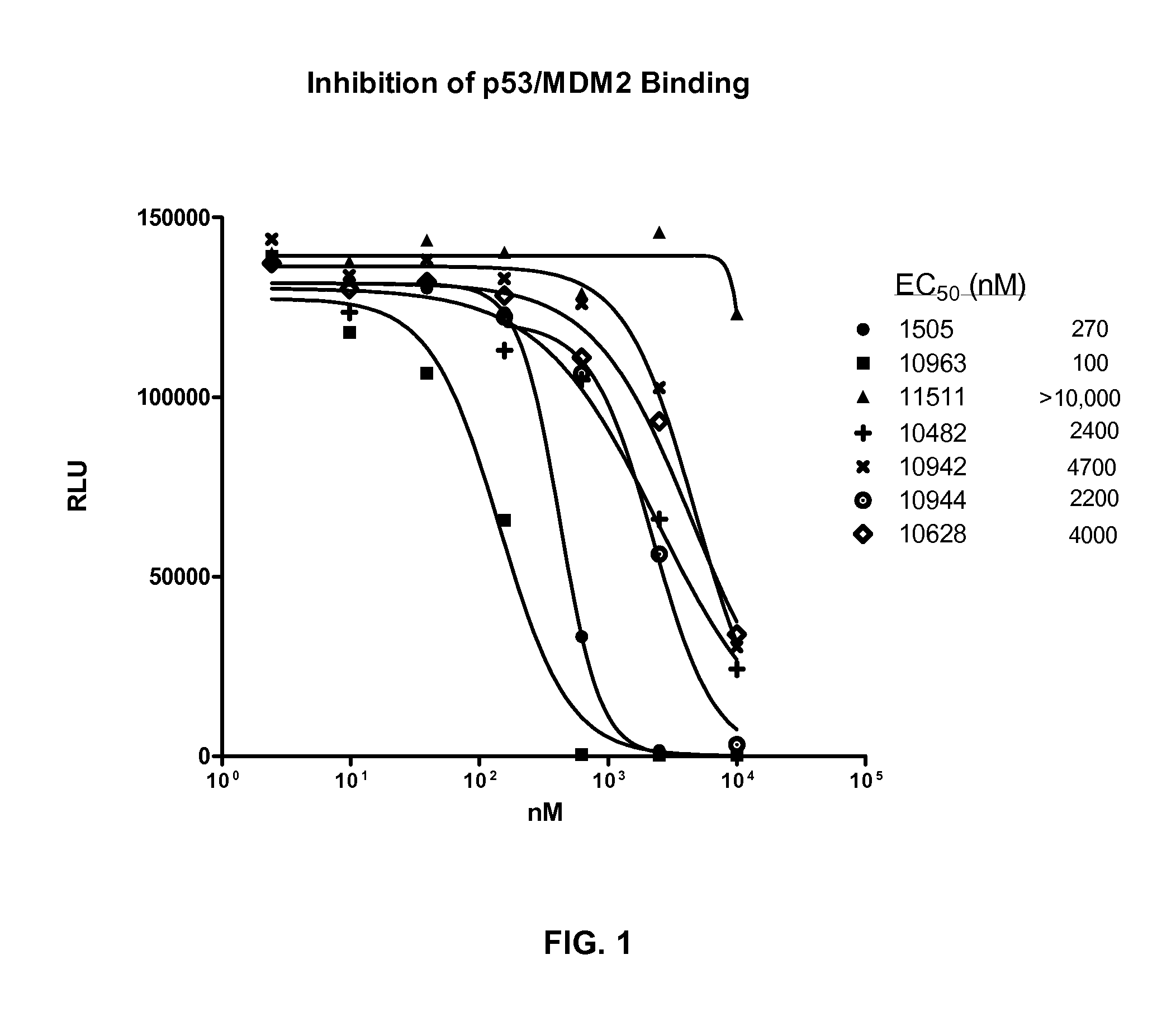
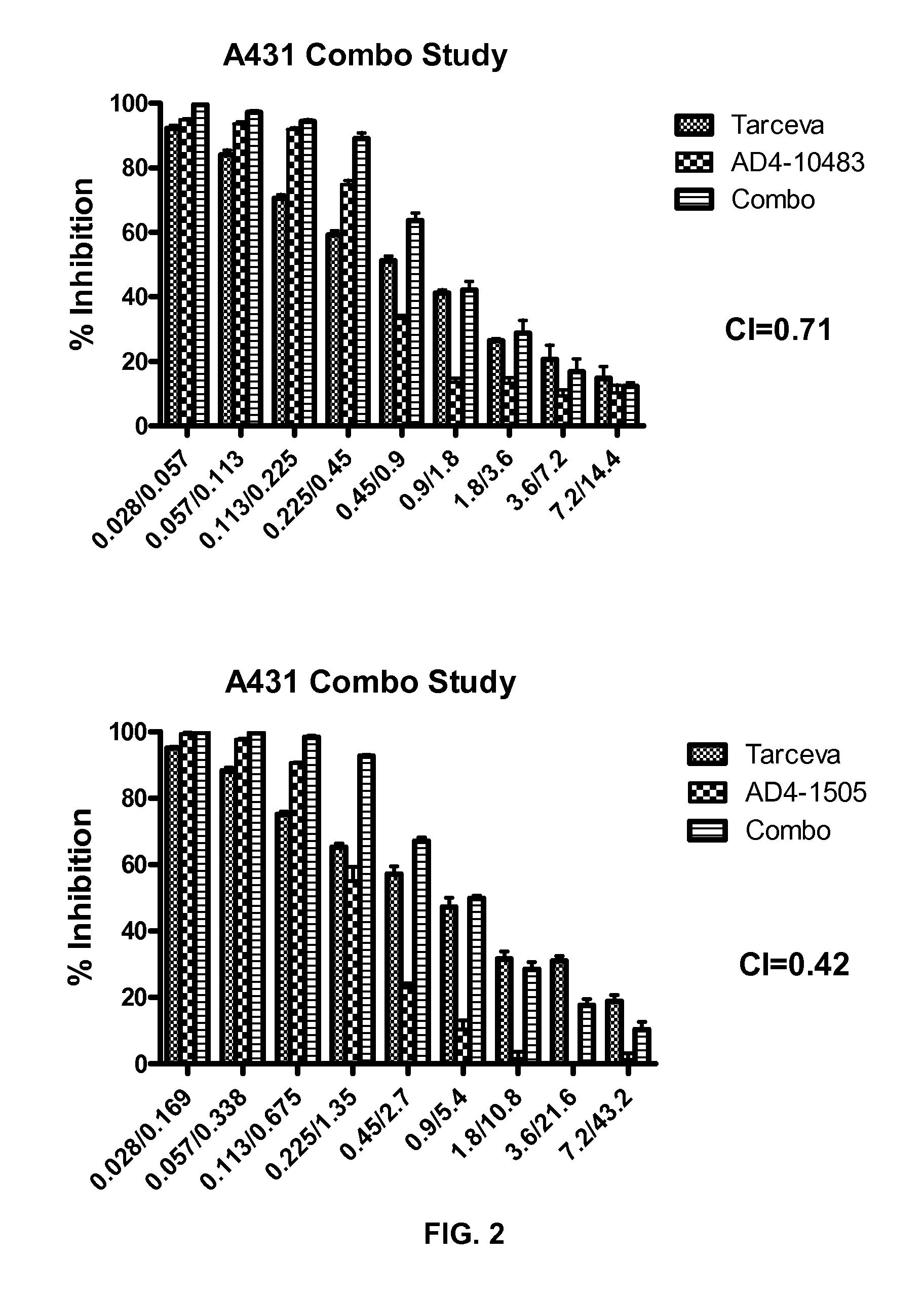
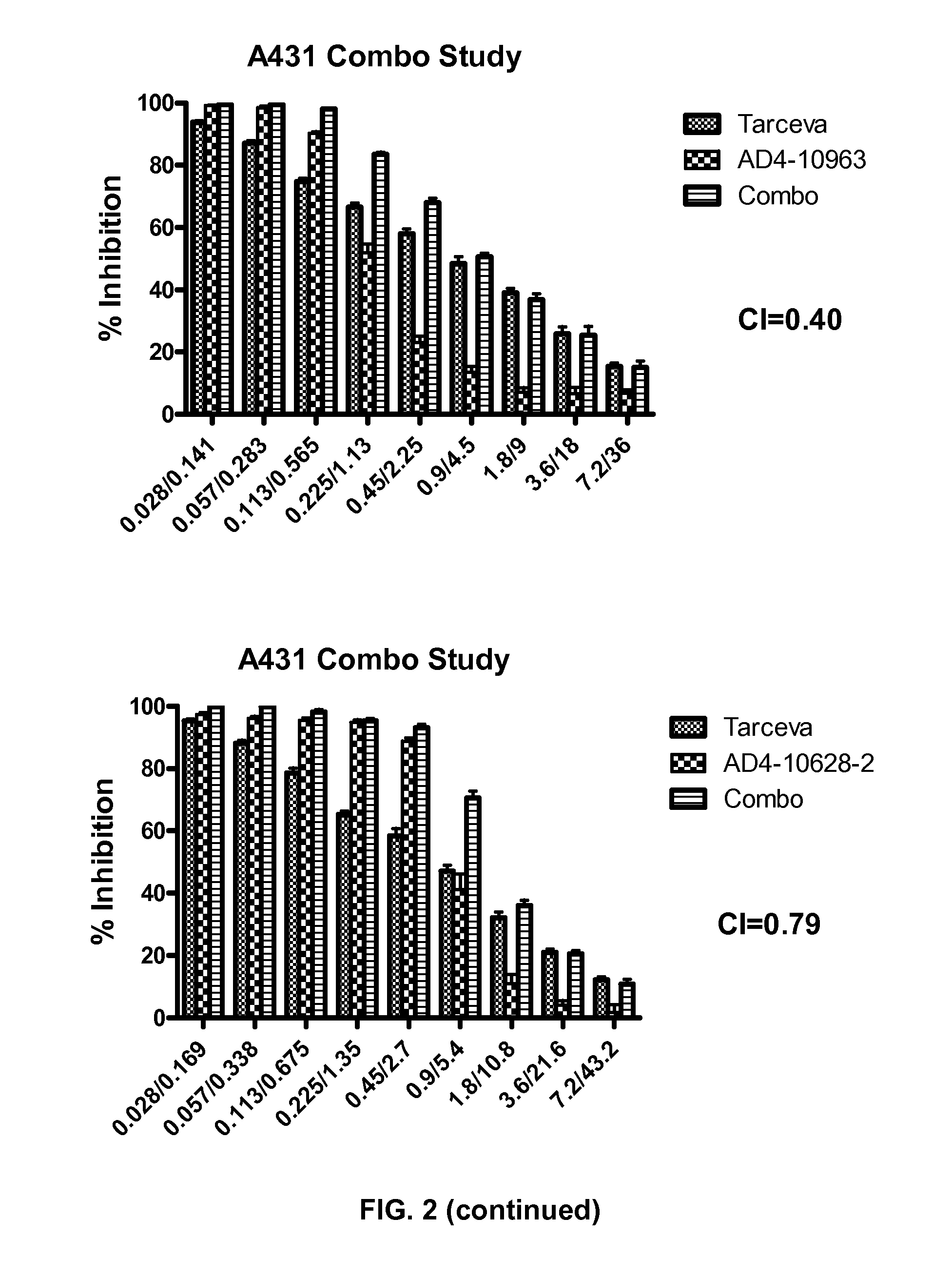
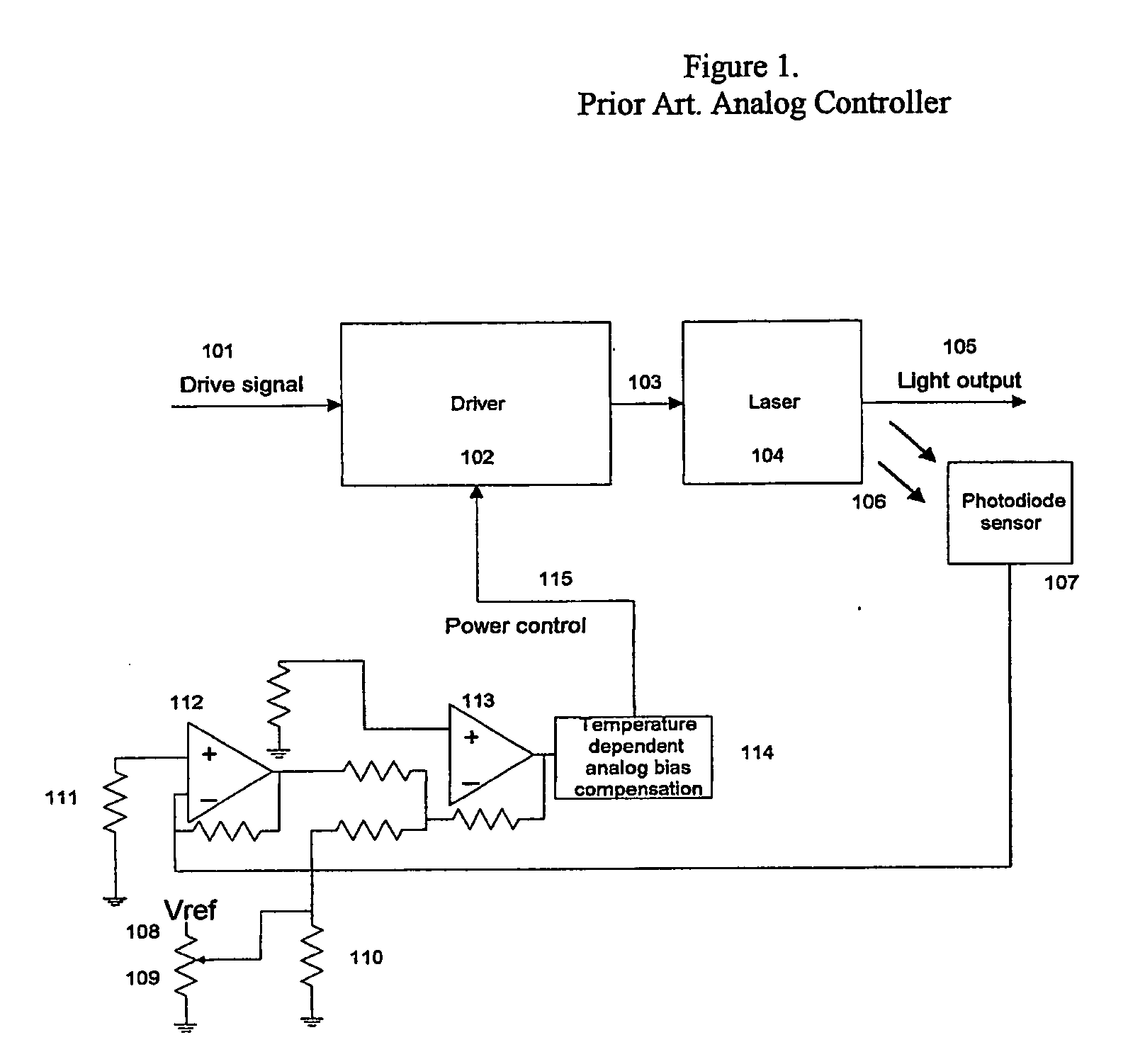
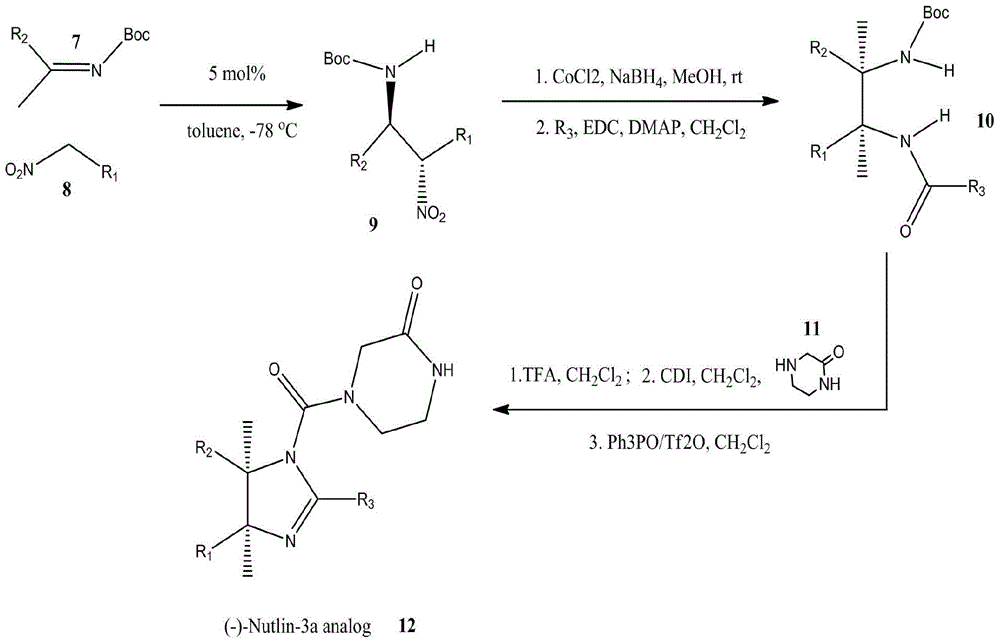
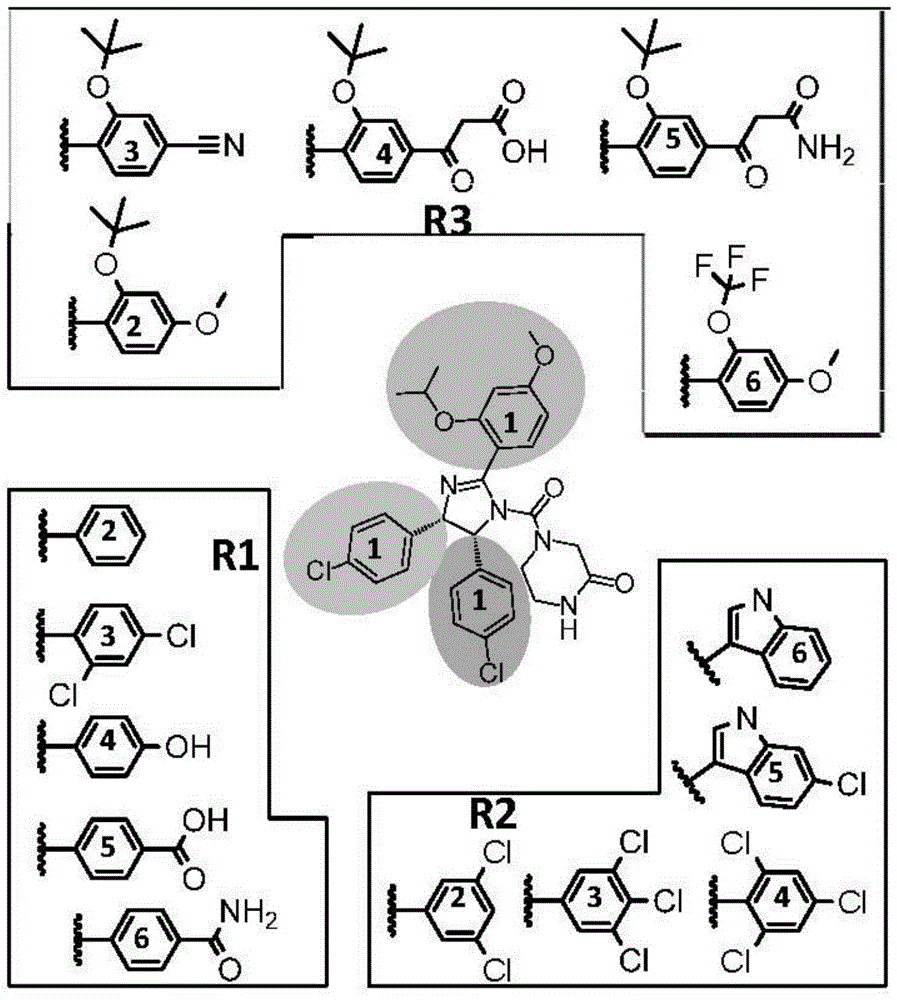
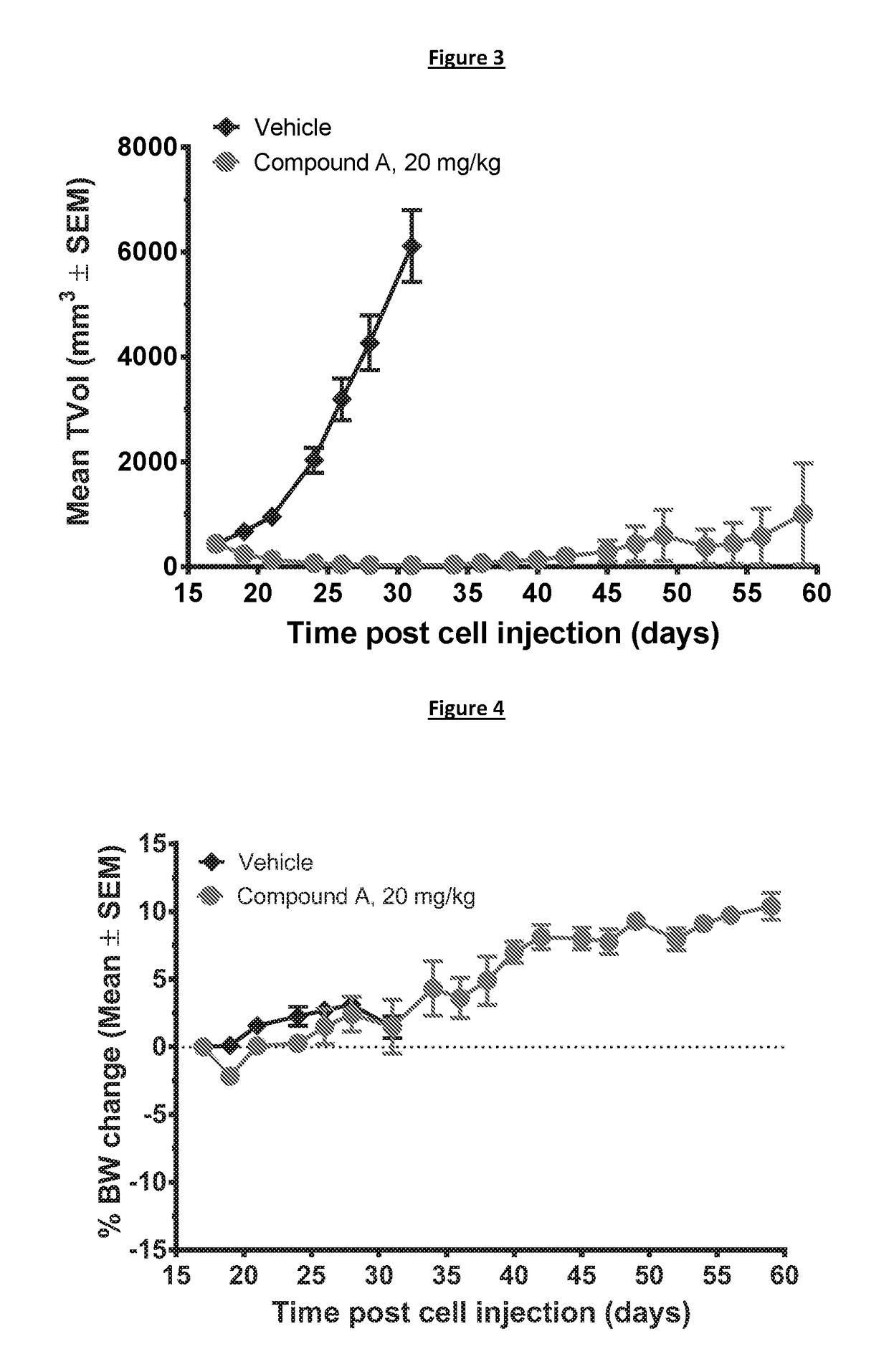
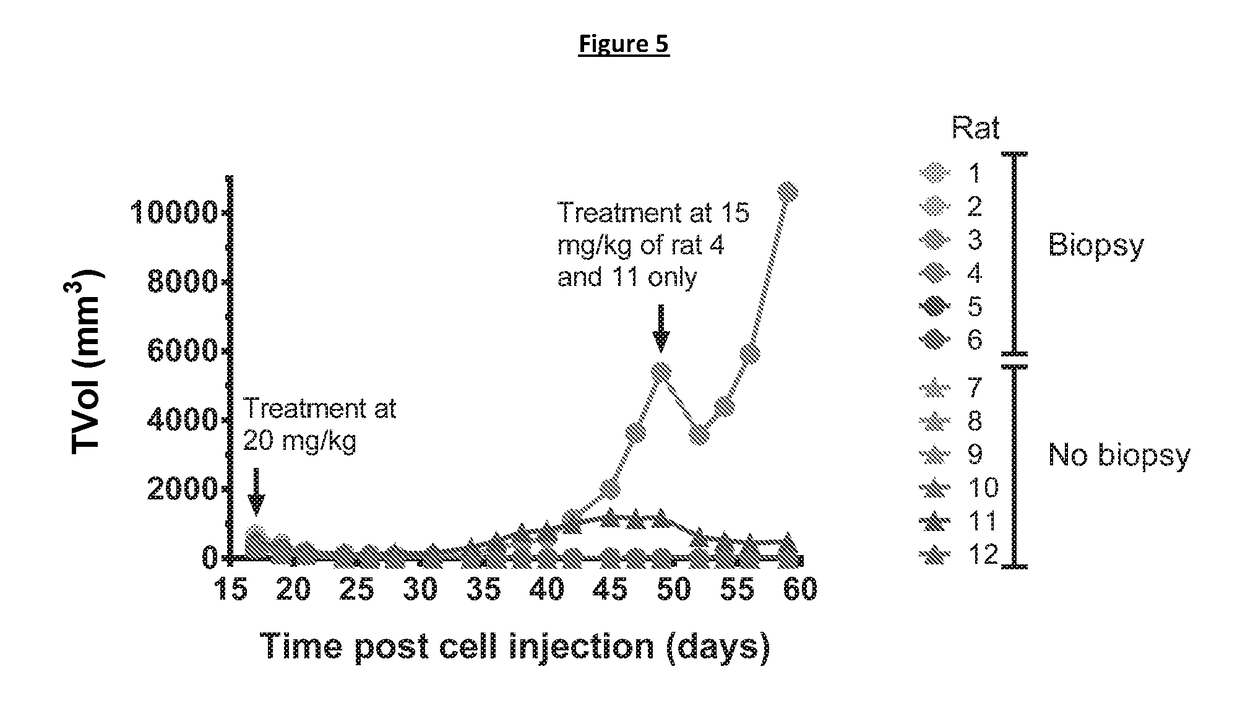
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
113 results about "Mdm2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mouse double minute 2 homolog (MDM2) also known as E3 ubiquitin-protein ligase Mdm2 is a protein that in humans is encoded by the MDM2 gene. Mdm2 is an important negative regulator of the p53 tumor suppressor. Mdm2 protein functions both as an E3 ubiquitin ligase that recognizes the N-terminal trans-activation domain (TAD) of the p53 tumor suppressor and as an inhibitor of p53 transcriptional activation.
Small molecule inhibitors of MDM2 and the uses thereof
InactiveUS7759383B2Extended half-lifeIncrease the function(s) of p53BiocideOrganic chemistrySensitized cellMdm2 Protein
Owner:RGT UNIV OF MICHIGAN
Potent d-peptide antagonists of mdm2 and mdmx for anticancer therapy
InactiveUS20120328692A1High expressionPeptide/protein ingredientsImmunoglobulinsPharmaceutical drugPharmacology
The present invention relates to a group of MDM2 and MDMX antagonists, namely, D-peptides, variants thereof, and stapled D-peptides, along with pharmaceutical compositions comprising the antagonists, and methods of treating conditions such as cancer using the antagonists.
