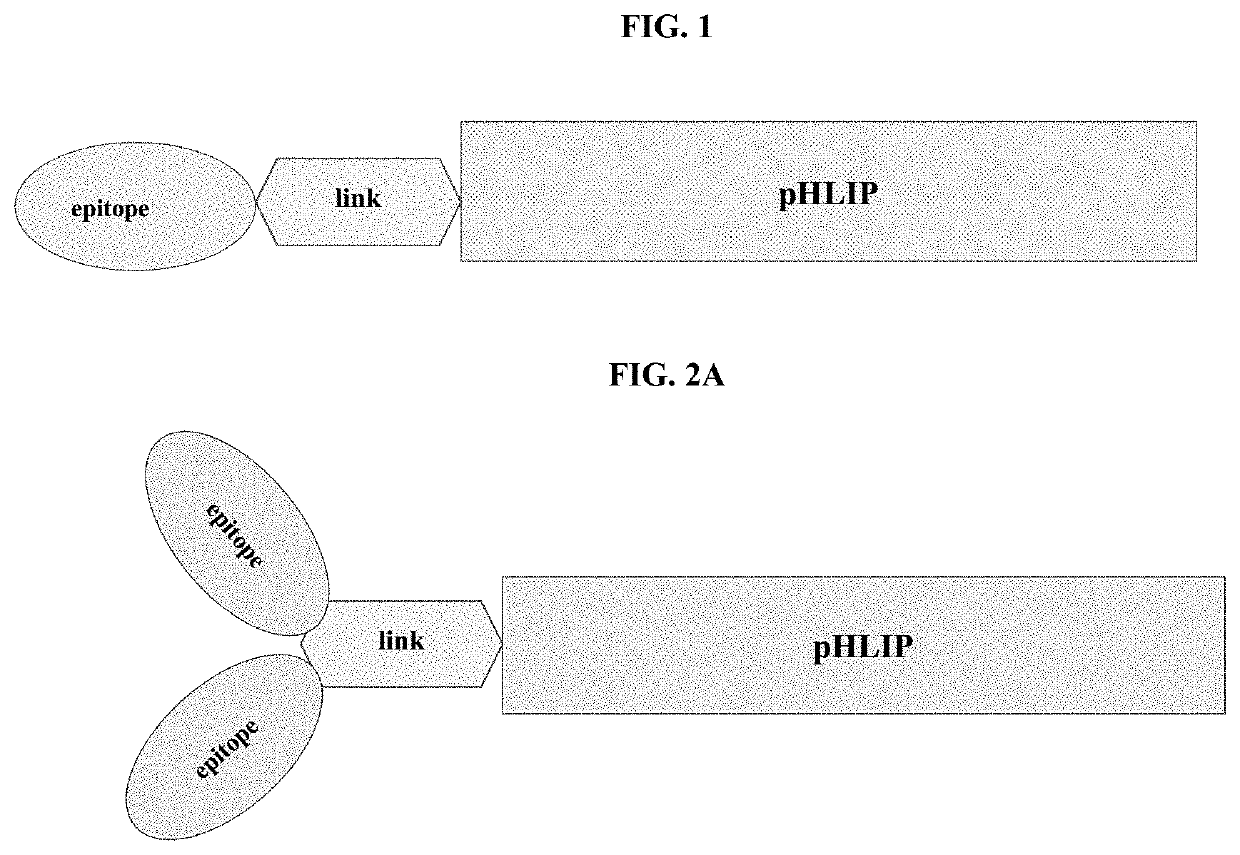
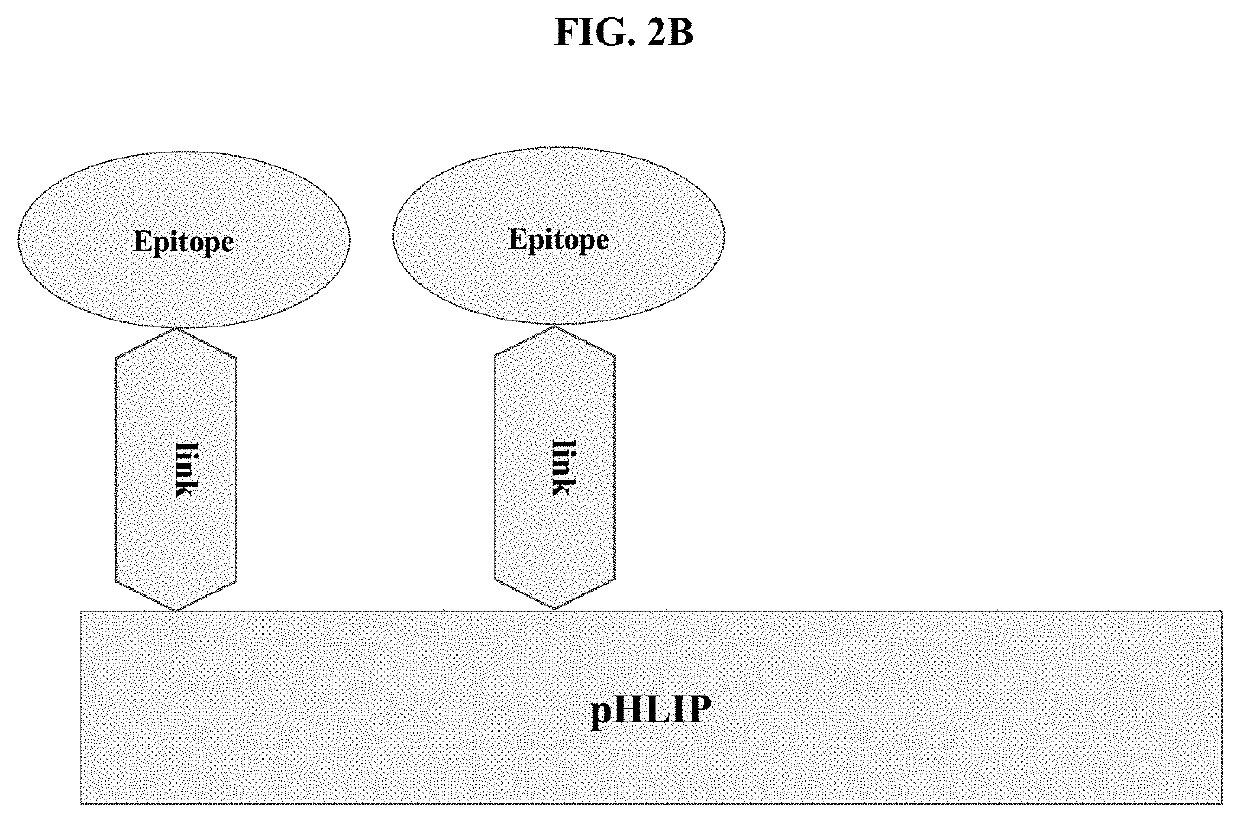
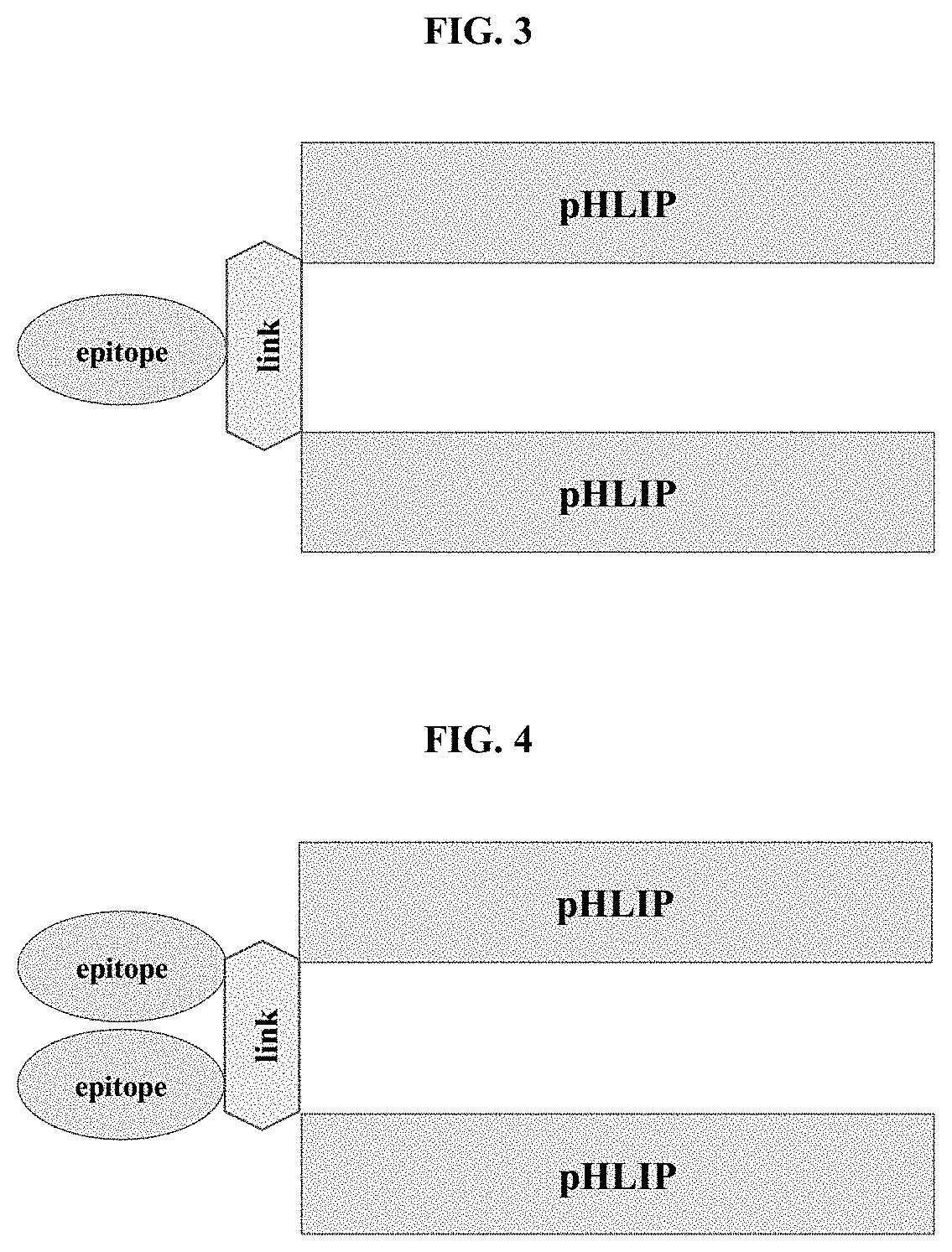
pHLIP® peptide-mediated epitope tethering at cell surfaces
a peptide and cell surface technology, applied in the field of immunotherapy, can solve the problems of limited use of peptides, achieve the effects of enhancing the use of developed monoclonal antibodies, efficient binding, and enhancing cell recruitmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Small Molecule Epitope Di-nitrophenyl (DNP) to Cancer Cells by pHLIP® Promoted Cell Killing
[0161]Three different pHLIP® constructs were synthesized with a DNP—(O—(2,4-dinitrophenyl)hydroxylamine):
i) DNP-pHLIP®, where DNP-malemide was conjugated with a single Cys residue at the N-terminal of the pHLIP® peptide;
ii) DNP-PEG4-pHLIP®, where DNP-PEG4-NHS was conjugated with a single Lys residue at the N-terminal of the pHLIP® peptide; and
iii) DNP-PEG12-pHLIP®, where DNP-PEG12-NHS as conjugated with a single Lys residue at the N-terminal of pHLIP® peptide.
