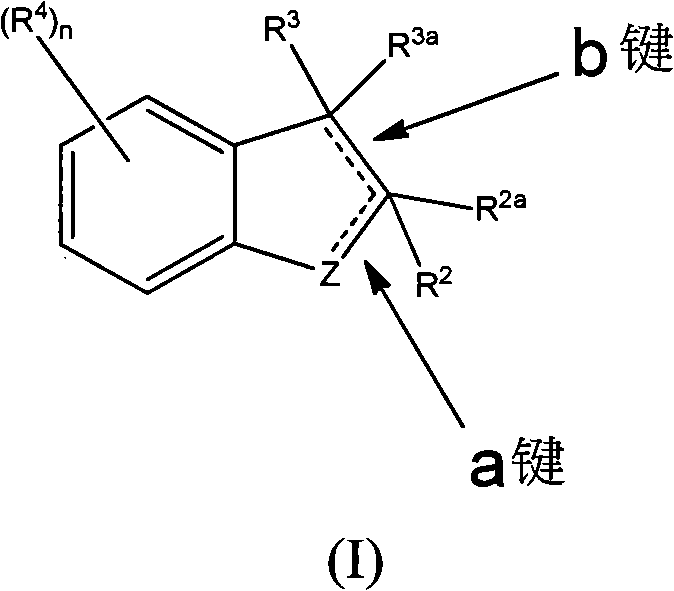
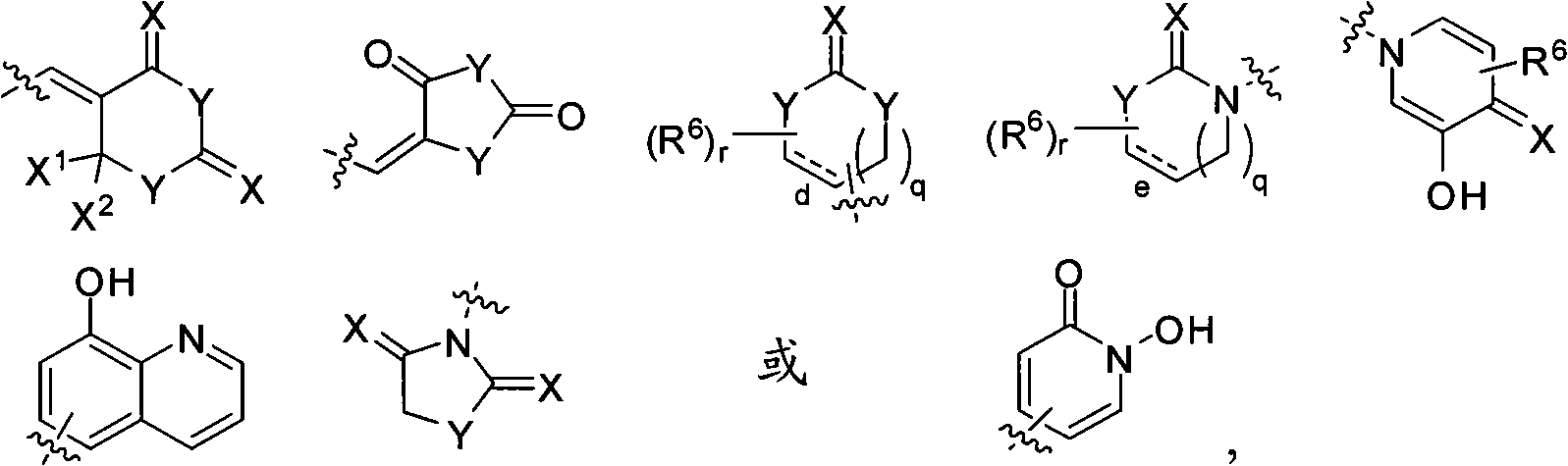
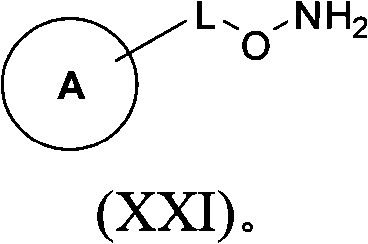Ido inhibitors
A diluent and pharmaceutical technology, applied in the direction of anti-inflammatory agent, antiviral agent, non-central analgesic agent, etc., can solve the problem of anatomical separation of fetal antigen immaturity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0834] Method A: Synthesis of allosteric dithiocarbamates with S-alkyl groups (Scheme 1)
[0835] Diagram 1.
