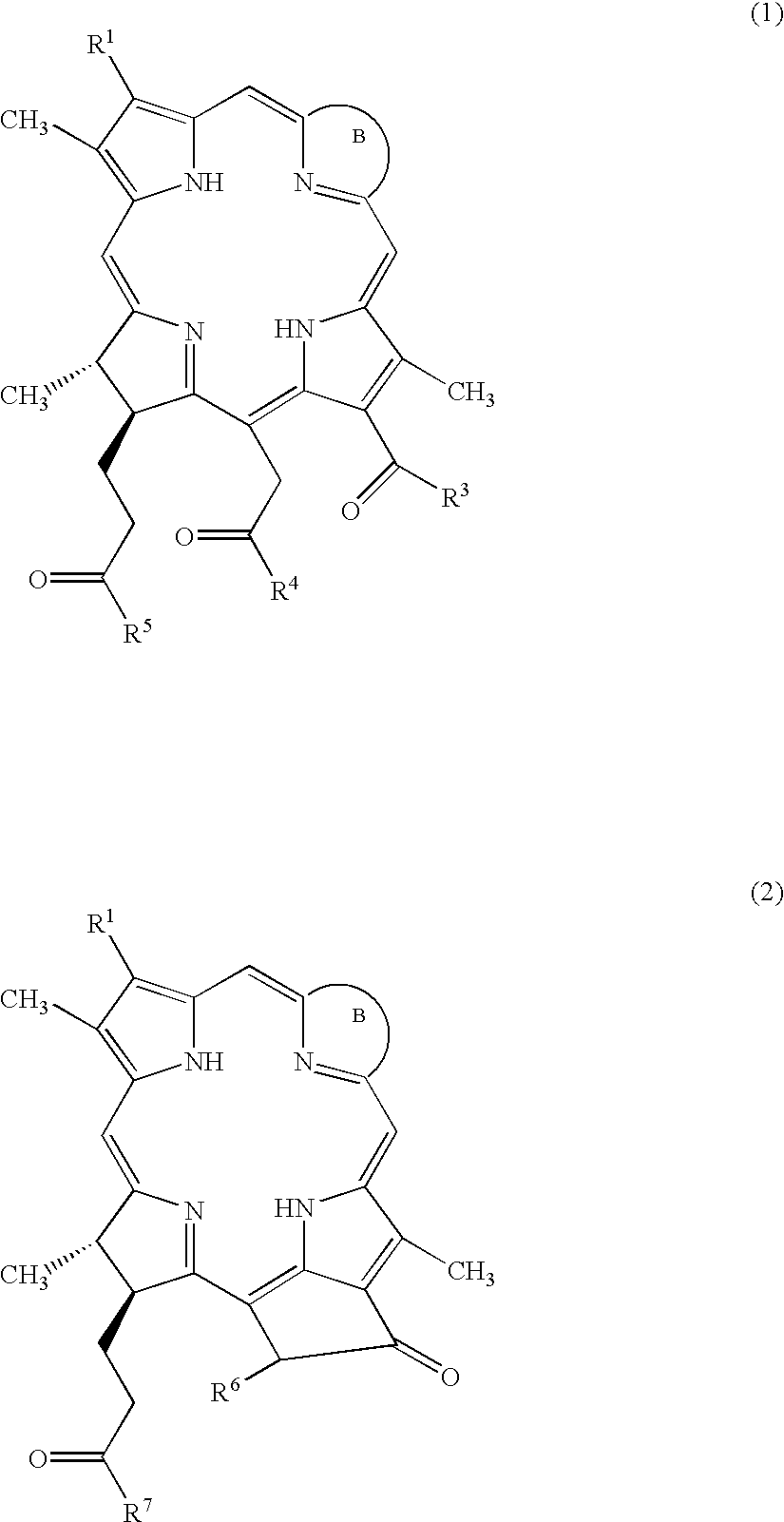
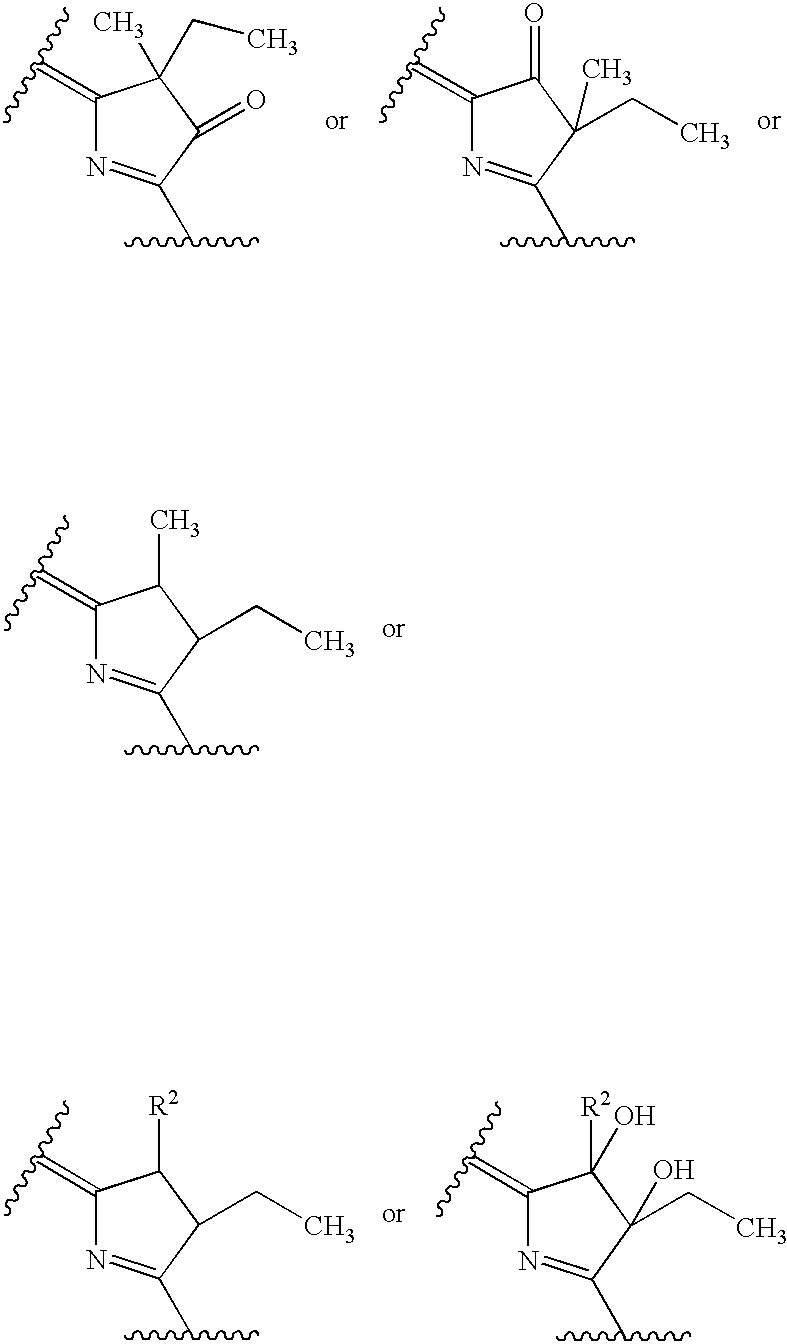
Water-soluble porphyrin derivatives for photodynamic therapy, their use and manufacture
InactiveUS6777402B2Easy and time-efficientBiocideEnergy modified materialsPorphyrinReversed-Phase Liquid Chromatography
Owner:BIOLITEC UNTERNEHMENSBETEILLIGUNGS II AG +1
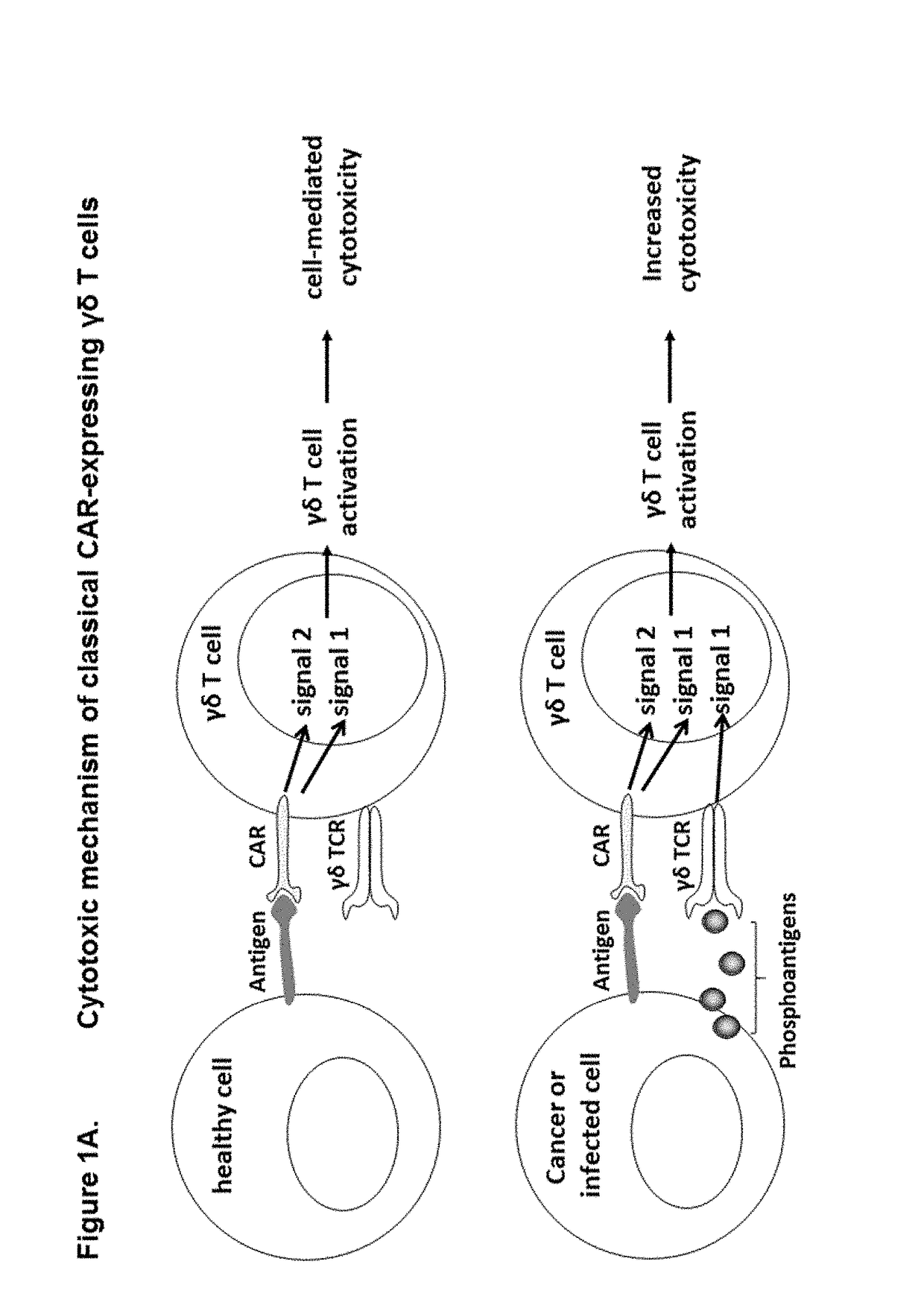
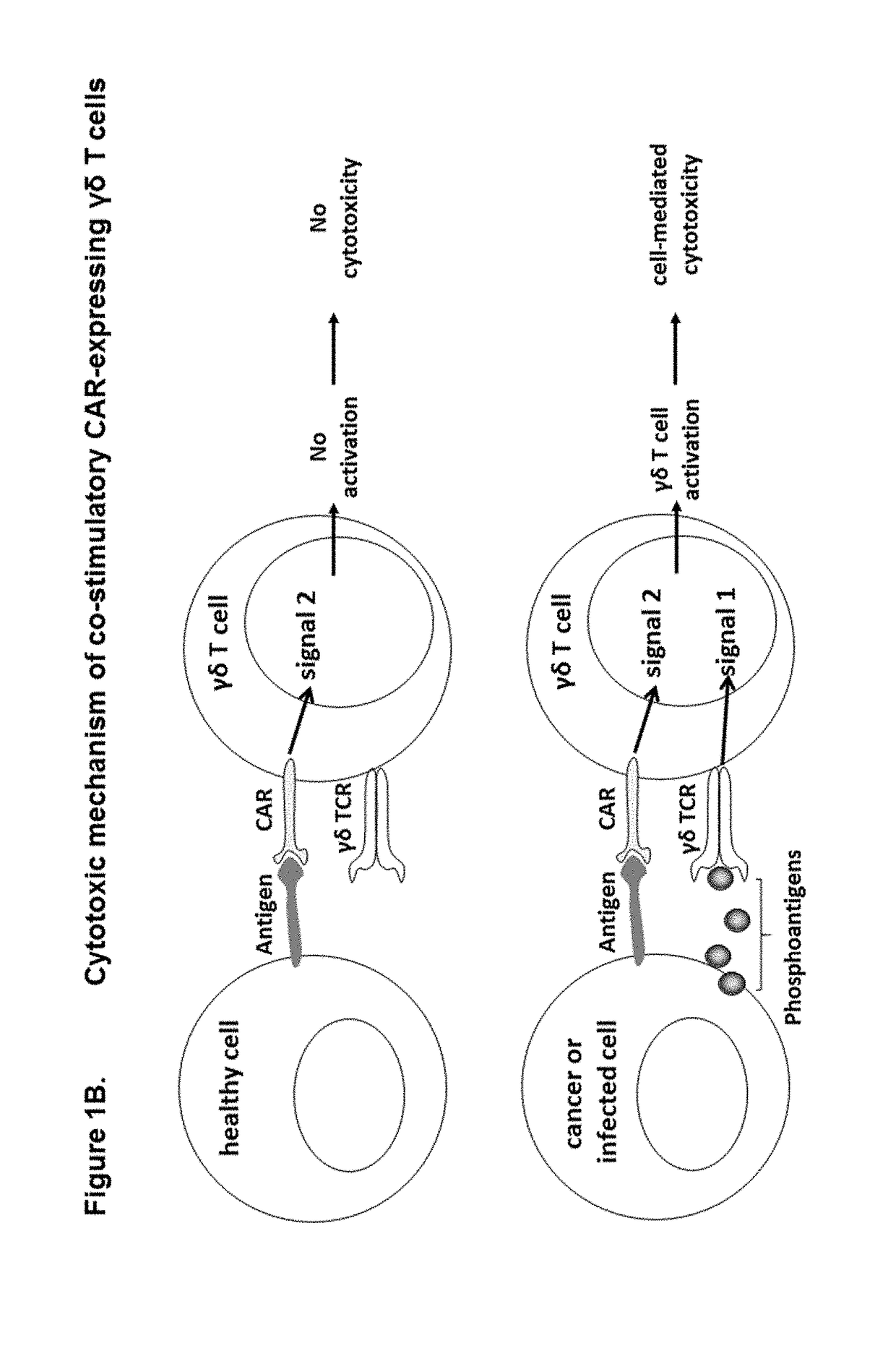
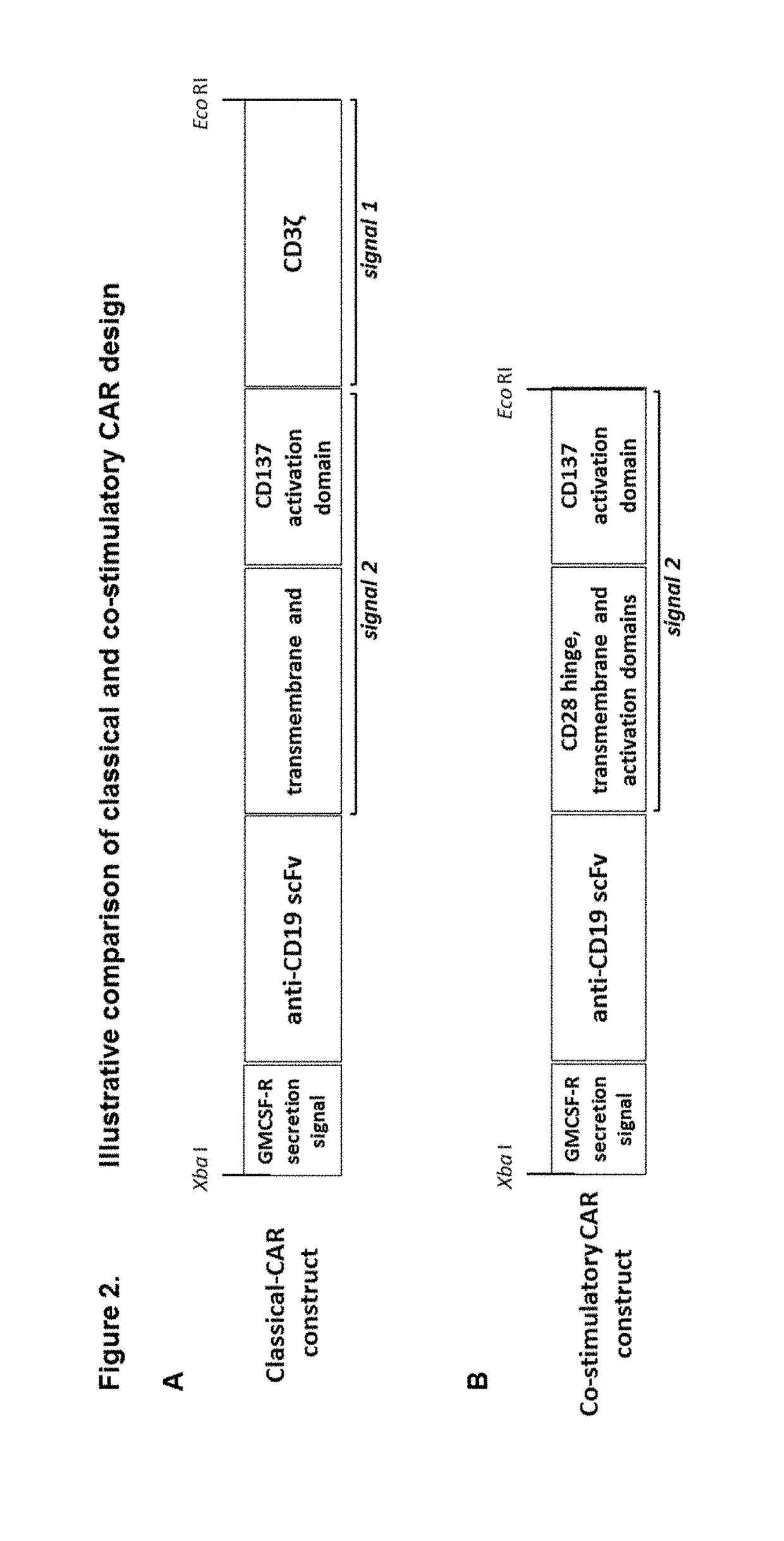
Modified gamma delta t cells and uses thereof
ActiveUS20180125889A1Antibacterial agentsOrganic active ingredientsGamma-delta T-Cell ReceptorAntigen receptors
The present invention provides composition and methods for the treatment of cancer or infectious diseases in a human. The invention includes the generation and administration of gamma delta T cells that express chimeric antigen receptors (CARs) comprising an antigen binding domain, a hinge domain, a transmembrane domain, a costimulatory signalling domain with the inclusion or not of a CD3 zeta signalling domain. Expression of CAR sequence omitting the CD3 zeta signalling domain in gamma delta T cells, provides for a CAR-T therapy in vivo, which will effect cytolysis only on target cells providing ligands for activation of the gamma delta T cell receptor (TCR).
