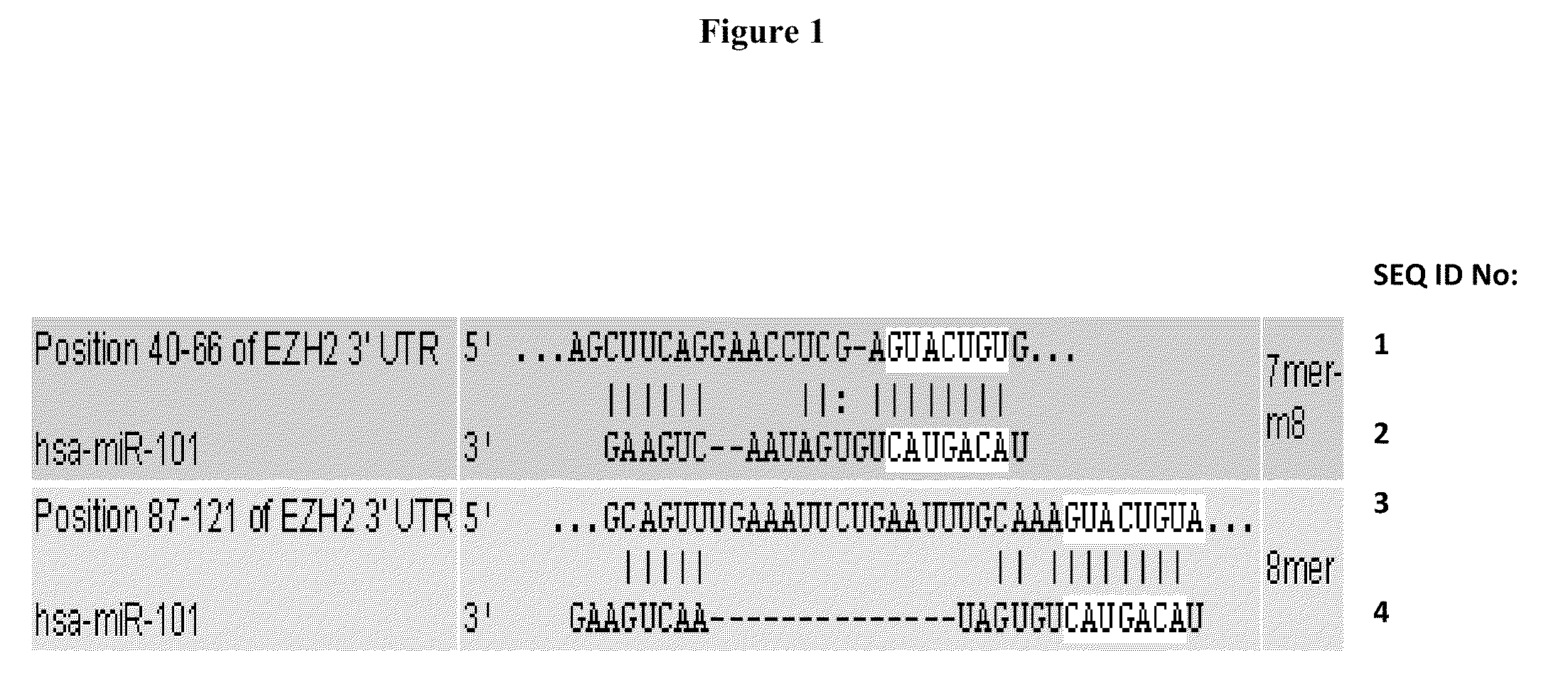
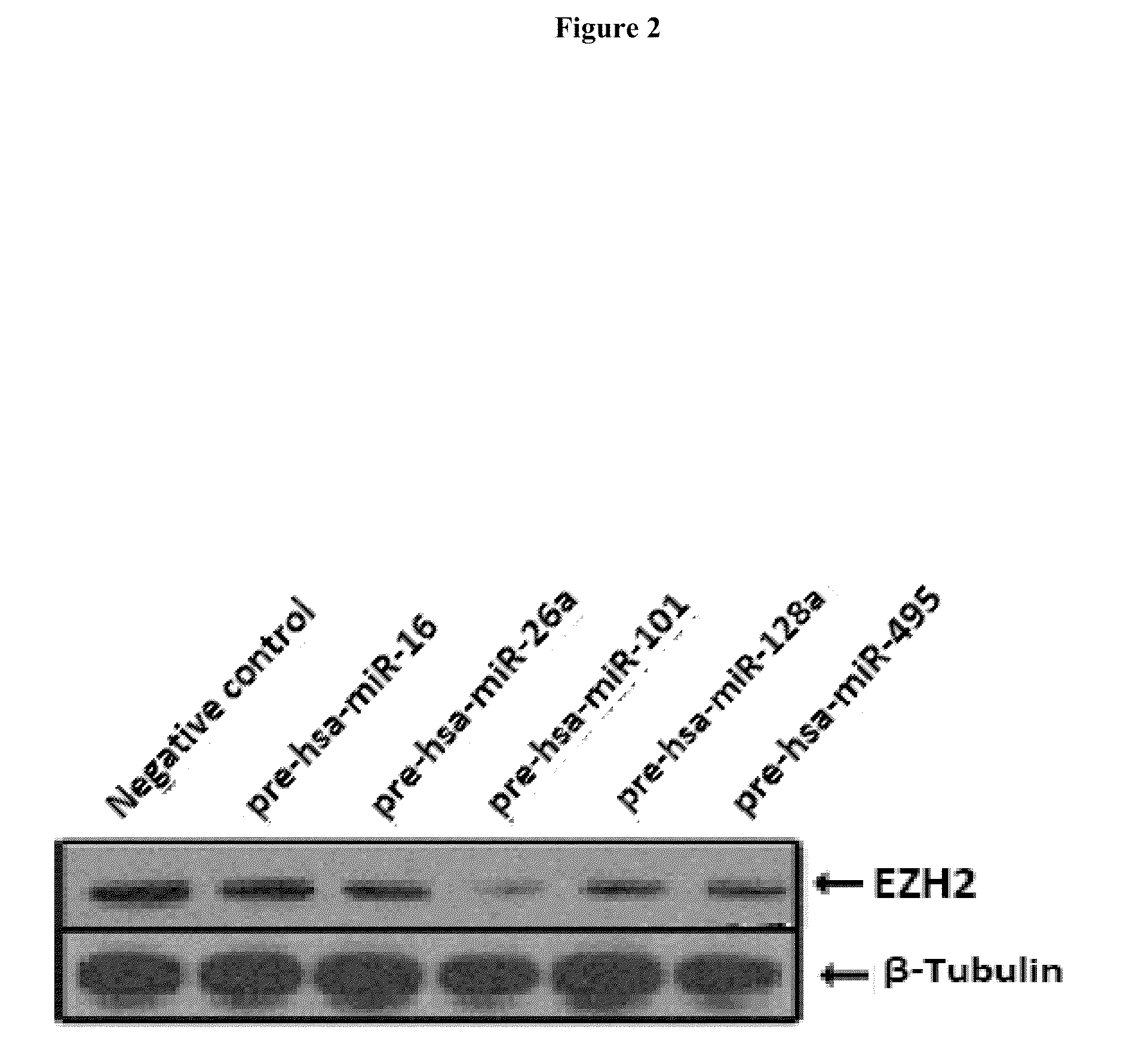
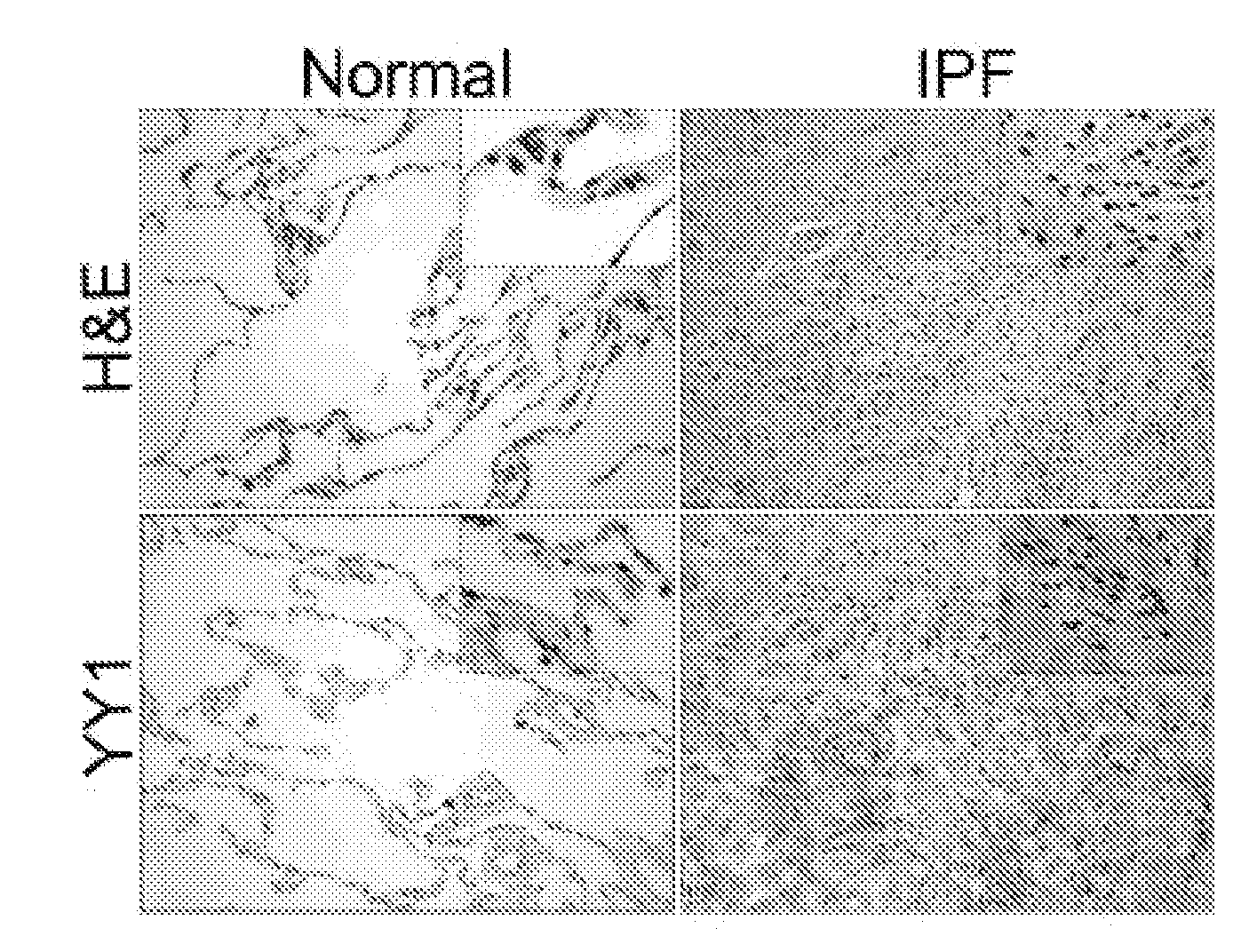
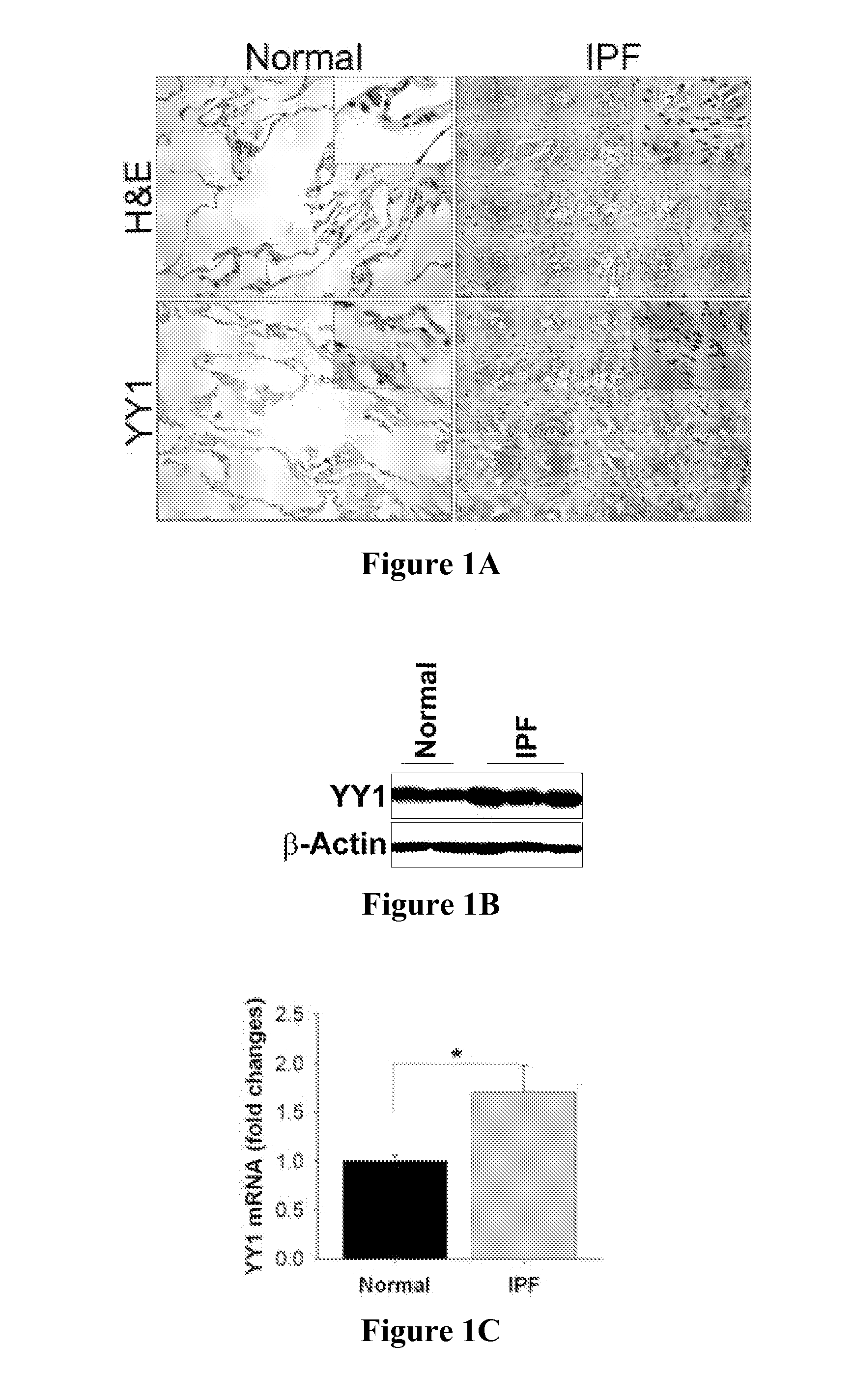
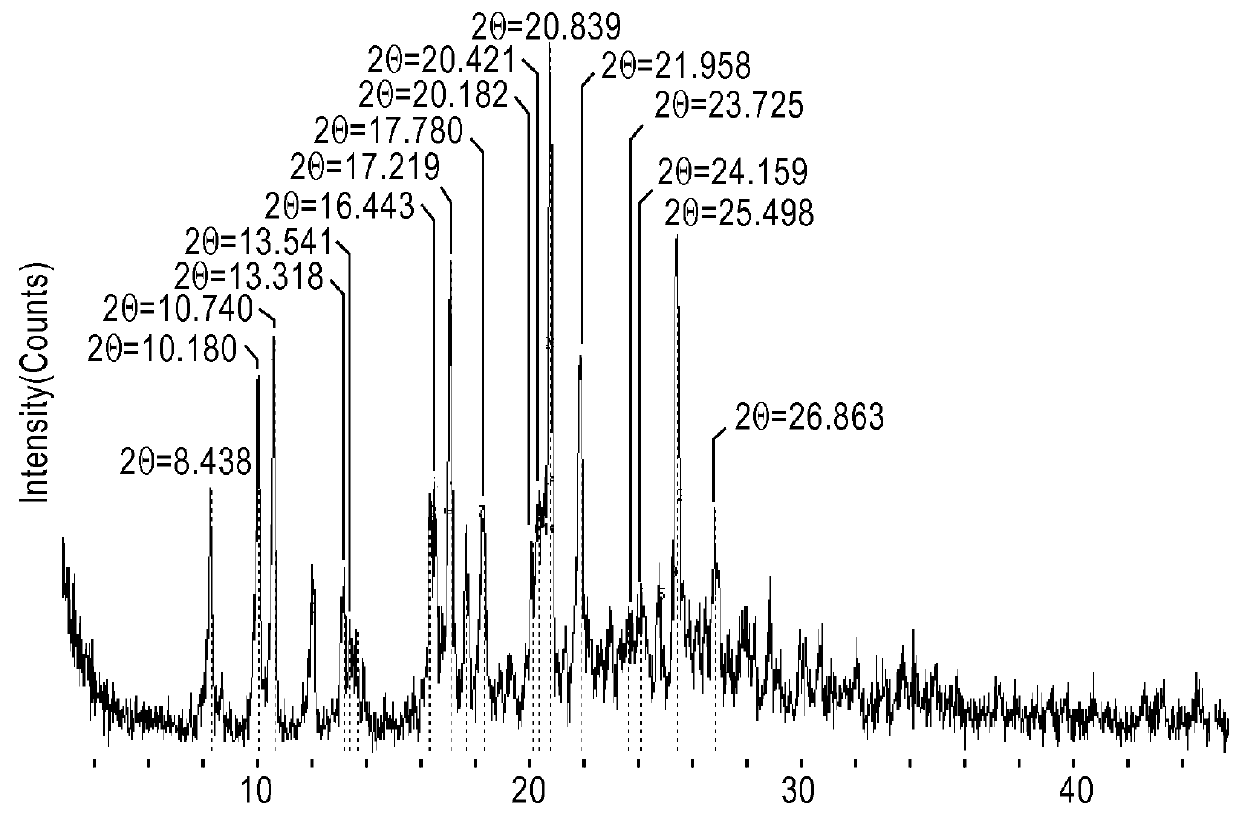
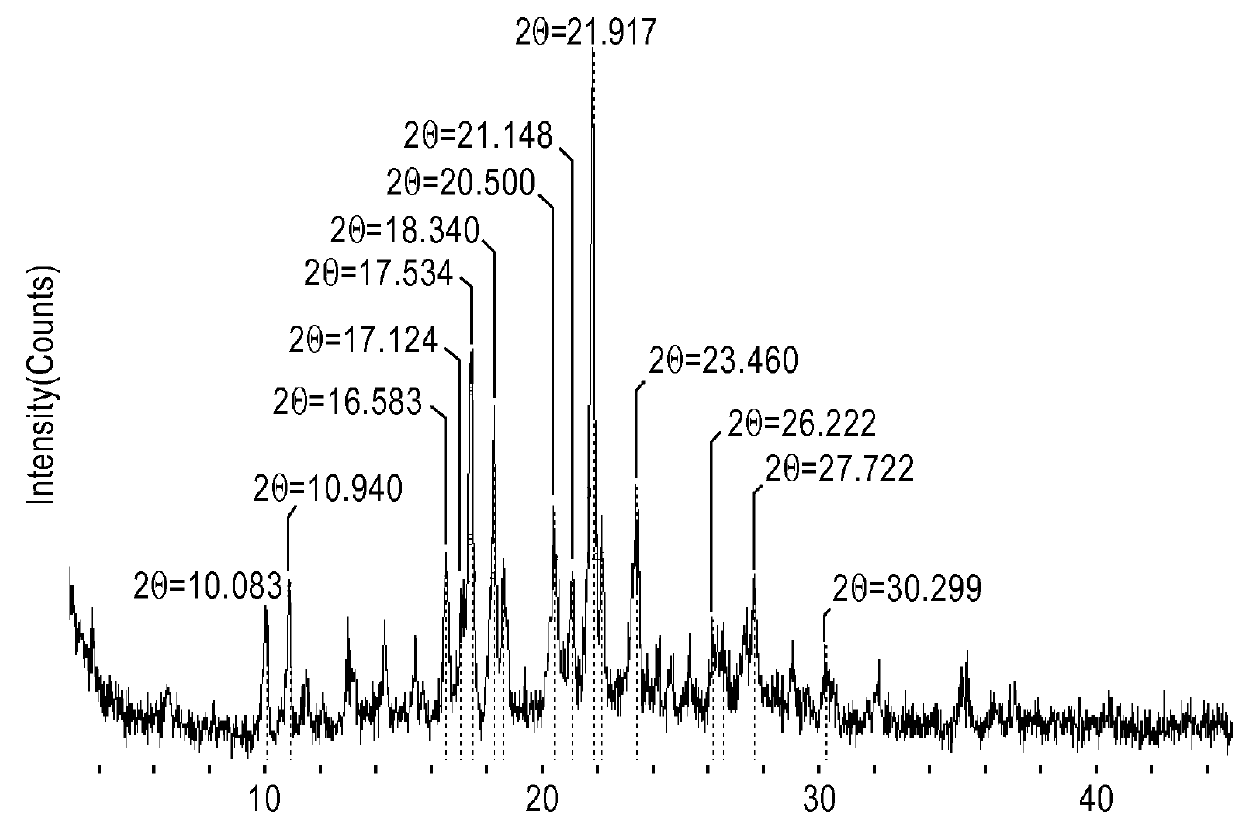
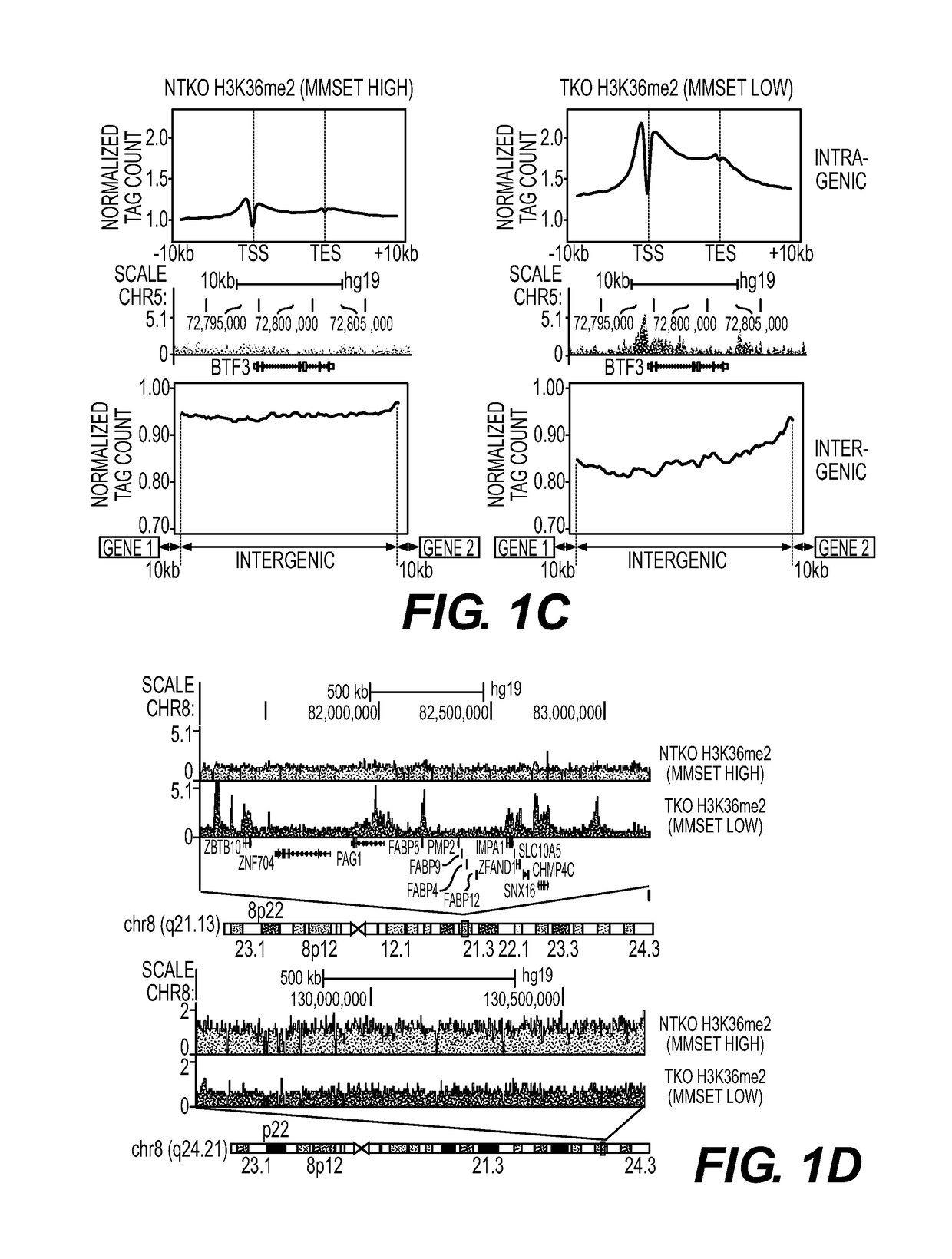
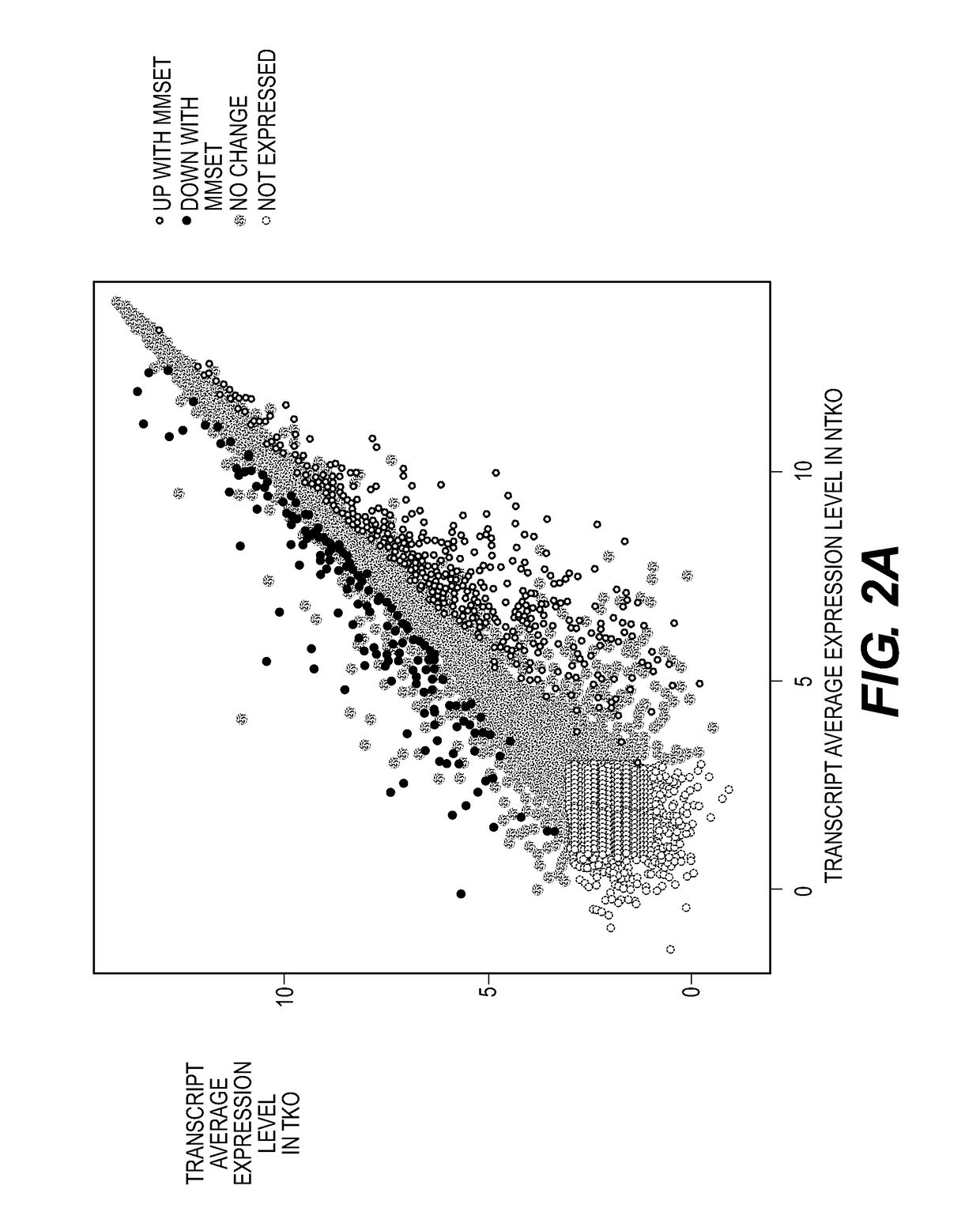
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "EZH2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enhancer of zeste homolog 2 (EZH2) is a histone-lysine N-methyltransferase enzyme (EC 2.1.1.43) encoded by EZH2 gene, that participates in histone methylation and, ultimately, transcriptional repression. EZH2 catalyzes the addition of methyl groups to histone H3 at lysine 27, by using the cofactor S-adenosyl-L-methionine. Methylation activity of EZH2 facilitates heterochromatin formation thereby silences gene function. Remodeling of chromosomal heterochromatin by EZH2 is also required during cell mitosis.
Compositions and methods for treatment of cancer
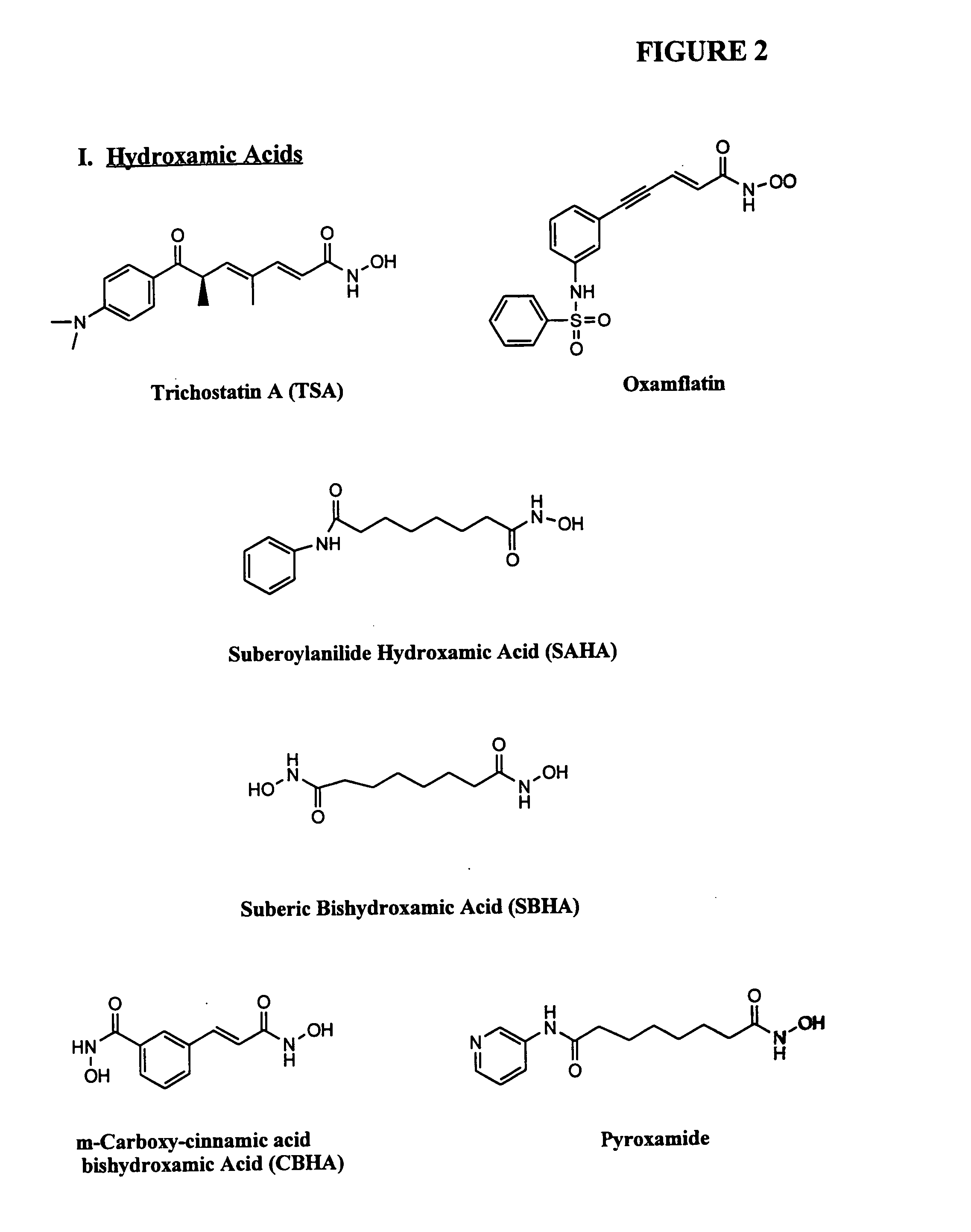
Compositions and methods for treatment of conditions related to the overexpression of EZH2, such as late stage prostate cancer, using a DNA methylation inhibitor and / or a histone deacetylase inhibitor, optionally in combination with an EZH2 antagonist and / or an antineoplastic agent, to specifically target diseases associated with EZH2 over-expression. Further provided are reagents and kits for treatment of EZH2 overexpression.
Owner:SUPERGEN
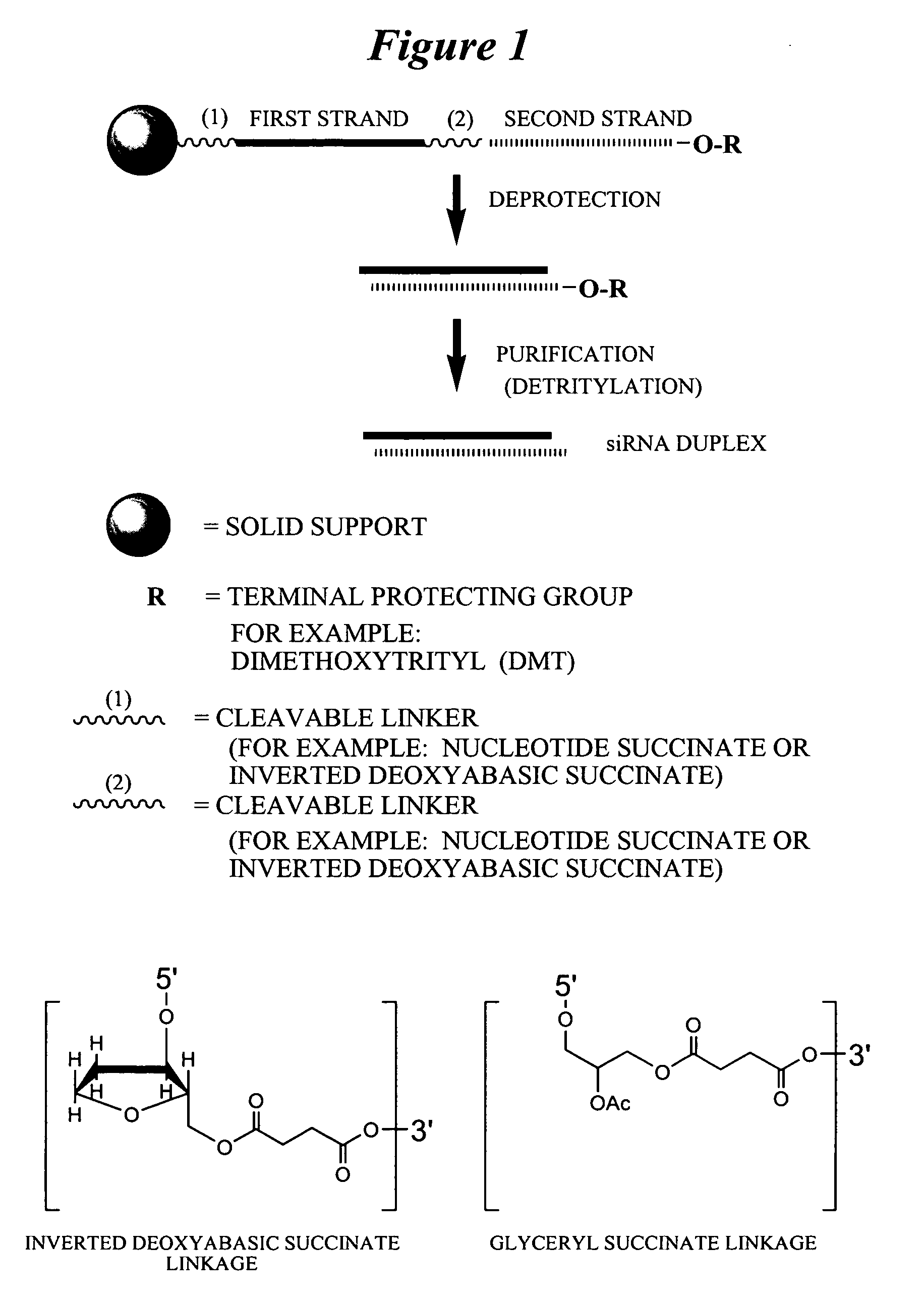
