Humanized breast cancer antigen and antibody thereof
A breast cancer and antigen technology, applied in the direction of antibodies, receptors/cell surface antigens/cell surface determinants, anti-tumor drugs, etc., can solve the problem of decreased self-resistance of patients, ineffective early detection of breast cancer, and harm to normal tissues And immune cells and other problems, to achieve the effect of high expression positive rate and broad prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of new human breast cancer recombinant protein antigen
[0040] Firstly, the c-terminal primer of the new human breast cancer antigen Hv1 was designed, the sequence is as follows:
[0041] C-Hv1BamH I-F: 5'CGCGGATCC AAGACACGTTCAGAACGGC (SEQ NO.2)
[0042] EcoR I-R: 5'CGGAATTCCTAGTTCACTTCACCAAGAAG (SEQ NO.3)
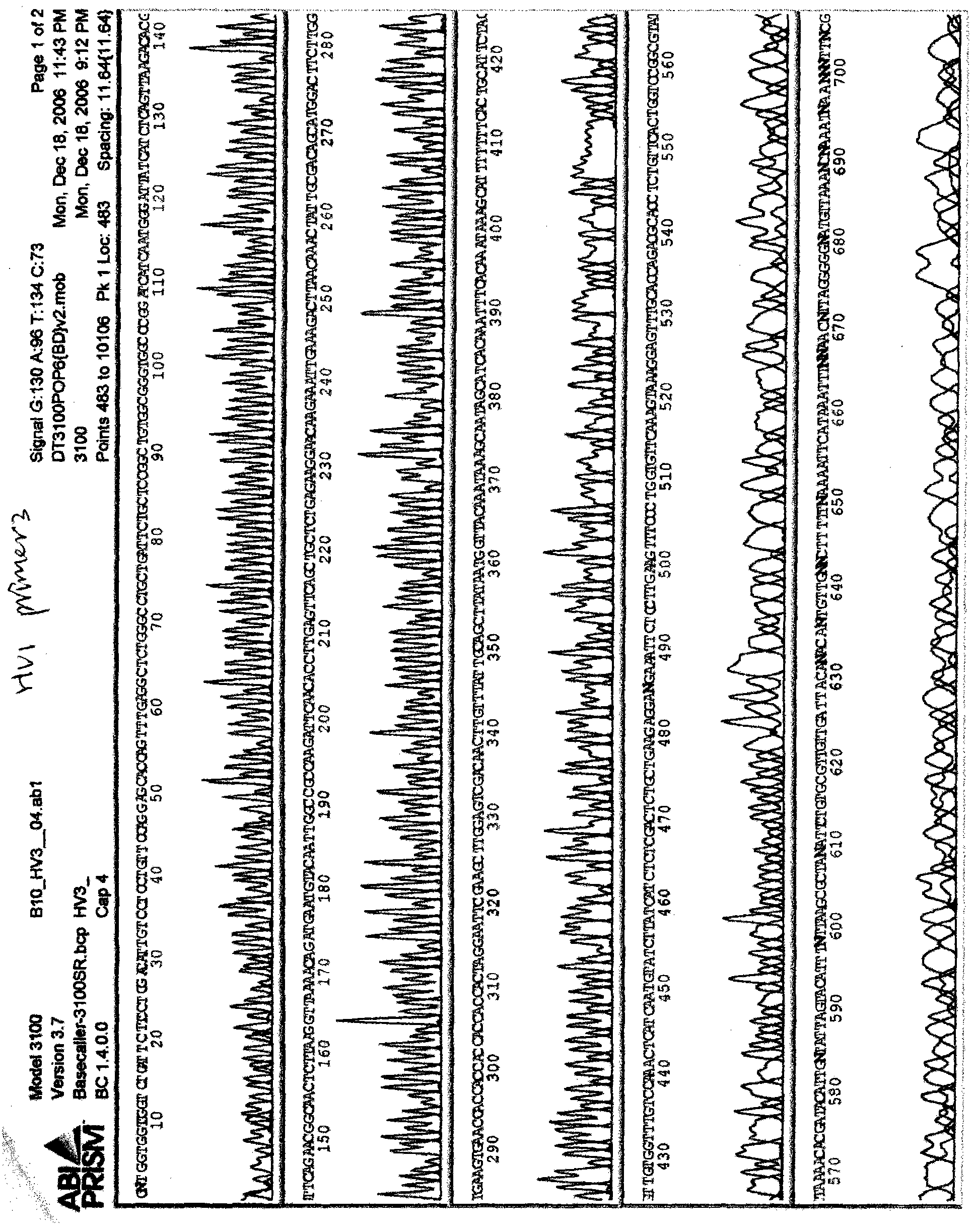
[0043] Through PCR amplification, the PCR product and the vector pGEX6p-1 were double digested and recovered, ligated to obtain a recombinant plasmid, and the sequencing results are shown in figure 1 . This clone is the clone of the recombinant expression plasmid containing the human breast cancer antigen DNA sequence of the present invention, and it is named pGEX6p-1-c-Hv1. pGEX6p-1-c-Hv1 was transformed into competent E.coli BL21(DE3) Escherichia coli, and 0.5mM IPTG inducer was used for protein expression. The recombinant protein antigen was purified by ion exchange column, and the amino acid sequence was shown in the sequence table SE...
Embodiment 2
[0044] Example 2: Preparation of anti-human breast cancer antigen Hv1 antibody
[0045] (1) Mouse polyclonal antibody
[0046] Using 50ug / recombinant protein antigen of human breast cancer antigen Hv1 mixed with complete Freund's adjuvant for multi-point subcutaneous immunization of Balb / c mice, 10 days later using 50ug / recombinant protein antigen of human breast cancer antigen Hv1 mixed without Balb / c mice were immunized subcutaneously with complete Freund's adjuvant for the second time. After the second immunization, the third immunization was carried out at intervals of one week. 10 days after the third immunization, blood was collected to measure the titer, when the titer was >1:10 6 , the animals were sacrificed to collect blood, the serum was collected by coagulation method, and centrifuged at low speed to remove the precipitate. Diluted with normal saline 1:2, purified on a Protein A affinity chromatography column, eluted with 0.1M glycine buffer at pH 2.8, neutraliz...
Embodiment 3
[0051] Preparation of anti-human breast cancer antigen Hv1 corresponding antibody derivatives
[0052] (1) Nuclide-antibody conjugates
[0053] (1) 188 Re-labeled anti-human breast cancer antigen Hv1 corresponding mouse monoclonal antibody
[0054] SnCl 2 The antibody was labeled by the direct reduction method, and the labeling efficiency and radiochemical degree of the antibody were measured immediately by rapid thin-layer chromatography (ITLC). After labeling, ITLC experiments show that: 188 The Re-antibody labeling rate is 90%, and the radiochemical purity is greater than 95%. 188 Re-antibody specific activity is 356MBq / mg. measured by ELISA 188 Re-antibody immunoactivity was 65%. The in vitro stability experiment of the labeled antibody showed that when the antibody was incubated at 37°C for 24 hours, no matter in normal saline or human serum albumin, the radioactive shedding was less than 5%, indicating that it was stable in vitro.
[0055] (2) 131 I-labeled anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com