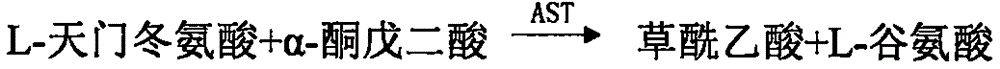Patents
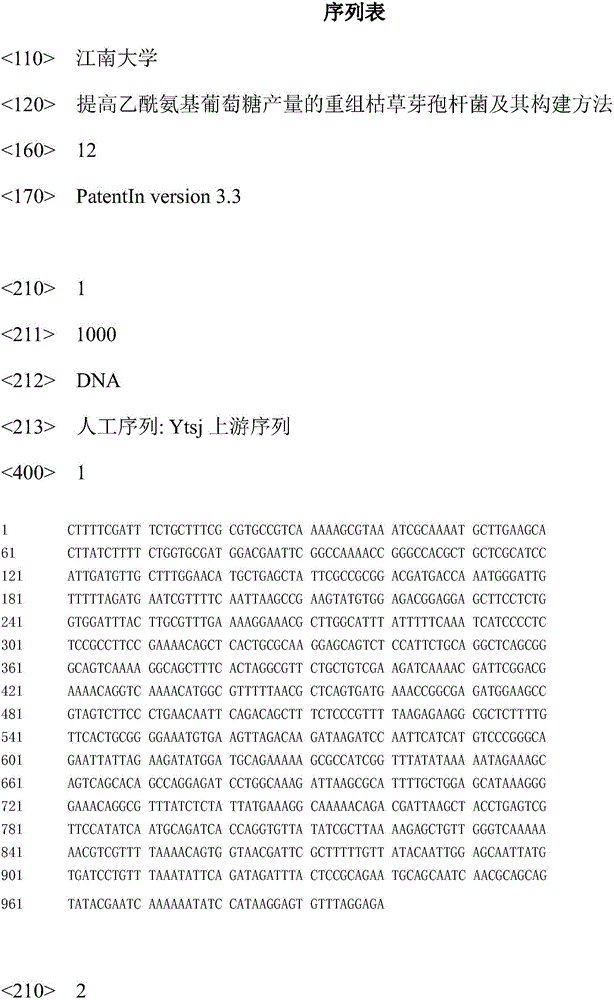
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Malic Dehydrogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Malic dehydrogenase. An enzyme in the Krebs cycle that catalyzes the conversion ofL-malic acid to oxaloacetic acid.
Method for synthesizing ursodeoxycholic acid through enzymatic method
The invention discloses a method for synthesizing ursodeoxycholic acid through an enzymatic method and a method for synthesizing the ursodeoxycholic acid by taking chenodeoxycholic acid as a raw material. The method takes the chenodeoxycholic acid as a base material and comprises the following steps: dissolving the chenodeoxycholic acid into a 50mM phosphate buffer solution; firstly, catalyticallyoxidizing the chenodeoxycholic acid by utilizing 7-alpha hydroxysteroid dehydrogenase in the presence of NAD, NOX2 and under the condition that oxygen is introduced, so as to obtain 7-ketolithocholicacid; then under the condition that the NAD, L-malic acid and malic dehydrogenase exist, catalytically reducing the 7-ketolithocholic acid by utilizing the 7-alpha hydroxysteroid dehydrogenase to obtain the ursodeoxycholic acid. According to the method disclosed by the invention, an organic solvent is not used and operation is simple; reaction conditions are moderate and easy to control and the utilization rate of the raw materials is high; the conversion rate reaches 99 percent or more.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
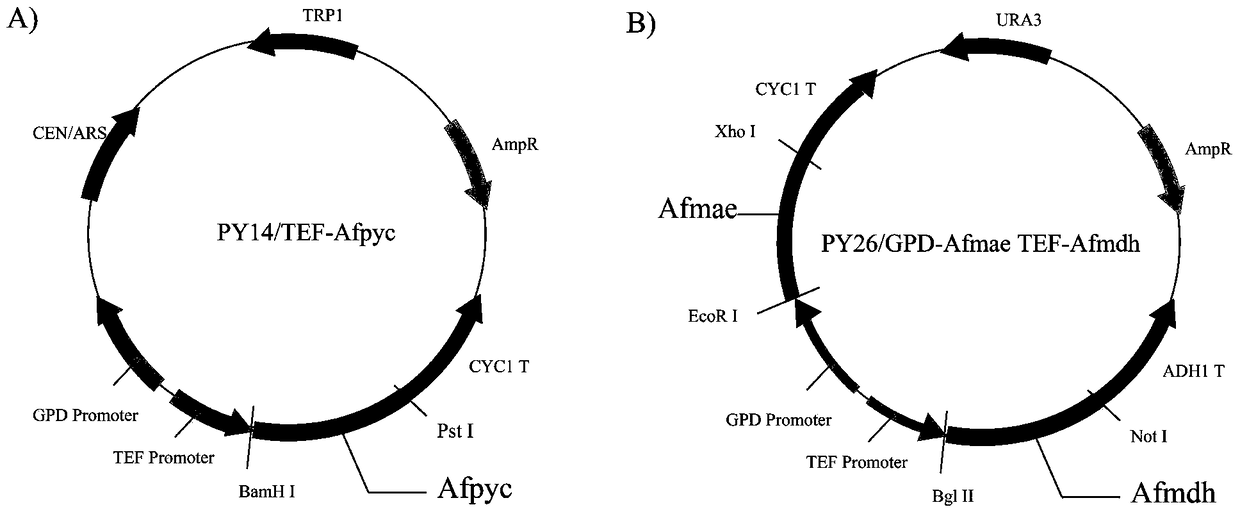
Establishment and application of brewing yeast engineering bacterium strain for producing L-malic acid
ActiveCN105400711ARealize accumulationFungiMicroorganism based processesPyruvate carboxylaseTrimeresurus flavoviridis
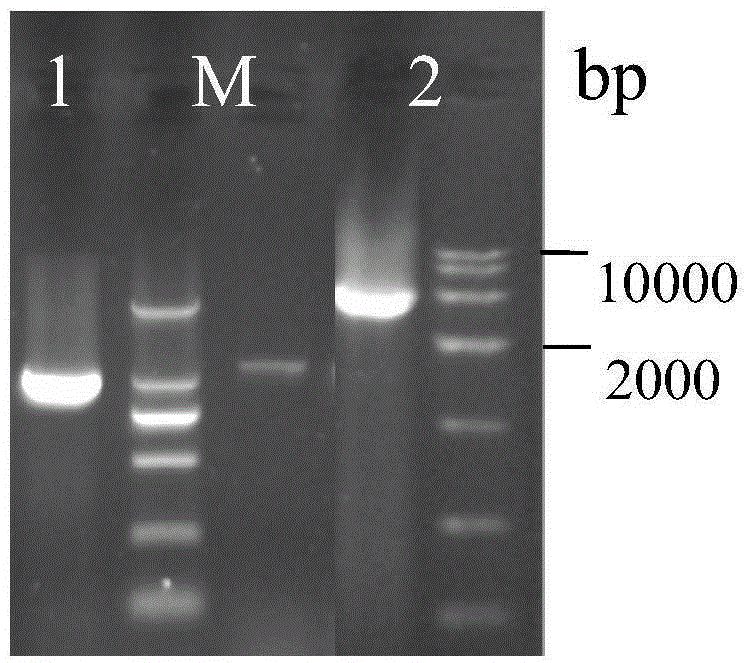
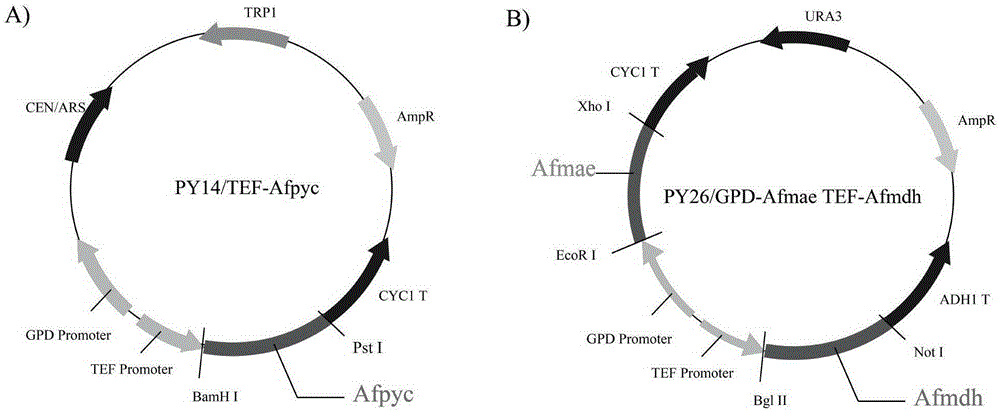
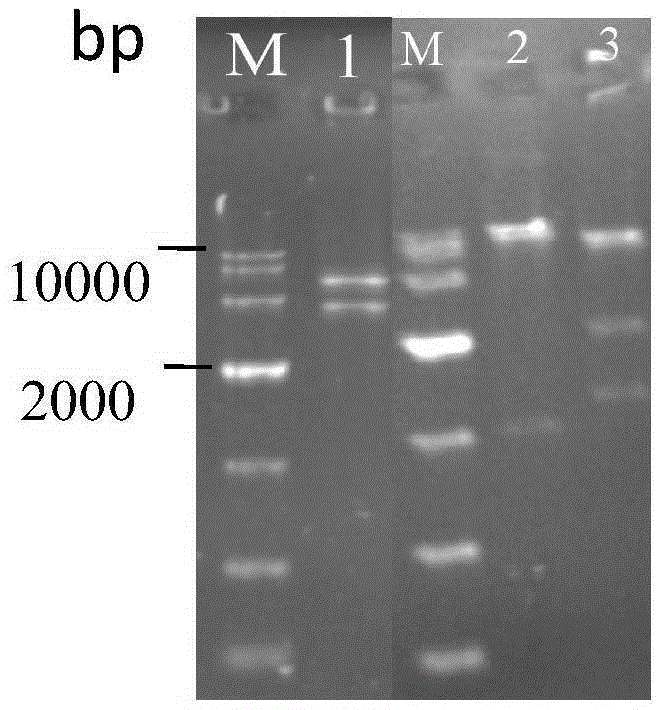
The invention discloses establishment and application of a brewing yeast engineering bacterium strain for producing L-malic acid, and belongs to the field of fermentation engineering. Genes of pyruvic carboxylase (Afpyc), malic dehydrogenase (Afmdh) and malic acid transport protein (Afmae) coming from Aspergillus flavus ATCC13697 are excessively and dissociatively expressed in the bacterium strain S.cerevisiae tTAM(delta)ura3(delta)trpl high in pyruvic acid yield, the malic acid accumulation path is established, and the bacterium W1101 is obtained. The bacterium strain is used for producing L-malic acid through fermentation; after fermentation is conducted for 84 h, the malic acid yield is 27.3 g / L; an original starting bacterium strain does not accumulate malic acid, the metabolism path of aspergillus flavus of the high-yield L-malic acid bacterium strain is successfully applied to brewing yeast, and a new strategy is provided for establishing the high-yield L-malic acid bacterium strain.