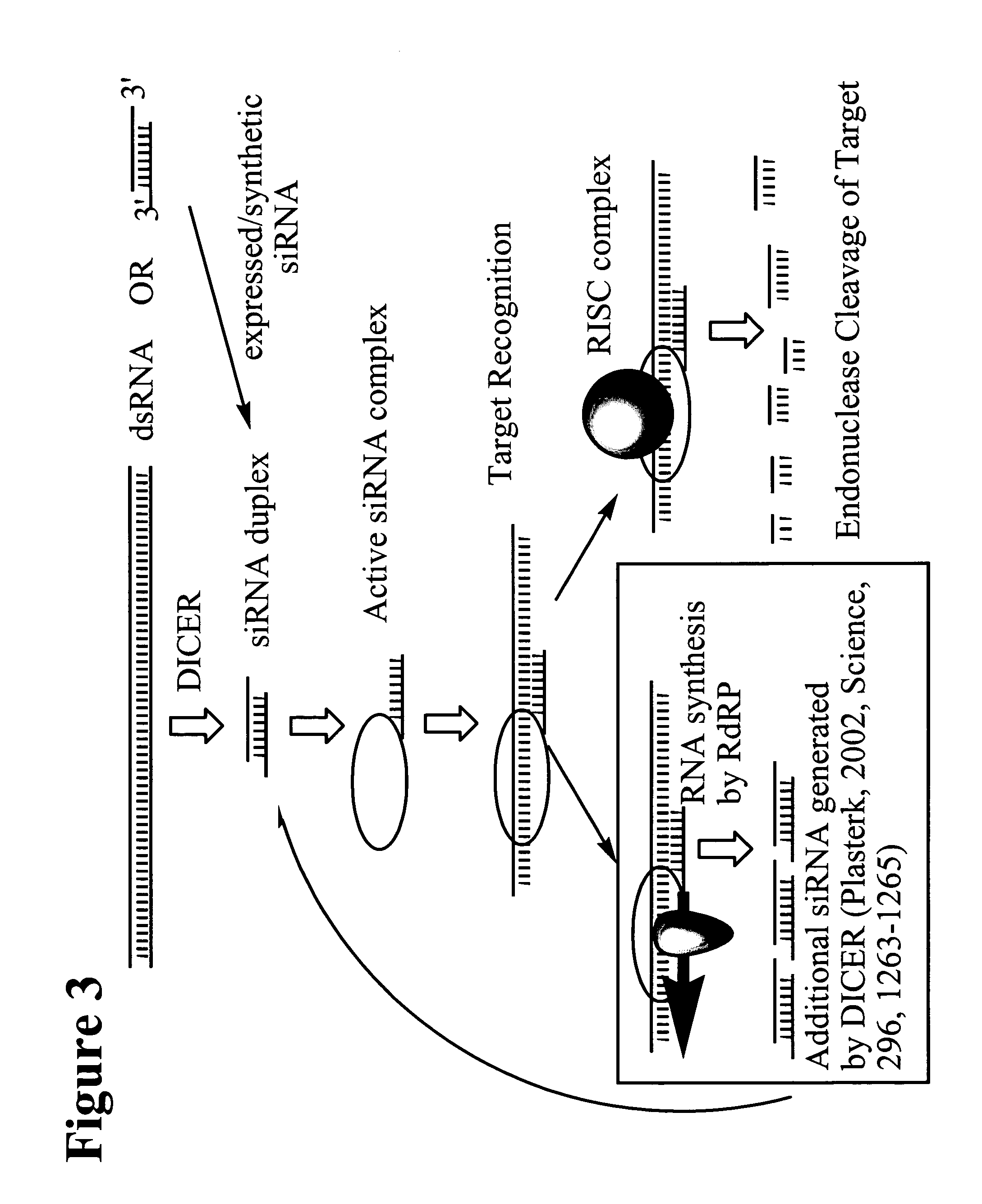
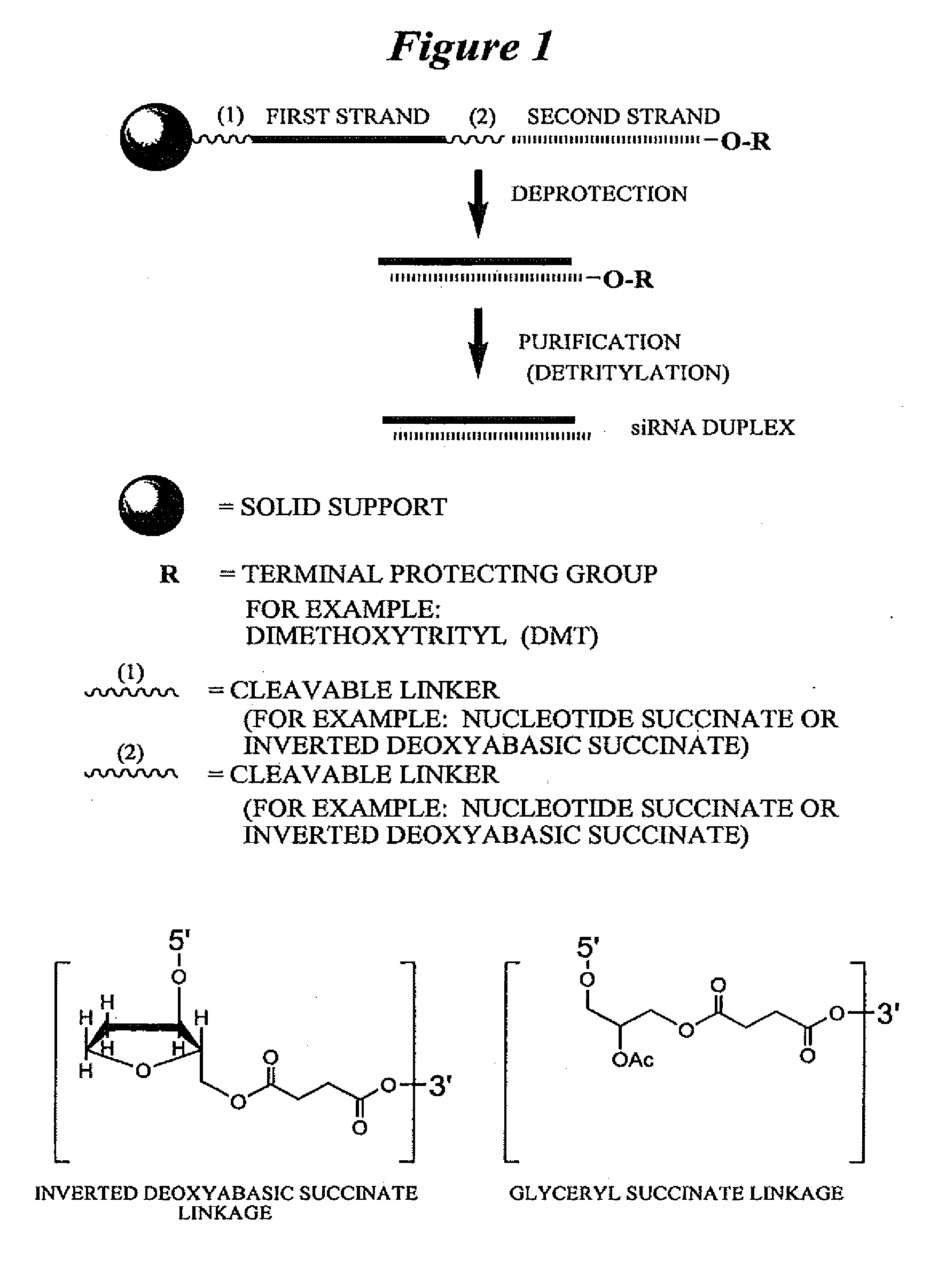
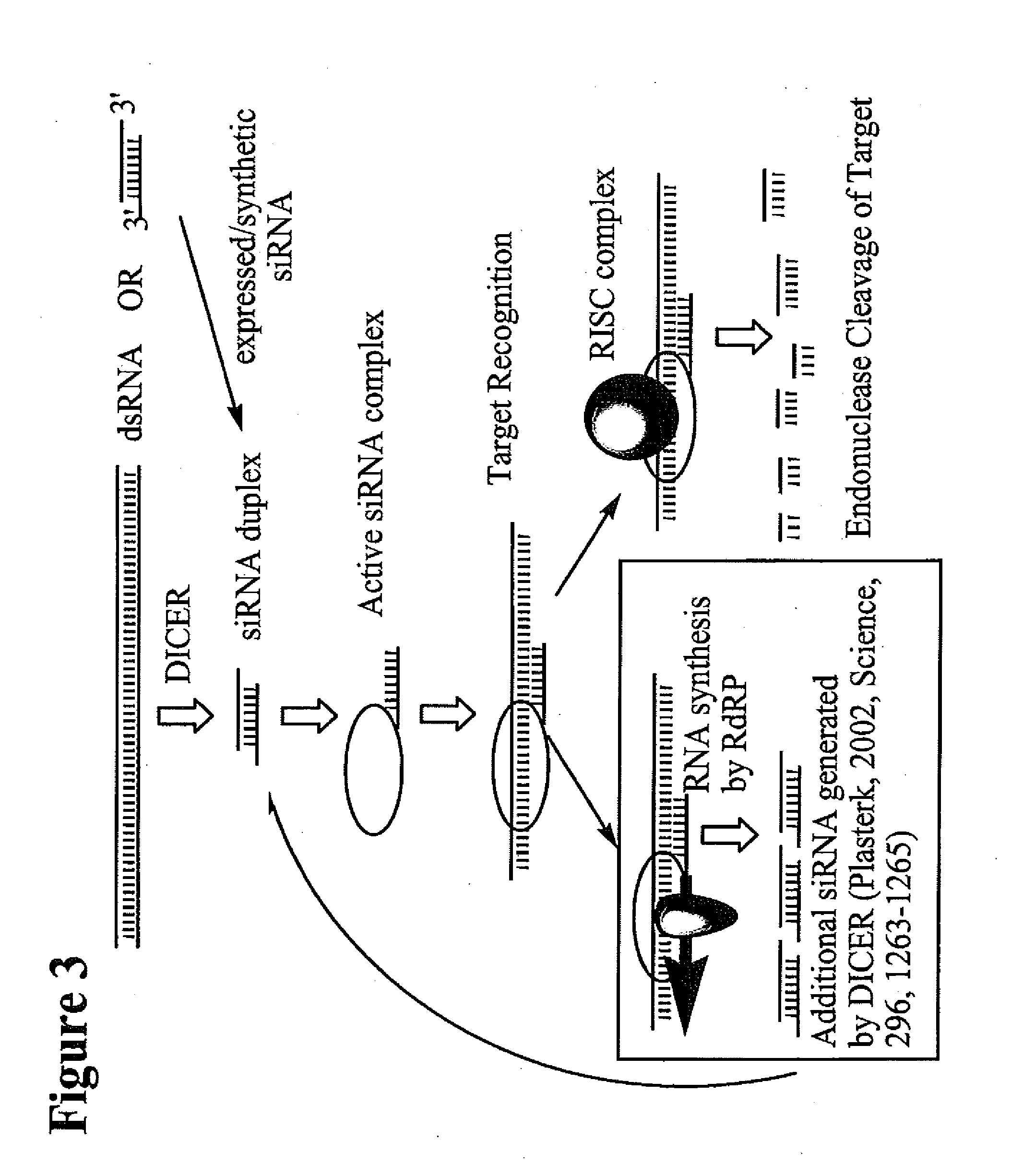
RNA interference mediated inhibition of polycomb group protein EZH2 gene expression using short interfering nucleic acid (siNA)
InactiveUS20050159382A1Improves various propertyImprove the immunityCompounds screening/testingSpecial deliveryEZH2Polycomb-group proteins
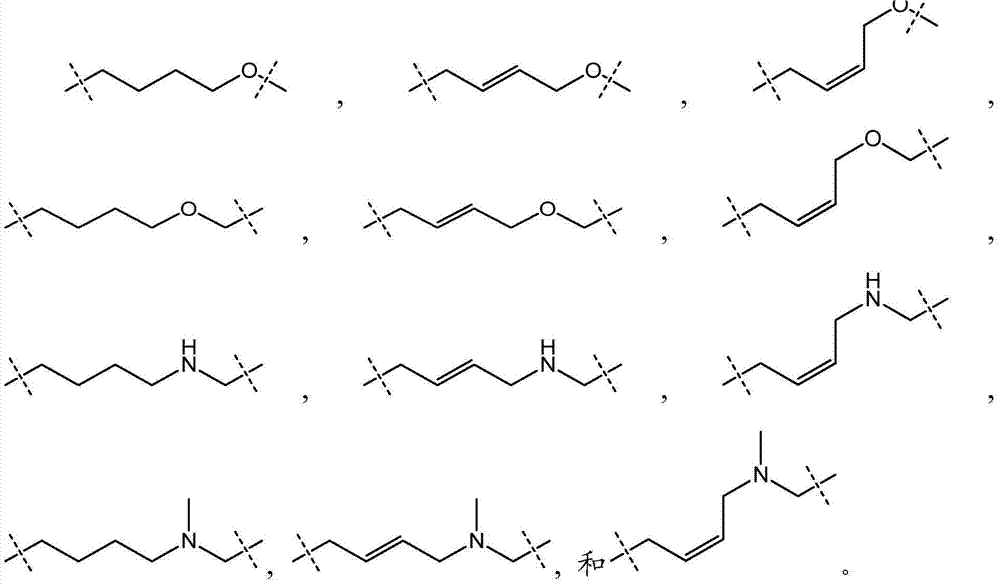
This invention relates to compounds, compositions, and methods useful for modulating polycomb group protein EZH2 gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of polycomb group protein EZH2 gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (mRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of polycomb group protein EZH2 genes, such as EZH2.
Owner:SIRNA THERAPEUTICS INC
Methods of diagnosing and treating fibrosis
InactiveUS20110286990A1Improve developmentPrevent and reduce disease progressionOrganic active ingredientsPeptide/protein ingredientsEZH2Fibrosis
The present invention is directed to methods of diagnosing and treating a fibrotic condition in a mammalian subject. These methods involve measuring the levels of trimethylation at lysine residue 27 of histone-3 and / or measuring the expression levels of EZH2 or YY-1. Agents useful for treating fibrosis or a fibrotic condition are also disclosed.
Owner:UNIVERSITY OF ROCHESTER
Methods and compositions for expansion of stem cells and other cells
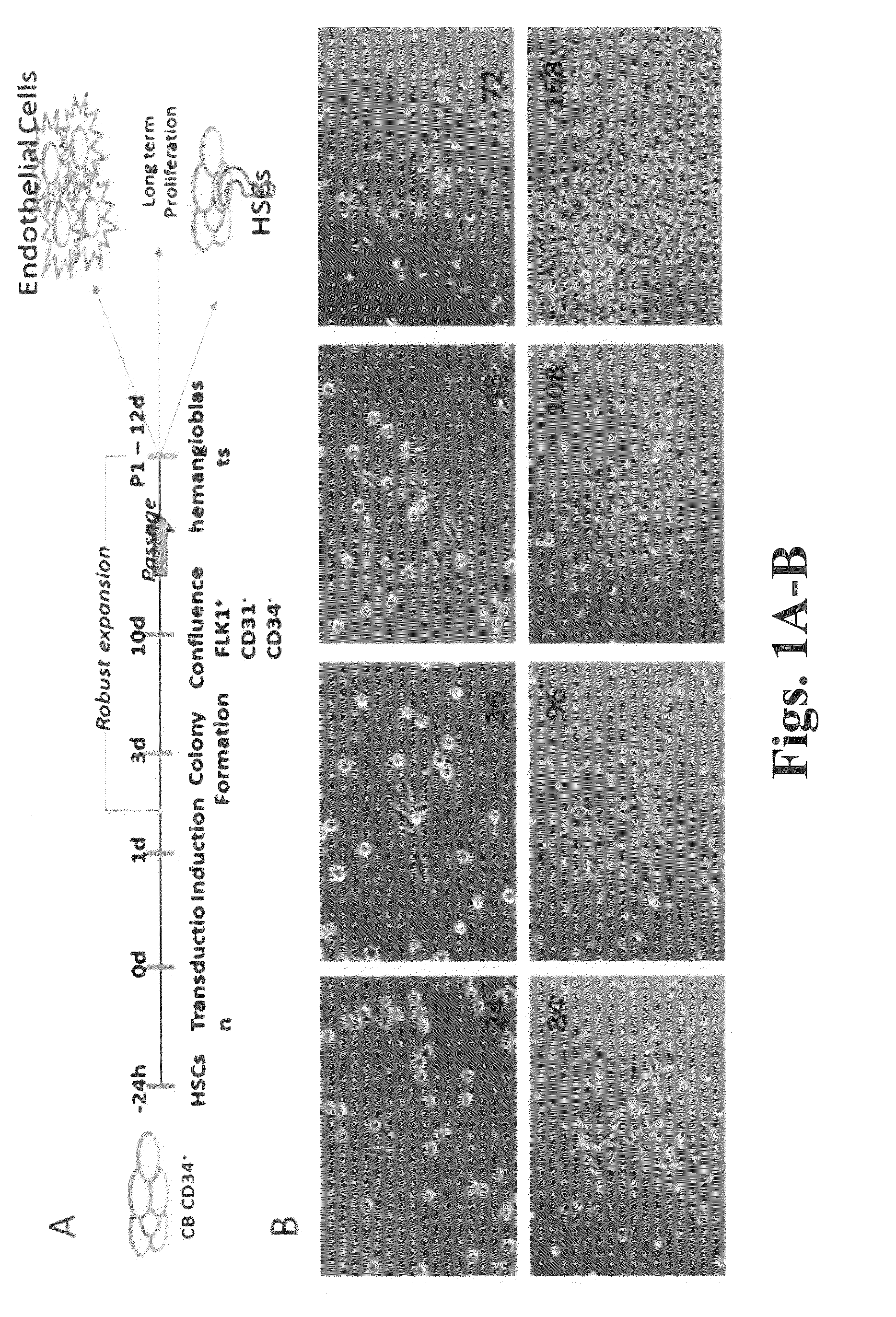