[0162]pHLIP® peptide with a single Cys residues used in the study for conjugation with DNP-malemide is the following: (ACDDQNPWRAYLDLLFPTDTLLLDLLWA (SEQ ID NO: ______) pHLIP® peptide with single Lys residue and acetylated N-terminus used in the study for conjugation with DNP-PEG4-NHS and DNP-PEG12-NHS is the following: Ac-AKDDQNPWRAYLDLLFPTDTLLLDLLWA (SEQ ID NO: ______)). Peptides were prepared by solid-phase synthesis. Progressions of co...
example 2
Two Peptide Epitopes by pHLIP® to Cancer Cells to Bind Two Heads of Ig Antibody
[0167]To enhance performance of antibodies and enhance immune response, it is important to promote binding of both heads of IgG with 2 epitopes coupled to the same pHLIP® peptide (see, e.g., FIG. 5B). The pHLIP® peptide with 2 Lys residues (bold and underlined) (Ac-AKQNDDQNKPWRAYLDLLFPTDTLLLDLLWA (SEQ ID NO: ______)) is conjugated with excess of NHS-PEG12-malemide and NHS-PEG24-malemide linkers, purified and, pHLIP-(PEG12)2 is coupled with an HA peptide epitope (YPYDVPDYAGGGCA (SEQ ID NO: ______)). pHLIP® and HA peptides are prepared by solid-phase synthesis. Progressions of both coupling reactions and purifications are performed using reverse-phase HPLC (RP-HPLC) (the gradient: water and acetonitrile with 0.05% TFA) followed by lyophilization. The concentration of the construct is measured by absorbance at 280 nm.