Owner:JIANGNAN UNIV
Aspartate transaminase mitochondrial isozyme detection kit
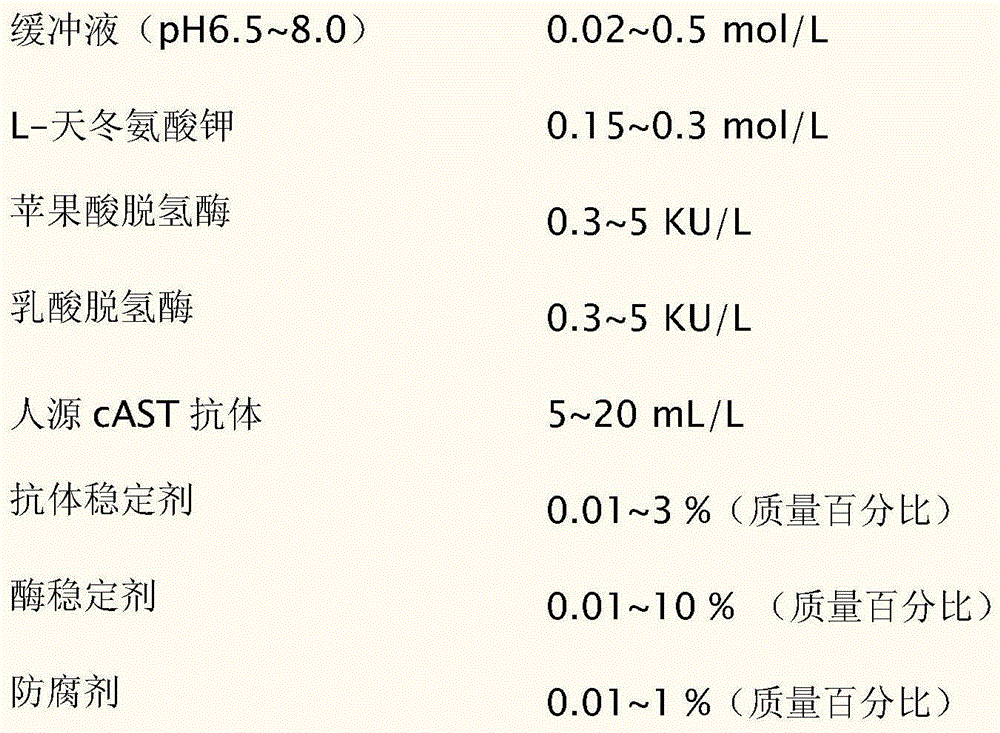
The invention discloses an aspartate transaminase mitochondrial isozyme detection kit, comprising: reagents 1: 0.02-0.5mol / L of buffer solution of which pH value is 6.5-8.0, 0.15-0.3mol / L of L-potassium aspartate, 0.3-5KU / L of malic dehydrogenase, 0.3-5KU / L of lactic dehydrogenase, 5-20mL / L of human cAST antibody, 0.01-3% of antibody stabilizer, 0.01-10% of enzyme stabilizer and 0.01-1% of preservative; and reagents 2: 0.02-0.5mol / L of buffer solution of which pH value is 7.0-9.0, 0.005-0.02mol / L of alpha-ketoglutarate, 0.1-0.5mmol / L of reduced coenzyme I, 0.01-1% of preservative and 0.01-10% of reduced coenzyme I stabilizer. The kit has the advantage of good stability.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Aspartate amino transferase detection kit
InactiveCN106644976AEliminate distractionsImprove accuracyColor/spectral properties measurementsAmidinotransferasesTransferase
The invention relates to an aspartate amino transferase (AST) detection kit and belongs to the technical field of clinic in vitro diagnostic reagents. The invention aims to provide the aspartate amino transferase detection kit with capability of effectively eliminating endogenous alpha-oxoglutarate interference and high stability in order to achieve more accurate and reliable determination results of aspartate amino transferase. The kit provided by the invention comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises the following components: Tris-HCl buffer solution, L-asparaginic acid, reduction coenzyme (NADH), malic dehydrogenase (MDH), enzyme protective agent, stabilizer and preservative, and the reagent 2 comprises the following components: Tris-HCl buffer solution, alpha-oxoglutarate, a stabilizer and a preservative. The enzyme protective agent and the stabilizer are added into the AST detection kit provided by the invention so that the stability of the aspartate amino transferase detection kit is promoted; the aspartate amino transferase detection kit is suitable for various full-automatic biochemical analyzers; the operation is simple and safe; the aspartate amino transferase detection kit has convenience in clinical application and popularization.
Owner:王贤俊
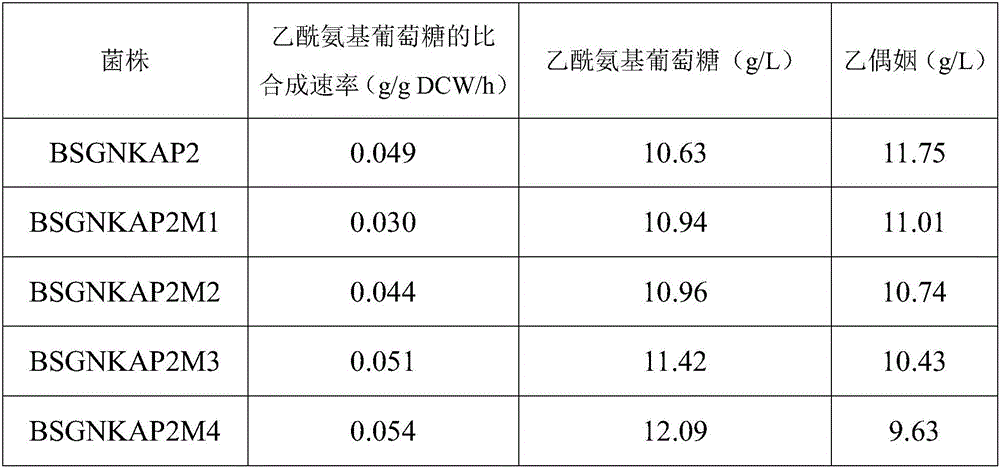
Recombinant bacillus subtilis for improving yield of acetylglucosamine and building method of recombinant bacillus subtilis
ActiveCN106148262AIncrease productionIncrease extracellular accumulationBacteriaStable introduction of DNABacillus subtilisAcetoin
The invention provides recombinant bacillus subtilis for improving the yield of acetylglucosamine. Malic dehydrogenase coding genes are knocked out from bacillus subtilis. The invention further provides a building method of the recombinant bacillus subtilis. The method comprises the steps that a malic dehydrogenase coding gene knock-out frame is built; malic dehydrogenase coding genes ytsJ, ywkA, malS and mleA are knocked out from a bacillus subtilis genome, and the recombinant bacillus subtilis for improving the yield of the acetylglucosamine is obtained. Compared with original strains, according to the recombinant bacillus subtilis, the acetylglucosamine extracellular accumulation amount is improved, and the yield of the by-product acetoin is reduced. The building method of the recombinant bacillus subtilis is simple, convenient to use and good in application prospect.
Owner:JIANGNAN UNIV
Construction method and use of fumaric acid producing candida glabrata engineering strain
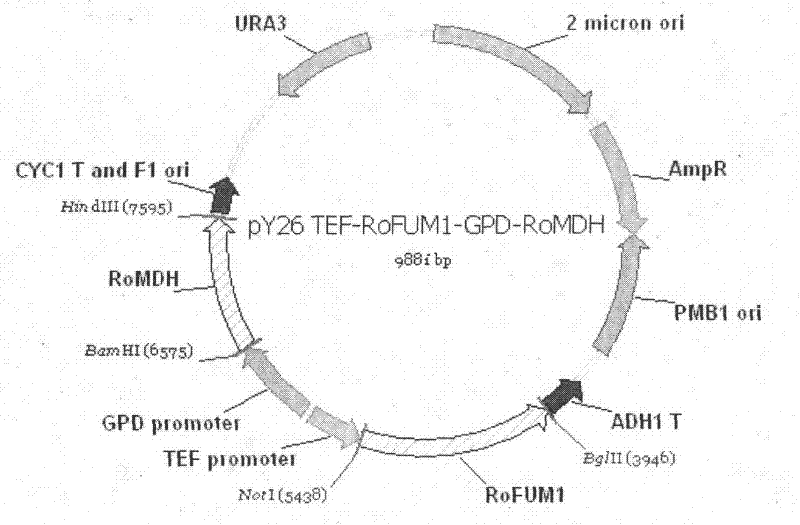
The invention discloses a construction method and use of a fumaric acid producing T.glabrata candida glabrata engineering strain and belongs to the field of fermentation engineering. In the invention, a recombinant T.glabrata FMME045 strain is obtained by overexpression of a malic dehydrogenase (RoMDH) gene and a fumarase (RoFUM1) gene from rhizopus oryzae in a uracil deficient (Ura) T.glabratadeltaura3 strain of a pyruvic acid producing strain T.glabrata in a free expression mode. When the strain is used for fermentation production of fumaric acid, the fumaric acid yield after the fermentation is performed for 60 hours is increased by 6 times to 35mg / L compared with that of a starting strain. Thus, a new approach is provided for producing fumaric acid by a microbial fermentation process. The method has a bright application prospect.