Owner:UNIV OF MARYLAND BALTIMORE
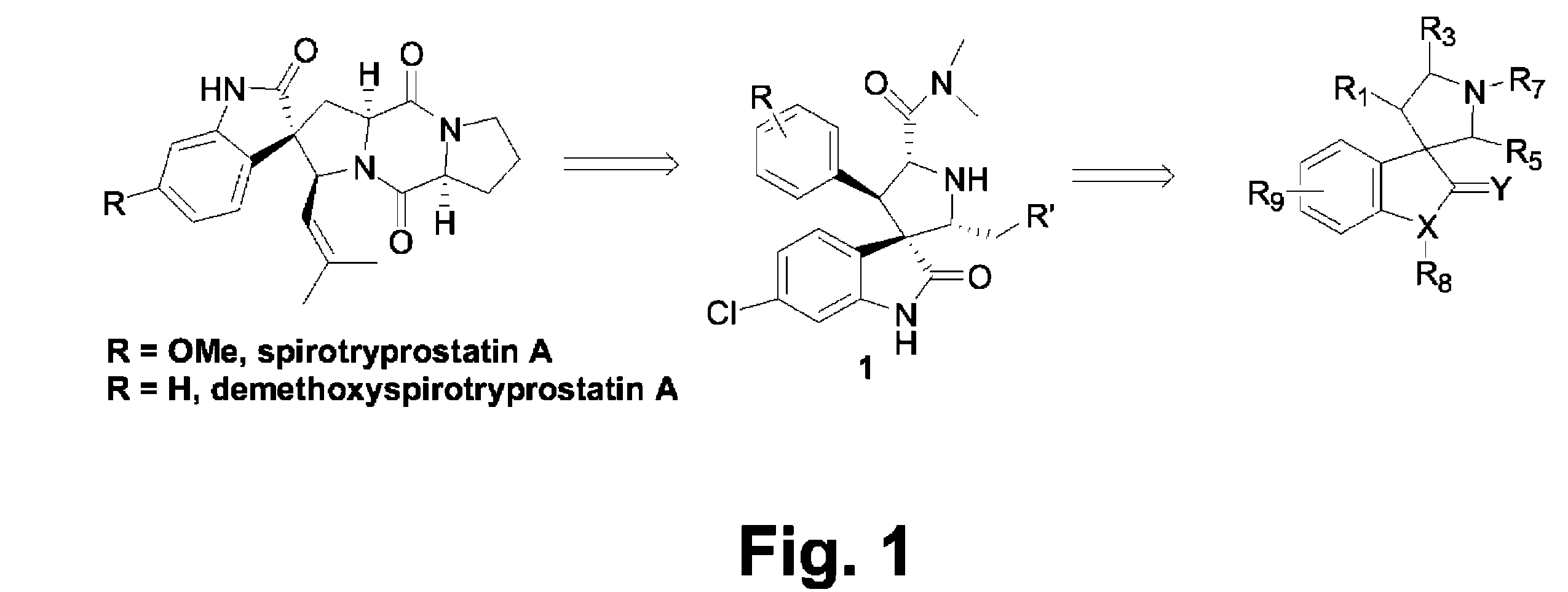
Spiro-oxindole mdm2 antagonists
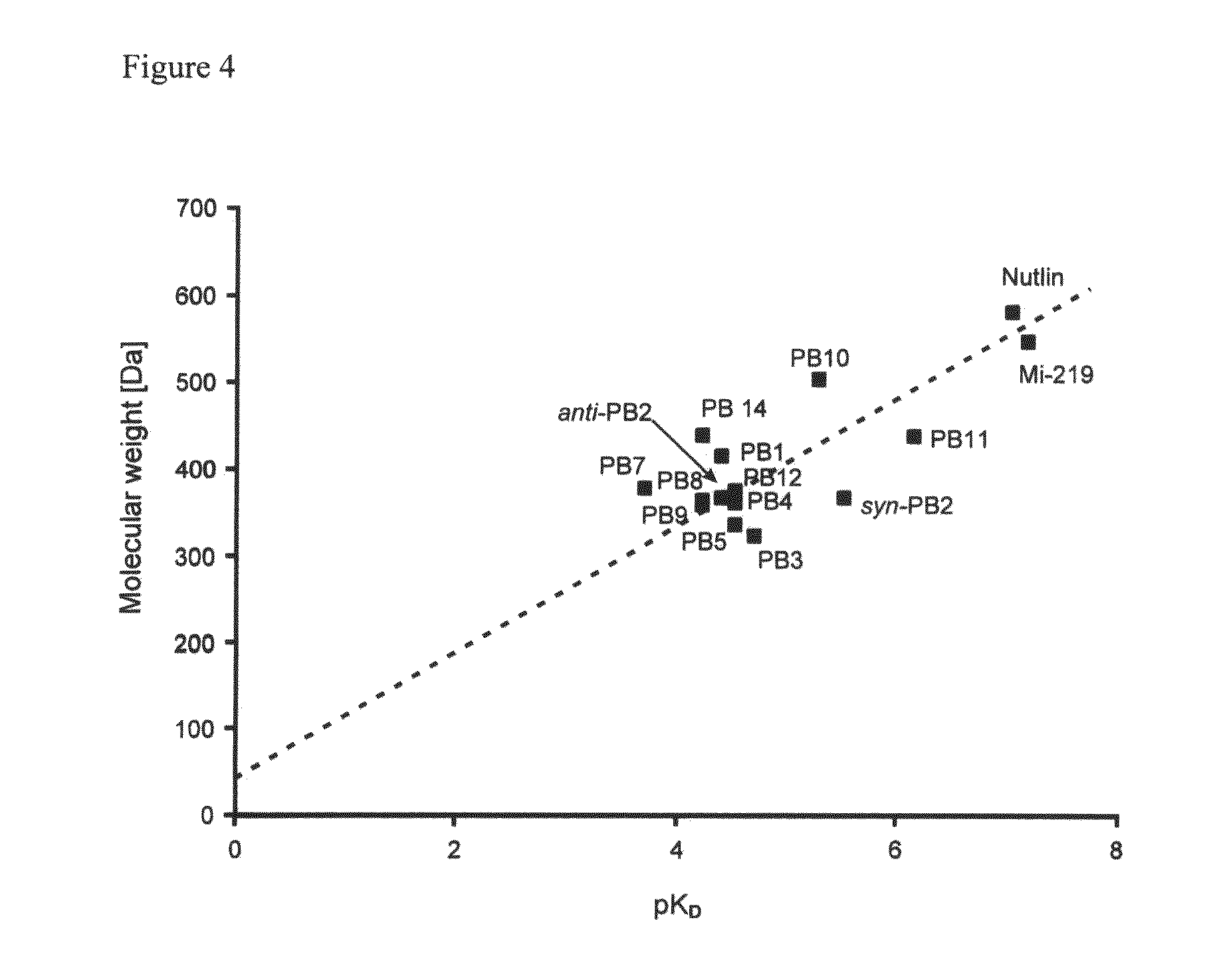
Provided herein are compounds, compositions, and methods in the field of medicinal chemistry. The compounds and compositions provided herein relate to spiro-oxindoles which function as antagonists of the interaction between p53 and MDM2, and their use as therapeutics for the treatment of cancer and other diseases.
Owner:RGT UNIV OF MICHIGAN +1
Combination therapy with mdm2 and efgr inhibitors
Provided is a method of treating a proliferative disease, condition, or disorder in a subject by administering a combination of an inhibitor of p53 and MDM2 binding and an EGFR inhibitor. Various embodiments of the disclosed methods provide a synergistic anti-proliferative or anti-apoptotic effect compared to administration of one agent alone.
Owner:ERRICO JOSEPH P
Method for cytoprotection through mdm2 and hdm2 inhibition
InactiveUS20060189511A1Easy interconnectionGood compatibilityBiocidePeptide/protein ingredientsBenzodiazepineCytotoxicity
The present invention is directed to a method of protecting one or more cells from programmed cytotoxic cell death by contacting the cells with a cytoprotective amount of an MDM2 and / or HDM2 inhibitor. The cytoprotective amount of inhibitor is typically used as a pulsed administration. Useful inhibitors include a class of 1,4-benzodiazepines, which act as inhibitors of MDM2-p53 interactions. The method of the invention can be employed as an adjunct to chemotherapy or radiation therapy. In addition, the methods of the invention can be employed to treat a disease or condition that involves excessive cell death.
Owner:3 DIMENSIONAL PHARMA
Substituted Heterocycles as Therapeutic agents for treating cancer
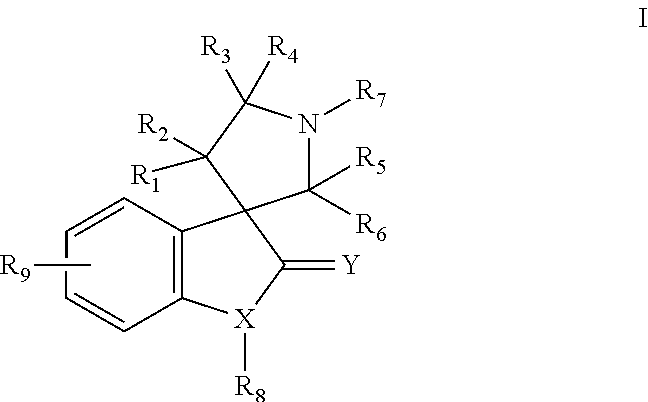
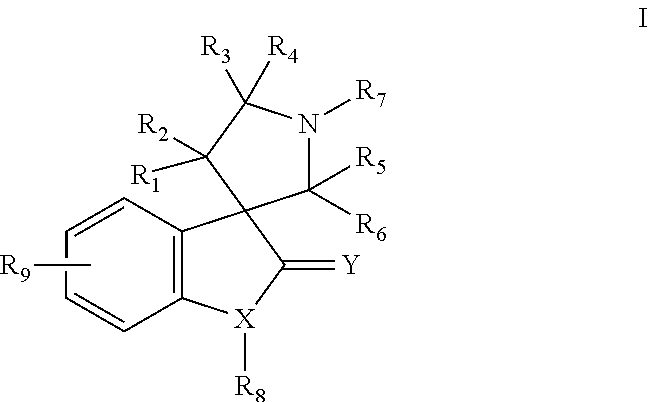
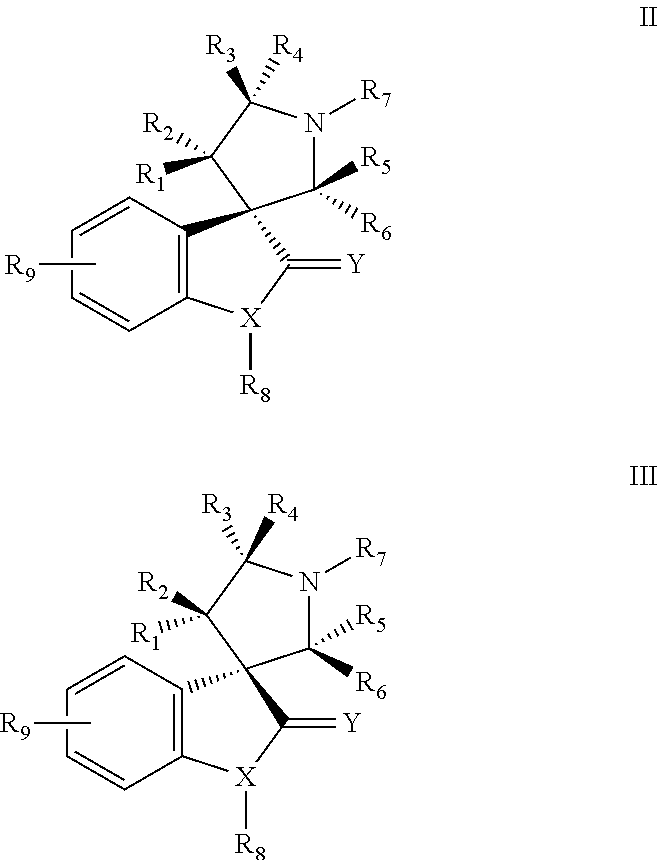
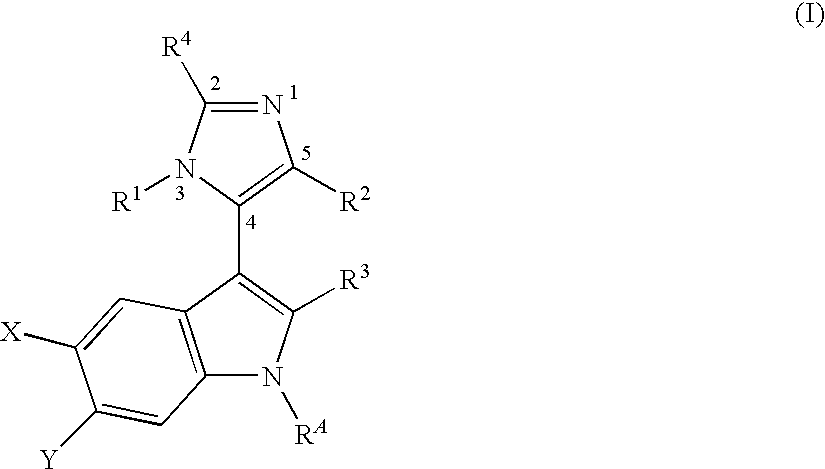
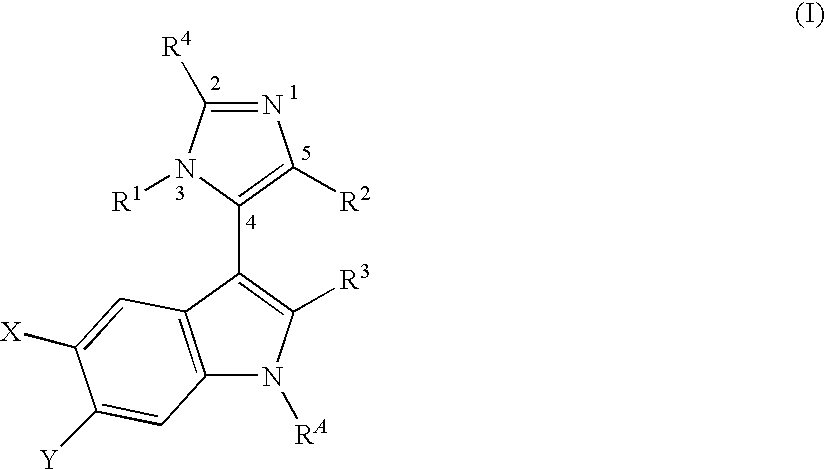
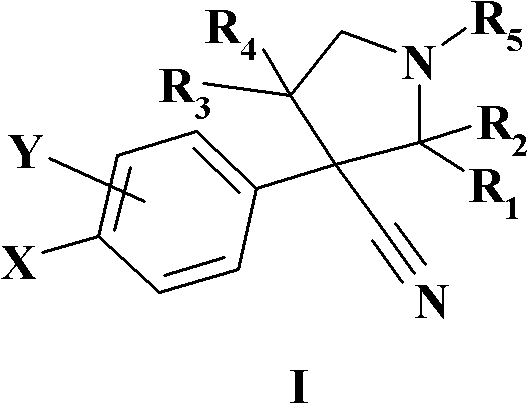
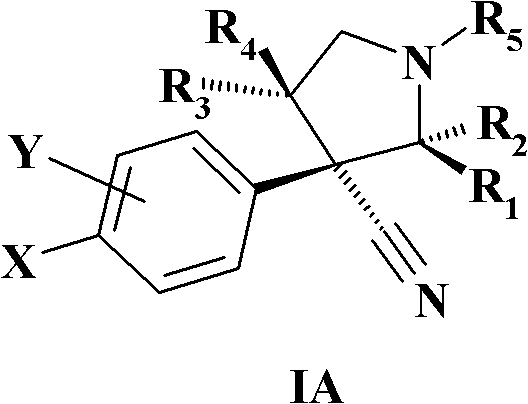
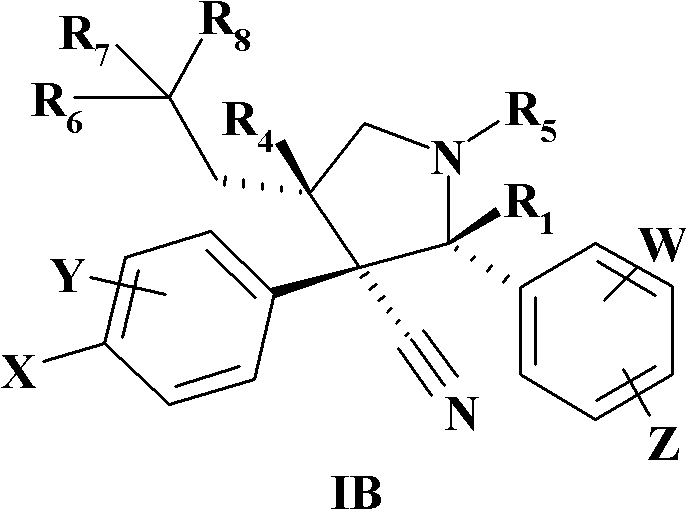
MDM2 and MDM4 proteins prevent apoptosis of cancer cells by negatively regulating the transcription factor p53. Compounds according to Formula Iare selective antagonists of MDM2 and MDM4 proteins, disrupting the p53 / MDM2 and p53 / MDM4 complex. These compounds therefore are candidate therapeutics for treating cancer as well as other cell proliferative disease states.