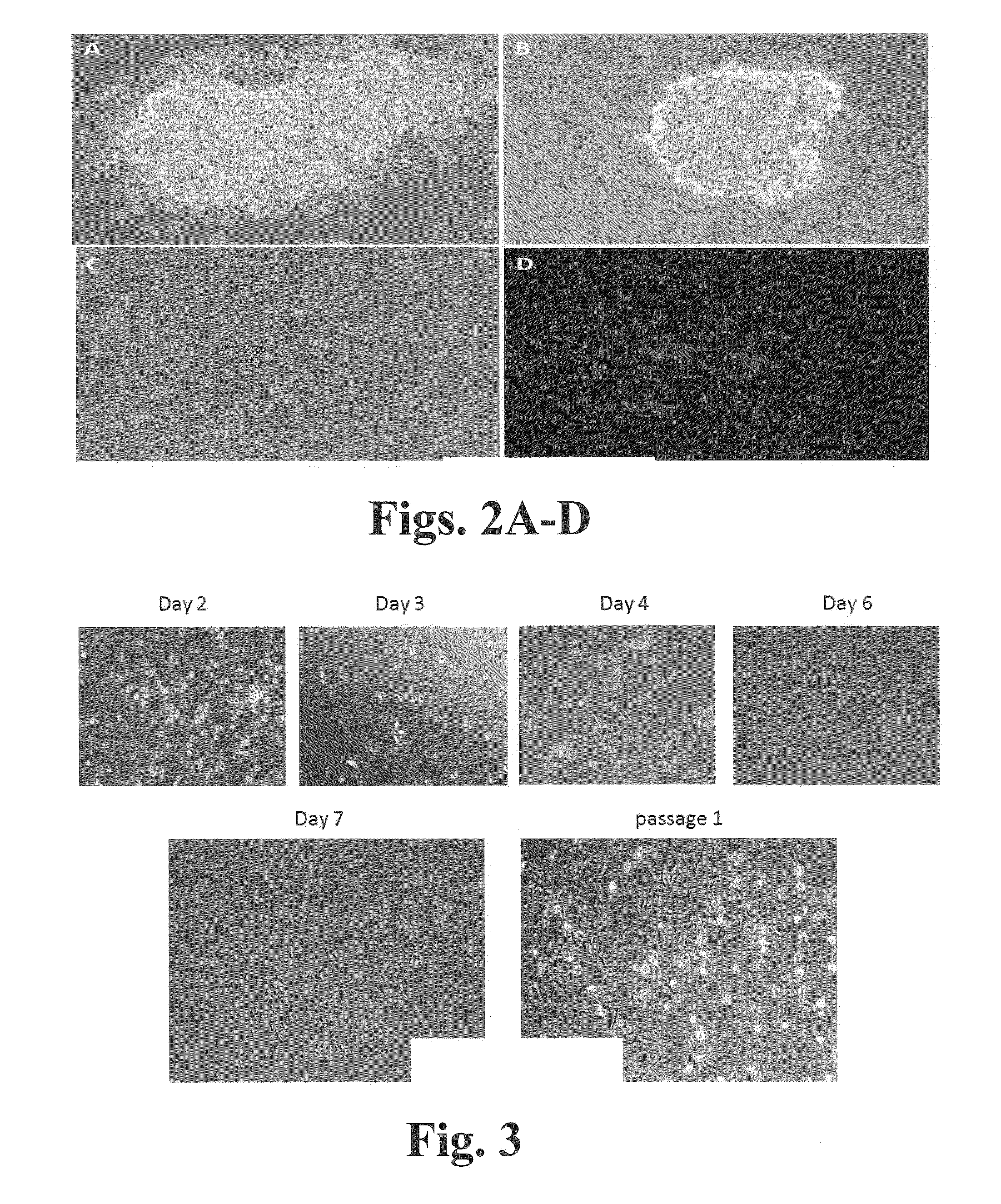
Presented herein are methods of generating a multipotent or immature cell from a mature somatic cell, involving contacting a mature somatic cell with one or more small molecule compounds selected from: a histone deacetylase (HDAC) inhibitor; a glycogen synthase kinase 3 (GSK-3) inhibitor; one or more transforming growth factor-beta receptor (TGF-βR) inhibitors; one or more lysine-specific demethylase 1 (LSD1) inhibitors; a cAMP agonist; a histone lysine methyltransferase (EZH2) inhibitor; and a histone methyltransferase (HMTase) G9a inhibitor; valproic acid. Also provided are methods of generating a multipotent or immature cell from a somatic cell, by driving expression of OCT4, or an OCT4 functional homolog or derivative, under the control of a high expressing promoter. Presented herein are also methods of stem cell expansion, stem cell regeneration and differentiation, which comprise contacting stem cells with one or more small chemical compounds.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Methods and compositions involving chitosan nanoparticles
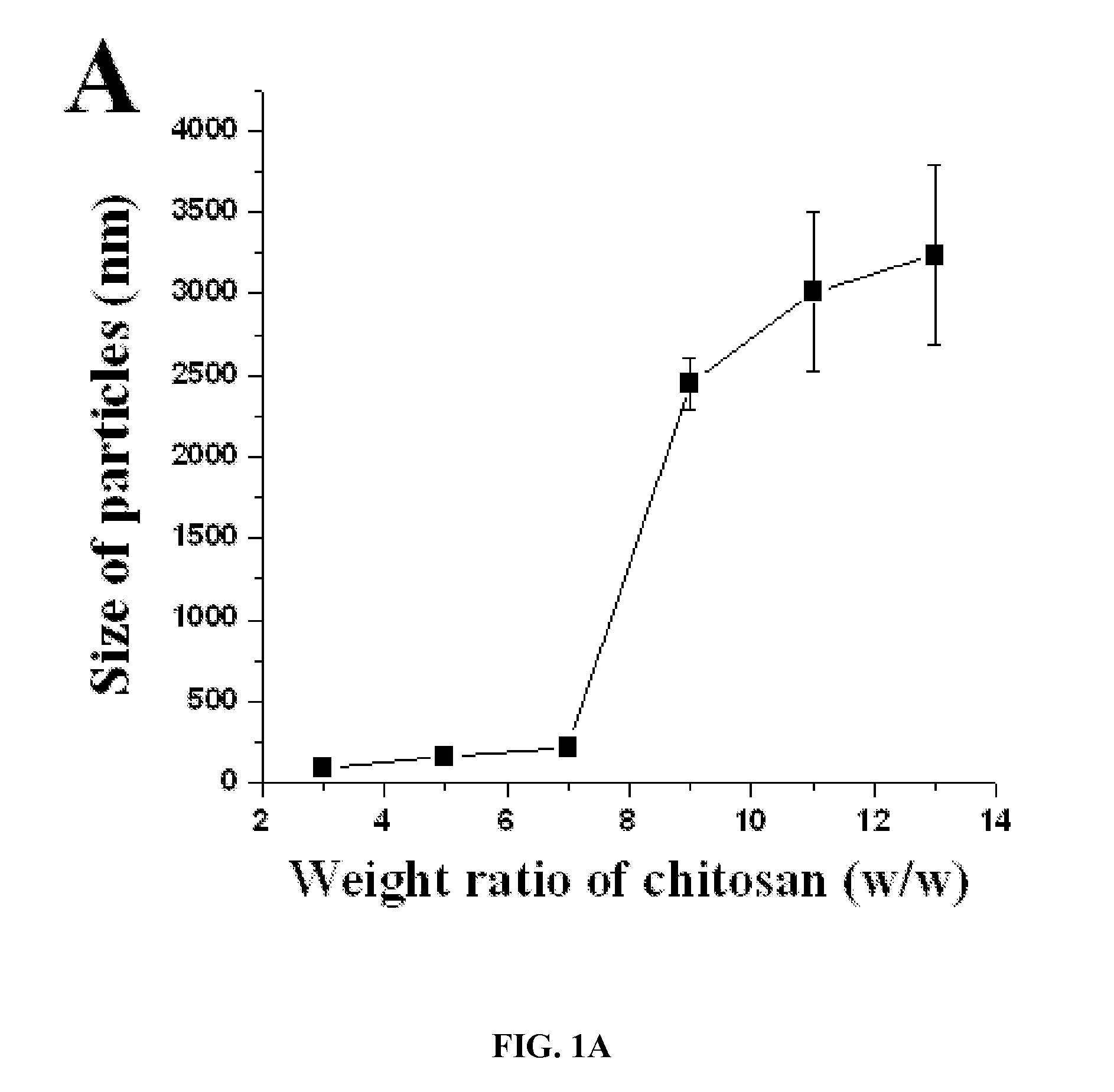
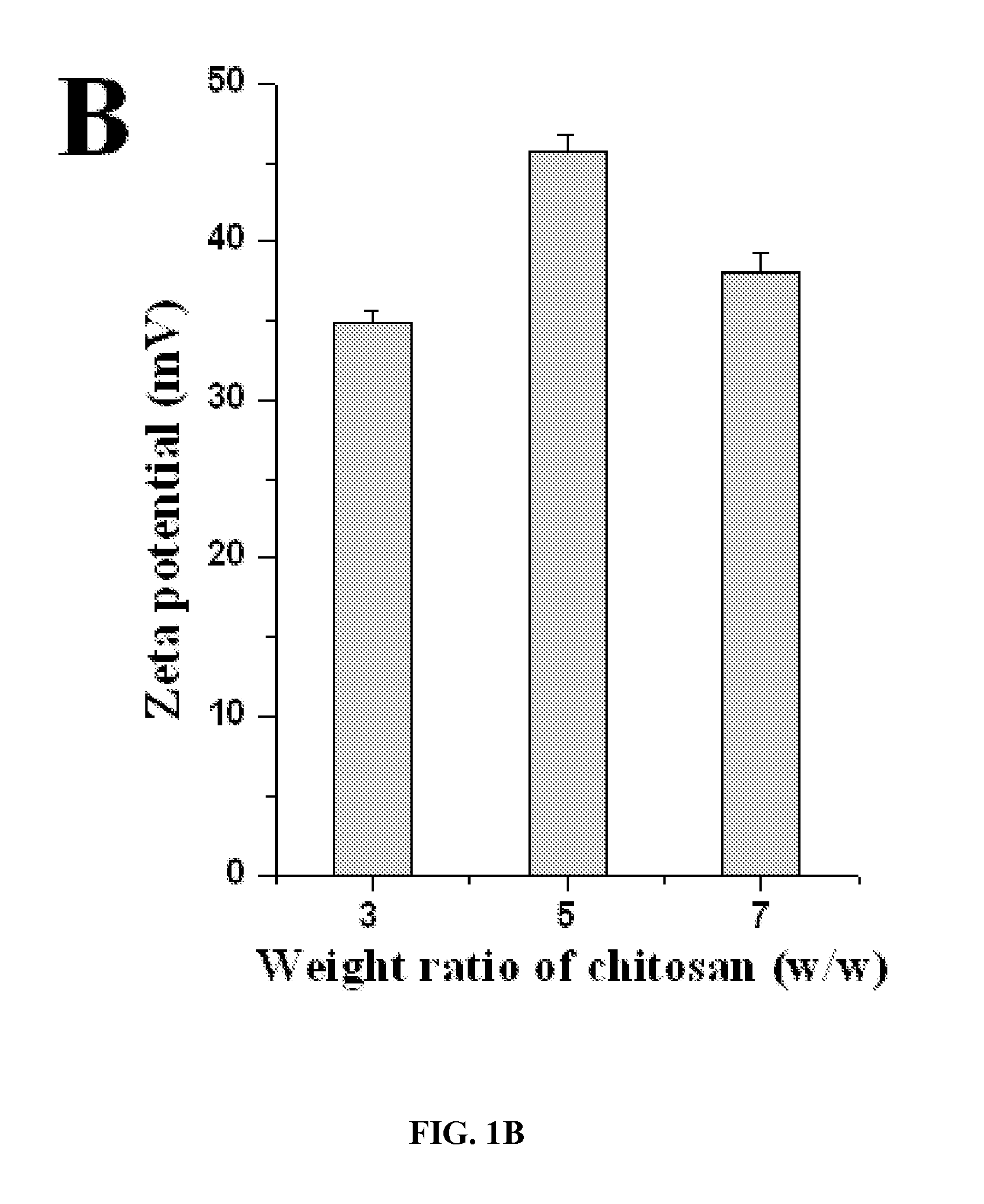
Disclosed are nanoparticles for the delivery of a therapeutic agent or a diagnostic agent to a subject that include a chitosan and a polyphosphate, wherein the weight ratio of the chitosan to the polyphosphate is about 1.0 or greater and the weight ratio of the polyphosphate to the therapeutic agent or diagnostic agent