Owner:TC BIOPHARM LTD
Recombinant cancer cell secreting modified heat shock protein-antigenic peptide complex
The present invention relates to methods for purifying immunogenic, prophylactically and therapeutically effective complexes of modified heat shock proteins noncovalently associated with antigenic peptides of cancer or infected cells. The claimed methods comprise the constructing of a nucleotide sequence encoding a secretable modified heat shock protein, expressing the sequence in an appropriate host cell, recovering the immunogenic complexes from the cell culture and the cells, and purifying the immunogenic complexes by affinity chromatography. Large amounts of such immunogenic complexes can be obtained by large-scale culturing of host cells containing the genetic sequence. The complexes can be used as a vaccine to elicit specific immune responses against cancer or infected cells, and to treat or prevent cancer or infectious diseases.
Owner:UNIV OF MIAMI
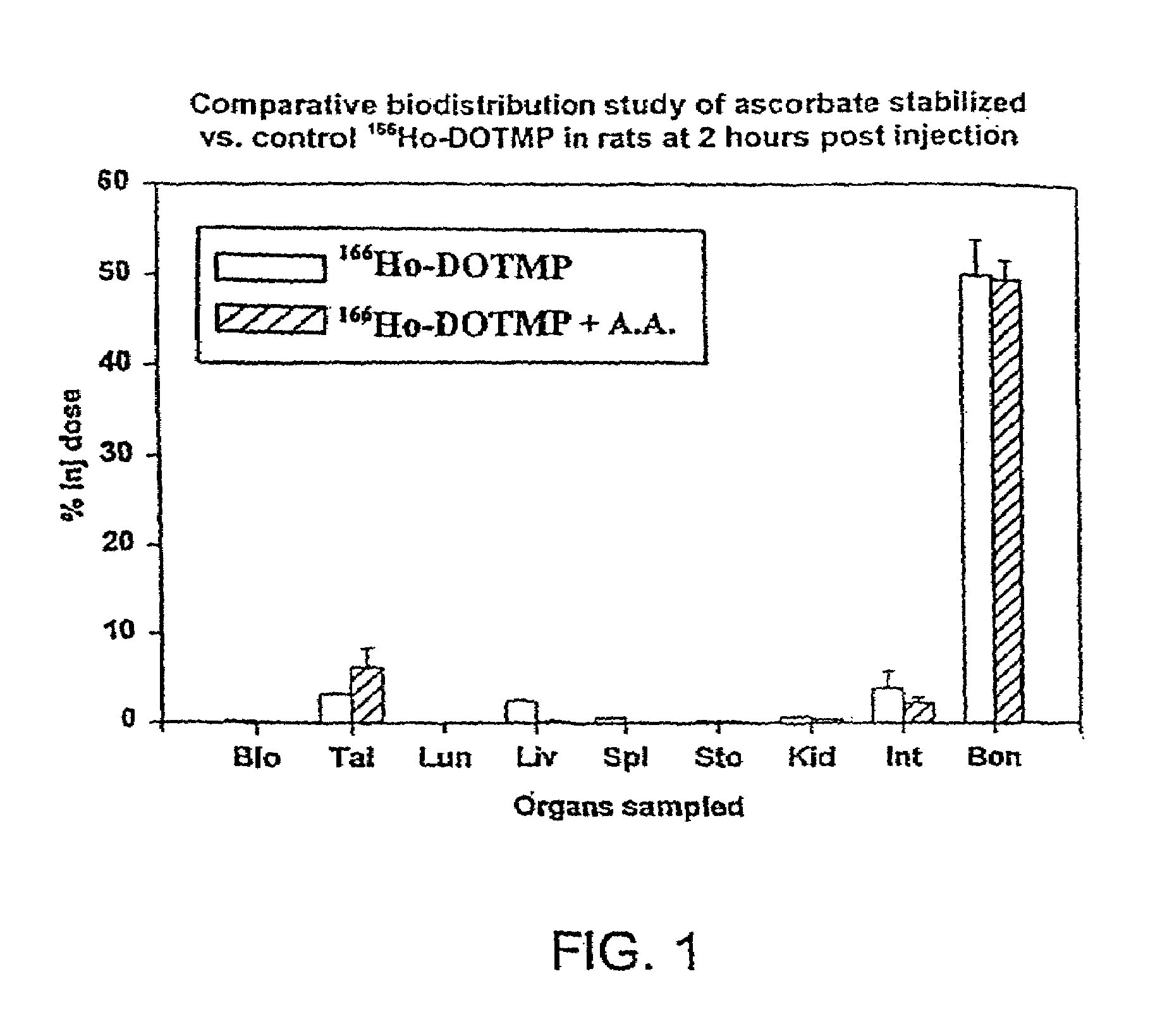
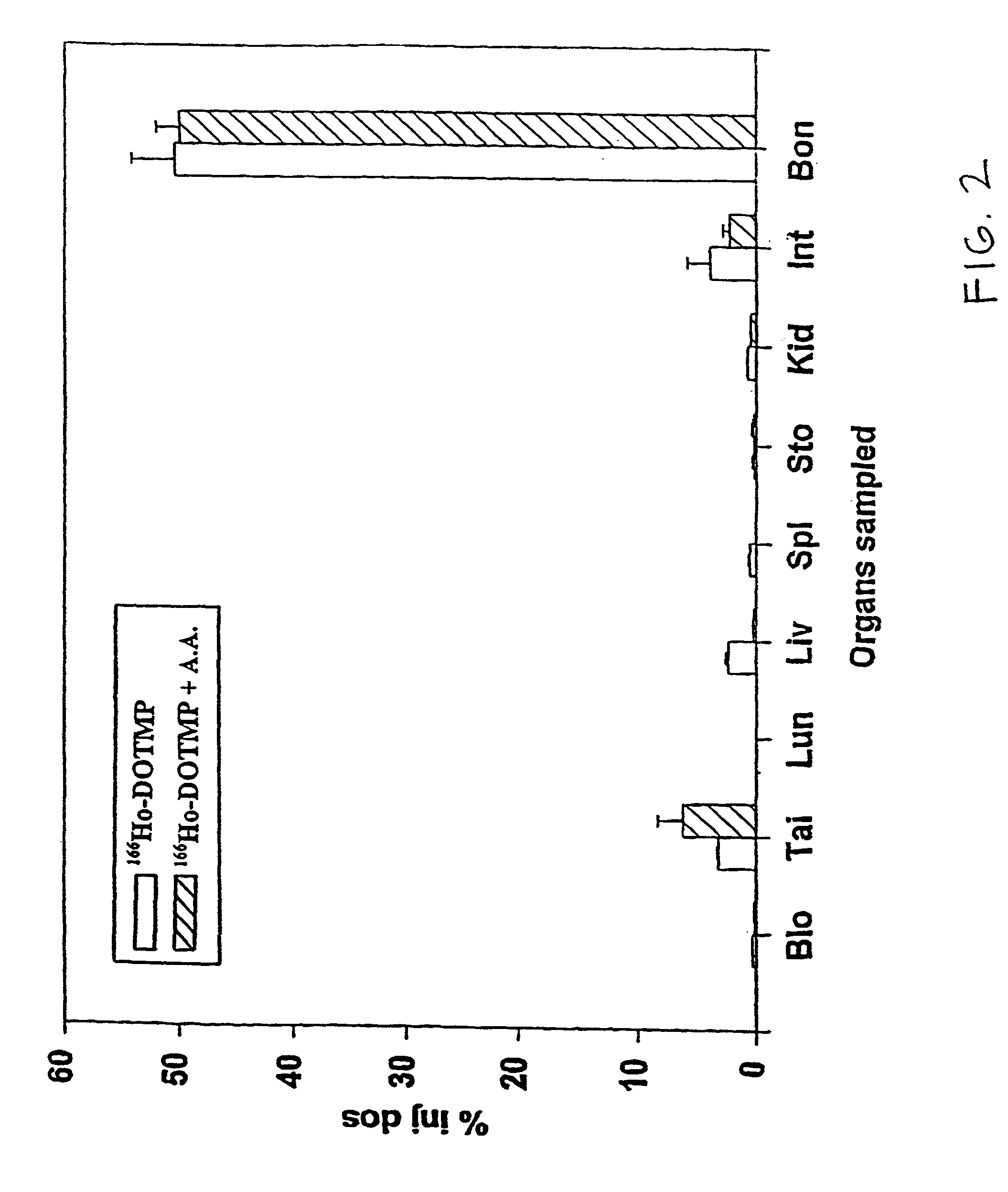
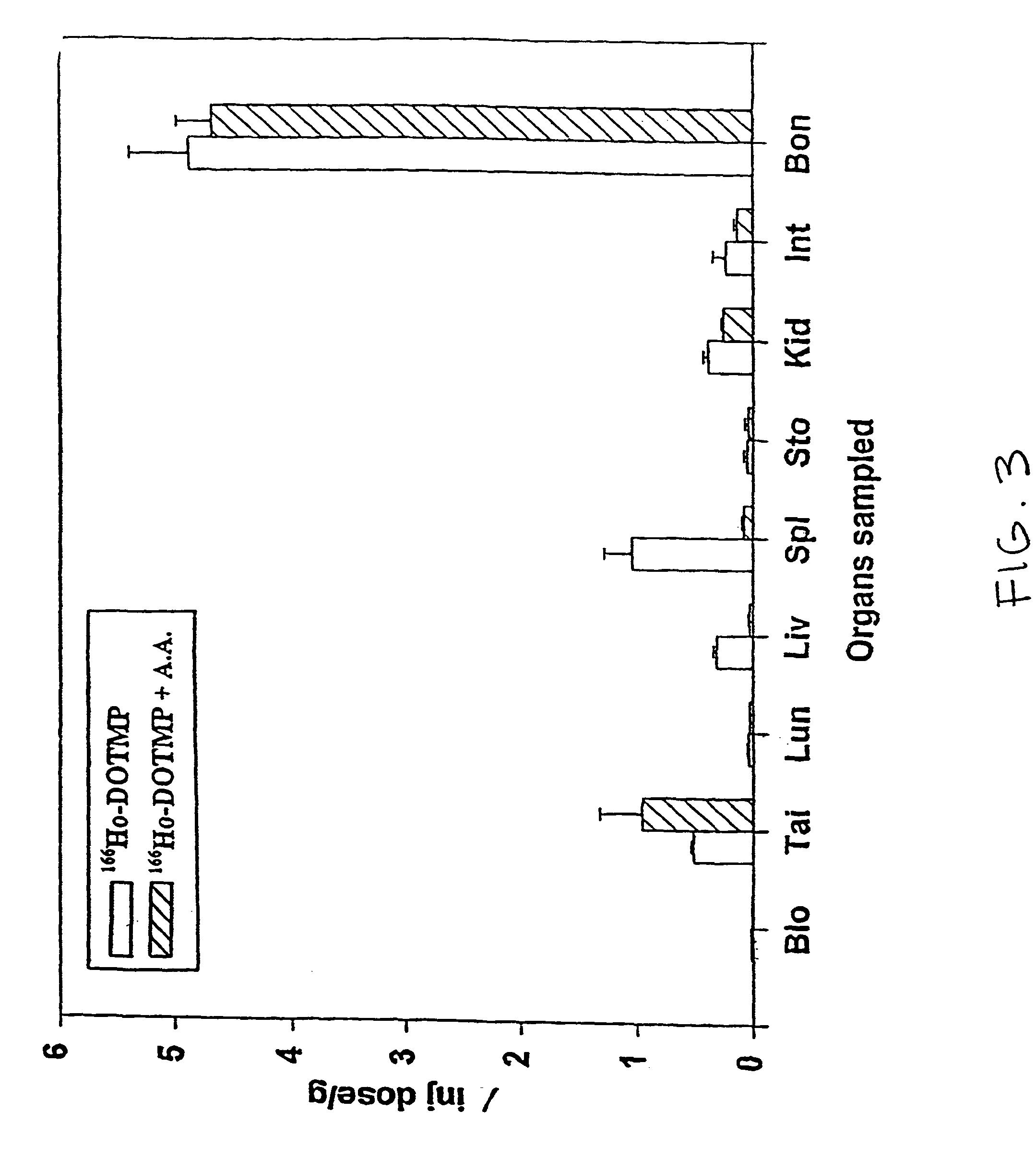
Skeletal-targeted radiation to treat bone-associated pathologies
InactiveUS7094885B2Promote resultsMinimal damageBiocideRadioactive preparation carriersAutoimmune diseaseBULK ACTIVE INGREDIENT
The present invention relates to a method of suppressing bone marrow (BM) and treating conditions that arise in or near bone such as cancer, myeloproliferative diseases, autoimmune diseases, infectious diseases, metabolic diseases or genetic diseases, with compositions having as their active ingredient a radionuclide complexed with a chelating agent such as macrocyclic aminophosphonic acid.