Owner:UNIVERSITY OF PITTSBURGH
Small molecule inhibitors of MDM2 and the uses thereof
InactiveUS8222288B2Growth inhibitionExtended half-lifeBiocideOrganic chemistrySensitized cellCell growth
Owner:RGT UNIV OF MICHIGAN
Peptidomimetic macrocycles
ActiveUS9505804B2Stabilize alpha-helical secondary structureImprove biological activityP53 proteinPeptide preparation methodsCross-linkDisease
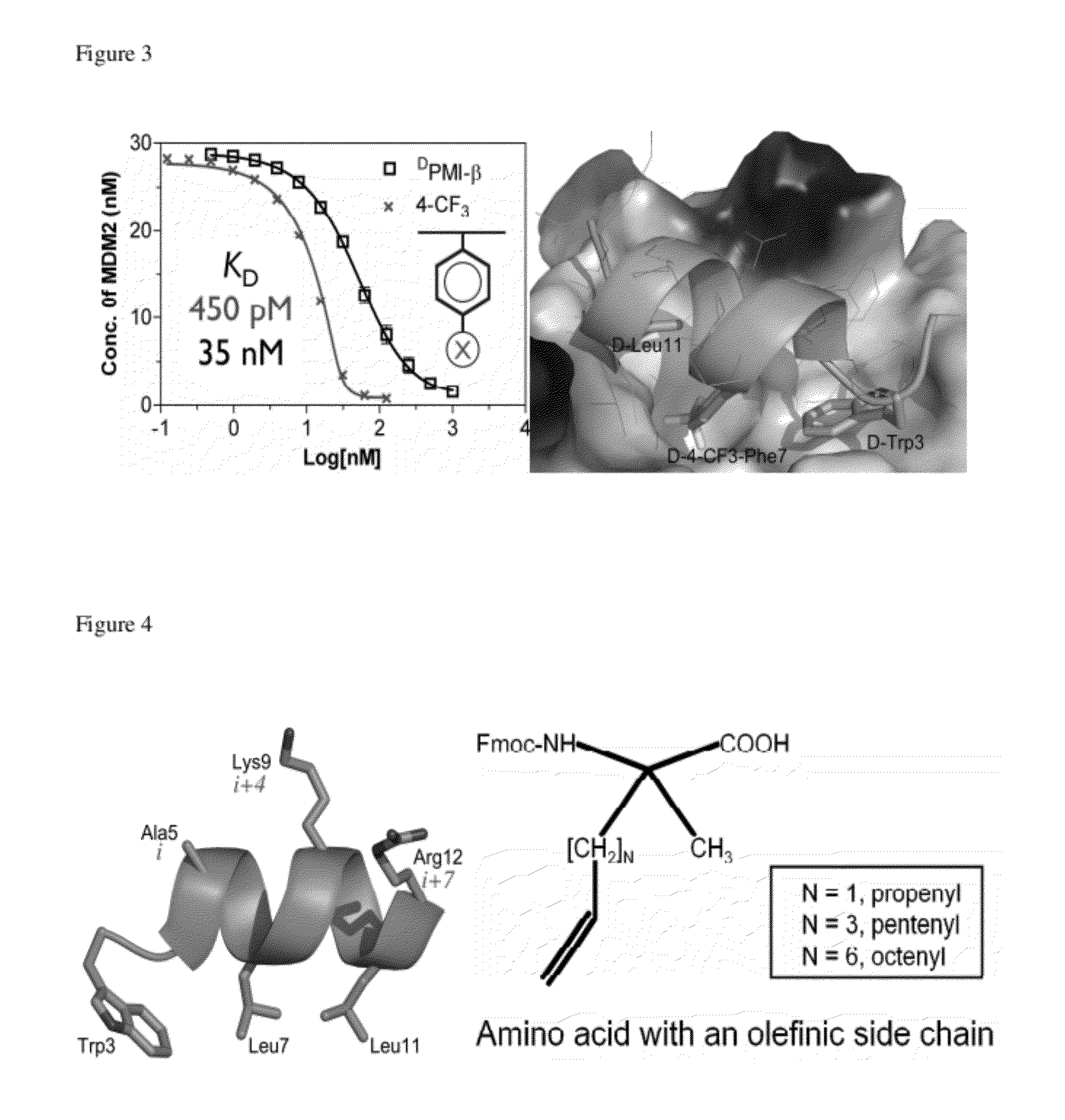
Provided herein are peptidomimetic macrocycles containing amino acid sequences with at least two modified amino acids that form an intramolecular cross-link that can help to stabilize a secondary structure of the amino acid sequence. Suitable sequences for stabilization include those with homology to the p53 protein. These sequences can bind to the MDM2 and / or MDMX proteins. Also provided herein are methods of using such macrocycles for the treatment of diseases and disorders, such as cancers or other disorders characterized by a low level or low activity of a p53 protein or high level of activity of a MDM2 and / or MDMX protein.
Owner:AILERON THERAPEUTICS INC
3-Imidazolyl-Indoles for the Treatment of Proliferative Diseases
Owner:NOVARTIS AG
Ubiquitin ligase inhibitors
InactiveUS20060160869A1Organic active ingredientsBiocidePharmaceutical drugUbiquitin Ligase Inhibitors
This invention describes compounds and pharmaceutical compositions useful as ubiquitin agent inhibitors, particularly ubiquitin ligase inhibitors. The compounds and pharmaceutical compositions of the invention are useful as inhibitors of the biochemical pathways of organisms in which ubiquitination is involved, such as signal transduction pathways. The invention also comprises the use of the compounds and pharmaceutical compositions of the invention for the treatment of conditions that require inhibition of ubiquitination. Furthermore, the invention comprises methods of inhibiting ubiquitination in a cell comprising contacting a cell in which inhibition of ubiquitination is desired with a compound or pharmaceutical composition according to the invention. Particularly, the compounds and pharmaceutical compositions are useful to inhibit the ubiquitin ligase activity of MDM2.