is about 15.0 or less. Also disclosed are nanoparticles that include a chitosan and an inhibitor of enhancer of Zeste homologue 2 (EZH2). Methods of delivering a therapeutic agent or a diagnostic agent to a subject for the treatment or prevention of a disease and methods of predicting prognosis of ovarian cancer in a subject that involve determining the expression and / or function of EZH2 in the subject are also disclosed.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
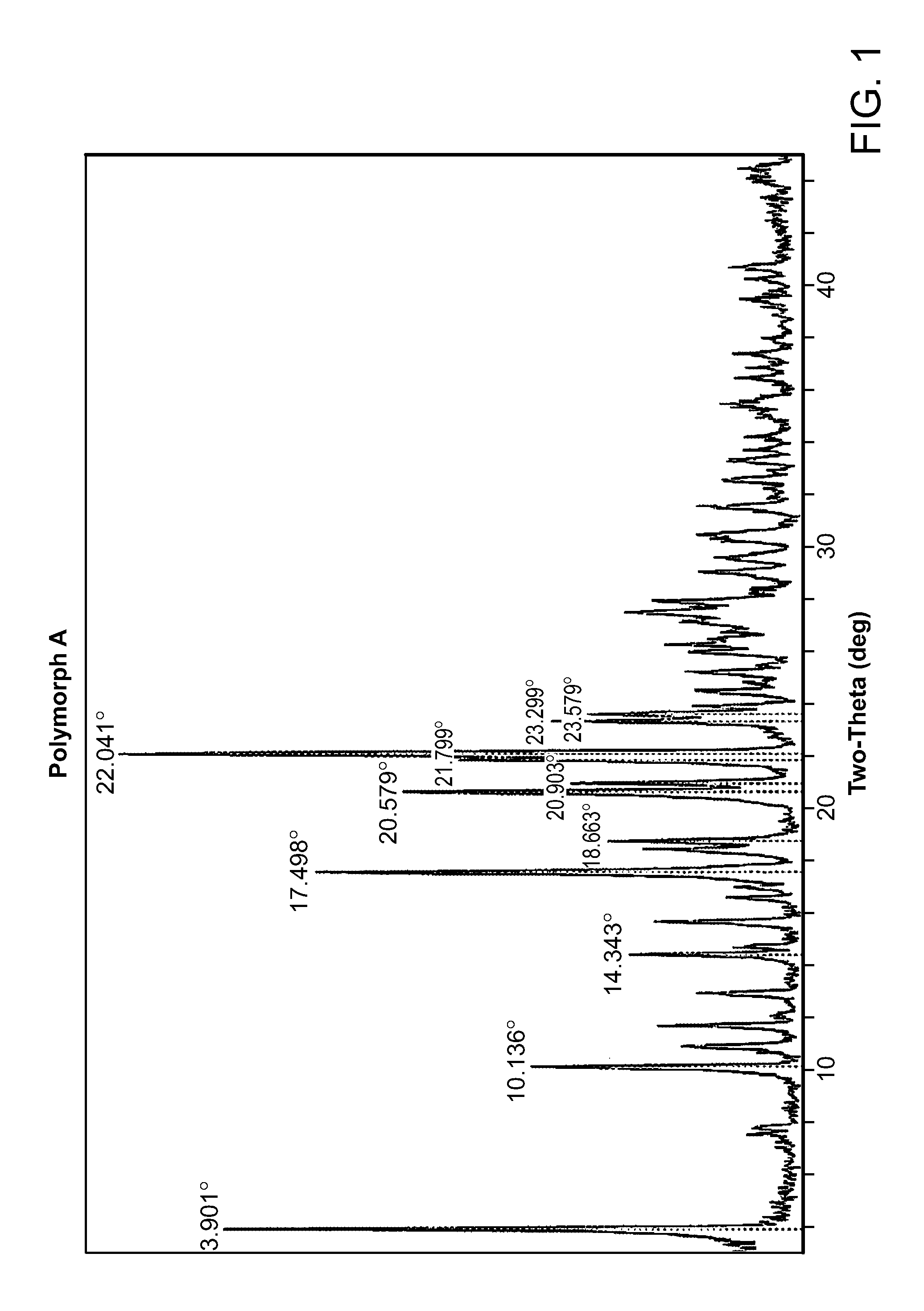
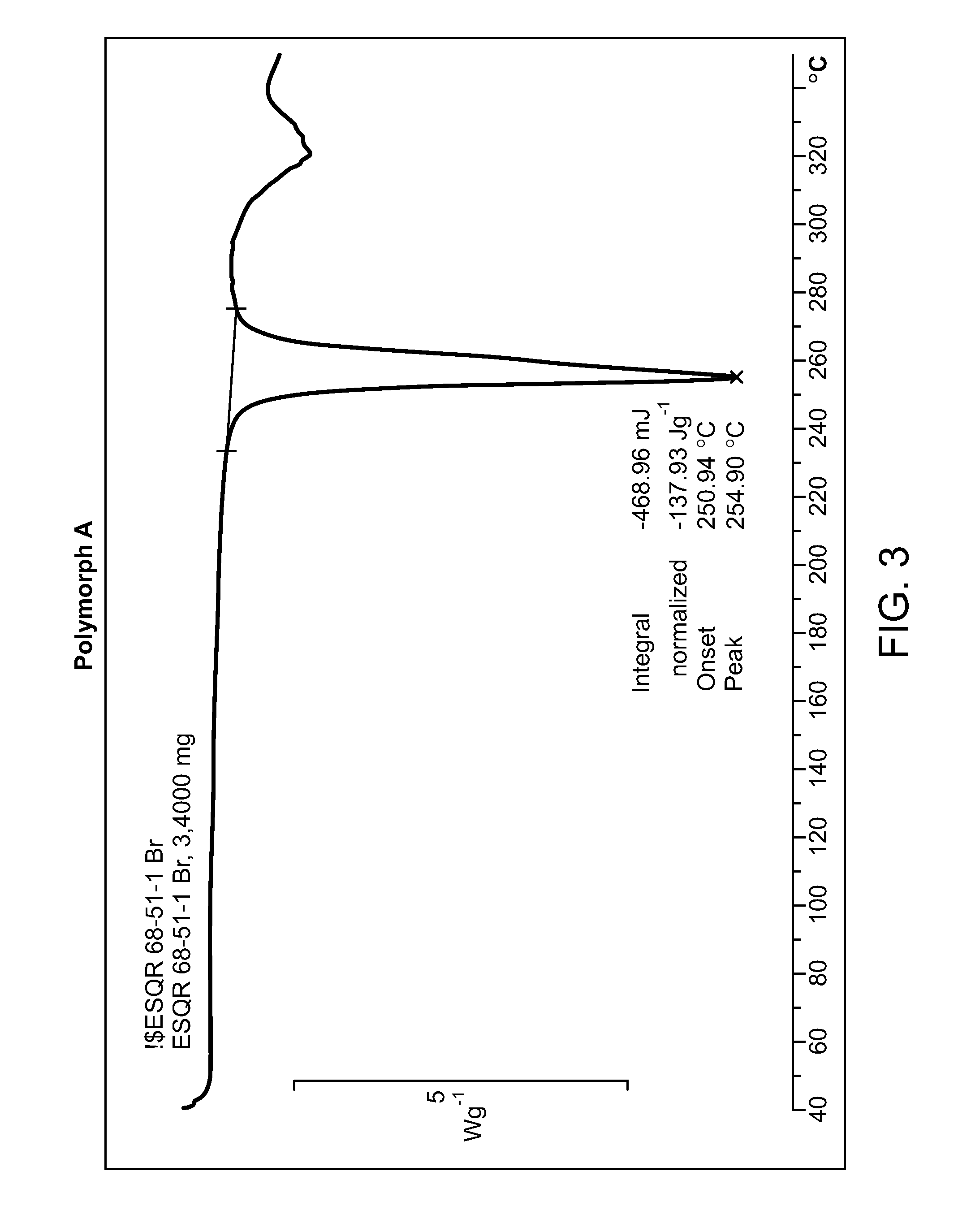
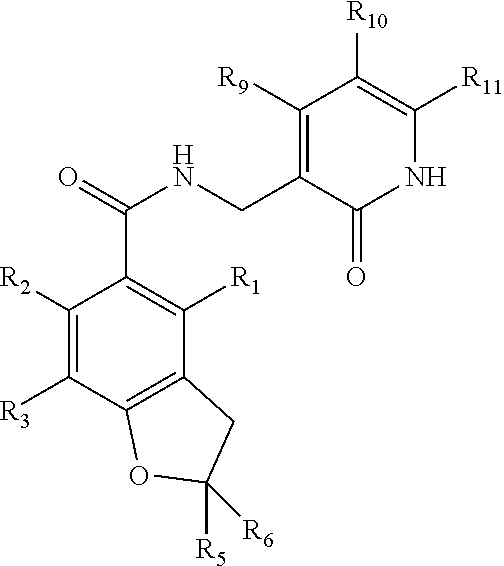
Salt form of a human histone methyltransferase EZH2 inhibitor
Provided herein is N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4′-(morpholinomethyl)-[1,1′-biphenyl]-3-carboxamide hydrobromide. Also provided herein is a particular polymorph form of this compound.
Owner:EISIA R&D MANAGEMENT CO LTD +1
Combination treatment of cancer
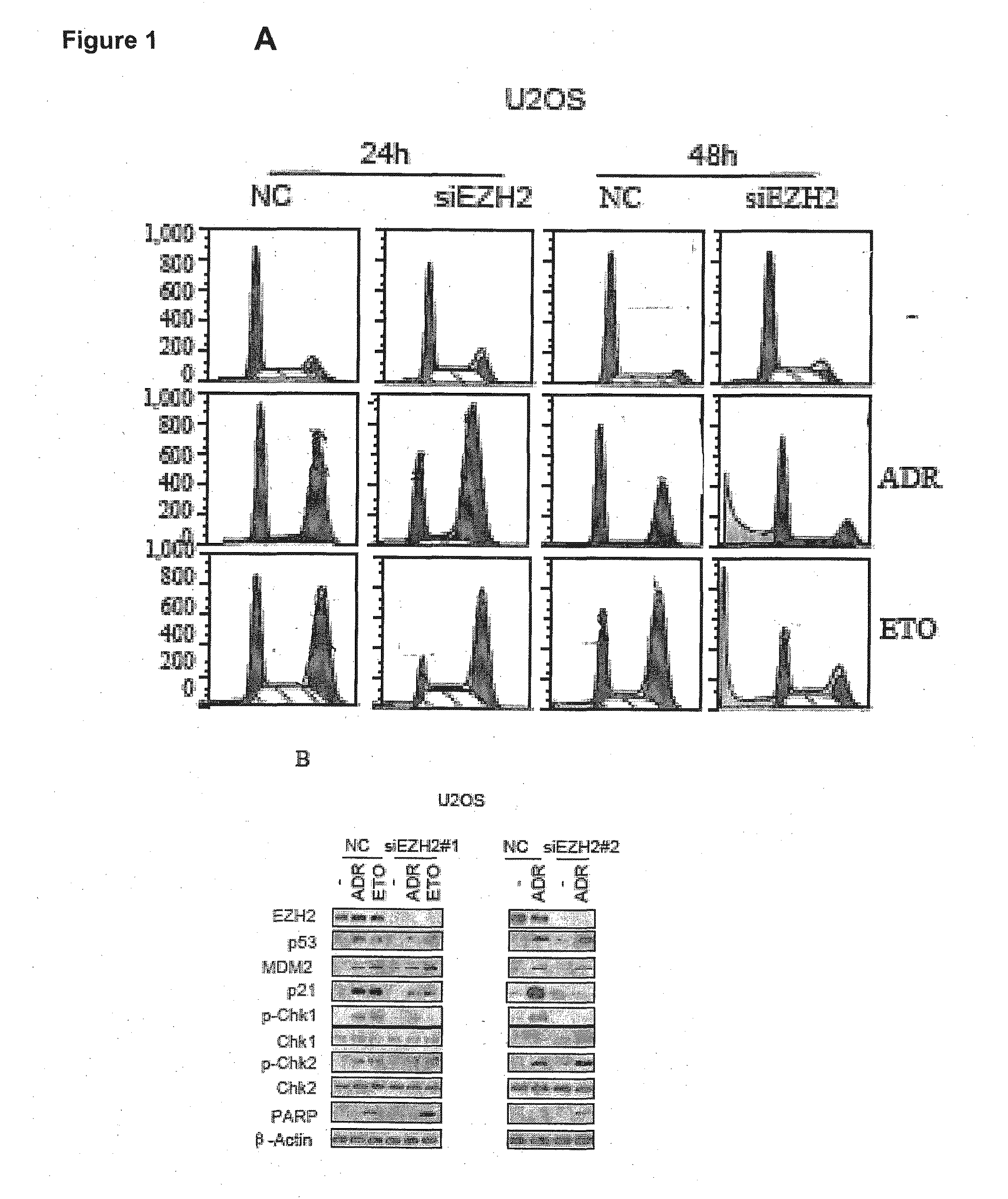
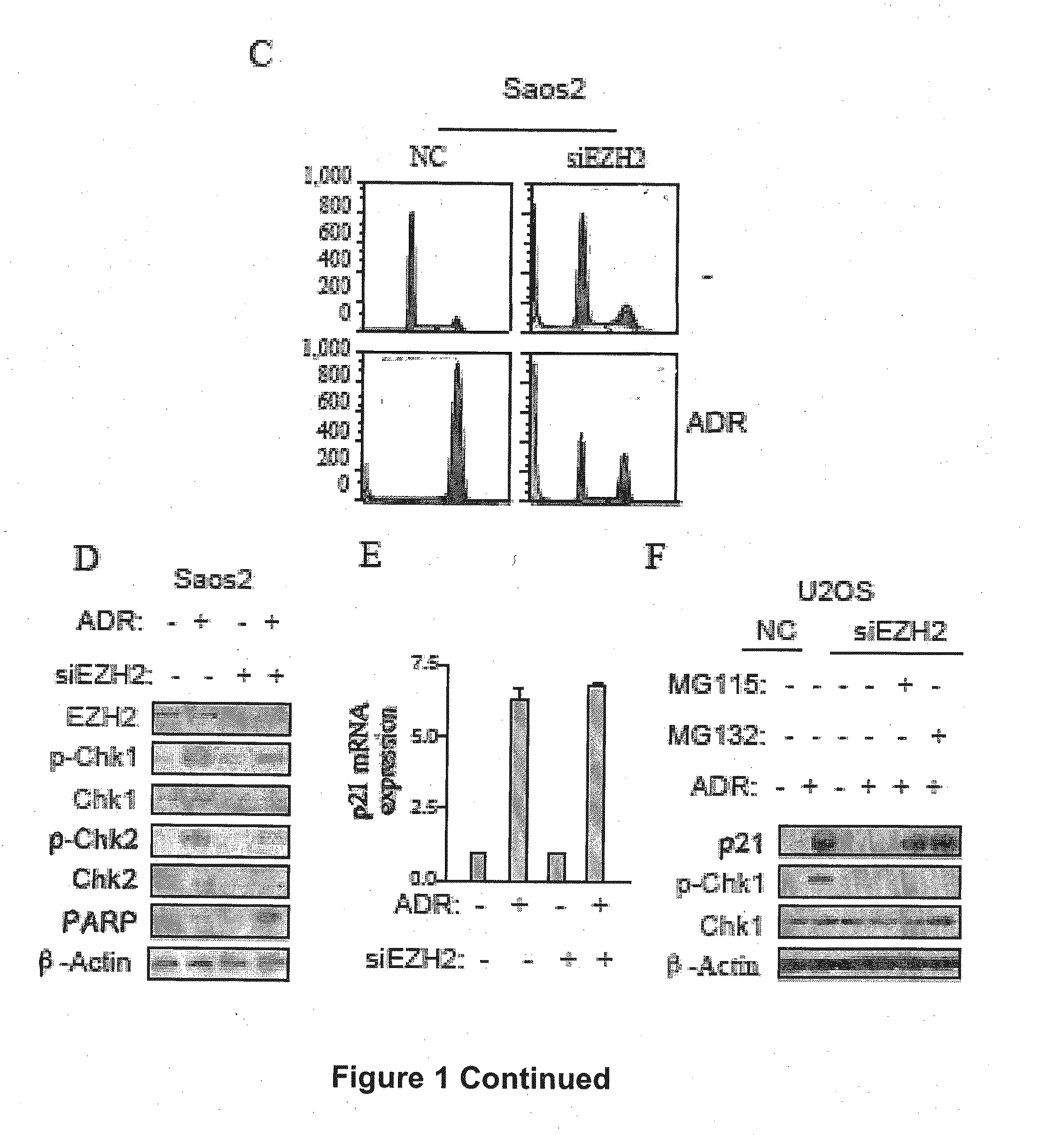
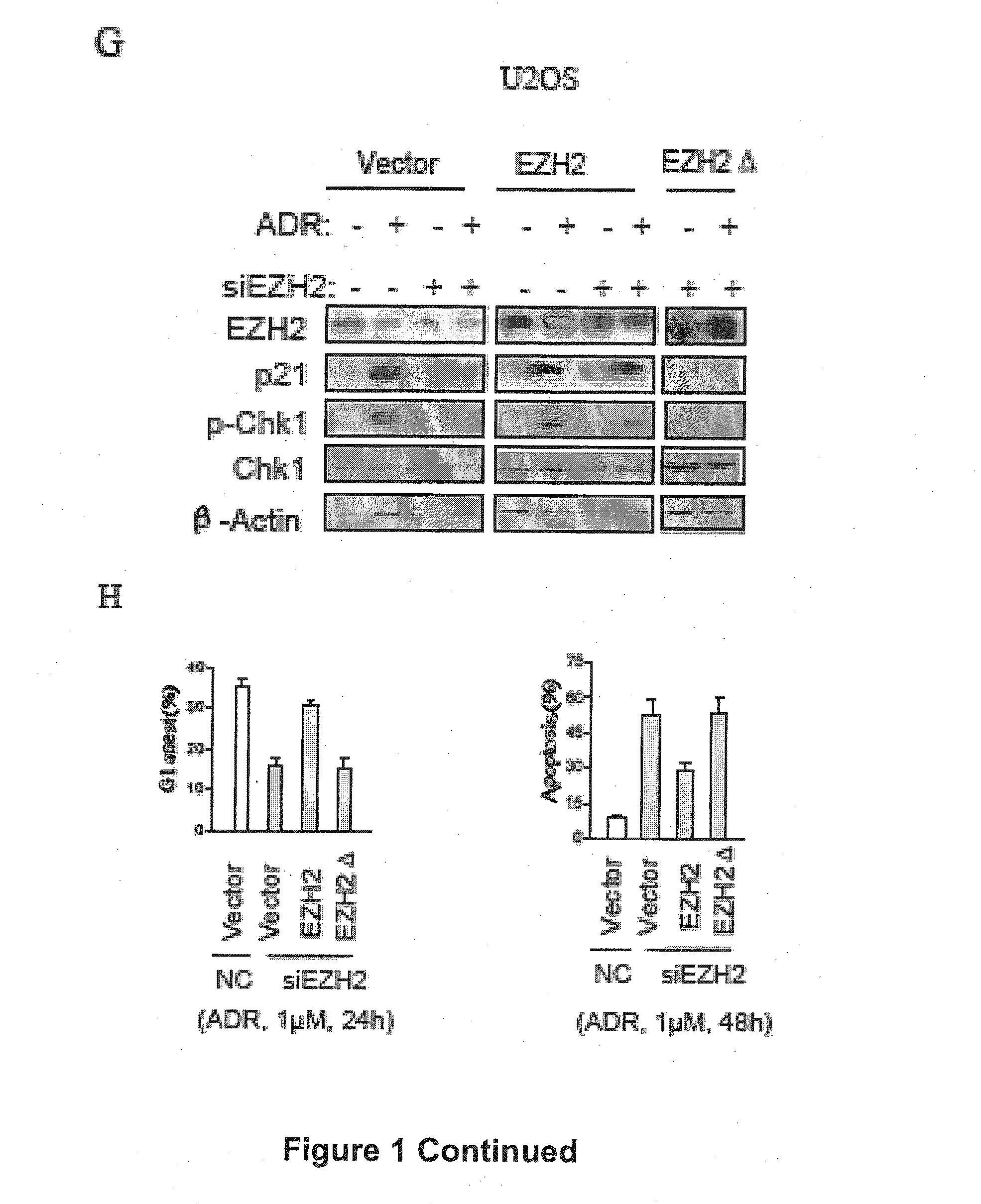
The present technology relates to a method of treating cancer by sensitizing human tumours to DNA damaging therapies through activating FBXO32 expression. Transactivation of FBXO32 through the inhibition of EZH2, a histone methyltransferase, decreases p21 protein induction which results in the sensitization of human tumours to chemotherapy. The method further provides a prognostic method to determine if a combination treatment would be effective.
