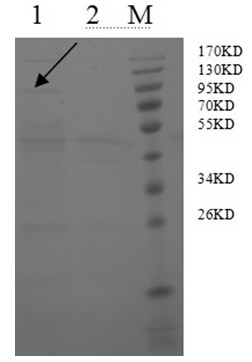
Preparation method of porcine epidemic diarrhea recombinant adenovirus vaccine
A technology of porcine epidemic diarrhea and recombinant adenovirus, which is applied in the field of biological vaccines, can solve the problems of low expression level, poor palatability of tobacco, and inability to modify the prokaryotic expression system, so as to prevent the occurrence of PED and have strong ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
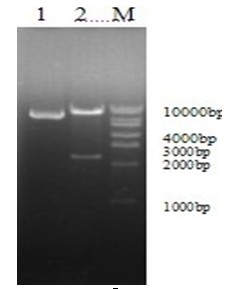
[0024] Example 1 Construction of recombinant adenovirus plasmid pAdeno-S1
[0025] 1.1 Linearization and purification of the recombinant shuttle plasmid pShuttle-CMV-S1 Add in a 0.5mL centrifuge tube: 20.0ul of the recombinant shuttle plasmid, 4.0ul of 10×NE Buffer4, 0.4ul of 100×BSA, 1.0ul of PmeⅠ, add sterilized double distilled water To a total volume of 40.0ul; 37°C for 4.0h, use the DNA gel recovery kit to recover the digested product;
[0026] 1.2 Preparation of BJ5183 Competent Cells Carrying Adenovirus Backbone Plasmid pAdEasy-1 For the method, see molecular cloning;
[0027] 1.3 Transformation of BJ5183 Competent Cells with Linearized Recombinant Shuttle Plasmid Take out BJ5183-AD-1 Competent Cells from the -80°C refrigerator, and after the ice bath melts, add 10ul linearized recombinant shuttle plasmid pShuttle-CMV-S1, mix gently, and place on ice Bath for 30min, then heat stress at 42°C for 90sec, and then ice bath for 5min; add 800μL fresh LB liquid medium preheat...
Embodiment 2
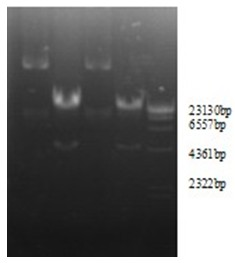
[0030] Example 2 Obtaining of recombinant adenovirus rAd-S1
[0031] 1.1 Linearization and purification of recombinant adenovirus plasmid pAdeno-S1 Add in a 0.5mL centrifuge tube: 30.0ul of recombinant adenovirus plasmid, 6.0ul of 10×NE Buffer1, 0.6ul of 100×BSA, 1.0ul of PacⅠ, add sterilized double distilled water To a total volume of 60.0ul; 37 ℃ water bath overnight, use the DNA gel recovery kit to recover the digested product;
[0032] 1.2 Recovery and subculture of AD-293 cells
[0033] 1.3 Transfect AD-293 cells with recombinant pAdeno-S1 linearized plasmid. Inoculate AD-293 cells on a 12-well cell culture plate at a density of 2×105 one day before transfection, and culture them in a 5% CO2 incubator at 37°C; Transfect when the cells grow to 80-90% full and the cells are in good condition. Discard the old cell culture medium before transfection and replace it with serum-free DMEM cell culture medium preheated at 37°C; in sterile 1.5mL Prepare the following solutions ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com