[0836]
[0837] at 0 °C in anhydrous CH 2 Cl 2 A solution of tryptamine 1 (1.1 equiv) was treated with triethylamine (1.1 equiv) followed by carbon disulfide (1.1 equiv) in (10 mL) and stirred for 30 minutes. After this time, alkyl bromide 2 (1.2 equiv unless otherwise stated) was added and the reaction was allowed to warm to room temperature and stirred overnight. The reaction mixture was then poured into 1M H 2 SO 4 and extracted with ethyl acetate (3 x 10 mL). The combined organic layers were washed with brine, in anhydrous Na 2 SO 4 Dry, then filter and concentrate. Purification by flash chromatography (silica, ethyl acetate / hexanes) afforded the desired product. pass 1H nuclear magnetic resonance spectroscopy observation, dithiocarbamate products generally exist in the form of about 7:3 tautomeric mixture, and listed spectral data.
Embodiment 2
[0839] Phenylethyl 2-(1H-indol-3-yl)ethyldithiocarbamate [Compound 00001]
[0840]
[0841] The reaction of 1 with (2-bromoethyl)benzene (1.2 eq.) was carried out as described in Method A. Purification by flash chromatography (silica, gradient 12% ethyl acetate / hexanes to 100% hexanes) afforded the product 00001 (0.148 g, 35%) as a yellow oil: 1 H nuclear magnetic resonance spectroscopy (500MHz, CDCl 3 ), 8.04(br s, 1H), 7.62(d, J=7.5Hz, 1H), 7.38(d, J=8.0Hz, 1H), 7.32-7.25(m, 2H), 7.25-7.20(m, 4H ), 7.16-7.13(m, 1H), 7.07-7.05(m, 1H), 6.92(br s, 1H), 4.11-4.06(m, 2H), 3.48-3.45(m, 2H), 3.13(t, J = 6.5Hz, 2H), 2.97-2.94(m, 2H) and signal due to minor tautomers (about 31%): 3.78-3.74(m), 3.59-3.55(m), 3.10-3.08 (m), 3.05-3.01(m); ESI MSm / z 341[M+H] + , HPLC (method 1)>99% (AUC), t R = 13.9 minutes.
Embodiment 3
[0843] 4-Fluorophenethyl 2-(1H-indol-3-yl)ethyldithiocarbamate [Compound 00003]
[0844]
[0845] Reaction of 1 with 4-fluorophenethyl bromide (0.6 equiv) was carried out as described in Method A. Purification by flash chromatography (silica, gradient 12% ethyl acetate / hexanes to 100% ethyl acetate) afforded the product 00003 (0.084 g, 31%) as a yellow oil: 1 H nuclear magnetic resonance spectroscopy (500MHz, CDCl 3 ), 8.04(br s, 1H), 7.63(d, J=8.0Hz, 1H), 7.39(d, J=8.0Hz, 1H), 7.24-7.13(m, 4H,), 7.08-7.04(m, 1H), 7.00-6.94(m, 2H), 6.92(br s, 1H), 4.11-4.07(m, 2H), 3.46-3.43(m, 2H), 3.14(t, J=7.0Hz, 2H), 2.94-2.91(m, 2H) and signal due to slight tautomers (about 31%): 3.79-3.75(m), 3.55-3.52(m), 3.11-3.09(m), 3.01-2.98( m); ESI MSm / z 359 [M+H] + , HPLC (method 1) 95.3% (AUC), t R = 13.7 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com