Owner:PONIARD PHARMA INC
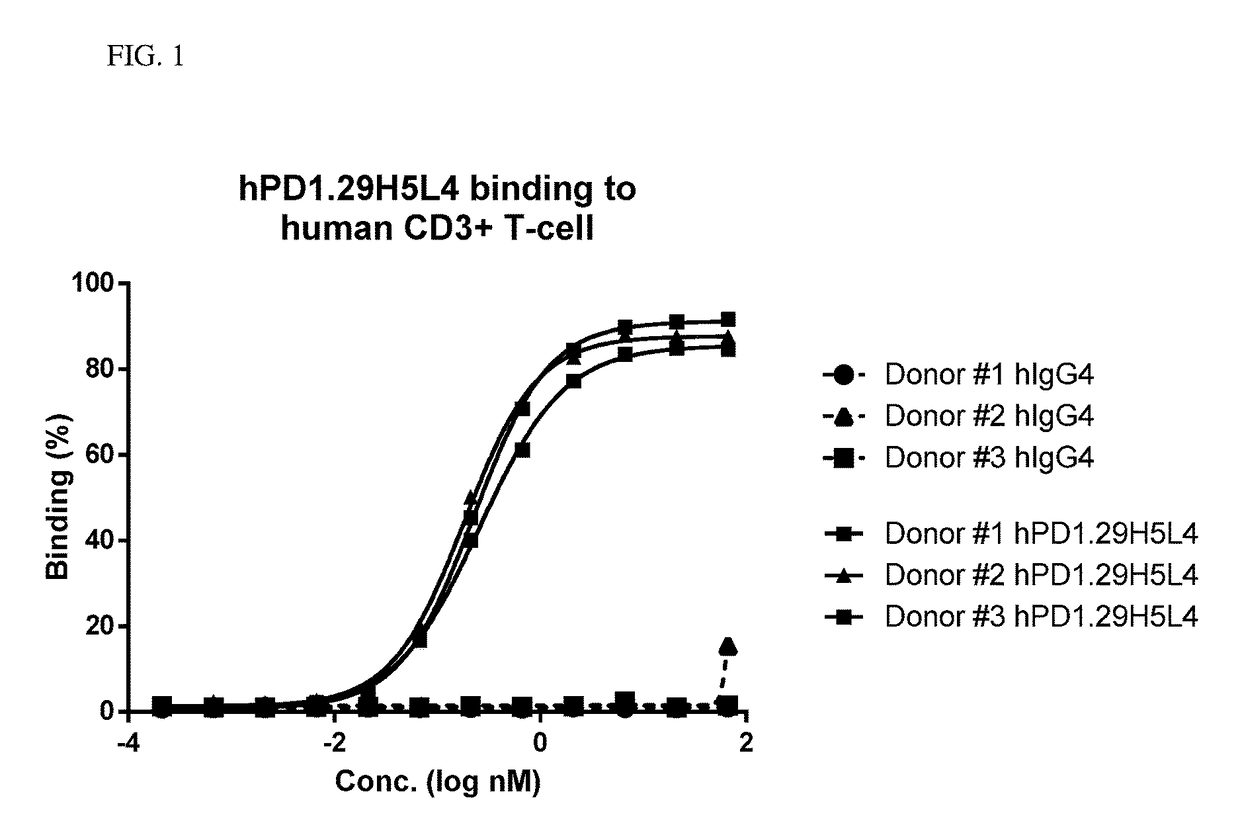
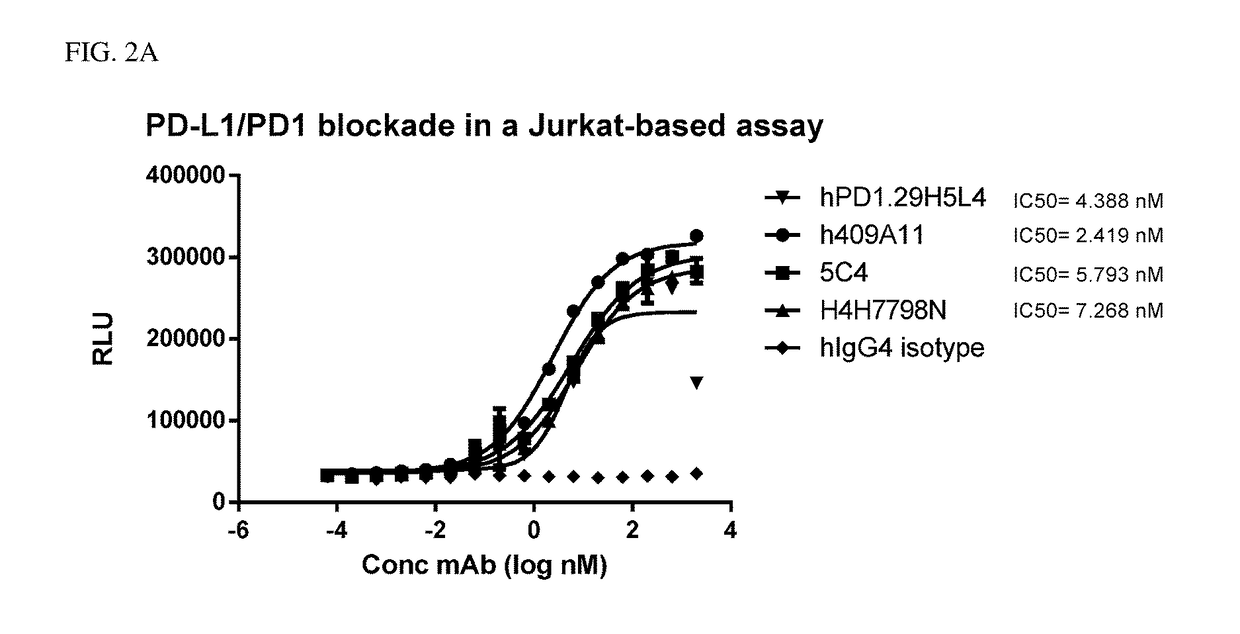
Anti-pd-1 antibodies
ActiveUS20180030137A1Bacteria material medical ingredientsBiological material analysisDiseaseNucleotide
The present invention relates to anti-PD-1 antibodies, as well as use of these antibodies in the treatment of diseases such as cancer and infectious disease. These antibodies have CDRs as provided in the enclosed sequences. Also part of the invention are nucleic acids encoding these antibodies, expression vectors comprising such nucleotide sequences and host cells that comprise said nucleotide sequences or expression vectors.
Owner:SAIROPA BV
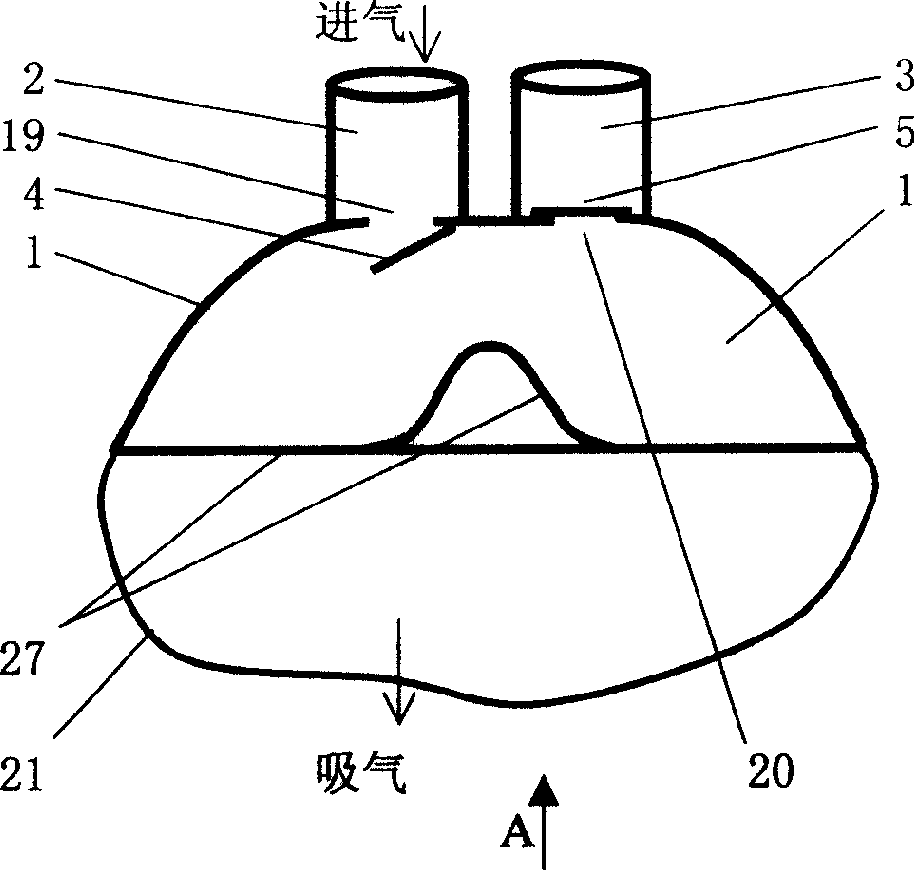
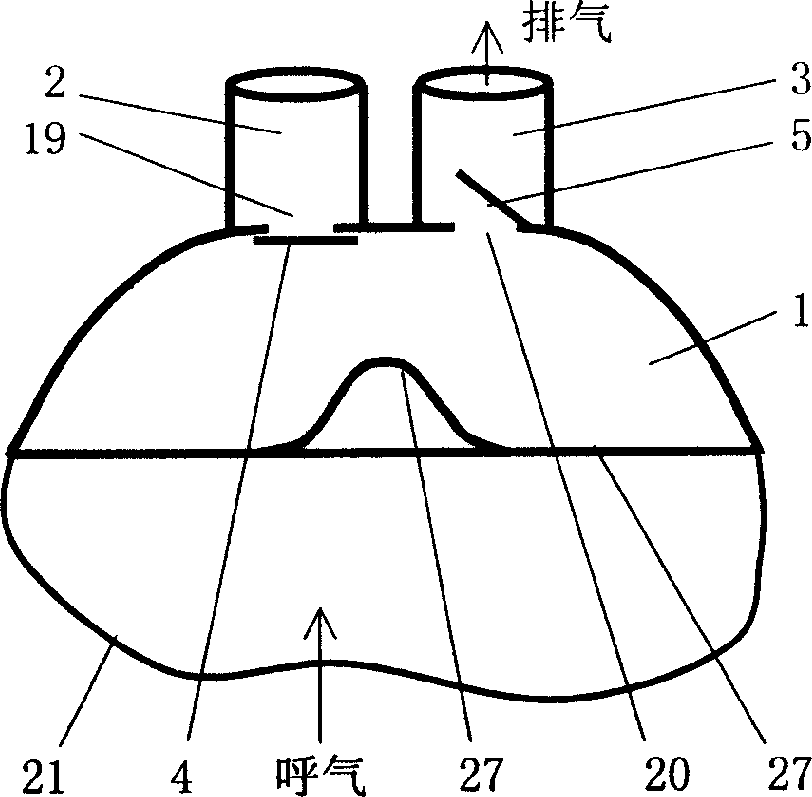
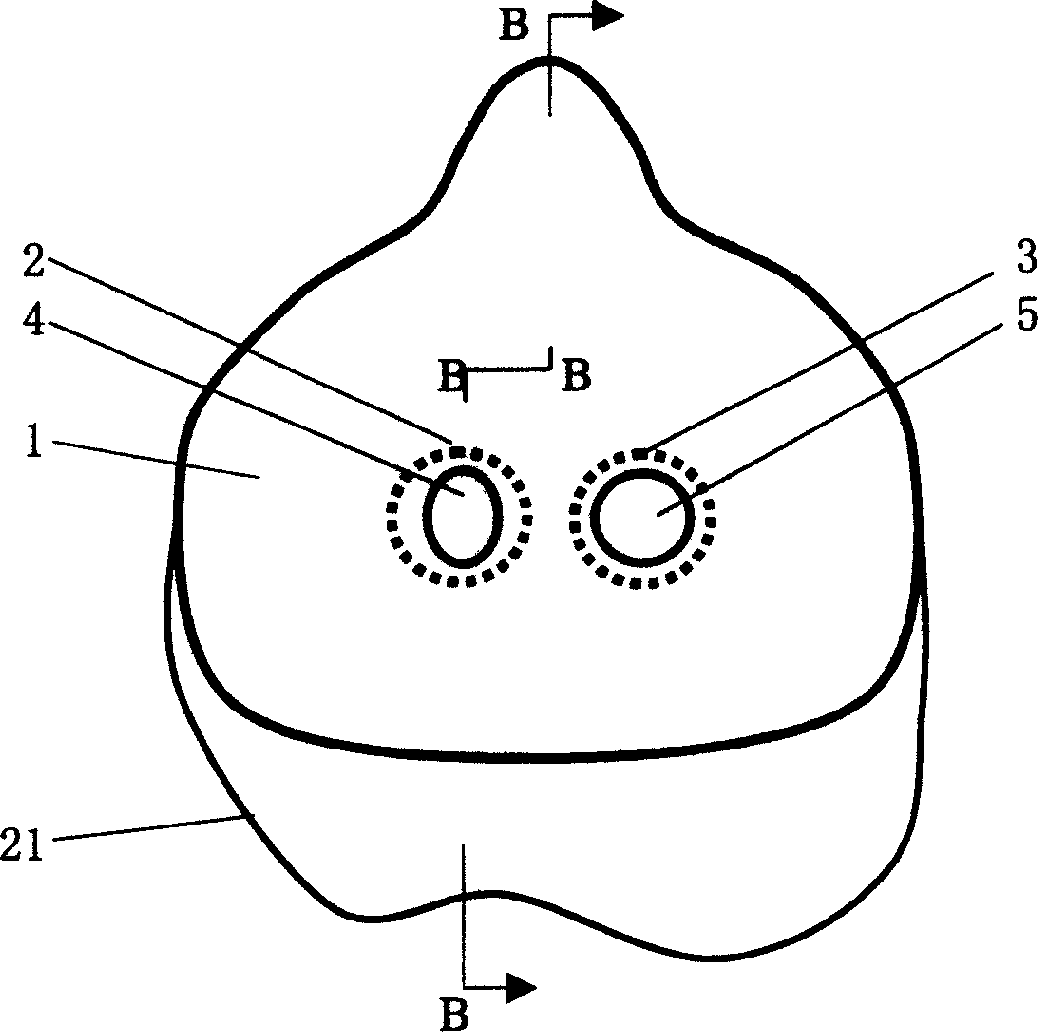
Mouth mask special for respiratory tract infectious disease sickrooms and its using method
InactiveCN1572338AReduce pollutionReduce cross infectionRespiratory masksPoison protectionDuring expirationExhalation
The special mouth mask includes a bowl shape mouth mask body and fixing belts. The mouth mask body is connected with an air suction passage and an exhalation passage. The air suction passage and the exhalation passage are respectively disposed with an air suction shutter and an exhalation shutter. The air suction shutter opens inward and the exhalation shutter opens outward. The using method for the mouth mask is to connect the air suction passage with an air intake pipe in sickroom and connect the exhalation passage with an air discharge pipe by soft pipes, the air intake and output pipes in each sickroom are connected to total air intake and discharge pipes to form the special mouth mask respiratory system. The respiratory system has three work modes