[0168]PEG12 and PEG24 are be stretched for 5 nm and 10 nm, respectively. The six residues (Q...
example 3
CXCL10 Protein Chemokine Epitope by pHLIP® to Cancer Cells to Promote NK-Cells Binding
[0170]Two fusion proteins with 2 different tags (His and cMyc) are expressed and purified:
CXCL10-mucin-2x-Myc-pHLIP ®SEQ ID NO: 531mnqtailicclifltlsgiqgvplsrtvrctcisisnqpvnprslekleiipasqfcprveiiatmkkkgekrclnpeskaiknllkavskerskrspgtfekqigevkprttpaaggmdesvvlepeatgesssleptpssqeaqralgtspelptgvtgssgtrlpptpkaqdggpvgtelfrvppvstaatwqssaphqpgpslwaeaktseapstqdpstqastasspapeenapsegqrvwgqgqsprpenslereemgpvpahtdafqdwgpgsmahvsvvpvssegtpsrepvasgswtpkaeepihatmdpqrlgvlitpvpdaqaatrrqeqkliseedleqkliseedladdqnpwrayidllfptdtllldllwCXCL10-mucin-6x-His-pHLIP ®SEQ ID NO: 532mnqtailicclifltlsgiqgvplsrtvrctcisisnqpvnprslekleiipasqfcprveiiatmkkkgekrclnpeskaiknllkavskerskrspgtfekqigevkprttpaaggmdesvvlepeatgesssleptpssqeaqralgtspelptgvtgssgtrlpptpkaqdggpvgtelfrvppvstaatwqssaphqpgpslwaeaktseapstqdpstqastasspapeenapsegqrvwgqgqsprpenslereemgpvpahtdafqdwgpgsmahvsvvpvssegtpsrepvasgswtpkaeepihatmdpqrlgvlitpvpdaqaatrrqhhhhhhaddqnpwra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com