Owner:JIANGNAN UNIV
Method for improving quality of loquat fruit by agricultural measures
InactiveCN101946673AReduce synthesisIncrease the amount of degradationSaving energy measuresCultivating equipmentsOrganic acidPyruvate carboxylase
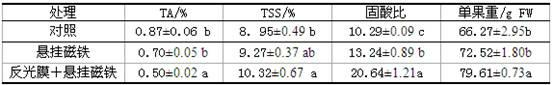
The invention provides a method for improving the quality of a loquat fruit by agricultural measures, which combines the reflection film spreading in an orchard and the suspension of magnets. The invention can accurately and effectively adjust and control key enzymes for forming and degrading the main organic acid (malic acid) of the loquat fruit. At the early growth stage of the fruit, the invention improves the activity of phosphoenolpyruvate carboxykinase and NAD-malic dehydrogenase in the pulp; and at the most critical stage for the accumulation of organic acid of the fruit, the invention can promote the activity decline of the PEPC in the pulp, obviously inhibit the activity of the NAD-MDH and enables the activity of NADP-ME in the pulp, reduce the synthesis of malic acid and enhance the degradation of malic acid, thereby obviously reducing the organic acid content of the fruit of the high-acid content loquat, and simultaneously increasing the TSS content of the mature pulp. The invention can obviously reduce the titratable acid (TA) in the fruit and increase the soluble solid (TTS) content of the mature pulp and the weight of a single mature fruit, thereby obviously improving the quality of the mature loquat fruit.
Owner:FUJIAN AGRI & FORESTRY UNIV
Method for measuring citric acid concentration and citric acid diagnose reagent kit
InactiveCN101082575AFree from pollutionHigh precisionMicrobiological testing/measurementColor/spectral properties measurementsCITRATE ESTERCoupling
The invention relates to a kind of enzyme colorimetric method and a method of the coupling method measuring the density of citric acid and a diagnosis reagent box applying the citrate synthetase and coupling malic dehydrogenase enzymatic reaction continues monitoring method / ratio colorimetric method. The reaction of citrate synthetase enzymolysis citric acid generates oxaloacetic acid and then through the effect of coupling malic dehydrogenase at last oxidizes the reduced coenzyme (there is a absorption peak at the site of 340nm) becoming the coenzyme (there is a absorption peak at the site of 340nm) so we can assay the degree / speed of the fall-way absorbance at the place of 340nm. It can measure and calculate the density of citric acid by measuring the degree / speed of the fall-way absorbance at the place of 340nm. The method has high specificity and it is not polluted by the endogenous and exogenous object and the test result is precise and accurate. The invention can get the array result by the ultraviolet / visible light analytic instrument so it is convenience to extend and apply.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
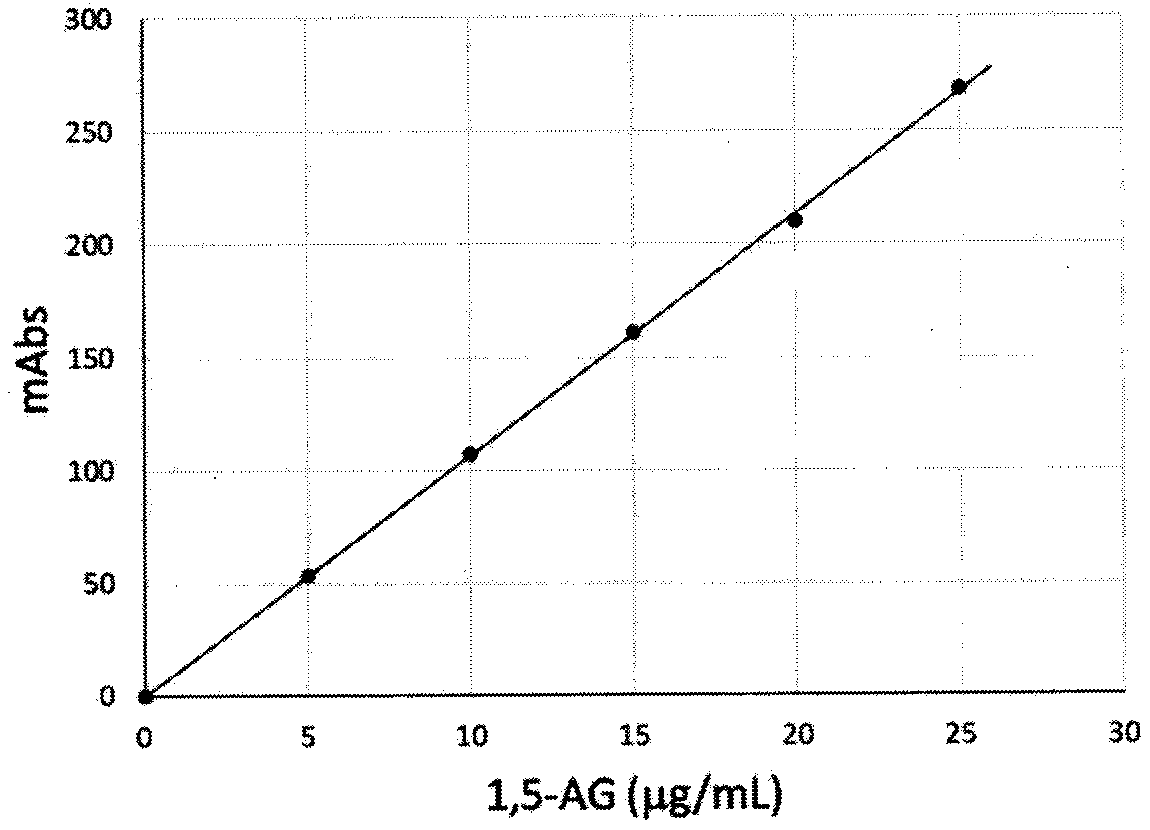
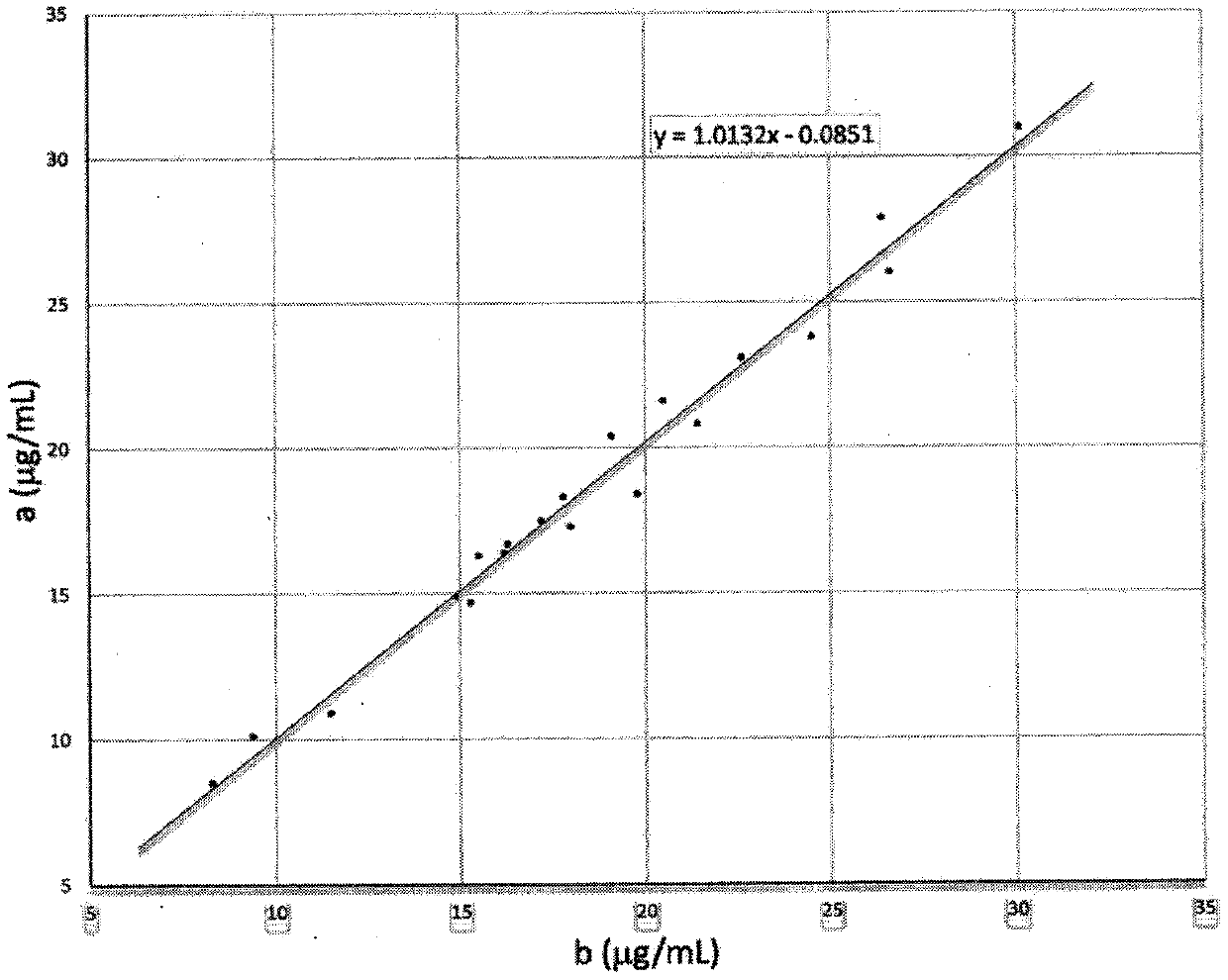
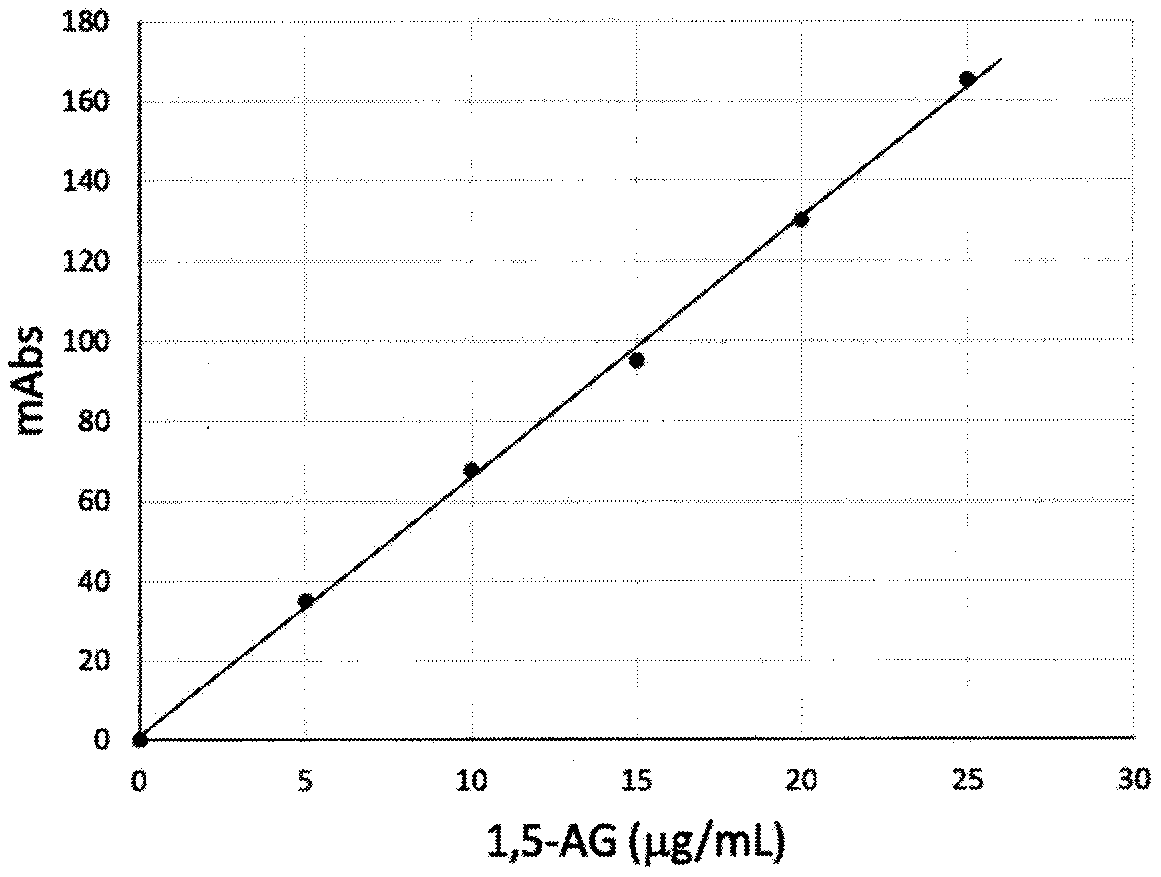
Zymologic quantification method for 1,5-anhydroglucitol, and quantification reagent adopted by zymologic quantification method
InactiveCN111575339AEliminate distractionsMicrobiological testing/measurementEnzyme systemBiochemistry