of natural respiration, ventilation respiration and compulsion respiration. The mouth mask can collect the gas including virus exhaled by patient, and discharge the gas after disinfection to the outdoor air, and reduce the sickroom pollution; the patient can suck outdoor fresh air; the operation operating medical person can reduce the rate of infection by wearing the mouth mask. The invention is simple in structure, low in manufacturing cost and can be easily actualized.
Owner:刘军屹
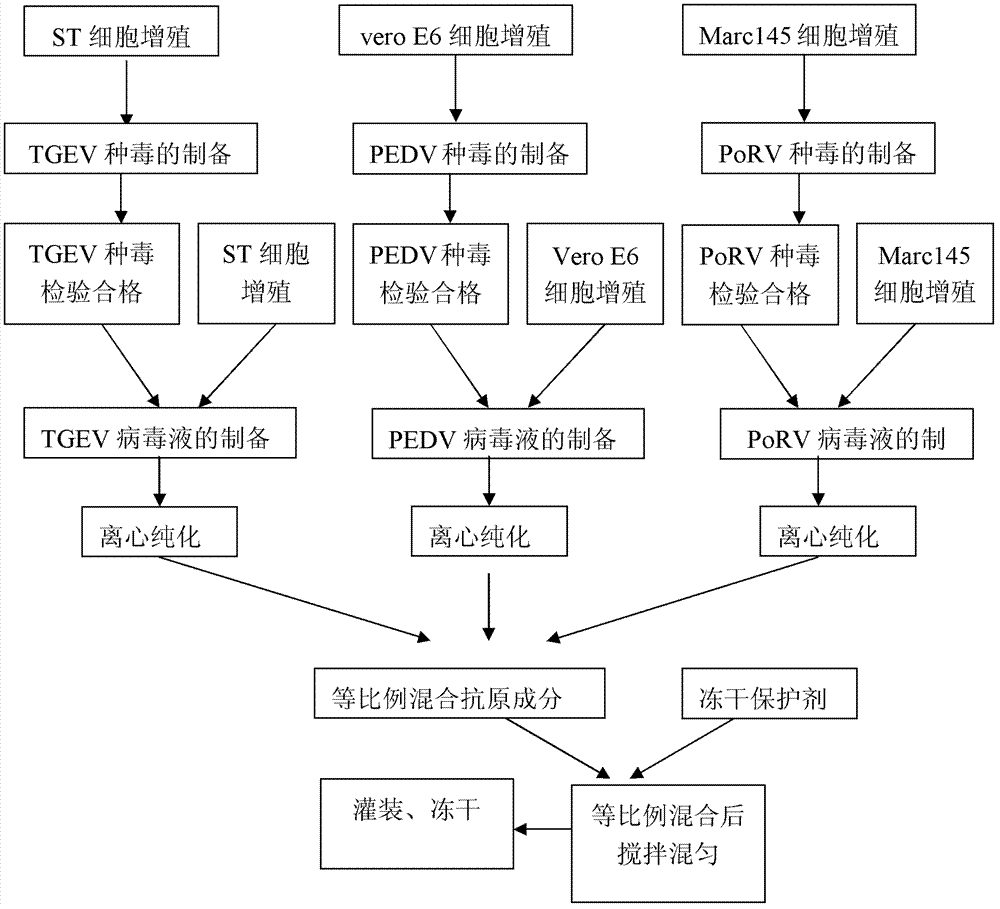
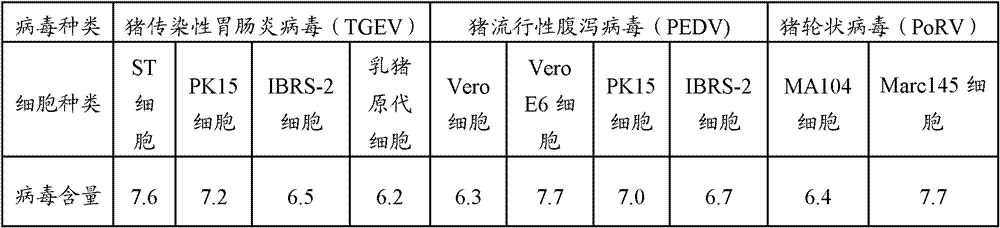
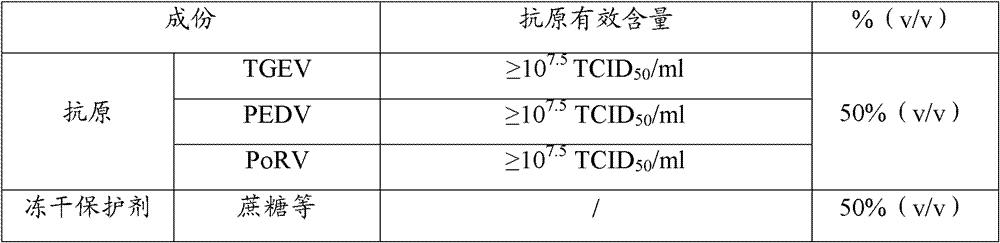
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Swine alpha-interferon gene synthesis, expression vector establishment and product preparing method
InactiveCN1611604AEfficient expressionBacteriaPeptide/protein ingredientsInfectious illnessAlpha interferon
One producing method of gene composition of pig a-interferon, expression vector structuring and its offspring includes synthesing pig a-interferon consisted of bacilluscoli preference codon and constructs highly active expression vector with this gene, and realizes the highly active expression in bacilluscoli of pig-a interferon, with 26% expression of the whole mycoprotein. At the same time, it provides a purely chemical engineering system of technique of induced expression, inclusion bodies separating and cracking, and complexity of albumen. Pig a-interferon has the functions of highly active antivirus and immunoregulation, and can be applied in preventing and treatment of pig contagion. This invention is able to produce efficient, safe, and cheap pig a-interferon which has no potential virus pollution. This lays a solid foundation for mass-production of pig a-interferon in our country.