Owner:AGENCY FOR SCI TECH & RES
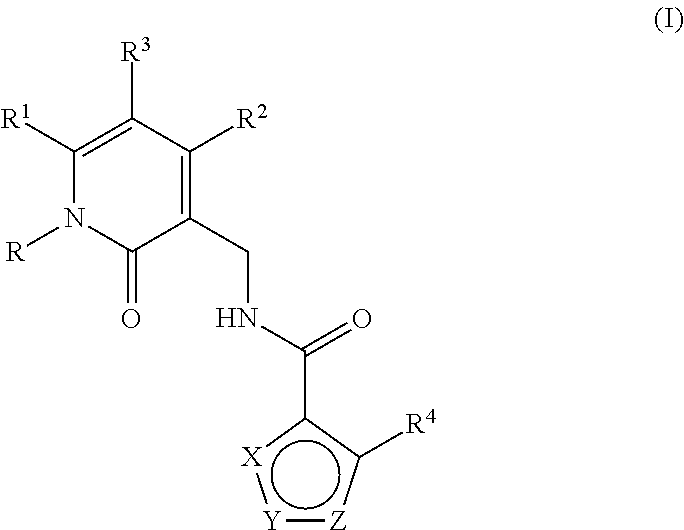
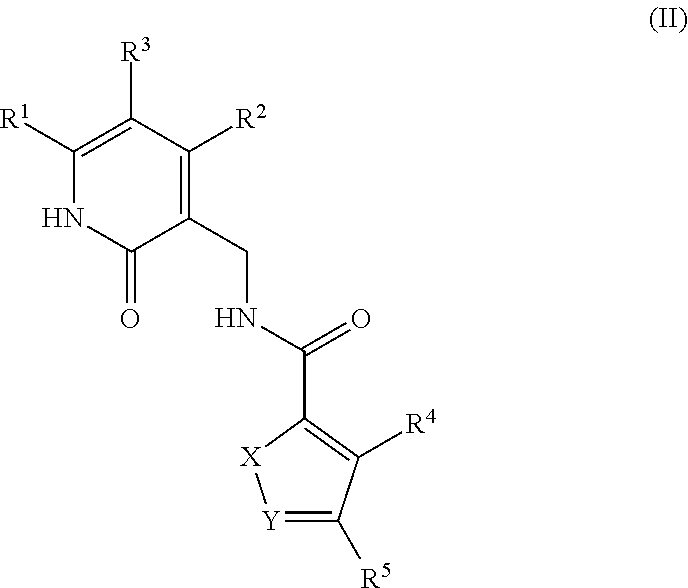
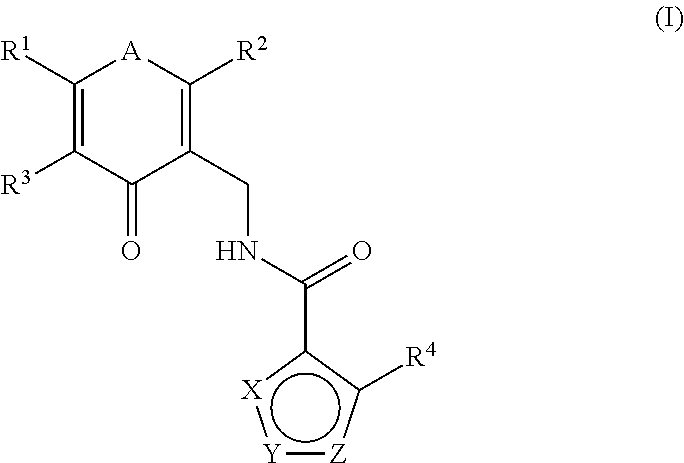
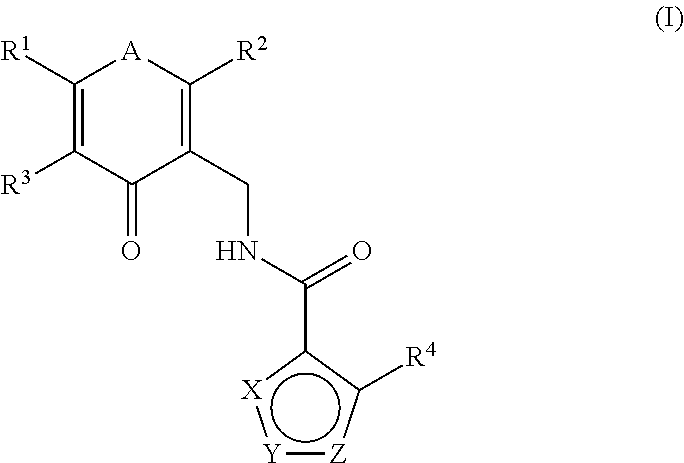
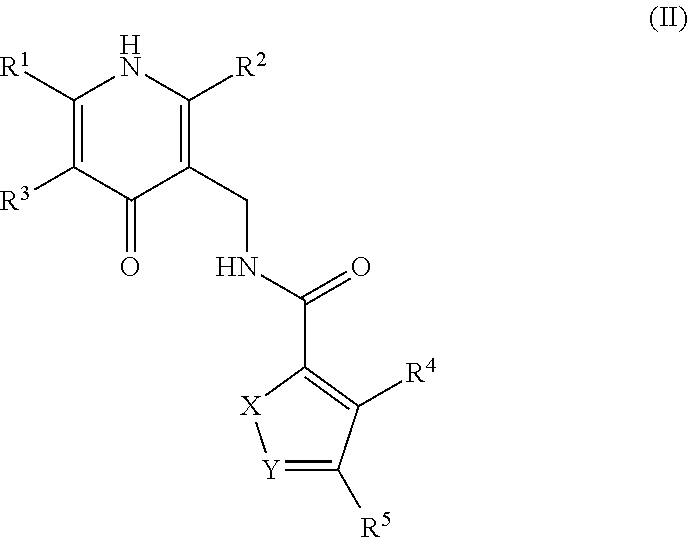
Enhancer of zeste homolog 2 inhibitors
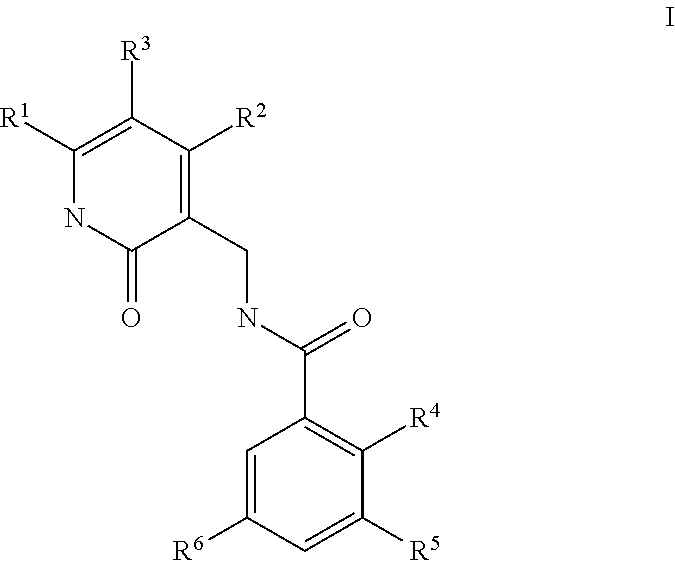
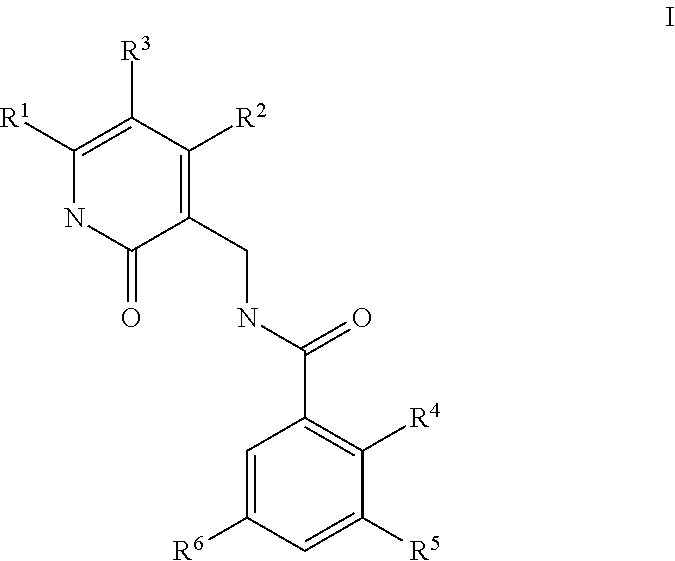
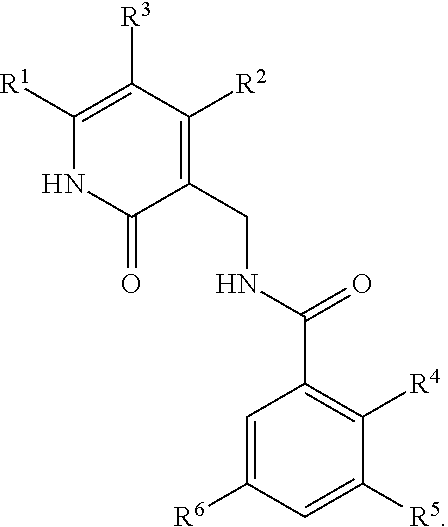
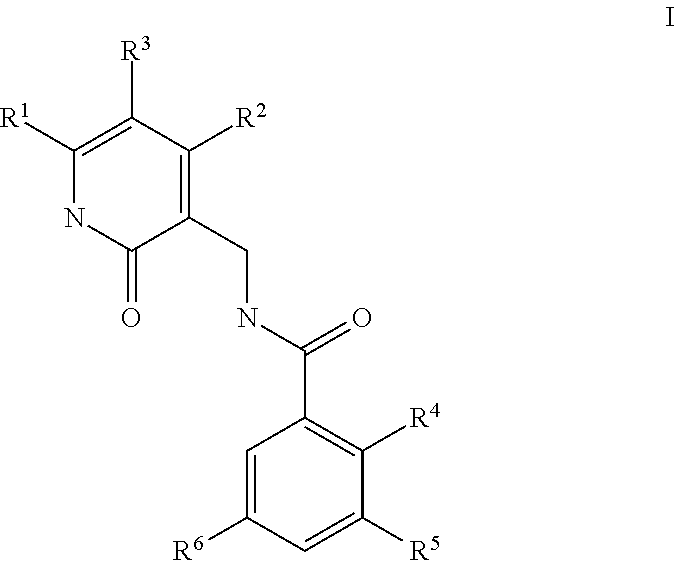
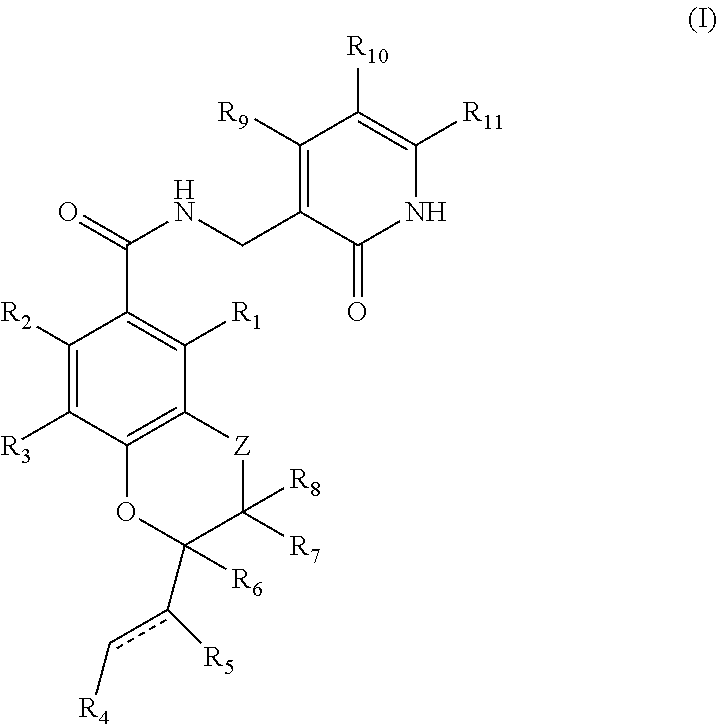
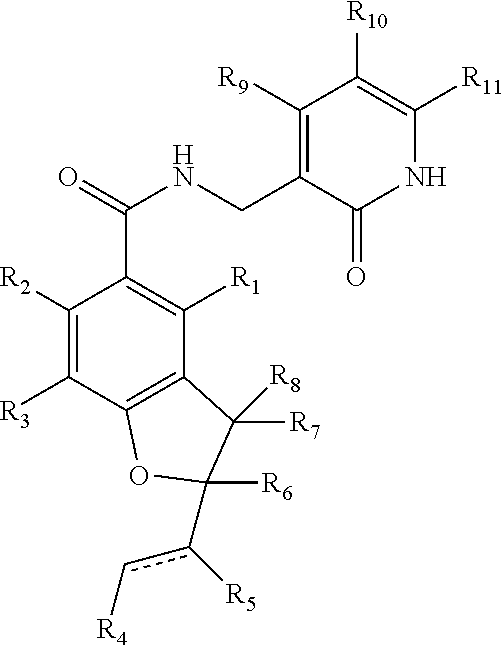
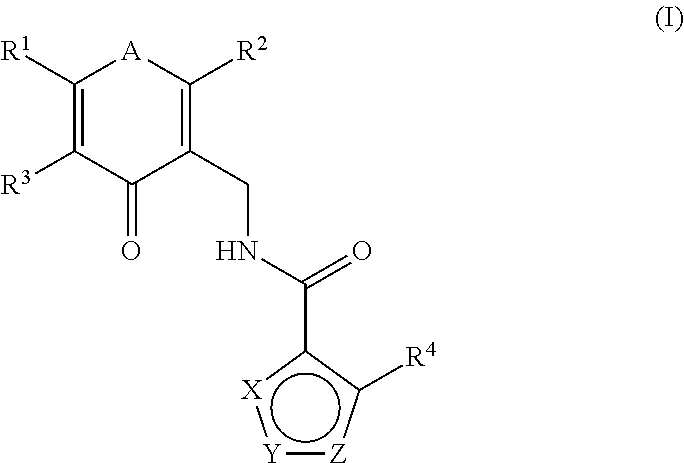
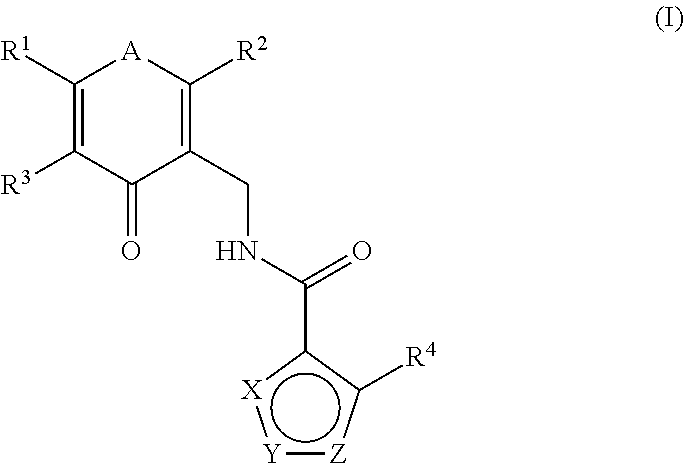
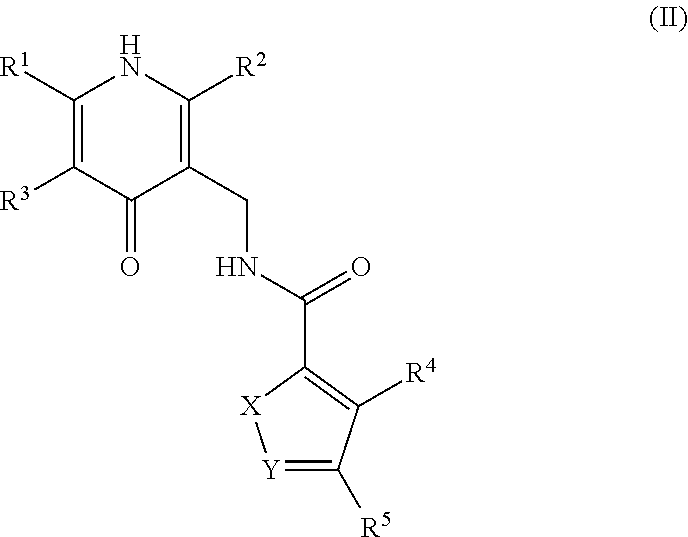
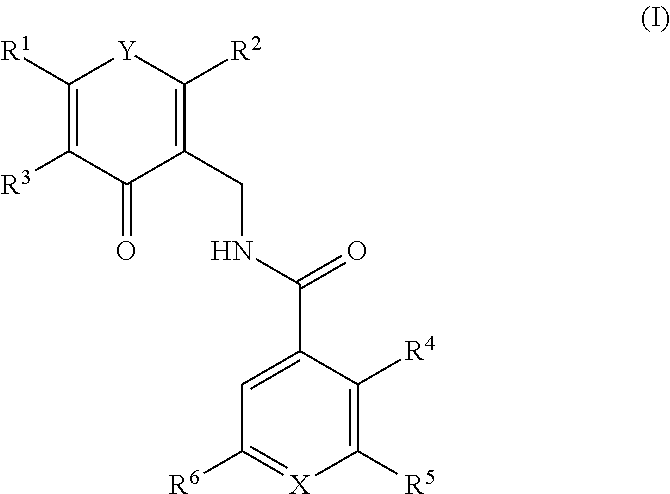
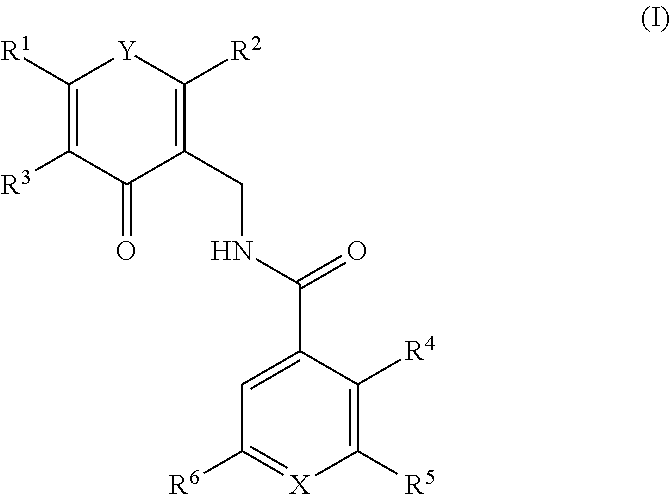
This invention relates to novel substituted benzamides according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXO SMITHKLINE LLC
Tumor susceptibility 62 genes and application thereof
InactiveCN105986031AImprove the detection rateEasy to identifyHealth-index calculationMicrobiological testing/measurementBAP1MAP2K4
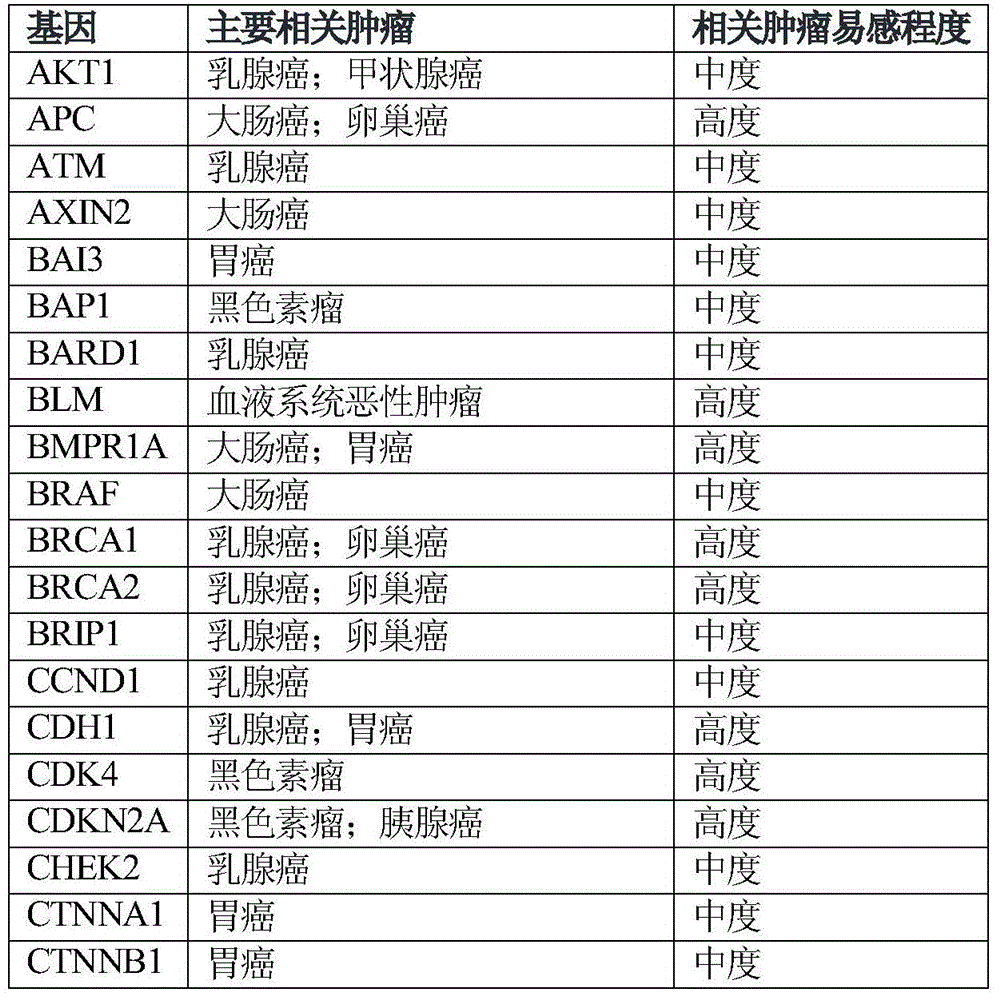
The invention relates to tumor susceptibility 62 genes and application thereof. The tumor susceptibility 62 genes comprise PTEN, STK11, CDH1, TP53, BRCA1, BRCA2, PALB2, CHEK2, ATM, BRIP1, NBN, RAD51C, MLH1, MSH2, MSH6, PMS2, BARD1, RAD51D, MRE11A, MUTYH, PMS1, RAD50, XRCC2, AKT1, PIK3CA, FANCC, RECQL, CCND1, ERBB2, ESR1, GATA3, FGFR1, MAP2K4, MAP3K1, BAI3, CTNNB1, BRAF, KRAS, CTNNA1, EPCAM, APC, BLM, SMAD4, BMPR1A, POLD1, POLE, AXIN2, MEN1, KIT, EGFR, EZH2, PRF1, CDKN2A, CDK4, BAP1, RB1, ERCC2, VHL, MET, FH, FLCN and RET. The detection of the genes can be used for evaluating tumor susceptibility.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Methods and Compositions For Detecting Mutation in the Human EZH2 Gene
Owner:ROCHE MOLECULAR SYST INC
Methods of treating cancer