The invention provides a quantification method for quickly measuring 1,5-anhydroglucitol in a glucose-containing sample, and a quantification reagent and a quantification-purpose kit used by the quantification method. The quantification method for the 1,5-anhydroglucitol in the glucose-containing sample is a method characterized in that a scavenger enzyme system consisting of a coenzyme I, glucosedehydrogenase and NAD (nicotinamide adenine dinucleotide)-dependent malic dehydrogenase is adopted to scavenge interference generated by glucose cross reaction in a test specimen, and then, a measurement enzyme system consisting of an oxidized coenzyme II and 1,5-anhydroglucitol-6-phosphate dehydrogenase is adopted to quantitatively measure a generated reduced coenzyme II.
Owner:王学忠
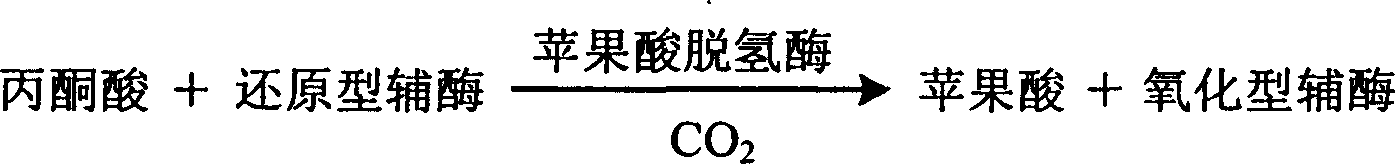
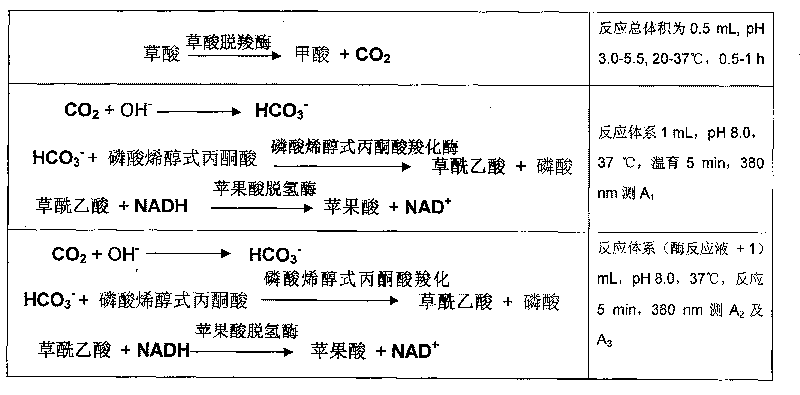
Process for determining content of carbon dioxide and kit for diagnosing carbon dioxide therefor
InactiveCN1786694AHighlight substantive featuresSignificant progressAnalysis by subjecting material to chemical reactionColor/spectral properties measurementsUltravioletAbsorbance
The invention relates to measuring method for carbon dioxide content and diagnosing kit. The invention directly uses carbon dioxide( bicarbonate radical) and pyruvic acid to produce malic acid by decarboxylation of malic dehydrogenase and oxidizes reduced coenzyme into oxidized type coenzyme, measuring the decent rate of dominant wavelength 340nm absorbance could quantitatively response the carbon dioxide content in sample, thus, the strength of bicarbonate radical could be extrapolated. The method has high specificity, has no pollution from inside and outside material, and accurate test result. The diagnose kit is made up into bi-agent or tri-agent that could reduce cross influence from each component and keeps stability of the agent that is convenient for long-term storage. The method could quickly test on commonness ultraviolet / visible light, analysis or semi-transfer / full automatically biochemical study instrument, and doesn't need particularity or extra instrument. The testing cost is cheap, and is convenient for popularization.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Method for increasing carbon source utilization rate in aspergillus oryzae L-malic acid synthesizing process
InactiveCN105969790AImprove conversion rateLow cost of industrializationFungiMicroorganism based processesEscherichia coliBiotechnology
The invention discloses a method for increasing the carbon source utilization rate in the aspergillus oryzae L-malic acid synthesizing process and belongs to the field of genetic engineering. According to the method, Aspergillus oryzae NRRL 3488 serves as an original strain, metabolic flux for synthesizing malic acid from oxaloacetic acid is enhanced by expressing pyruvic carboxylase and malic dehydrogenase in aspergillus oryzae cytoplasm, and the shake flask yield of L-malic acid is increased to 50.5+ / -1.06 g / L and is increased by 38.4%; then, the synthesizing efficiency of oxaloacetic acid from phosphoenolpyruvic acid in aspergillus oryzae cytoplasm is improved by expressing pyruvic carboxylase and pyruvate carboxykinase of Escherichia coli in the converter Aspergillus oryzae p16, finally, the yield of L-malic acid reaches 63.2+ / -1.32 g / L, the concentration of succinic acid is 8.88+ / -0.45 g / L, the production intensity is also improved to 0.7 g / (L.h) from original 0.24 g / (L.h) of Aspergillus oryzae NRRL 3488, and a foundation is laid for further metabolic engineering reform of Aspergillus oryzae for producing L-malic acid.