Owner:RIGEL PHARMA
MDM2 inhibitors and therapeutic methods using the same
ActiveUS9745314B2Easy to solveImprove anti-tumor activityOrganic active ingredientsOrganic chemistryDiseaseCancer research
Owner:RGT UNIV OF MICHIGAN
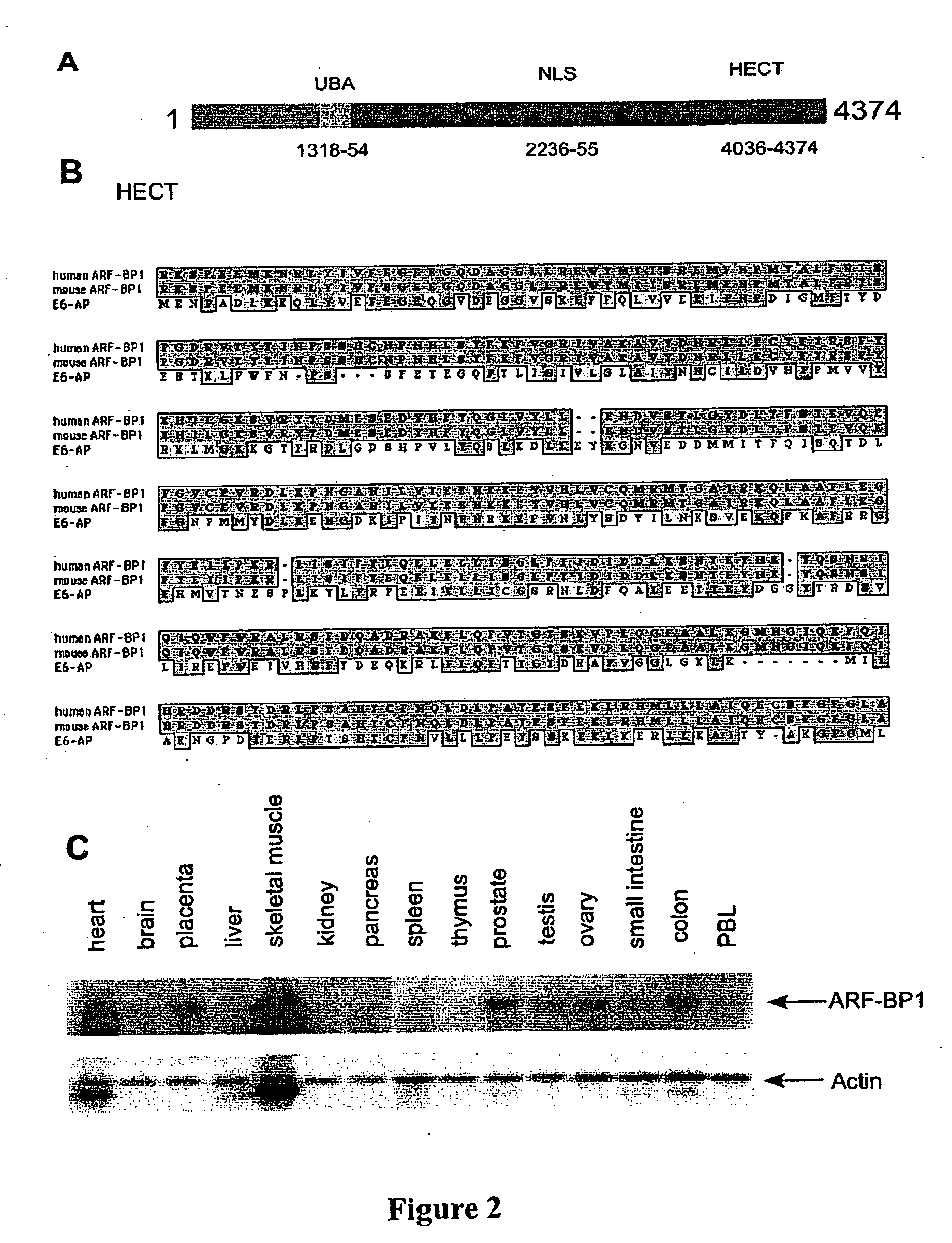
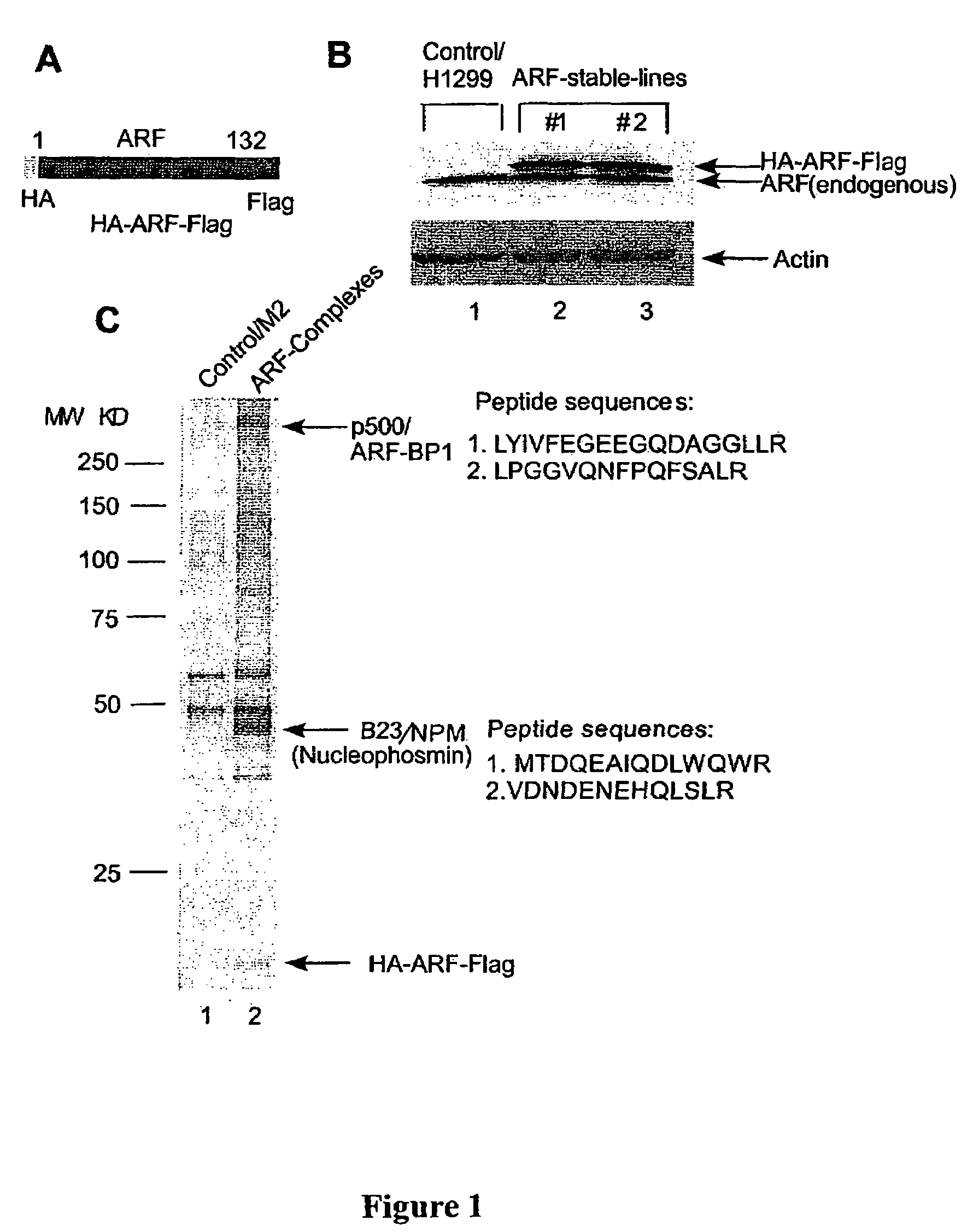
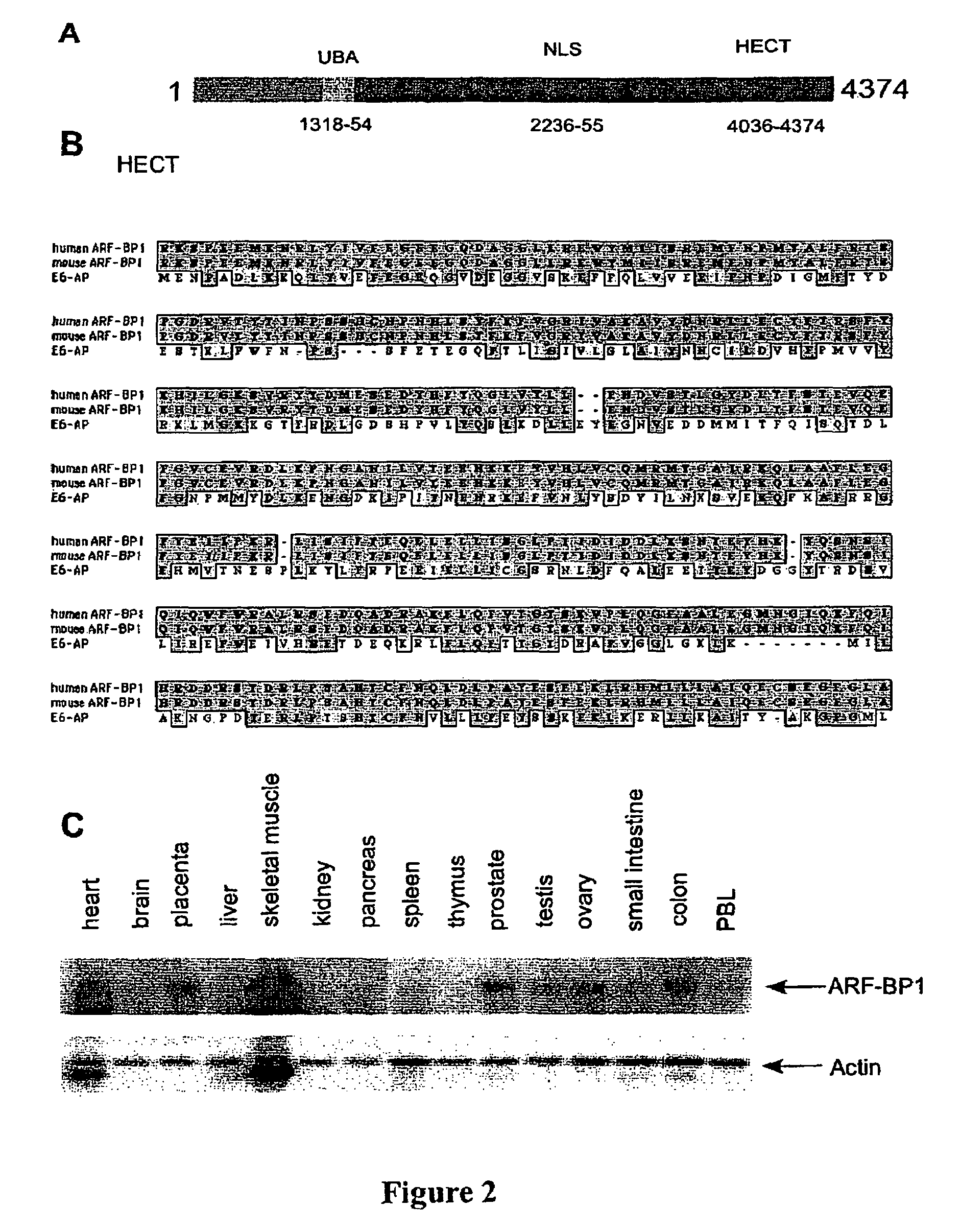
ARF-BP1 as mediator of p53-dependent and independent tumor suppression and uses thereof
InactiveUS20060088847A1Assessing abilityAvoid interactionHydrolasesTissue cultureUbiquitin ligaseSomatic cell
The present invention relates to the mechanism of ARF-mediated cell growth suppression. ARF-BP1 is identified as a novel ubiquitin ligase, and a major component of ARF-containing nuclear complexes in human cells. The present invention discloses a novel mechanism of ARF-mediated p53 activation and that ARF-BP1 is a critical mediator of both p53-independent and p53-dependent tumor suppression functions of ARF. Inactivation of ARF-BP1 in normal cells stabilizes p53 and induces p53-dependent apoptosis. Inactivation of ARF-BP1, but not Mdm2, in p53-wildtype cells promotes cell growth inhibition in a manner reminiscent of ARF induction. ARF-BP1 directly binds and ubiquitinates p53 and inactivation of endogenous ARF-BP1 is crucial for ARF-mediated p53 stabilization in Mdm2-null cells. ARF-BP1 is advantageous over Mdm2 as a target for suppressing tumor cell growth regardless of p53 status.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Pyrrolopyrrolidinone compounds