Owner:BAOYITE BIOTECH INST QINGDAO
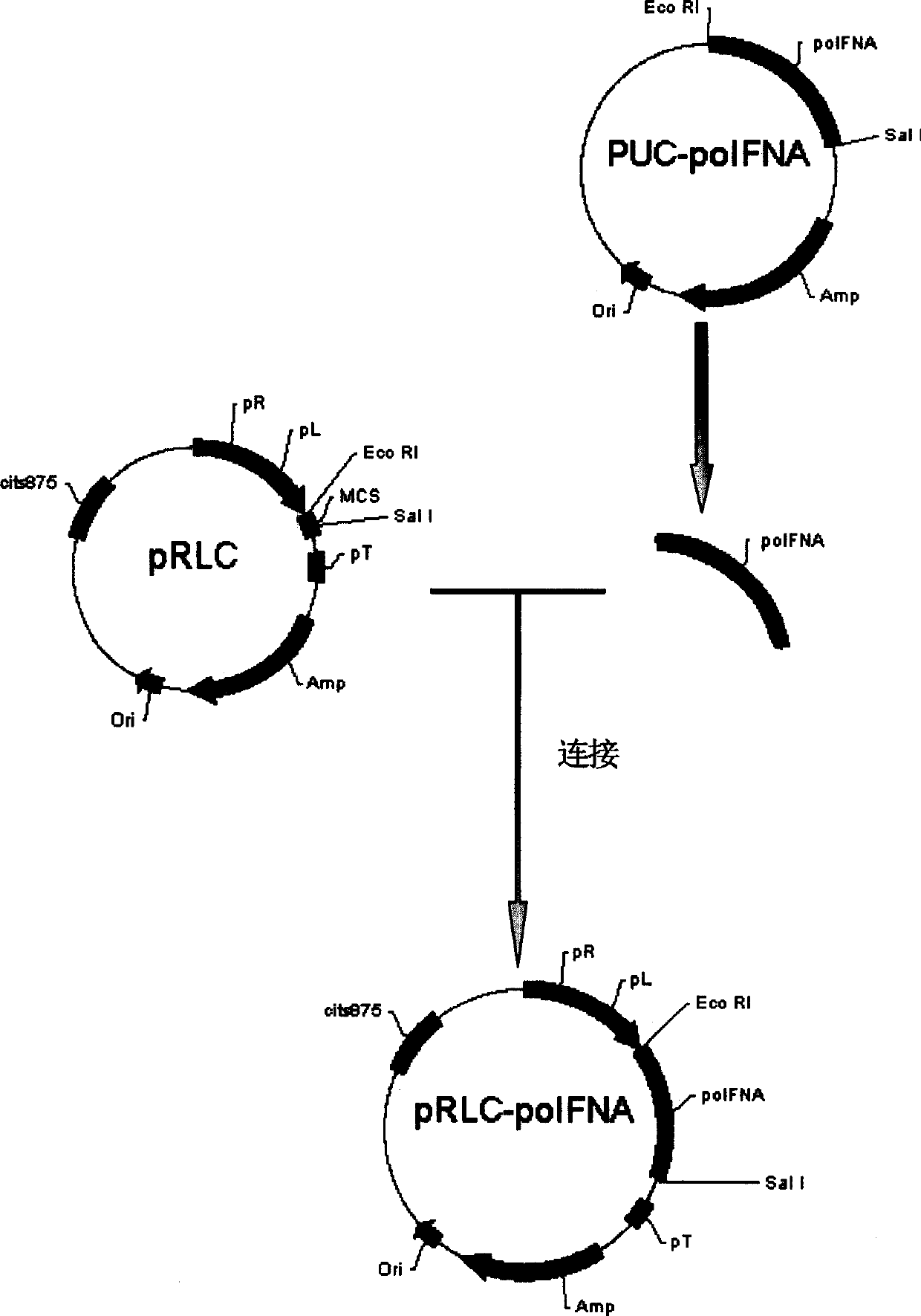
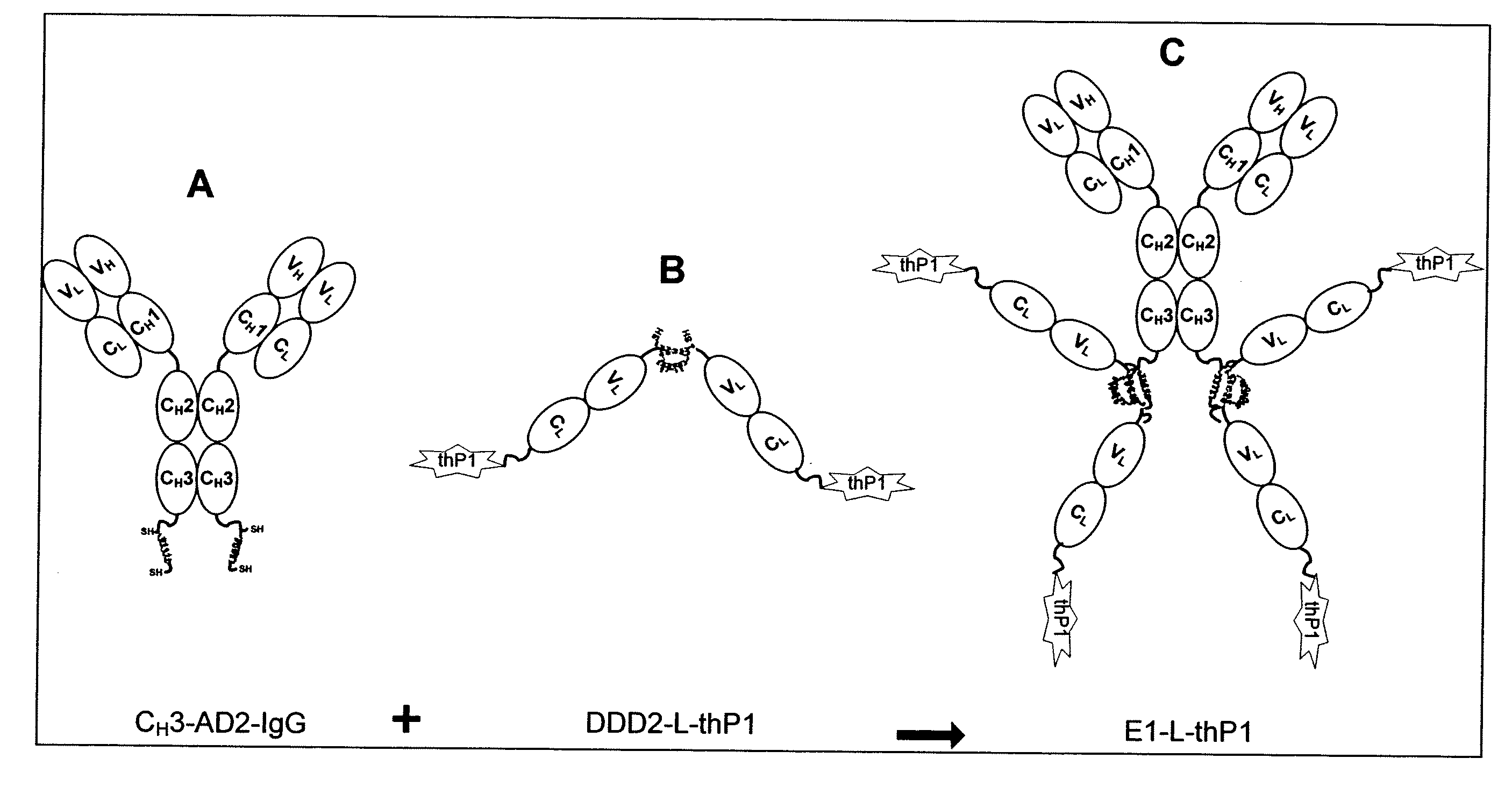
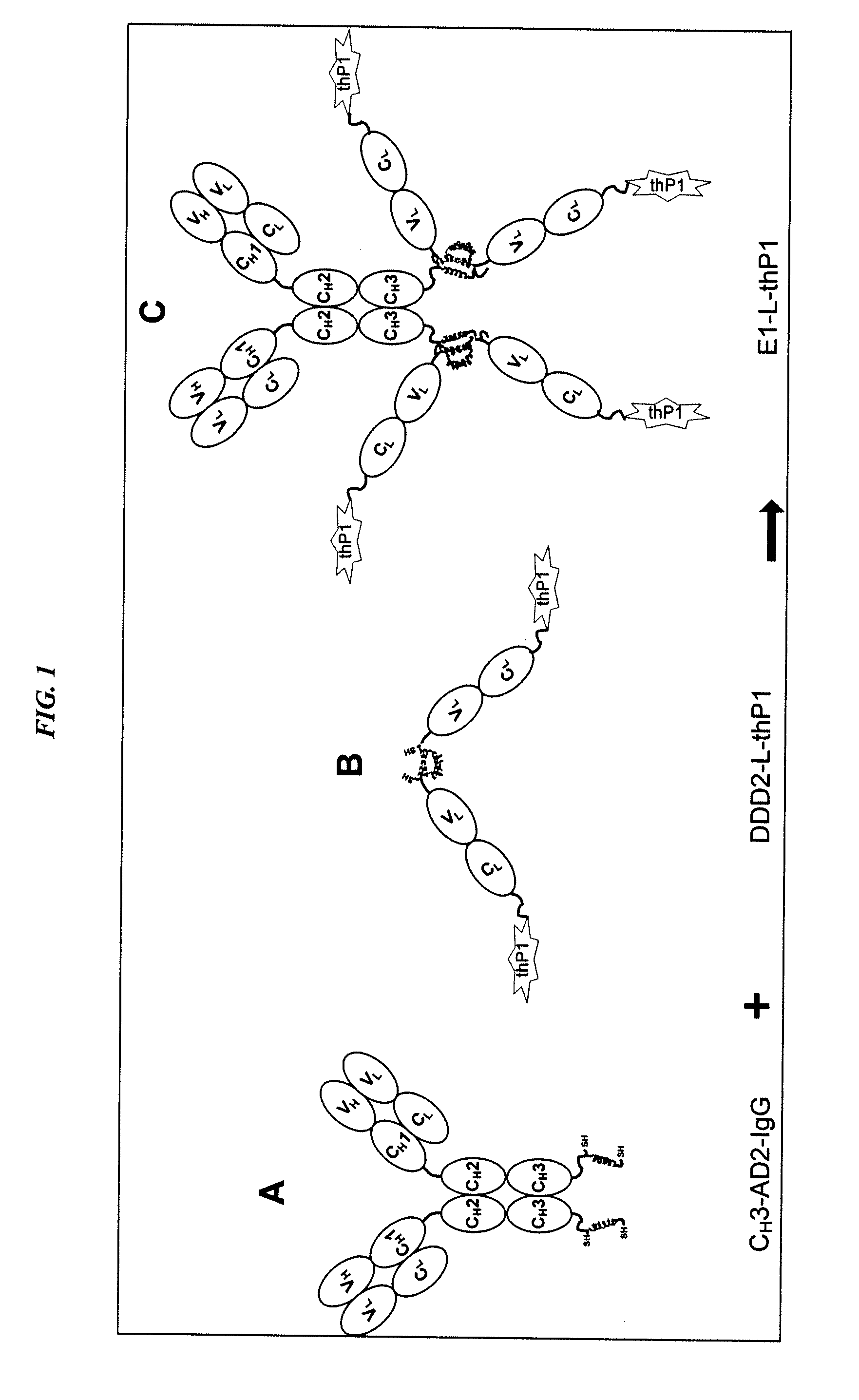
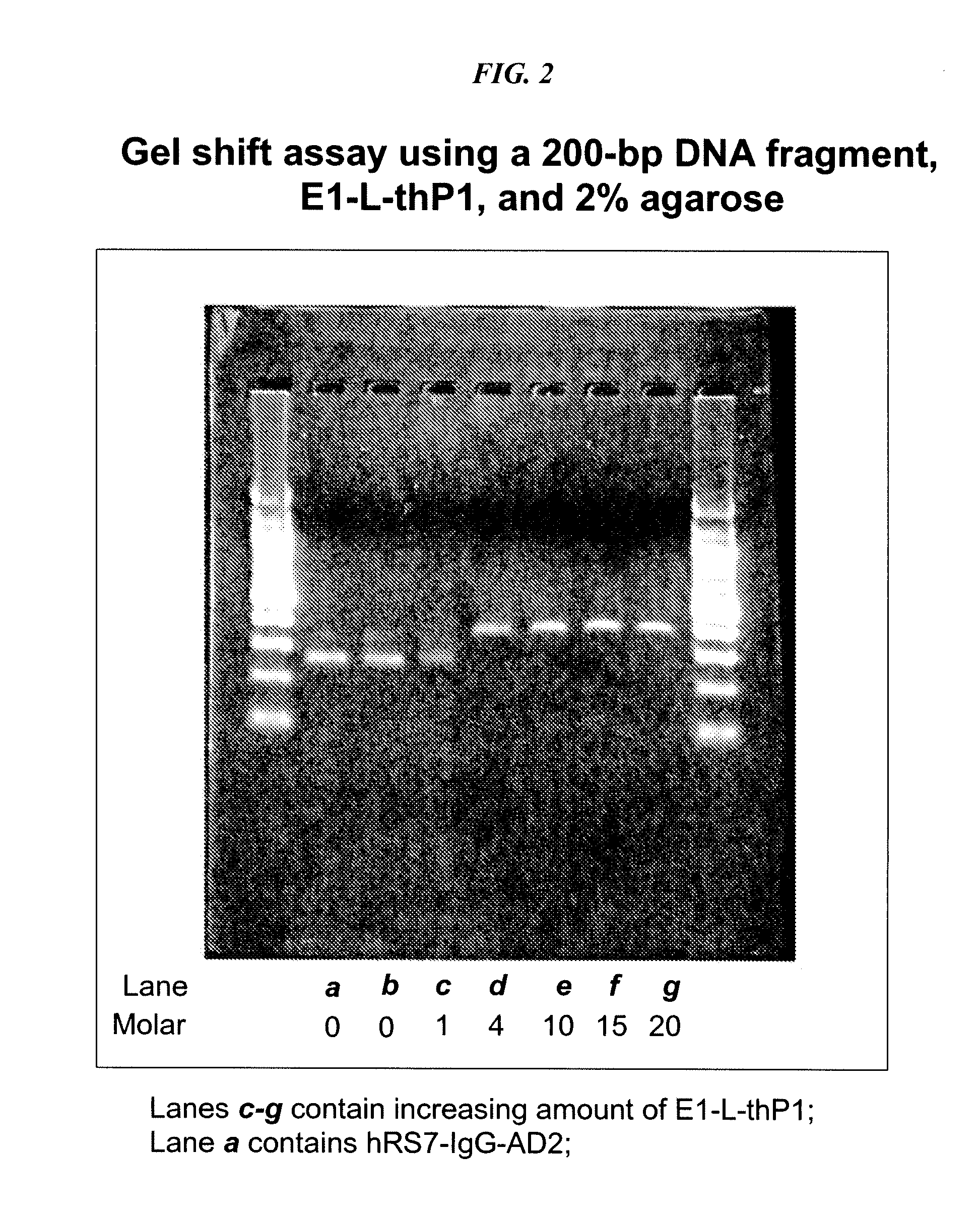
Dock-and-Lock (DNL) Complexes for Delivery of Interference RNA
ActiveUS20110123436A1Efficient deliveryHighly selective and specific deliveryNervous disorderAntibody mimetics/scaffoldsDendrimerAutoimmune responses
Described herein are compositions and methods of use of targeted delivery complexes for delivery of siRNA to a disease-associated cell, tissue or pathogen. The targeted delivery complex comprises a targeting molecule, such as an antibody or fragment thereof, conjugated to one or more siRNA carriers. In preferred embodiments the siRNA carrier is a dendrimer or protamine and the targeting molecule is an anti-cancer antibody, such as hRS7. More preferably, the antibody or fragment is rapidly internalized into the target cell to facilitate uptake of the siRNA. Most preferably, the targeted delivery complex is made by the DNL technique. The compositions and methods are of use to treat a variety of disease states, such as cancer, autoimmune disease, immune dysfunction, cardiac disease, neurologic disease, inflammatory disease or infectious disease.
Owner:IBC PHARMACEUTICALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com