Owner:JIANGNAN UNIV
Method for determining citric acid concentration and citric acid determining reagent kit
InactiveCN101324505AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsLuminosityEnzyme catalysis
The invention relates to a kit for diagnosing / mensurating citric acid by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for mensurating the concentration of the citric acid, and belongs to the technology field of environment / food inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, citrate cleavage enzyme,malic dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the degree / velocity of the decrease in absorbance at 340nm of the dominant wavelength is detected, thereby mensurating the concentration of the citric acid.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
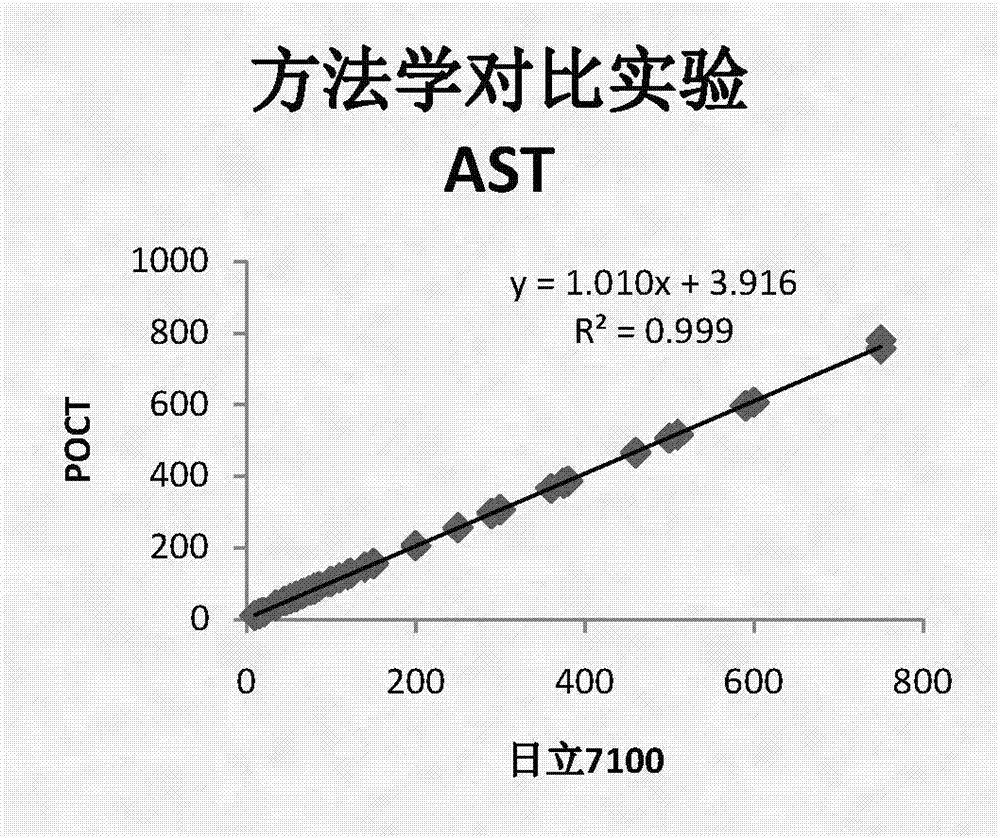
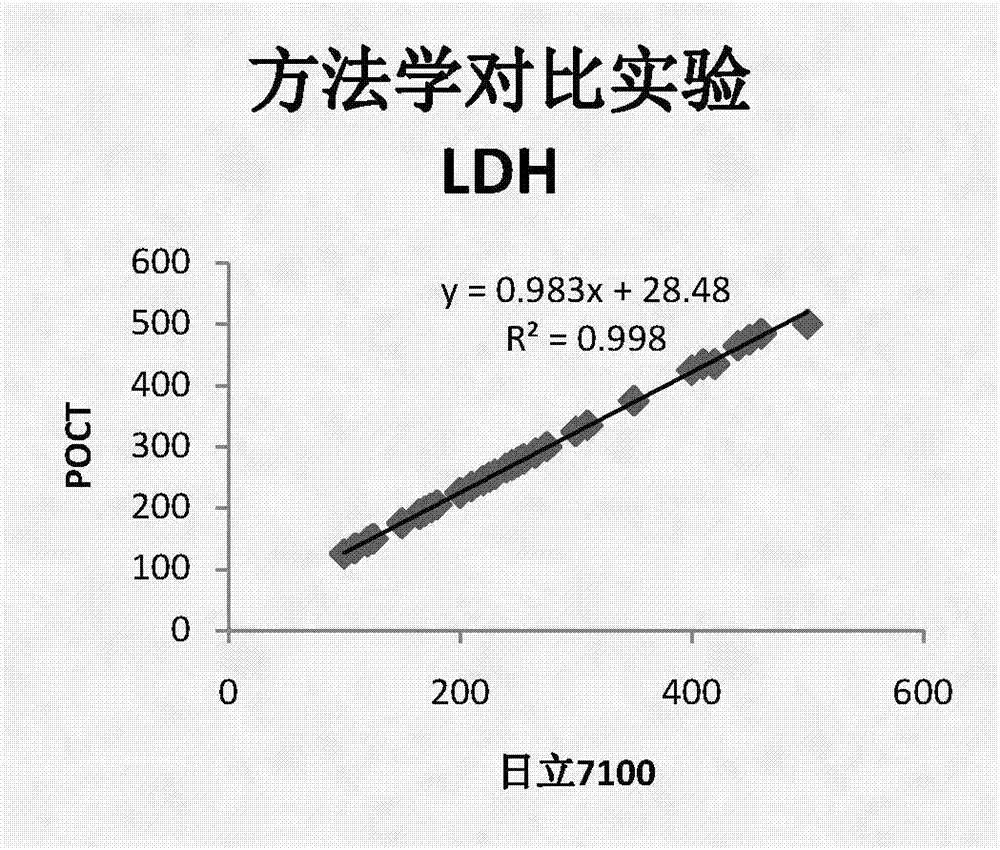
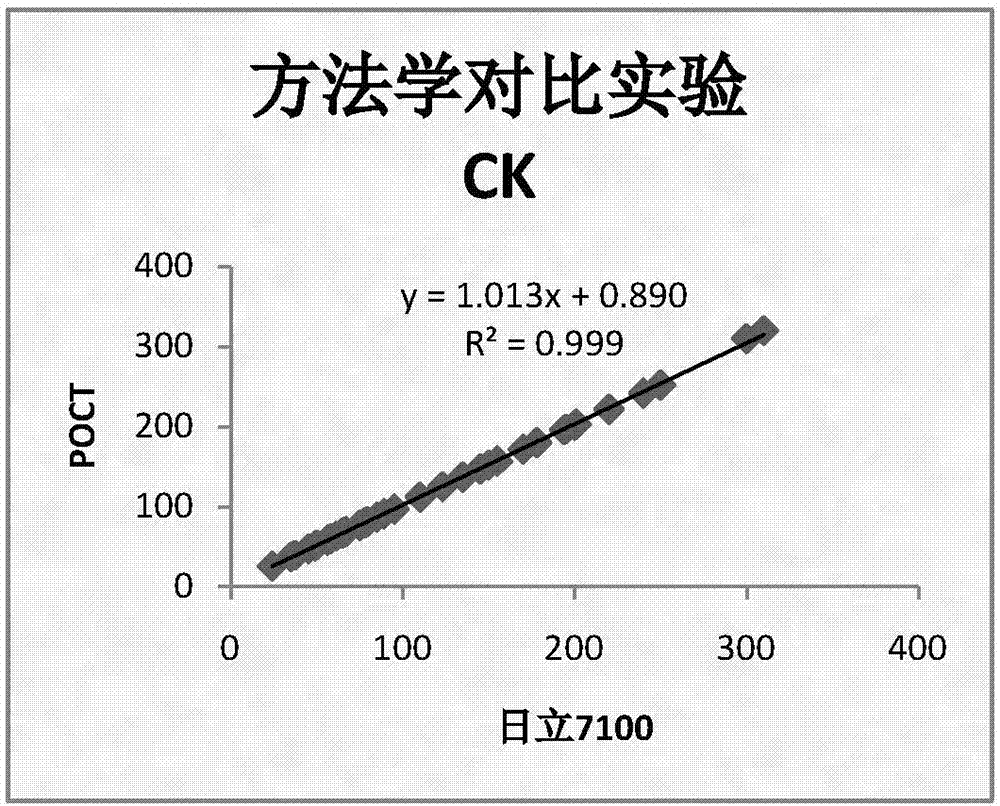
CK, CKMB, LDH and AST combined detection reagent
ActiveCN107988315APlay a role in de-interferenceMicrobiological testing/measurementBiological material analysisVitamin CL-Aspartate
The invention discloses a CK, CKMB, LDH and AST combined detection reagent which comprises a diluent 1, a diluent 2 and 4 freeze-dried balls. The diluent 1 comprises trismetyl aminomethane, a surfactant, a dehydrobilirubin interference agent, vitamin C oxidase and a preservative; the diluent 2 comprises a buffer solution ad an inhibitory CK-M antibody; the freeze-dried ball 1 comprises a buffer solution, alpha-ketoglutaric acid, a reduced coenzyme I, malic dehydrogenase, L-aspartate and a freeze-drying protective additive; the freeze-dried ball 2 comprises a buffer solution, L-lithium lactate,an oxidized coenzyme I and a freeze-drying protective additive; the freeze-dried balls 3 and 4 comprise a buffer solution, adenosine diphosphate, glucose-6-phosphate dehydrogenase, hexokinase, D-glucose, phosphocreatine, an enzyme activator and a freeze-drying protective additive. The combined detection reagent is applicable to a multifunctional full-spectrum POCT (Point-of-care Testing) biochemical analyzer, has good correlation to detection results of clinically common liquid reagents on a large biochemical analyzer, and has the advantages of being simple and convenient to operate, convenient in reagent preservation and transport, low in detection cost and the like.