InactiveUS20140378470A1Improve the level ofOrganic active ingredientsNucleotide librariesEZH2Tyrosine
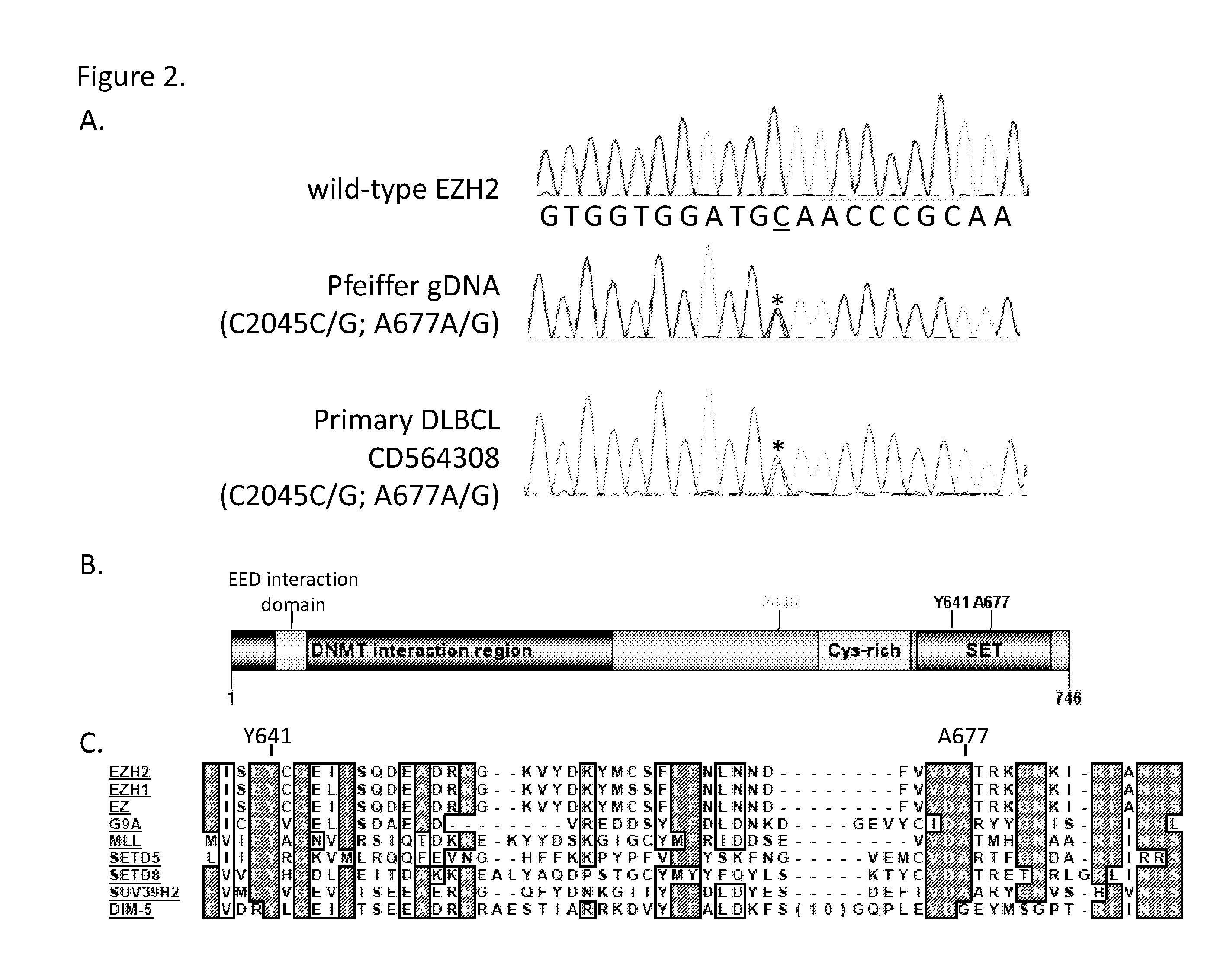
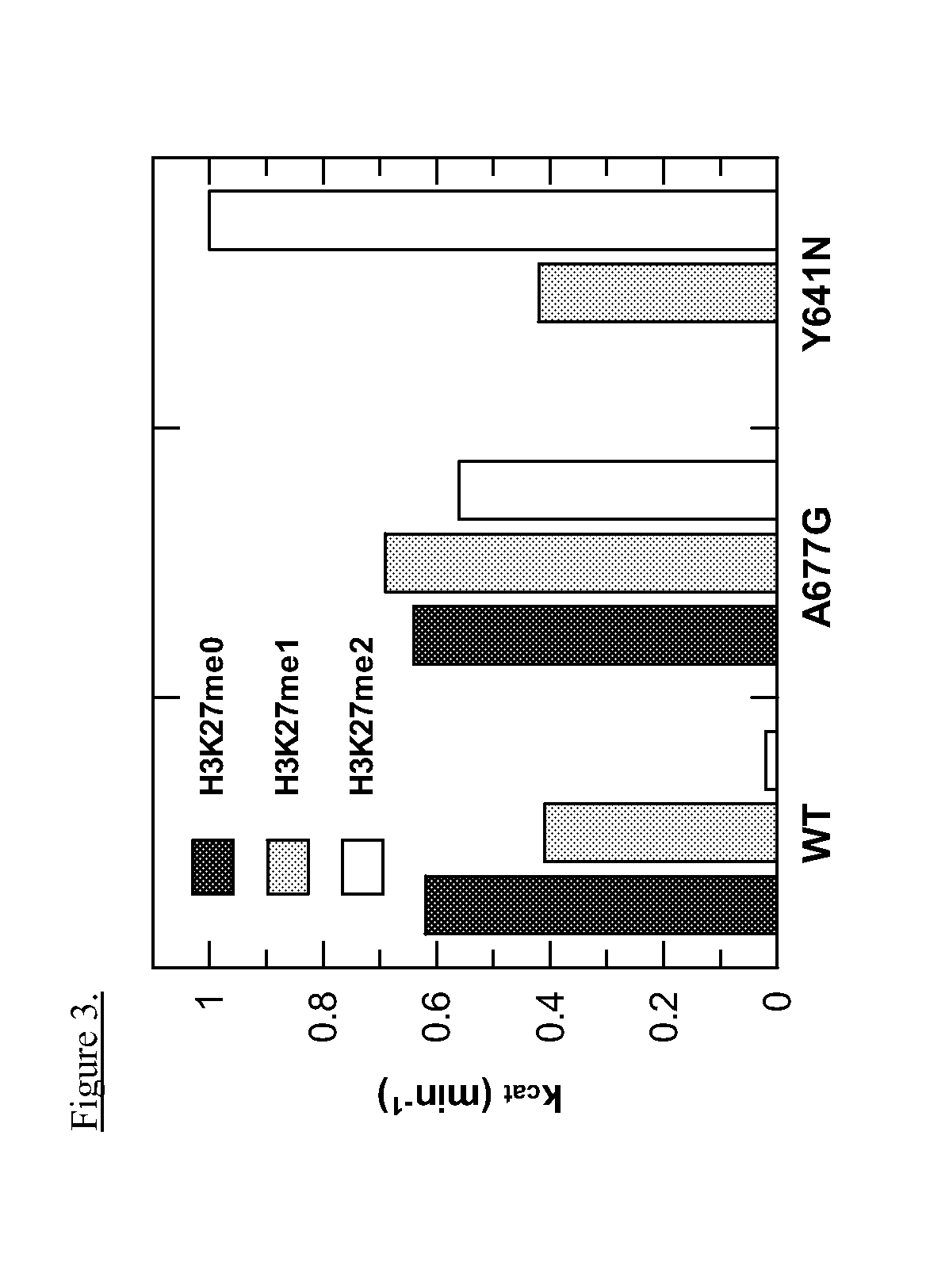
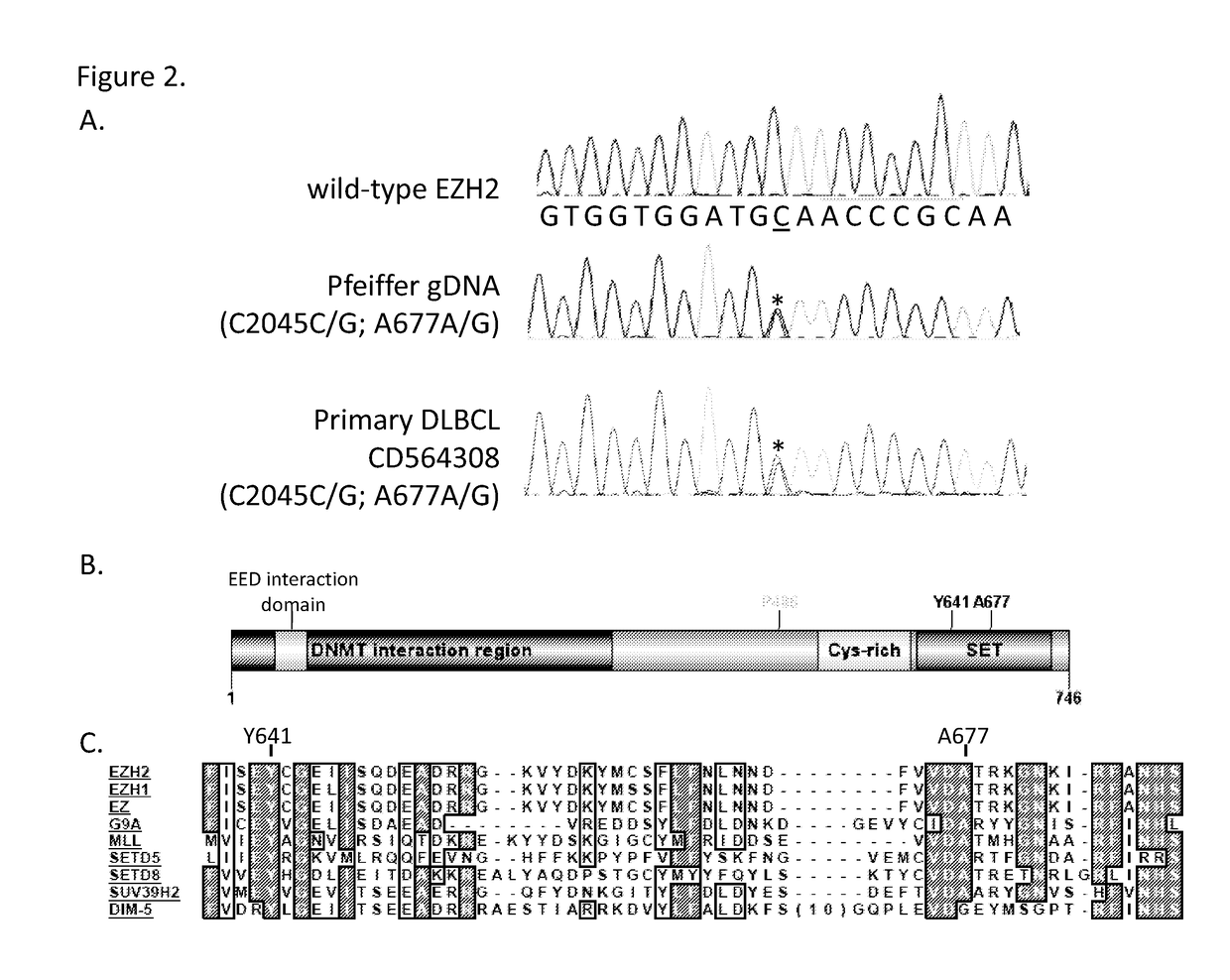
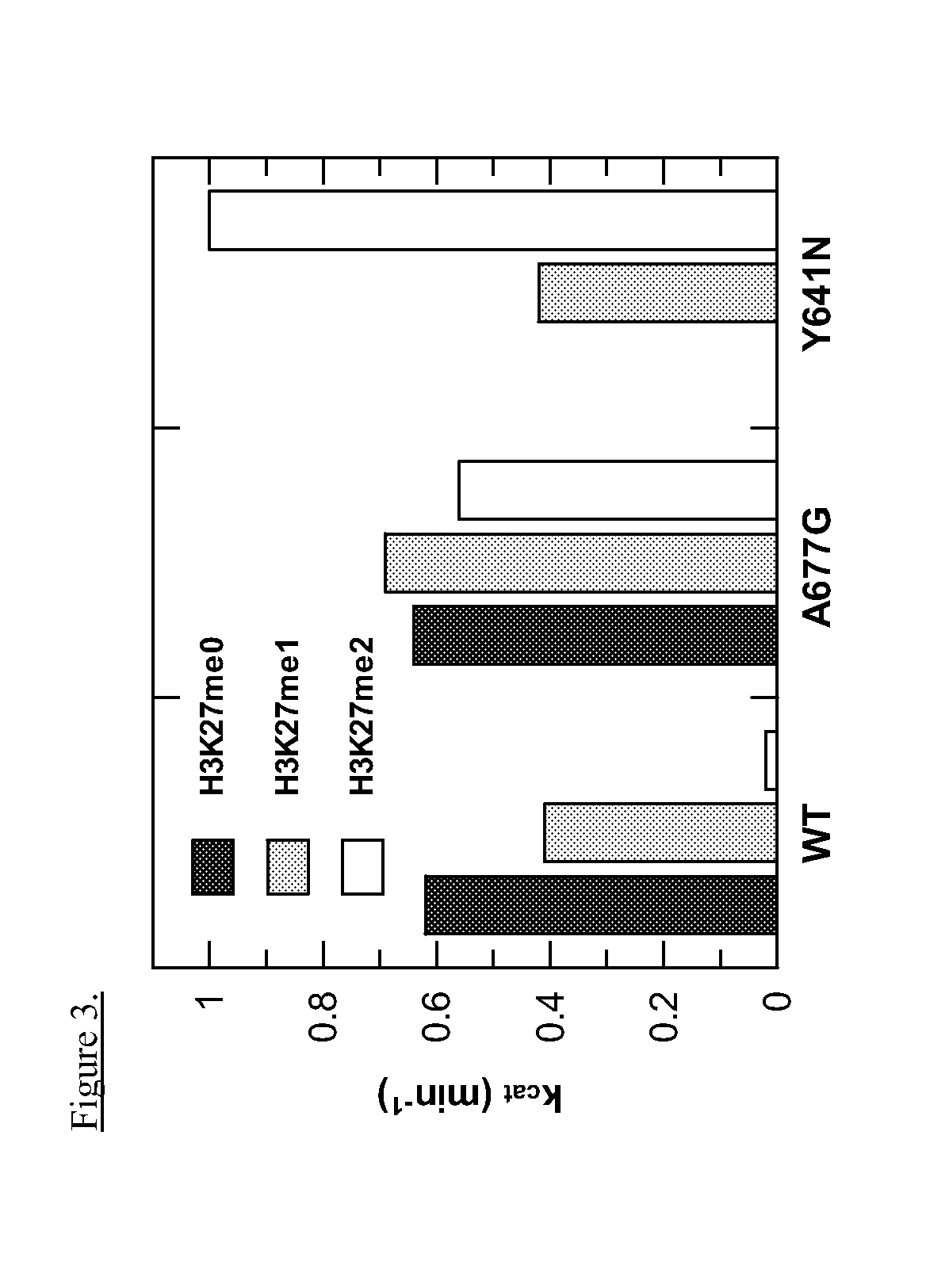
This invention relates to methods of treating cancer in a subject such as a human and determining at least one of the following in a sample from the subject, such as a human: (a) the presence or absence of a mutation at the alanine 677 (A677) residue in EZH2; or (b) the presence or absence of a mutation at the tyrosine 641 (Y641) residue in EZH2; or (c) the presence or absence of an increased level of H3K27me3 as compared to a control, and administering to said human an effective amount of an EZH2 inhibitor or a pharmaceutically acceptable salt thereof if at least one of the A677 mutation, Y641 mutation, or increased level of H3K27me3 is present in the sample.
Owner:GLAXO SMITHKLINE LLC
Enhancer of zeste homolog 2 inhibitors
This invention relates to novel substituted benzamides according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXO SMITHKLINE LLC
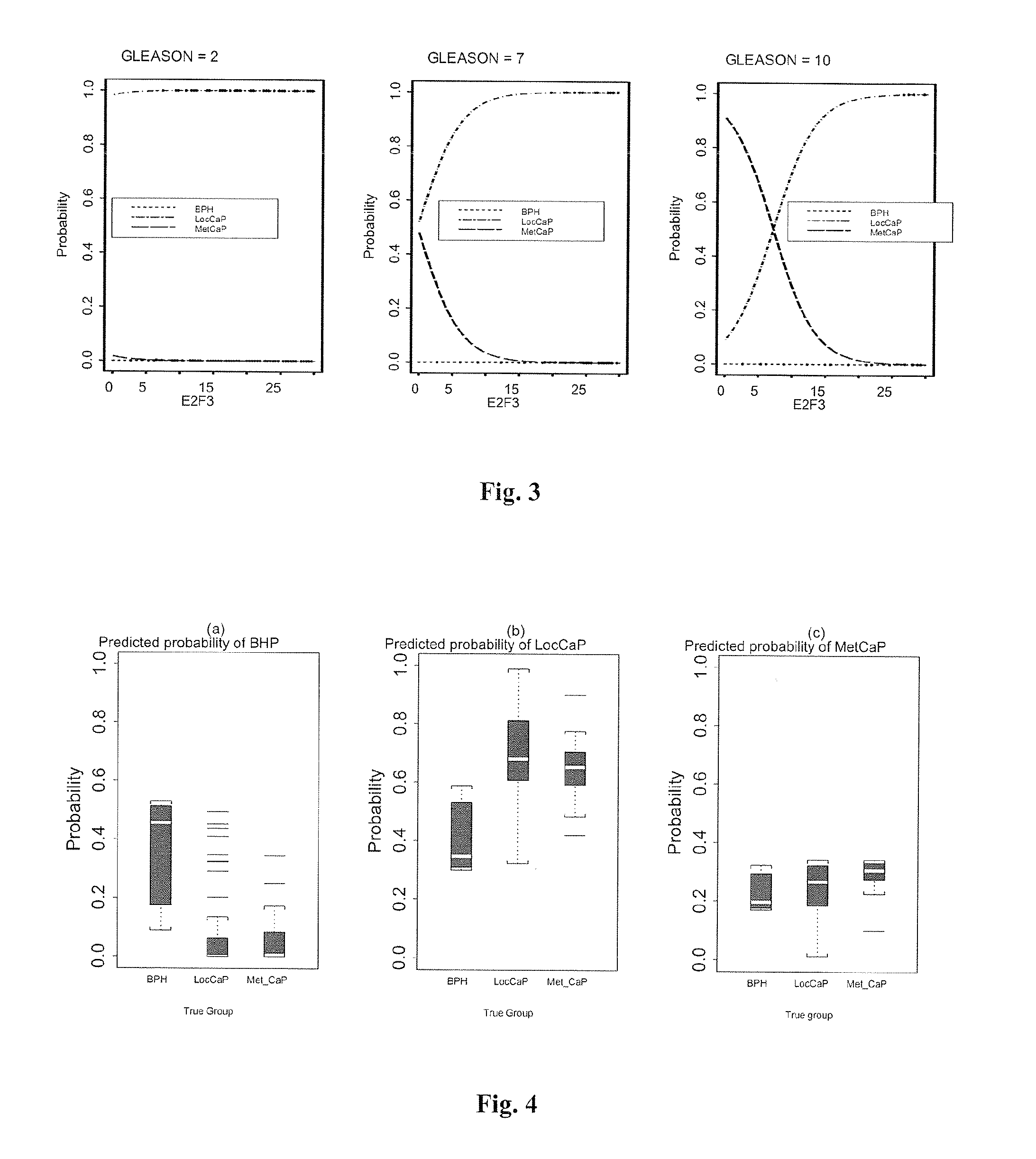
Diagnosis of prostate cancer
InactiveUS20100143247A1Marker is correctIn-vivo radioactive preparationsMicrobiological testing/measurementProstate cancerKinin
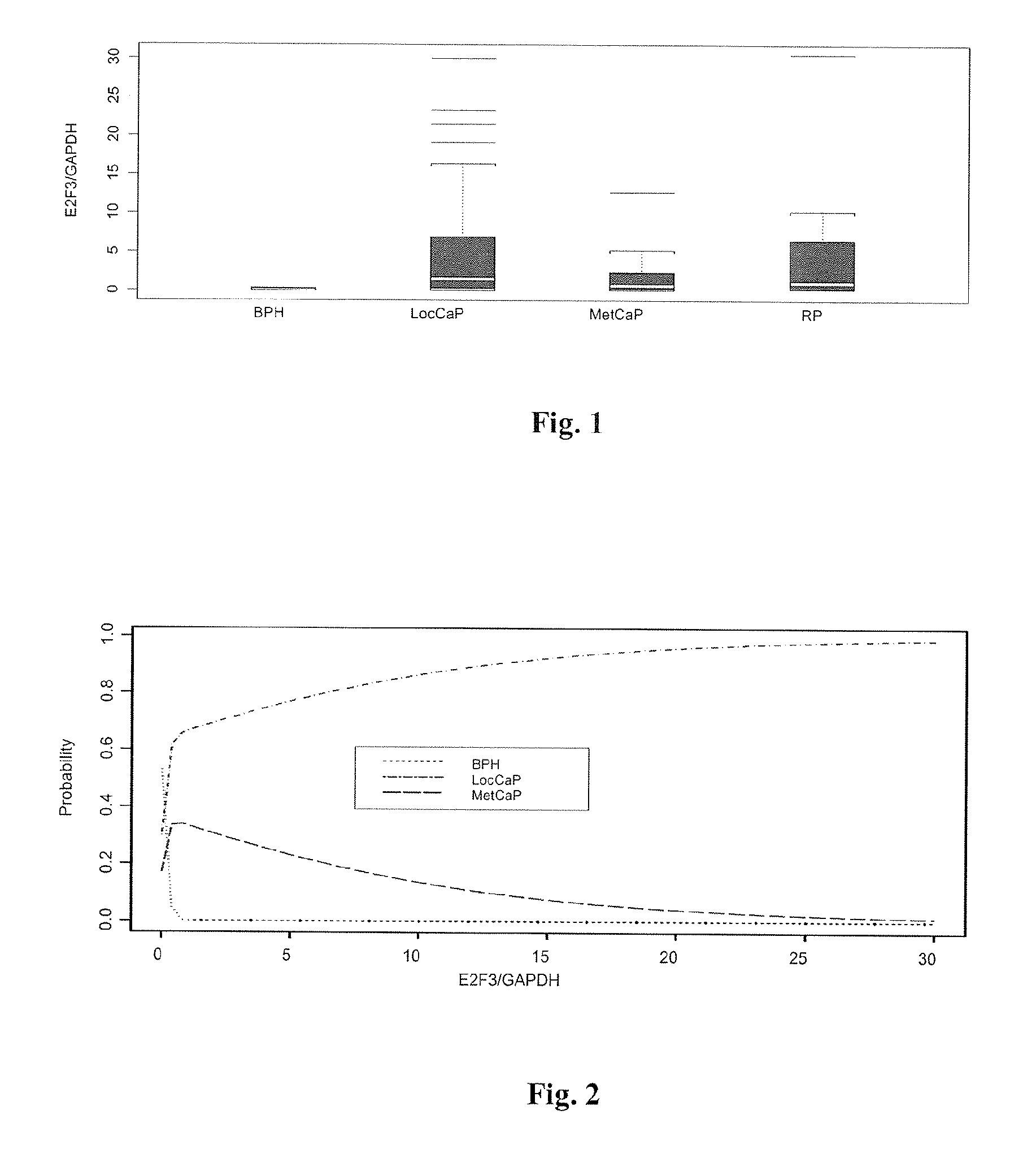
The present invention provides a method for determining the presence of prostate cancer in a subject which method comprises determining the level of expression of one or more markers in a blood sample from the subject, wherein said one or more markers comprise at least one of E2F3, c-met, pRB, EZH2, e-cad, CAXII, CAIX, HIF-1α, Jagged, PIM-1, hepsin, RECK, Clusterin, MMP9, MTSP-1, MMP24, MMP15, IGFBP-2, IGFBP-3, E2F4, caveolin, EF-1A, Kallikrein 2, Kallikrein 3 and PSGR.