The invention relates to compounds of formula (I):as described herein, pharmaceutical preparations comprising such compounds, uses and methods of use for such compounds in the treatment of a disorder or a disease mediated by the activity of MDM2 and / or MDM4, and combinations comprising such compounds.
Owner:NOVARTIS AG
Gene detection kit for prognosing gastric cancer metastasis and use method of gene detection kit
InactiveCN106636366AThe result is objectiveOptimal treatment timeMicrobiological testing/measurementBreast cancer metastasisRAD51
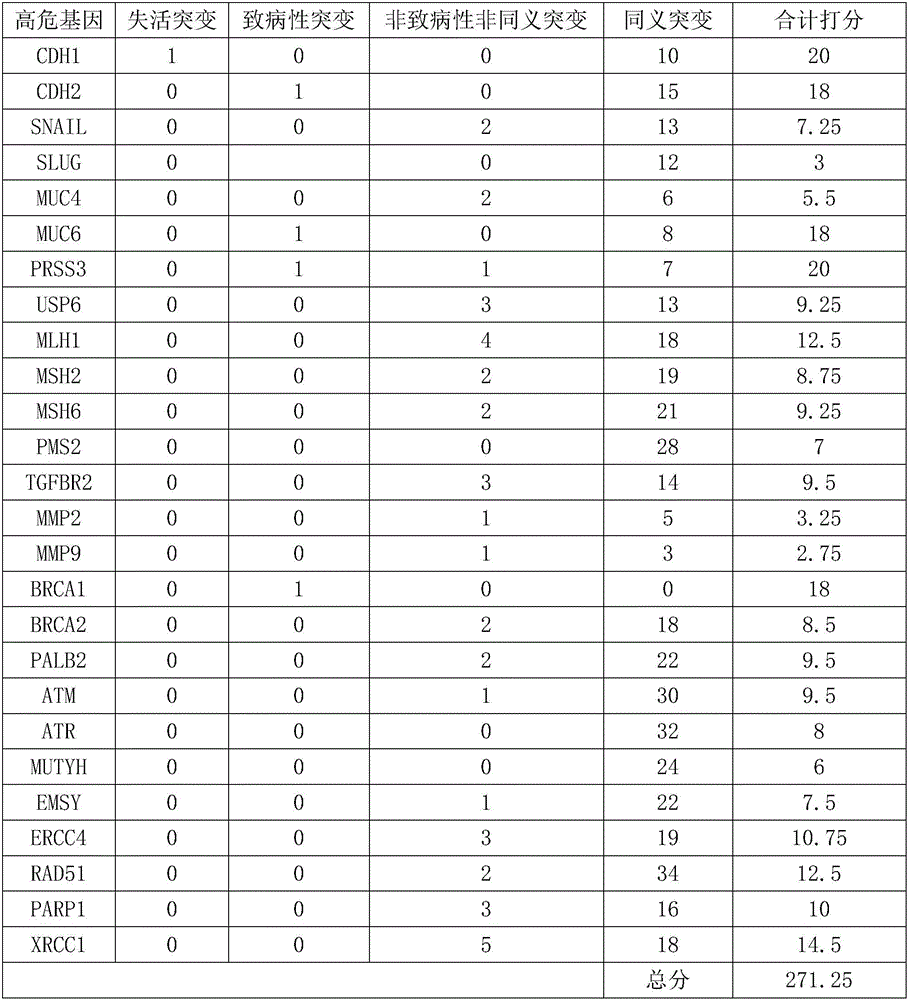
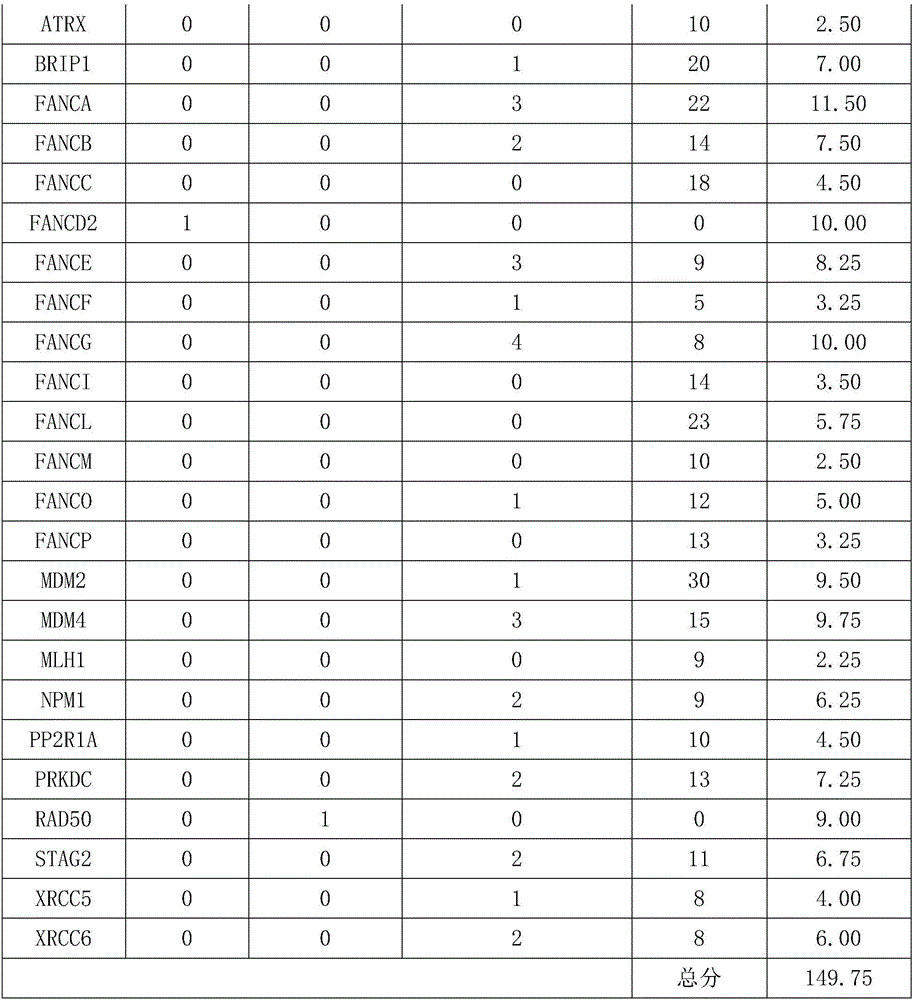
The invention relates to a gene detection kit for prognosing gastric cancer metastasis. The kit comprises a DNA library building kit, wherein the DNA library building kit comprises a high-risk gene probe and a low-risk gene probe; the high-risk gene probe comprises CDH1, CDH2, SNAIL, SLUG, MUC4, MUC6, PRSS3, USP6, MLH1, MSH2, MSH6, PMS2, TGFBR2, MMP2, MMP9, BRCA1, BRCA2, PALB2, ATM, ATR, MUTYH, EMSY, ERCC4, RAD51, PARP1 and XRCC1; the low-risk gene probe comprises ATRX, BRIP1, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, FANCO, FANCP, MDM2, MDM4, MLH1, NPM1, PP2R1A, PRKDC, RAD50, STAG2, XRCC5 and CRCC6. The invention further discloses a use method of the kit. The use method comprises the following steps: extracting cfDNA in a blood sample; building a library for the cfDNA through the DNA library building kit, and then sequencing the DNA to obtain a gene overall length sequence; carrying out gene mutation analysis on the gene overall length sequence.