Owner:NINGBO MEIKANG BAOSHENG BIOMEDICAL ENG
Glycine diagnosis/measuring reagent kit and glycine concentration determination method
InactiveCN101464273AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAbsorbanceFood inspection
The invention relates to a kit for diagnosing / measuring glycine by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the concentration of the glycine, and belongs to the technical field of medical / food inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, methyl sulfate phenazine, a pyruvic acid, hydrogen cyanide synthetase, malic dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the degree / velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of the glycine.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
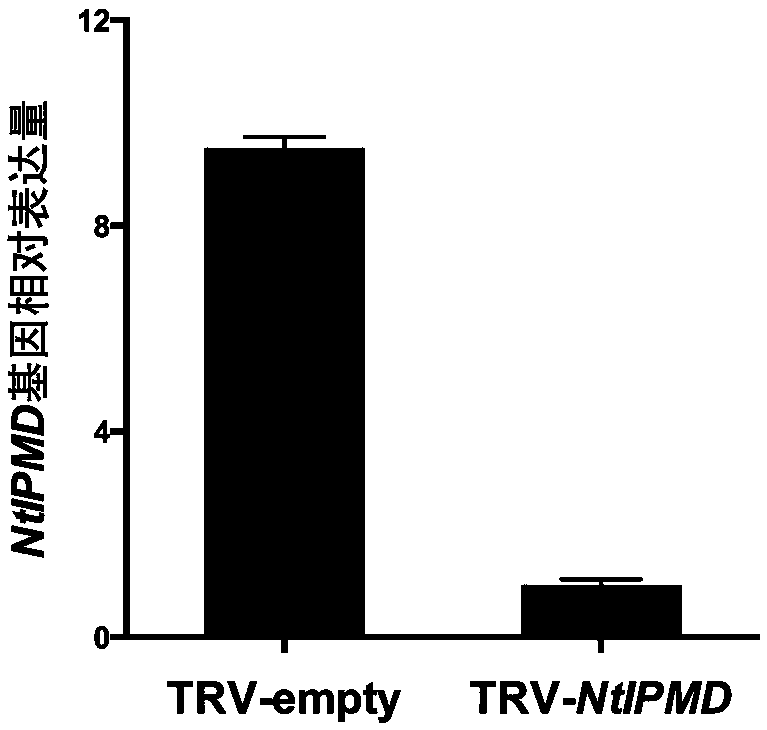
NtIPMD gene affecting differentiation of tobacco axillary bud
The invention belongs to the field of tobacco gene engineering, and particularly relates to a NtIPMD gene affecting differentiation of a tobacco axillary bud. The gene comprises 1218bp basic group with the specific nucleotide sequence shown as SEQ ID NO. 1, wherein the nucleotides at the 68-403 sites are specific nucleic acid segments. Isopropyl malic dehydrogenase NtIPMD coded by the gene has thecharacteristic that isopropyl malic dehydrogenase NtIPMD comprises 405 amino acid. The primary research on the NtIPMD gene shows that the gene is related to development of the axillary bud of a plant, after the gene is silenced, development of a lateral branch can be promoted, by taking use of the characteristic, the gene can be silenced or overexpressed through gene silencing or overexpression techniques, accordingly adjustment of the plant shape can be achieved at the molecular level, meanwhile, the gene has the important effect on culturing new species of tobacco, and therefore the gene has the more significant practical value.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Lithium diagnosis/measuring reagent kit and lithium concentration determination method
InactiveCN101464311AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsEnzymatic ColorimetryAbsorbance
The invention relates to a kit for diagnosing / measuring lithium by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay, wherein, the activity of the kit can be inhibited by lithium. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the concentration of lithium, and belongs to the technical field of medical / industrial / environmental inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, magnesium chloride, inositol-1-phosphate, an oxaloacetic acid, a pyruvic acid, inositol-1-phosphatase, phosphoenolpyruvate carboxykinase, malic dehydrogenase and a stabilizer. Through mixing a check sample and a lithium sample respectively with the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, the degree / velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, and the difference in the degree / velocity of the decrease between the check sample and lithium sample is compared, thereby measuring the concentration of lithium.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Carbon dioxide diagnosis/measuring reagent kit and carbon dioxide concentration determination method
InactiveCN101464270AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsPhosphoenolpyruvate carboxylaseEnzymatic Colorimetry
The invention relates to a kit for diagnosing / measuring carbon dioxide by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the concentration of the carbon dioxide, and belongs to the technical field of medical / food / environmental inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, a phosphoenolpyruvic acid, phosphoenolpyruvate carboxykinase, malic dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the degree of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of the carbon dioxide.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Construction and application of an engineered strain of Saccharomyces cerevisiae producing l-malic acid
ActiveCN105400711BRealize accumulationFungiMicroorganism based processesPyruvate carboxylaseAspergillus flavus
The invention discloses establishment and application of a brewing yeast engineering bacterium strain for producing L-malic acid, and belongs to the field of fermentation engineering. Genes of pyruvic carboxylase (Afpyc), malic dehydrogenase (Afmdh) and malic acid transport protein (Afmae) coming from Aspergillus flavus ATCC13697 are excessively and dissociatively expressed in the bacterium strain S.cerevisiae tTAM(delta)ura3(delta)trpl high in pyruvic acid yield, the malic acid accumulation path is established, and the bacterium W1101 is obtained. The bacterium strain is used for producing L-malic acid through fermentation; after fermentation is conducted for 84 h, the malic acid yield is 27.3 g / L; an original starting bacterium strain does not accumulate malic acid, the metabolism path of aspergillus flavus of the high-yield L-malic acid bacterium strain is successfully applied to brewing yeast, and a new strategy is provided for establishing the high-yield L-malic acid bacterium strain.
Owner:JIANGNAN UNIV
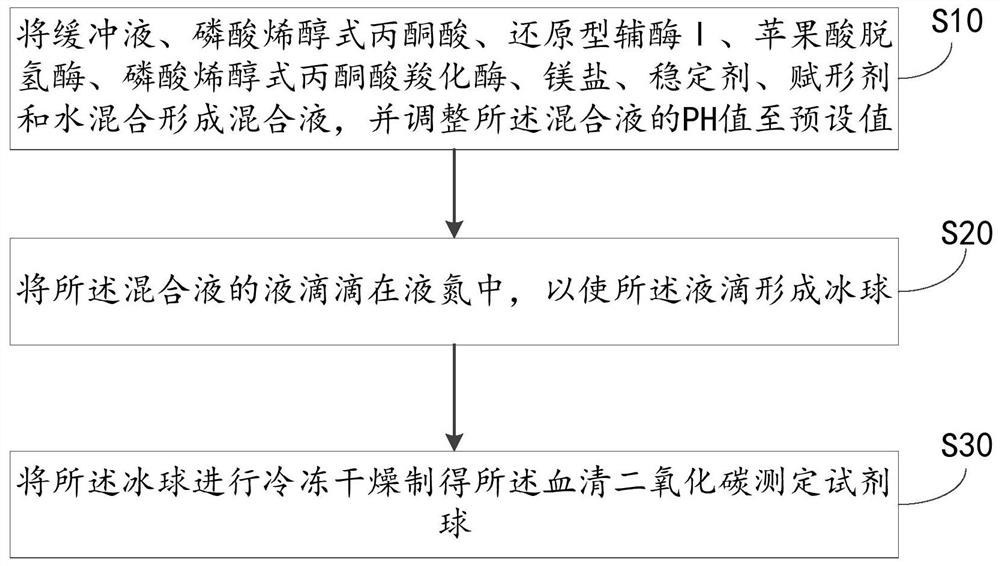
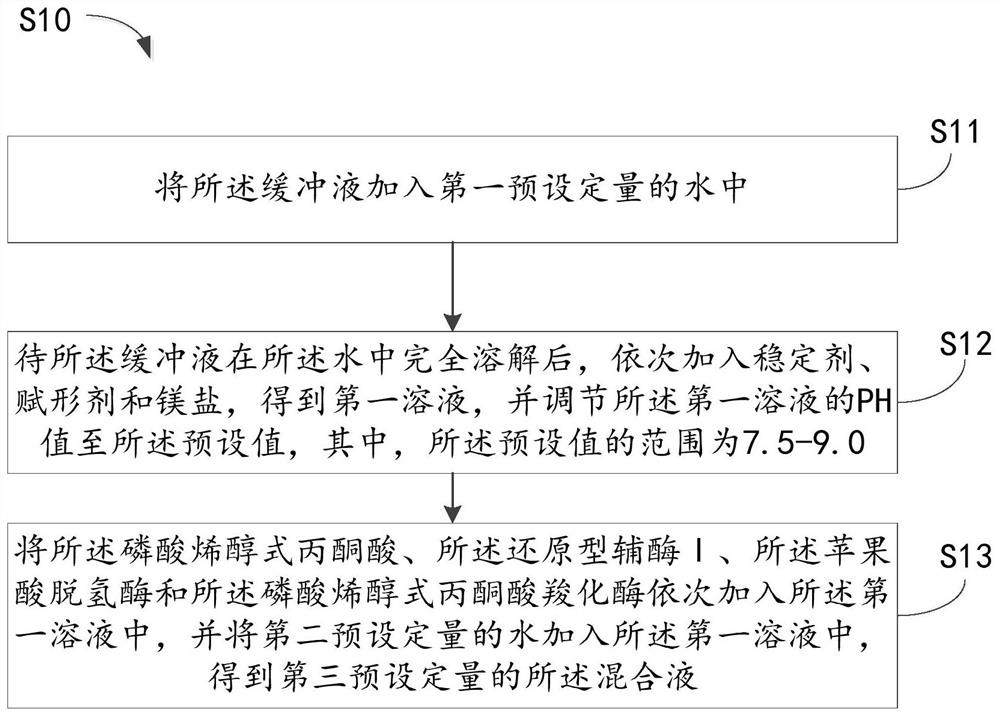
Preparation method of serum carbon dioxide determination reagent ball and reagent ball
PendingCN113278680AFully formedGuaranteed SolubilityMicrobiological testing/measurementBiological material analysisPhosphoenolpyruvate carboxylasePhosphoric acid
The embodiment of the invention relates to the technical field of medical immune in-vitro diagnosis, and particularly relates to a preparation method of a serum carbon dioxide determination reagent ball and the reagent ball. The method comprises the following steps of: dripping a mixed solution into liquid nitrogen in a liquid drop form, so that liquid drops form an ice ball; and then freezing and drying the ice ball to prepare the serum carbon dioxide determination reagent ball, wherein the mixed solution is prepared from the following components: 5 to 150mmol / L of a buffering solution, 5 to 20g / L of phosphoenolpyruvic acid, 0.1 to 10g / L of reduced coenzyme I, 5 to 20KU / L of malic dehydrogenase, 0.1 to 5KU / L of phosphoenolpyruvate carboxylase, 0.1 to 10g / L of a stabilizer and 10 to 100g / L of an excipient. The excipient with the dosage range can ensure the shape and re-melting solubility of the reagent ball and the activity of reactants is protected; the excipient is good for completely freezing and drying the reagent ball. Namely, the serum carbon dioxide determination reagent ball prepared by the method has relatively good shape and re-melting solubility and can be completely frozen and dried, so that the serum carbon dioxide determination reagent ball has relatively high stability and precision and can realize in-time diagnosis.