Owner:ST GEORGES ENTERPRISES
Enhancer of zeste homolog 2 inhibitors
This invention relates to novel compounds according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Enhancer of Zeste homolog 2 inhibitors
This invention relates to novel compounds according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
RNA INTERFERENCE MEDIATED INHIBITION OF POLYCOMB GROUP PROTEIN EZH2 GENE EXPRESSION USING SHORT INTERFERING NUCLEIC ACID (siNA)
InactiveUS20090137508A1Improve bioavailabilityMinimize the possibilitySenses disorderNervous disorderEZH2Polycomb-group proteins
This invention relates to compounds, compositions, and methods useful for modulating polycomb group protein EZH2 gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of polycomb group protein EZH2 gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of polycomb group protein EZH2 genes, such as EZH2.
Owner:MERCK SHARP & DOHME CORP
Long chain non-encoding RNA sequence for early diagnosis of human prostatic cancer and its application
ActiveCN107699564AImprove accuracyStrong specificityOrganic active ingredientsGenetic material ingredientsProstate cancer cellEZH2
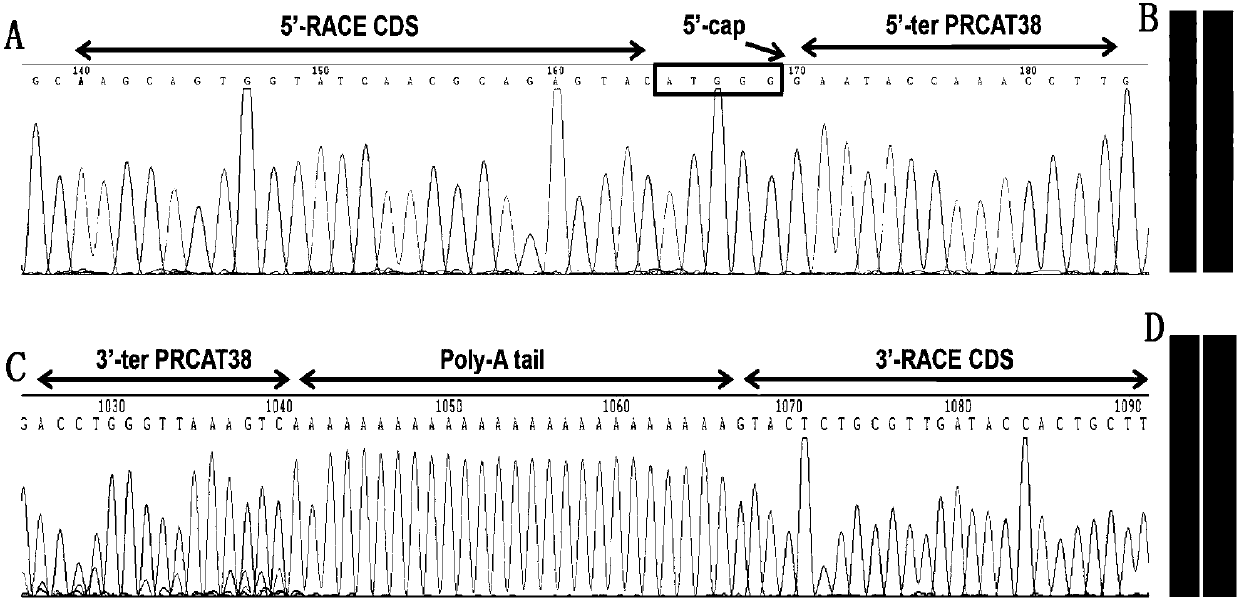
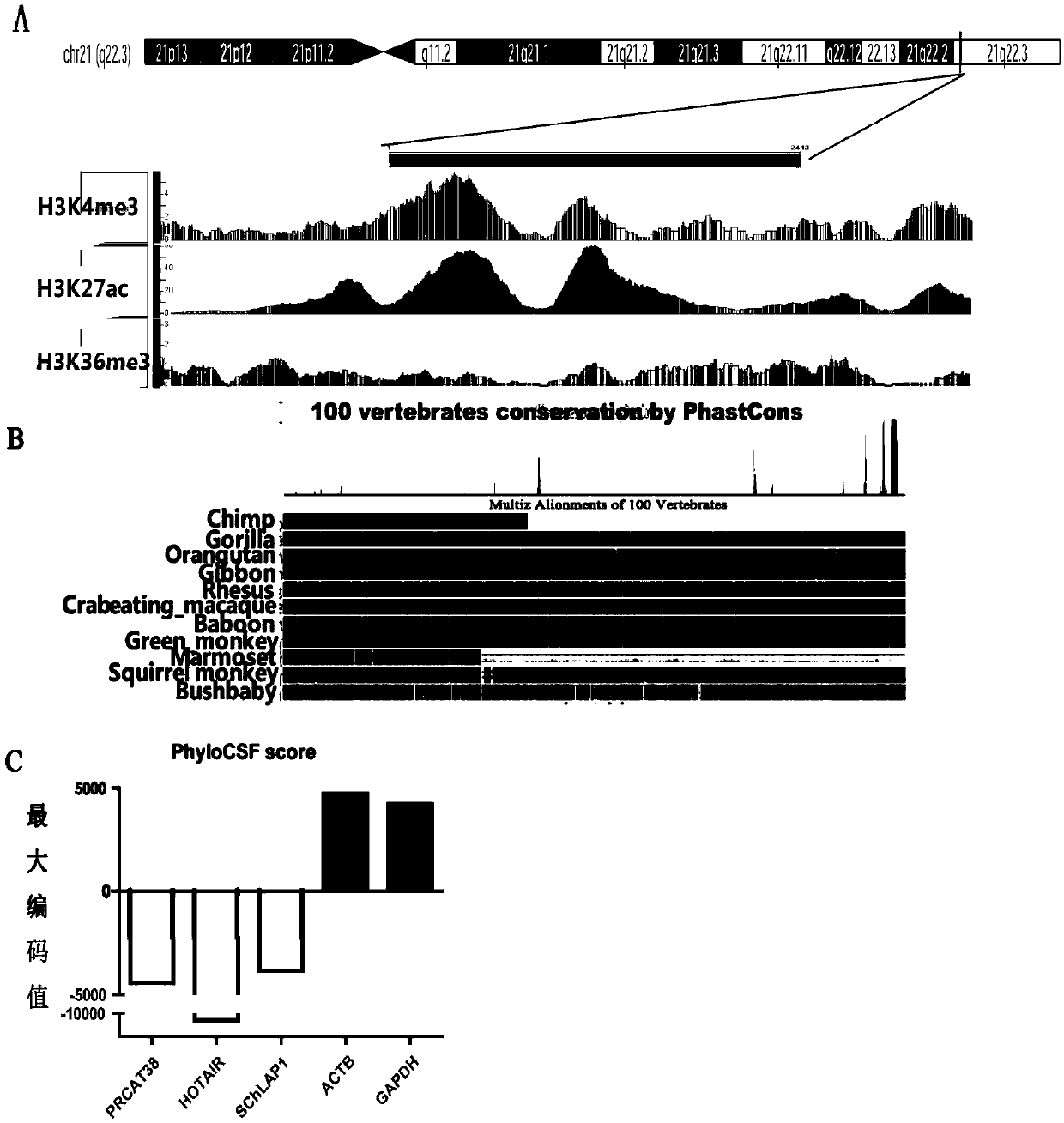
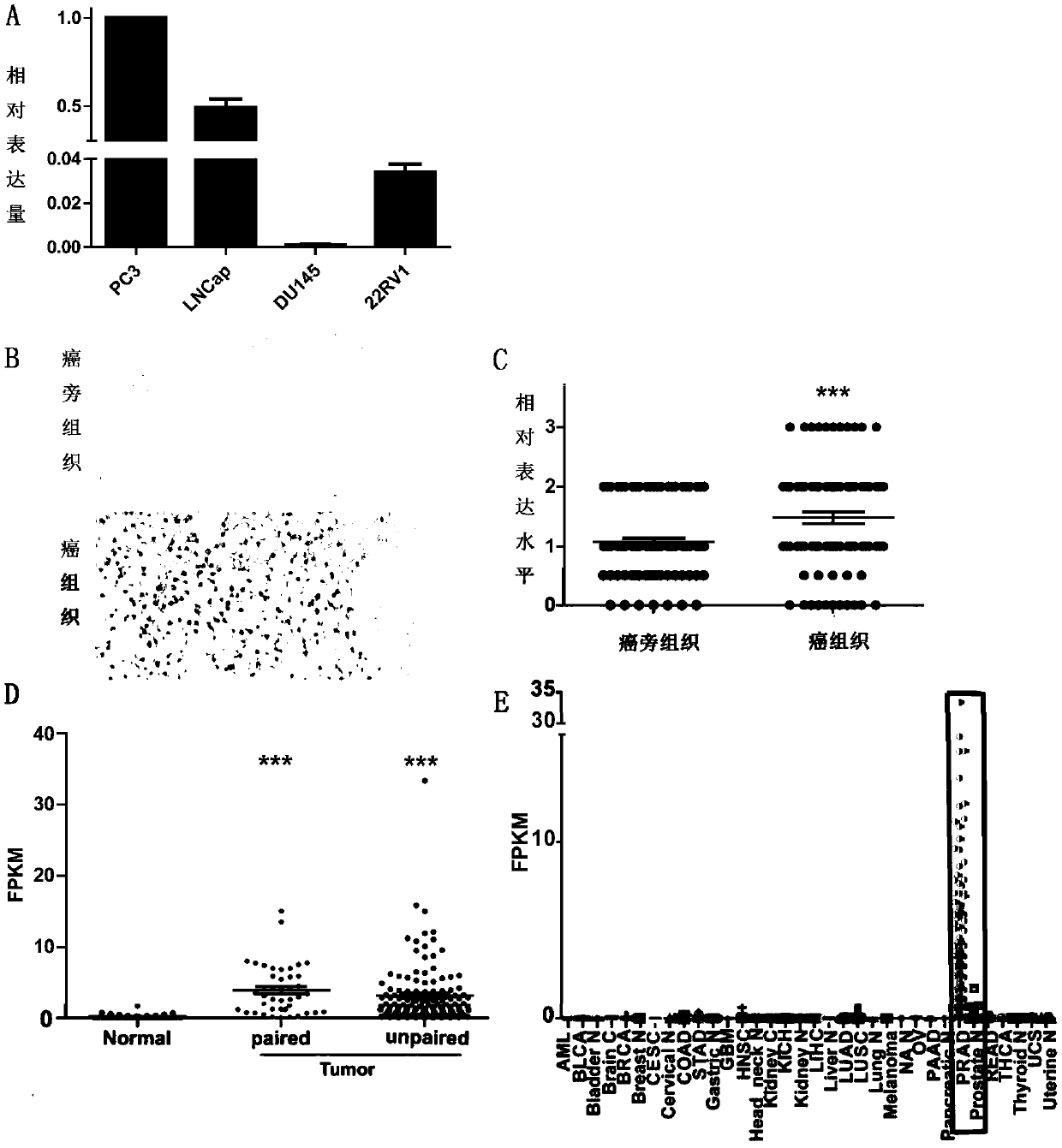
The invention provides a full-length cDNA sequence of long chain non-encoding RNA for early diagnosis of human prostatic cancer and its application. An accurate sequence of the PRCAT38 is cloned for the first time, and a foundation for further studying the function of the PRCAT 38 in the generation and development processes of the prostatic cancer is laid. The specificity high expression of the PRCAT 38 in the prostatic cancer cells and tissues can influence the proliferation, clone formation and migration of the prostatic cancer cells, and the PRCAT 38 can be applied to prepare the product for the early diagnosis, prognosis evaluation and / or treatment of the prostatic cancer. The invention provides a cancer diagnosis marker, which comprises PRCAT 38, EZH2 and miR-24-2, the marker has highsensitivity and high specificity to detect prostate. The invention provides an expression inhibitor of the long chain non-encoding RNA, which comprises siRNA and / or shRNA; the expression inhibition can inhibit the expression of PRCAT38 and EZH2 at the same time, and is good for the gene treatment and clinical drug research and development of the prostatic cancer.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
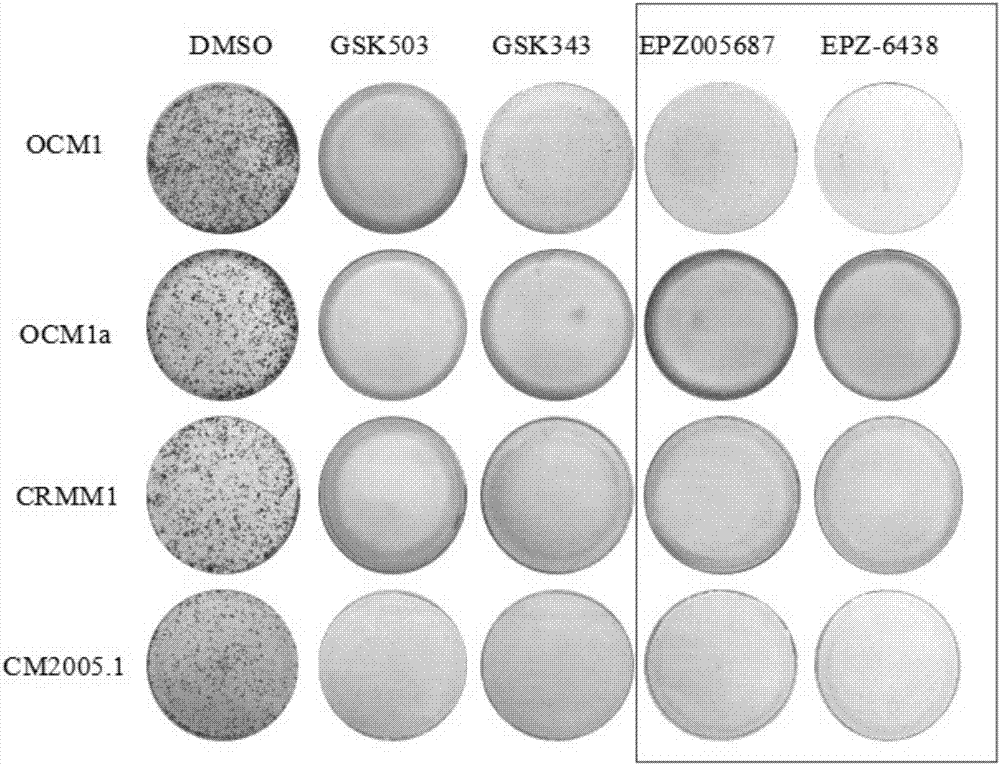
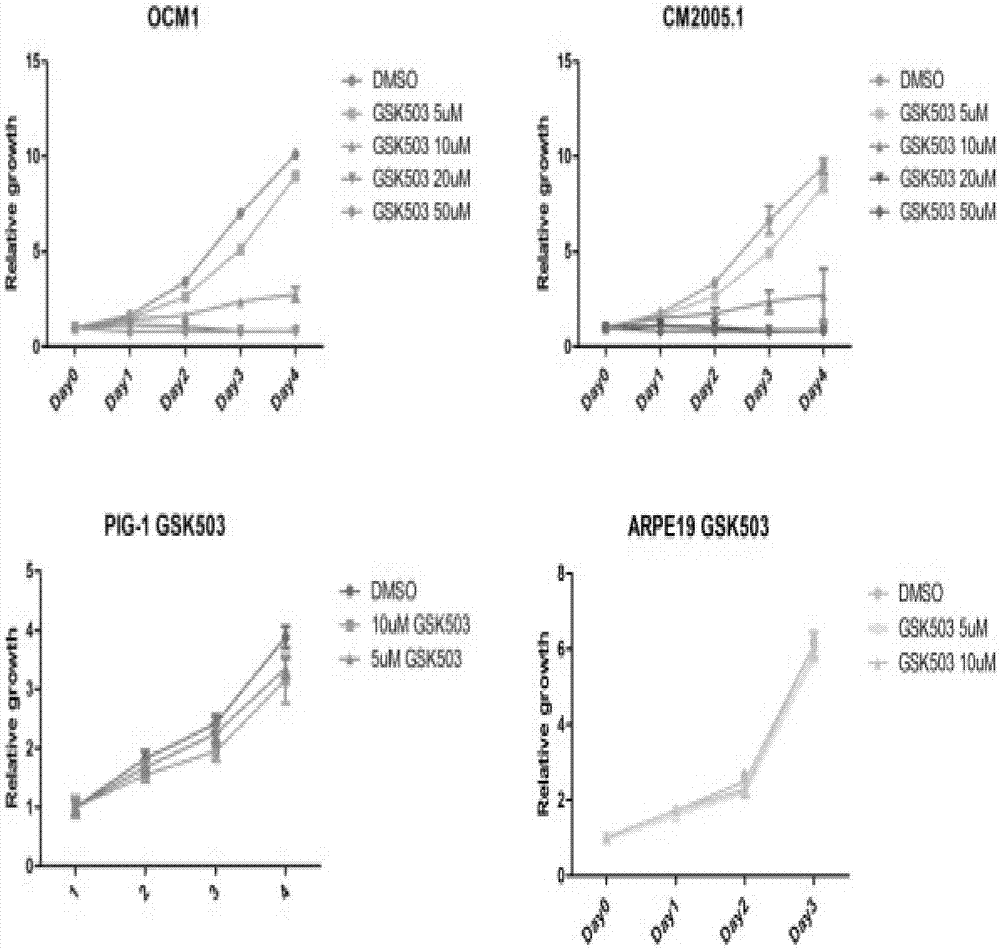
Application of EZH2 (enhancer of zeste homolog 2) inhibitor compounds in preparation of drugs for treating eye melanoma
ActiveCN106963765AGrowth inhibitionDecreased methylationOrganic active ingredientsAntineoplastic agentsEZH2Melanoma
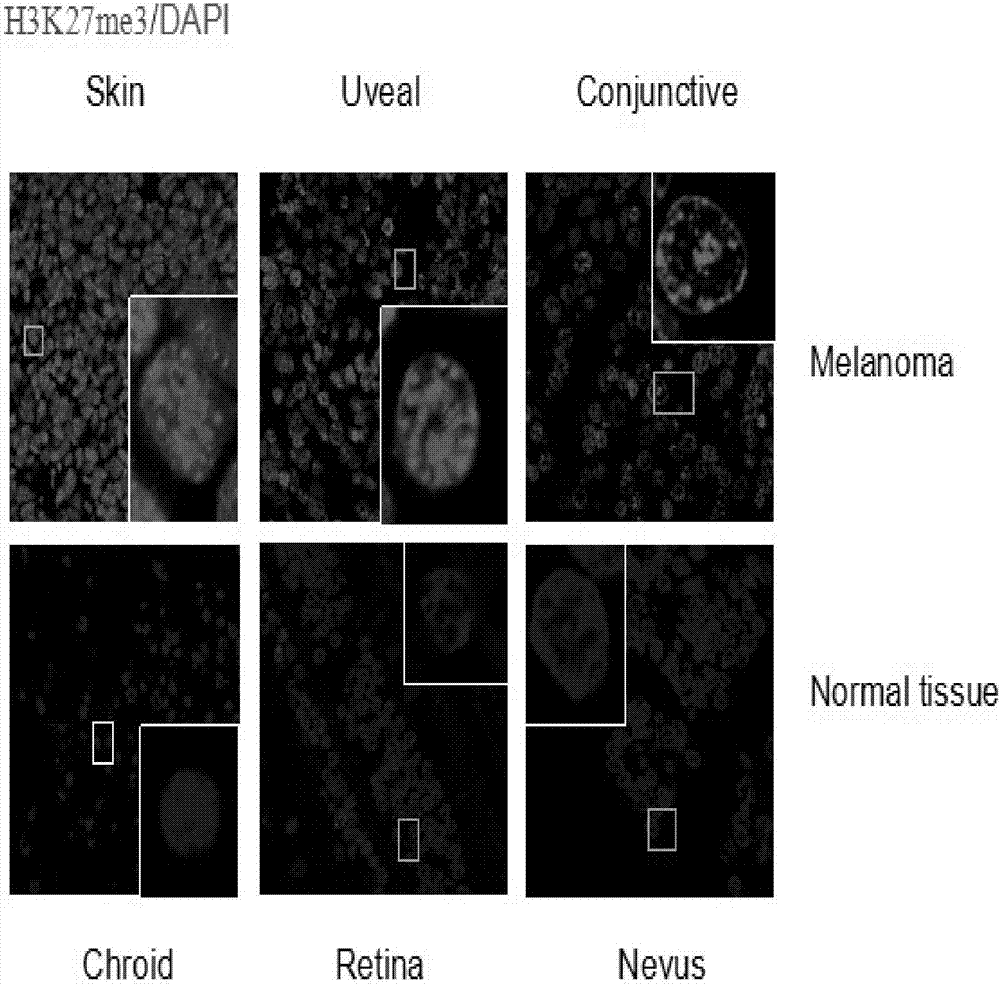
The invention discloses application of EZH2 (enhancer of zeste homolog 2) inhibitor compounds in the preparation of drugs for treating eye melanoma; the EZH2 inhibitor compounds include GSK503, GSK343, EPZ005687 and EPZ-6438, having molecular formulas of C31H38N6O2, C31H39N7O2, C34H44N4O4 and C32H37N5O3, and molecular structural formulas which are shown in the description. The invention is intended to provide novel targets and drugs for the clinical treatment of eye melanoma, treatment effectiveness is improved, eye ball extraction rate is reduced, vision prognosis of patient is improved, and a novel field is exploited unprecedentedly.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Inhibitors of lysine methyl transferase
Owner:BRISTOL MYERS SQUIBB CO
Enhancer of zeste homolog 2 inhibitors
This invention relates to novel compounds according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Enhancer of zeste homolog 2 inhibitors
This invention relates to novel compounds according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Enhancer of Zeste Homolog 2 inhibitors
This invention relates to novel compounds according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXO SMITHKLINE LLC
Hydrochloride salt form for EZH2 inhibition
Provided herein are novel solid forms of N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4′-(morpholinomethyl)-[1,1′-biphenyl]-3-carboxamide hydrochloride, and related compositions and methods.