Owner:GENRUI BIOTECH INC
Adenosine deaminase activity determination method and adenosine deaminase diagnosi kit
InactiveCN1769478AStrong specificityImprove test accuracyMicrobiological testing/measurementPhosphoric acidPyruvate synthesis
The invention relates to a method for determining the activity of adenosine deaminase, and also the reagent kit for adenosine deaminase diagnosis. The reagent kit comprises cushioning solution, adenosine, glutacid, deacidized type coenzyme, adenosine triphosphate, pyroracemic acid, phosphoenolpyruvate phosphatase, glutamine synthetase, pyruvic oxidase, phosphoenolpyruvate pyruvate carboxylase, malic dehydrogenase, and stabilizer. By mixing sample and reagent of a predetermiend volumetric ratio, generating coupling reaction between them, subjecting the final reactant to biochemiscal analyser, the main wavelength absorbancy variance ratio (speed) can be detected, and the activity of the adenosine deaminase can thus be measured. The method of the invention can be used to obtain the needed measurement result purely through biochemical analytic instruments, and advantages of the method include higher sensibility, better accuracy, less susceptibility to contamination of internal or external materials, and easy application.
Owner:王尔中
Inorganic phosphorus (phosphate radical) diagnostic and measuring kit and method for measuring inorganic phosphorus (phosphate radical) concentration
InactiveCN101609033AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsPhosphateGlutamate decarboxylase
The invention relates to an inorganic phosphorus (phosphate radical) diagnostic and measuring kit utilizing technologies of an enzymatic-colorimetric method and an enzyme-link method, also relates to a method and a principle of measuring inorganic phosphorus (phosphate radical) concentration and compositions and components of reagents, and belongs to the technical field of testing and measuring of medical science, food, and environment. The kit mainly comprises the following compositions: buffer solution, reduced coenzyme, glutamine, adenosine diphosphate, pyruvic acid, glutamine synthetase, glutamate decarboxylase, malic dehydrogenase, and stabilizer; samples are mixed with the reagents in certain volume ratio to perform a series of enzymatic reactions; then reactants are placed under a UV / visible analyzer; and the descending level of absorbance is tested at the position where dominant wave length is 340nm so as to measure and calculate the inorganic phosphorus (phosphate radical) concentration.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Ammonia ion diagnosis/measuring reagent kit and ammonia ion concentration determination method
InactiveCN101464377AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsEnzymatic ColorimetryPyrophosphate
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Cordyceps sinensis hirsutella-sinensis malic dehydrogenase B, encoding gene and application of two
InactiveCN104130986AExpand biological applicationsEnhance expressive abilityBacteriaMicroorganism based processesNucleotideTricarboxylic acid
The invention relates to malic dehydrogenase A which comes from corbrin-capsule-producing strain cordyceps sinensis hirsutella sinensis, participates tricarboxylic acid cycle and is used for citric acid synthesis, a gene encoding the malic dehydrogenase B, and application of the above two. The amino acid sequence of the malic dehydrogenase B and protein shown as SEQ ID No. 1 have 90% or more of homology, and the nucleotide sequence of the encoding gene and a sequence shown as SEQ ID No. 2 have 90% or more of homology. A cloned DNA of the provided nucleotide sequence can be transferred into an engineering bacterium through a transduction, transformation or conjugal transferring method. By adjusting expression of the encoding gene corresponding to the enzyme catalyzing malic acid for preparing corresponding oxobutanedioic acid, a host is endowed with property of highly expressing malic dehydrogenase B, an effective approach is provided for expanding biological application of malic dehydrogenase A, and important application prospect is provided.
Owner:ZHEJIANG UNIV OF TECH +1
Malic dehydrogenase PbMDH as well as coded sequence and application thereof
ActiveCN109337879AIncreased specific enzyme activityEfficient expressionContaminated soil reclamationOxidoreductasesNucleotide sequencingBiology
The invention provides malic dehydrogenase PbMDH derived from Pseudomonas beteli, as well as a coded gene and an application of the malic dehydrogenase. Cloned plasmid with the malic dehydrogenase PbMDH nucleotide sequence can be transferred into engineering bacteria by transduction, transformation and conjugal transfer, the malic dehydrogenase PbMDH can be efficiently expressed by adjusting expression of the coded gene, and an effective way is provided for production of the malic dehydrogenase. The marine-derived malic dehydrogenase is resistant to salt and high temperature and has importantindustrial application prospects.
Owner:XIAMEN UNIV
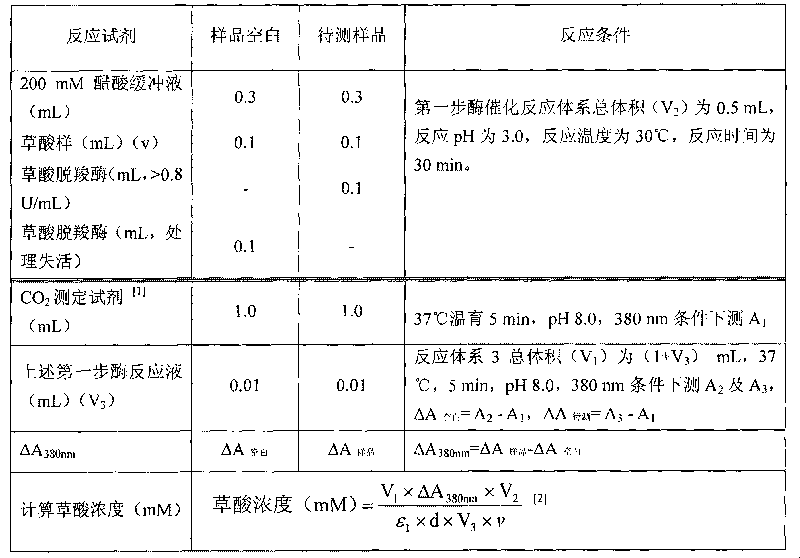
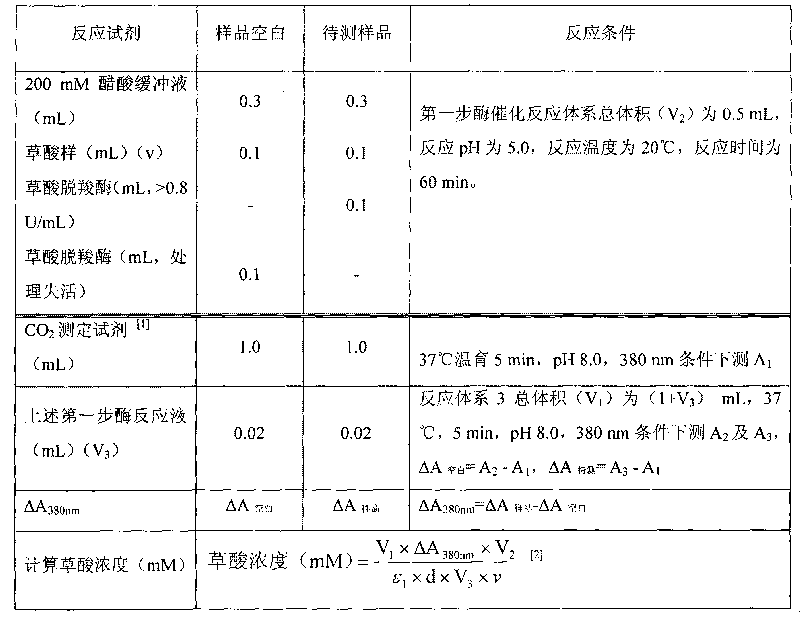
Method for determining concentration of oxalic acid by determining concentration of carbon dioxide through enzymatic method
InactiveCN101691601ASimple and fast operationLow costMicrobiological testing/measurementColor/spectral properties measurementsPhosphoenolpyruvate carboxylaseBuffer solution
The invention relates to a method for determining the concentration of oxalic acid by determining the concentration of carbon dioxide through an enzymatic method. The method comprises the following steps: (1) carrying out reaction of the mixture of oxalic acid, oxalate decarboxylase and acetate buffer solution for 30-60min at a temperature of 20-37 DEG C; (2) preparing Tris-HCI buffer solution containing phosphoenolpyruvate, phosphoenolpyruvate carboxylase, NADH, malic dehydrogenase, with a pH value of 7.3-8.5, incubating at a temperature of 37 DEG C and determining A1 at the position of 380nm; (3) adding the reaction solution prepared in the step (1) to the solution prepared in the step (2) for reaction for 5min at a temperature of 37 DEG C and determining A2 and A3 at the position of 380nm; and (4) calculating the decrement of NADH according to the formula: delta A 380nm = delta A to be determined - delta A blank, and calculating the concentration of the generated carbon dioxide according to a reaction principle so as to calculate the concentration of the oxalate decarboxylase. The method has simple operation, low cost and high sensitivity; the enzyme reagent adopted in the method is cheaper; and the method has similar accuracy compared with that of the existing kits for determining oxalic acid and has good practicability.