Owner:EISIA R&D MANAGEMENT CO LTD +1
Methods of treating cancer
Disclosed are methods for treating cancer such as a myeloid malignancy such as multiple myeloma in a human using EZH2 inhibitors in human populations having a translocation MMSET or a decreased level of a functional UTX protein or both.
Owner:NORTHWESTERN UNIV +1
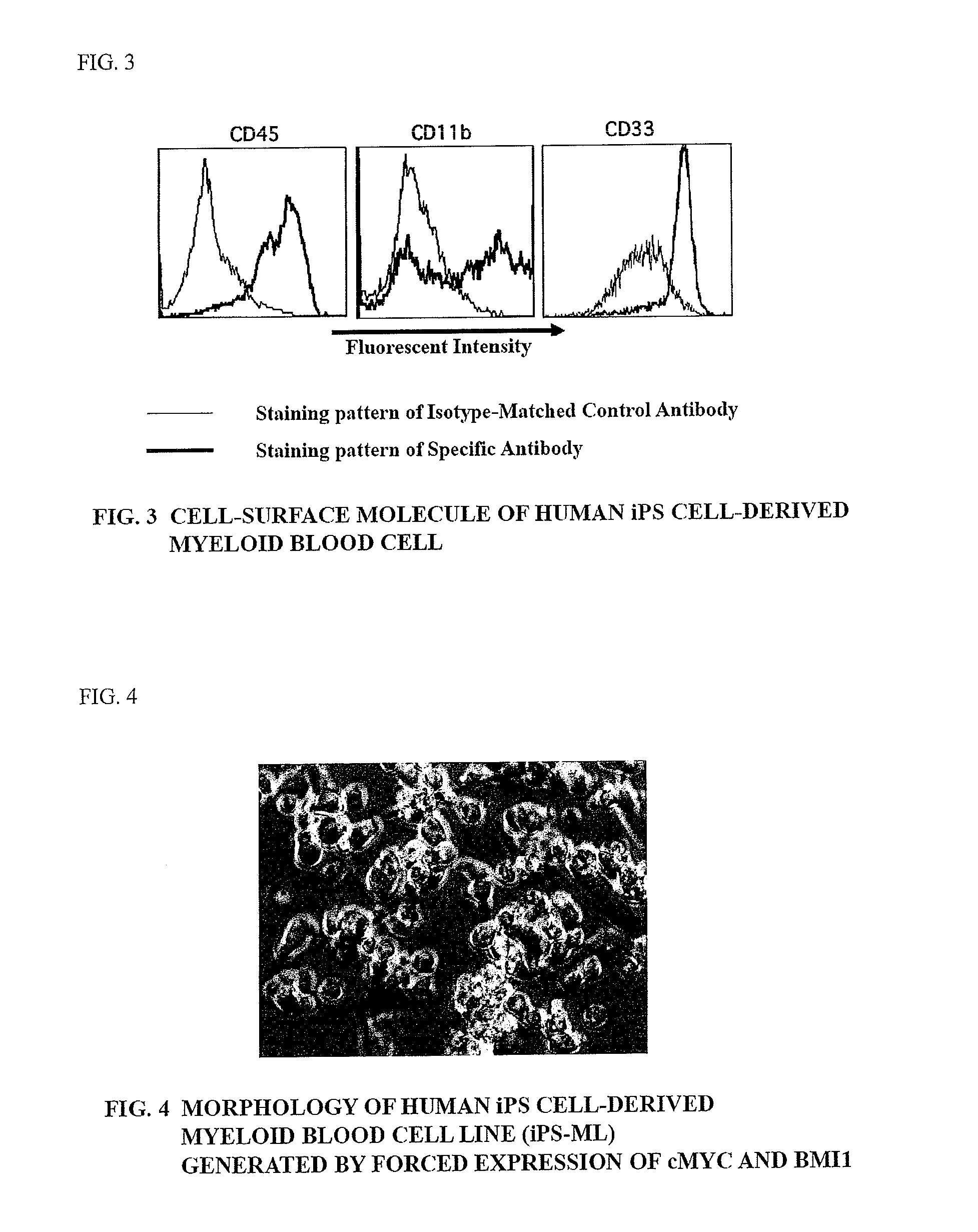
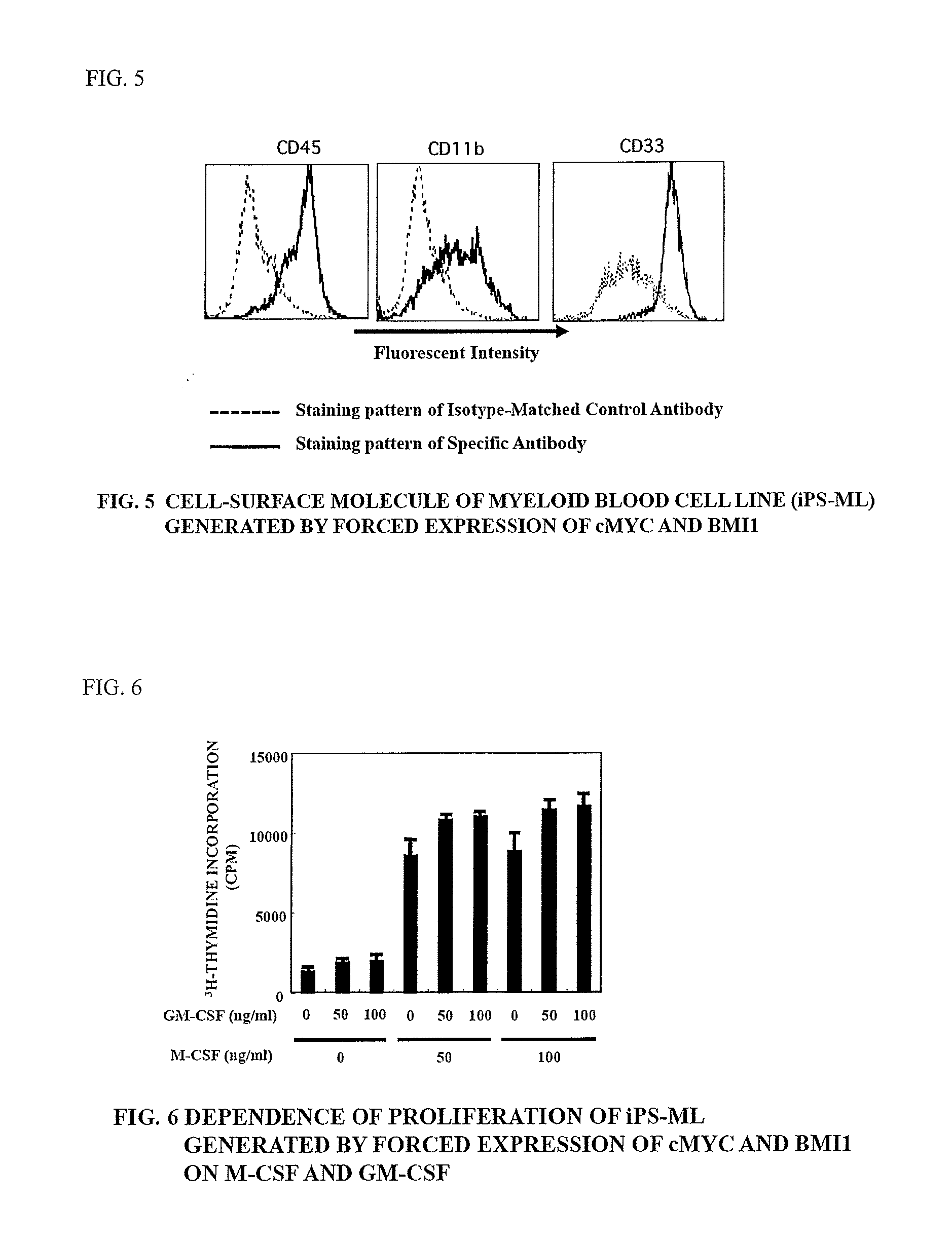
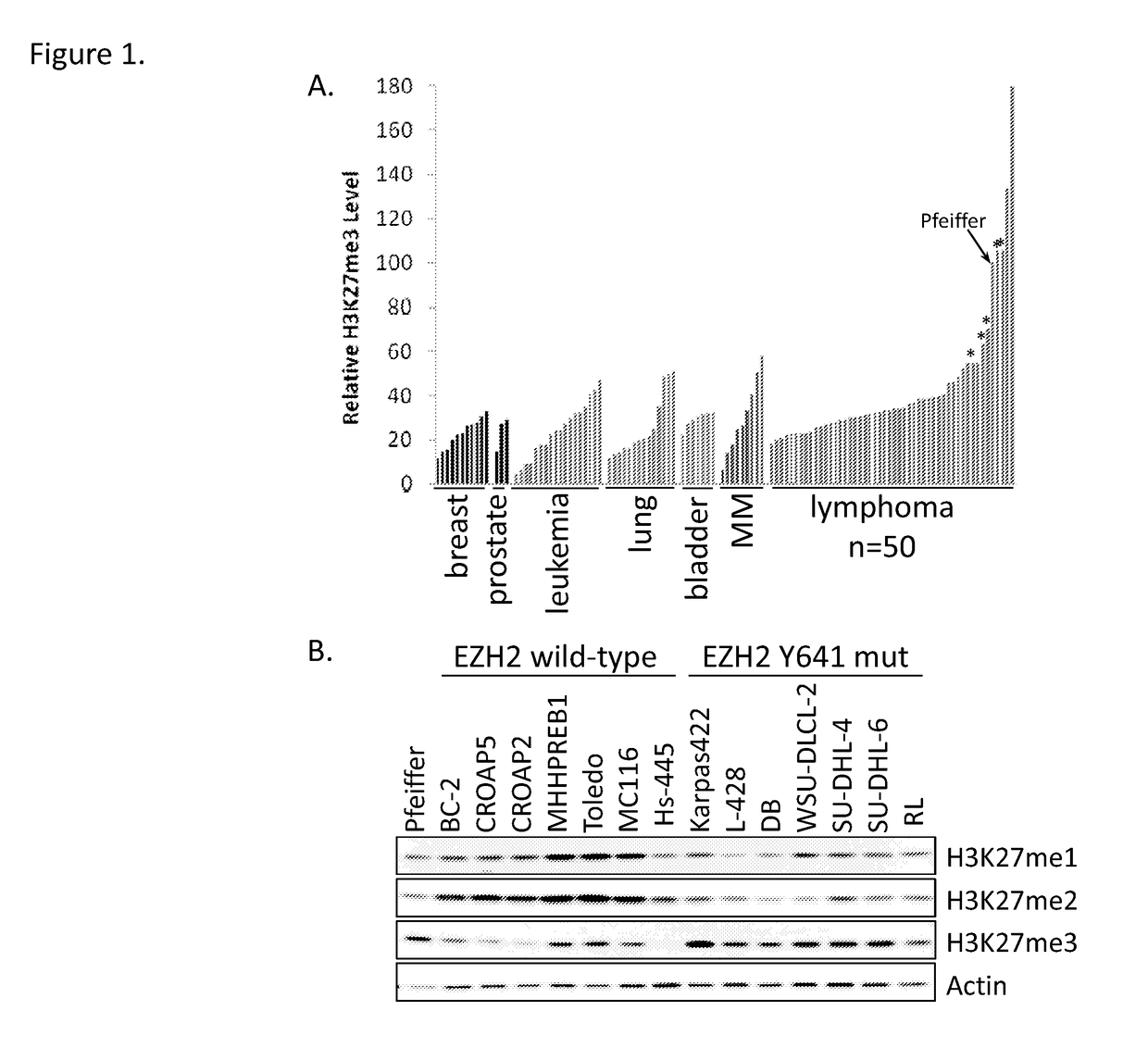
Method of producing myeloid blood cells
An object of the present invention is to provide a method of producing a myeloid blood cell possessing a proliferative capability. According to the present invention, provided is a method of producing a myeloid blood cell possessing a proliferative capability, including forcedly expressing (A) a cMYC gene, and (B) at least one gene selected from the group consisting of a BMI1 gene, an EZH2 gene, an MDM2 gene, an MDM4 gene, and an HIF1A gene in a myeloid blood cell.
Owner:NAT UNIV CORP KUMAMOTO UNIV
Methods of treating cancer
This invention relates to methods of treating cancer in a subject such as a human and determining at least one of the following in a sample from the subject, such as a human: (a) the presence or absence of a mutation at the alanine 677 (A677) residue in EZH2; or (b) the presence or absence of a mutation at the tyrosine 641 (Y641) residue in EZH2; or (c) the presence or absence of an increased level of H3K27me3 as compared to a control, and administering to said human an effective amount of an EZH2 inhibitor or a pharmaceutically acceptable salt thereof if at least one of the A677 mutation, Y641 mutation, or increased level of H3K27me3 is present in the sample.
Owner:GLAXO SMITHKLINE LLC
Enhancer of zeste homolog 2 inhibitors
This invention relates to novel compounds according to Formula (I) which are inhibitors of Enhancer of Zeste Homolog 2 (EZH2), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy for the treatment of cancers.
Owner:GLAXO SMITHKLINE LLC
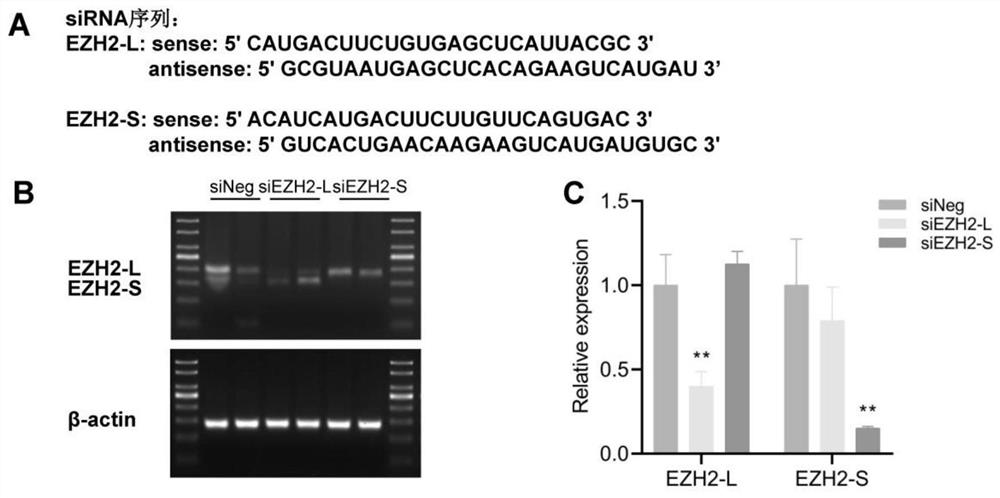
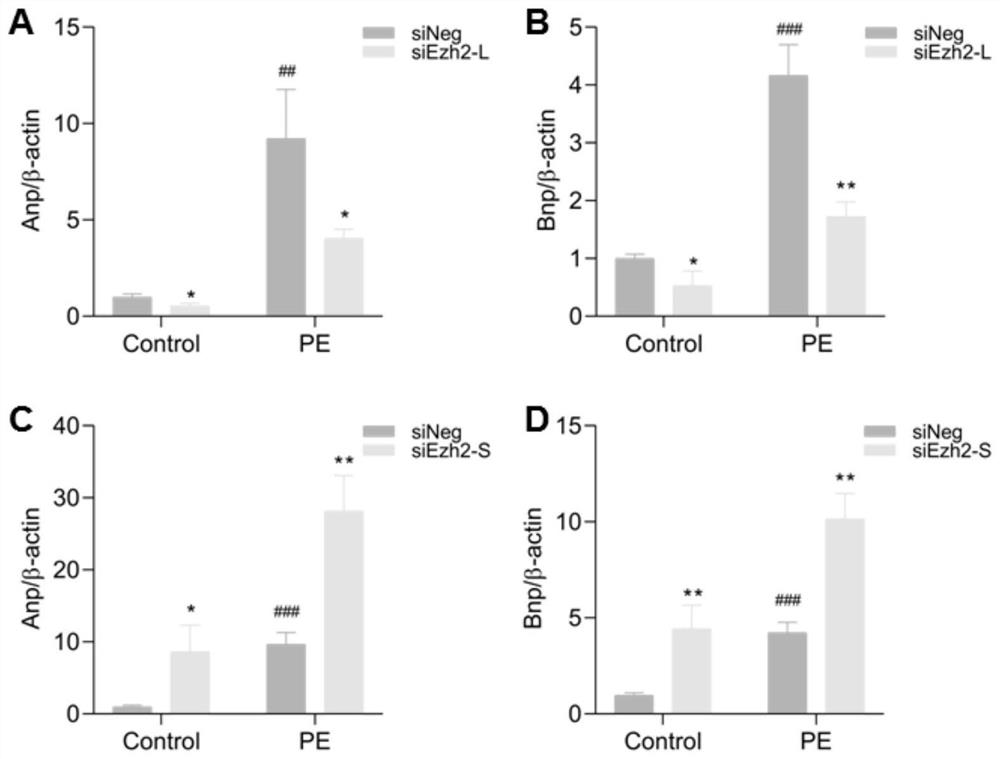
EZH2 variable spliceosome and application thereof
The invention belongs to the technical field of biology, and particularly relates to an EZH2 variable splicing site and an application thereof. Specific siRNA is used for knocking down two subtypes ofEZH2-L and EZH2-S respectively, and myocardial hypertrophy and heart failure models are established at the cellular level and the animal level respectively. Results show that after the EZH2-L is knocked down, the expression level of a myocardial hypertrophy pathological gene is obviously reduced, the myocardial cell area is reduced, and the heart weight is reduced; and after the EZH2-S is knockeddown, the expression level of the myocardial hypertrophy pathological gene is remarkably increased, the myocardial cell area is increased, the heart weight is increased, and the situation prompts that the EZH2-L and the EZH2-S have the effects of promoting and inhibiting myocardial hypertrophy and heart failure respectively. In addition, after the EZH2-L is knocked down, tumor cell proliferationis inhibited, and knock-down of the EZH2-S has no influence on tumor cell proliferation. The invention provides a new target and a new strategy for research and development of medicines for treating EZH2 gene related diseases.
Owner:WUHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com