Owner:DONGHUA UNIV
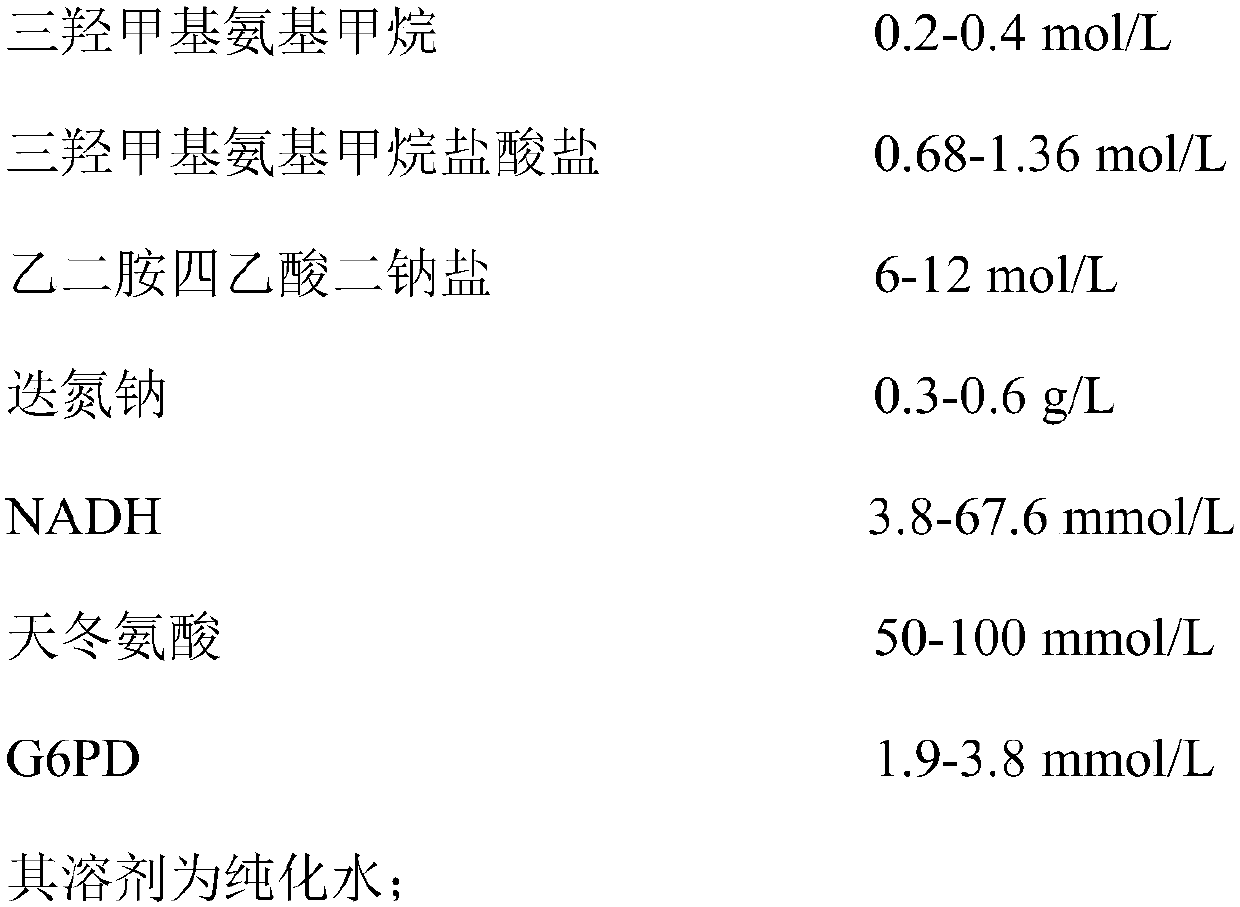
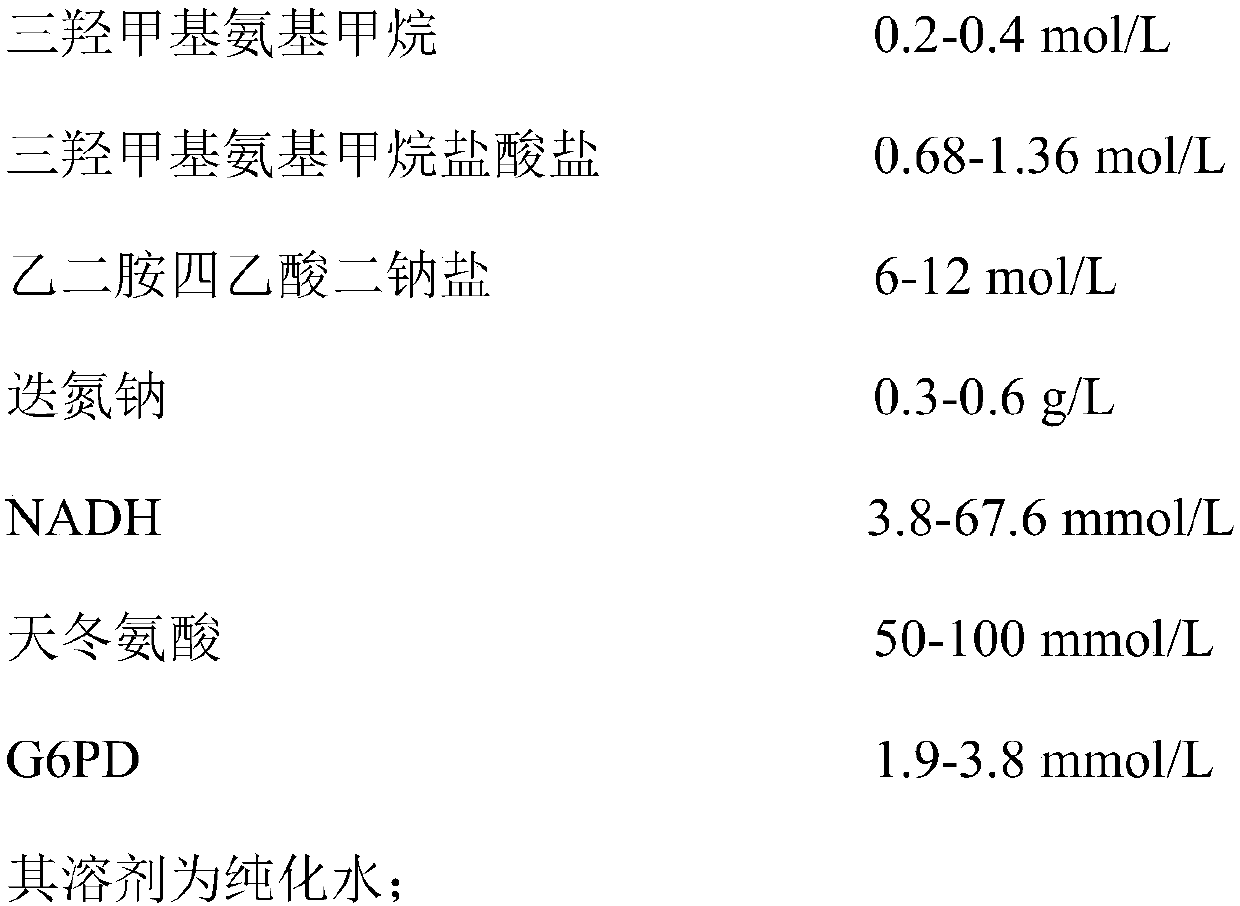
Aspartic acid aminotransferase detection kit as well as preparation and use method thereof
The invention discloses an aspartic acid aminotransferase detection kit which comprises a reagent R1, including trismetyl aminomethane, trismetyl aminomethane hydrochloride, disodium ethylenediamine tetraacetic acid, sodium azide, NADH (Nicotinamide-Adenine Dinucleotid), aspartic acid and G6PD, and a reagent R2, including trismetyl aminomethane, trismetyl aminomethane hydrochloride, alpha-oxoglutarate and malic dehydrogenase. The invention further discloses a preparation and use method of the aspartic acid aminotransferase detection kit. The kit has the advantages that firstly, the kit is simple to prepare, convenient to operate and low in cost, secondly, the kit is rapid in detection speed, thirdly, influence of environment factors to activity of reaction enzymes is retarded through G6PD,and thus the problems of long-term storage and degradation in the use process of the kit are solved.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Kalium ion diagnosis/measuring reagent kit and kalium ion concentration determination method
InactiveCN101464302AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsSodium bicarbonatePotassium
The invention relates to a kit for diagnosing / measuring potassium (ions) by utilizing the technologies of the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the concentration of the potassium (ions), and belongs to the technical field of medical / food / environmental inspection and measurement. The main components of the kit include a buffer solution, reduced coenzyme, adenosine diphosphate, a phosphoenolpyruvic acid, sodium bicarbonate, pyruvate oxidase, oxaloacetic decarboxylase, malic dehydrogenase and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the velocity of the decrease in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the concentration of potassium (ions).
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
A kind of malate dehydrogenase pbmdh and its coding sequence and application
ActiveCN109337879BIncreased specific enzyme activityEfficient expressionContaminated soil reclamationOxidoreductasesNucleotideMicrobiology
The invention provides malic dehydrogenase PbMDH derived from Pseudomonas beteli, as well as a coded gene and an application of the malic dehydrogenase. Cloned plasmid with the malic dehydrogenase PbMDH nucleotide sequence can be transferred into engineering bacteria by transduction, transformation and conjugal transfer, the malic dehydrogenase PbMDH can be efficiently expressed by adjusting expression of the coded gene, and an effective way is provided for production of the malic dehydrogenase. The marine-derived malic dehydrogenase is resistant to salt and high temperature and has importantindustrial application prospects.
Owner:XIAMEN UNIV
Determination method of ammonia (ammonia ions) and diagnosis/determination kit for ammonia (ammonia ions)
InactiveCN102565332AMicrobiological testing/measurementColor/spectral properties measurementsCarbamyl PhosphateEnzymatic Colorimetry
The invention relates to a determination method of ammonia (ammonia ions) content by means of technologies of enzymatic colorimetry and enzyme-linked assay, and composition and components of a reagent. The technical principle of determination is based on a series of catalytic reactions of ammonia kinase, carbamyl phosphate synthetase, and malic dehydrogenase. The invention further relates to a diagnosis / determination kit for ammonia (ammonia ions). The determination method provided by the invention has high sensitivity and small error. Therefore, the determination method and the kit provided by the invention can be widely applied to clinical medical / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Aspartate aminotransferase mitochondrion isozyme detection kit and application
The invention relates to an aspartate aminotransferase mitochondrion isozyme detection kit. The aspartate aminotransferase mitochondrion isozyme detection kit comprises the following components: the reagent 1 comprising 50 mmol / L of Tris buffer solution, 15 mmol / L of L-aspartic acid, 0.5 mmol / L of lactate dehydrogenase, more than 1 KU / L of the goat anti-human ASTs antibody, and more than 0.8 KU / Lof the malic dehydrogenase; the reagent 2 comprising 130 mmol / L of alpha-ketoglutaric acid and 130 mmol / L of NADH.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com