Owner:苏州首度基因科技有限责任公司 +1
Mdm2 inhibitors and therapeutic methods using the same
ActiveUS20150299211A1Easy to solveImprove anti-tumor activityBiocideOrganic chemistryDiseaseMdm2 Protein
Owner:RGT UNIV OF MICHIGAN
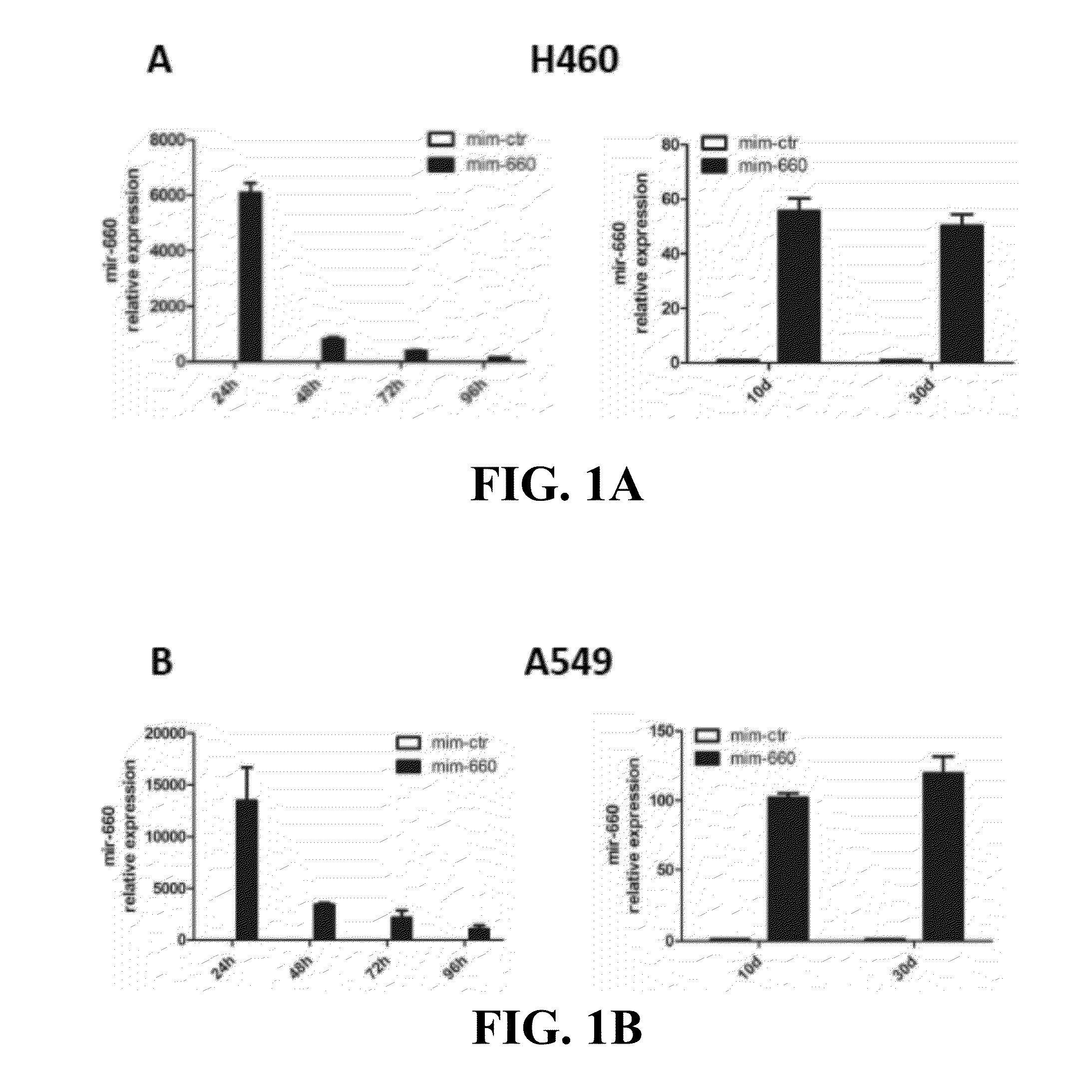
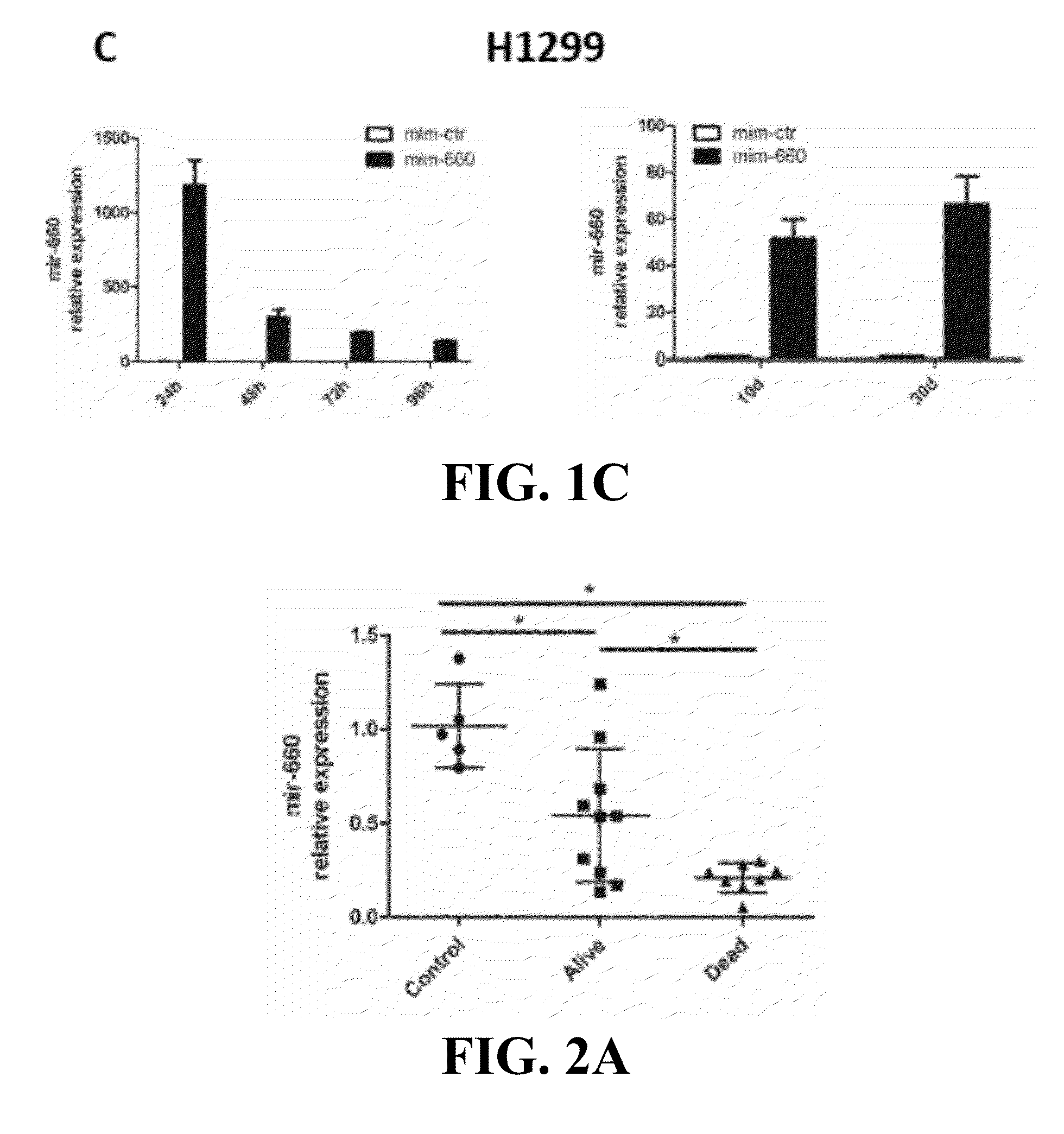
Lung cancer diagnostics and therapeutics with mir-660
InactiveUS20160108405A1Microbiological testing/measurementFermentationFavorable prognosisUbiquitin-Protein Ligases
Provided are methods of treating lung cancer in a patient in need thereof. The method includes administration to the patient a composition comprising a therapeutically effective amount of a compound that reduces the expression level of E3 ubiquitin-protein ligase MDM2. The compound in certain instances is a miR-660 miRNA, or a functional variant thereof. The patient in need of treatment in certain instances expresses miR-660 in a lung tissue sample or biological fluid sample at a level lower as compared to a control level derived from a subject or plurality of subjects that do not have lung cancer, or as compared to a control level derived from a lung cancer patient or plurality thereof, that have been given a favorable prognosis; expresses MDM2 at a higher level in a lung tissue sample or biological fluid sample as compared to a control level derived from a subject or plurality of subjects that do not have lung cancer, or as compared to a control level derived from a lung cancer patient or plurality thereof that have been given a favorable prognosis; and / or expresses p53 in a lung tissue sample or a biological fluid sample below a control level derived from a lung tumor tissue sample, or plurality thereof, or a biological fluid sample, or plurality thereof, obtained from a patient that has a favorable lung cancer prognosis; or a control level derived from a healthy subject.
Owner:BIOMIRNA HLDG
Companion diagnostic for CDK4 inhibitors
ActiveUS9889135B2Organic active ingredientsMicrobiological testing/measurementCancer cellBiomarker (petroleum)
The present invention relates to the use of one or more biomarkers to evaluate the likelihood that a CDK4 inhibitor would produce an anti-cancer effect in a subject. It is based, at least in part, on the discovery that cancer treatment with a CDK4 inhibitor is more effective where treated cancer cells undergo cellular senescence rather than a transient cell cycle arrest, where cellular senescence is associated with decreased MDM2 protein level. Accordingly, in non-limiting embodiments, the present invention provides for methods, compositions, and kits for a companion diagnostic for CDK4 inhibitors, and in particular, to the use of MDM2 expression as a biomarker for the likelihood that a cancer can be successfully treated by CDK4 inhibition.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
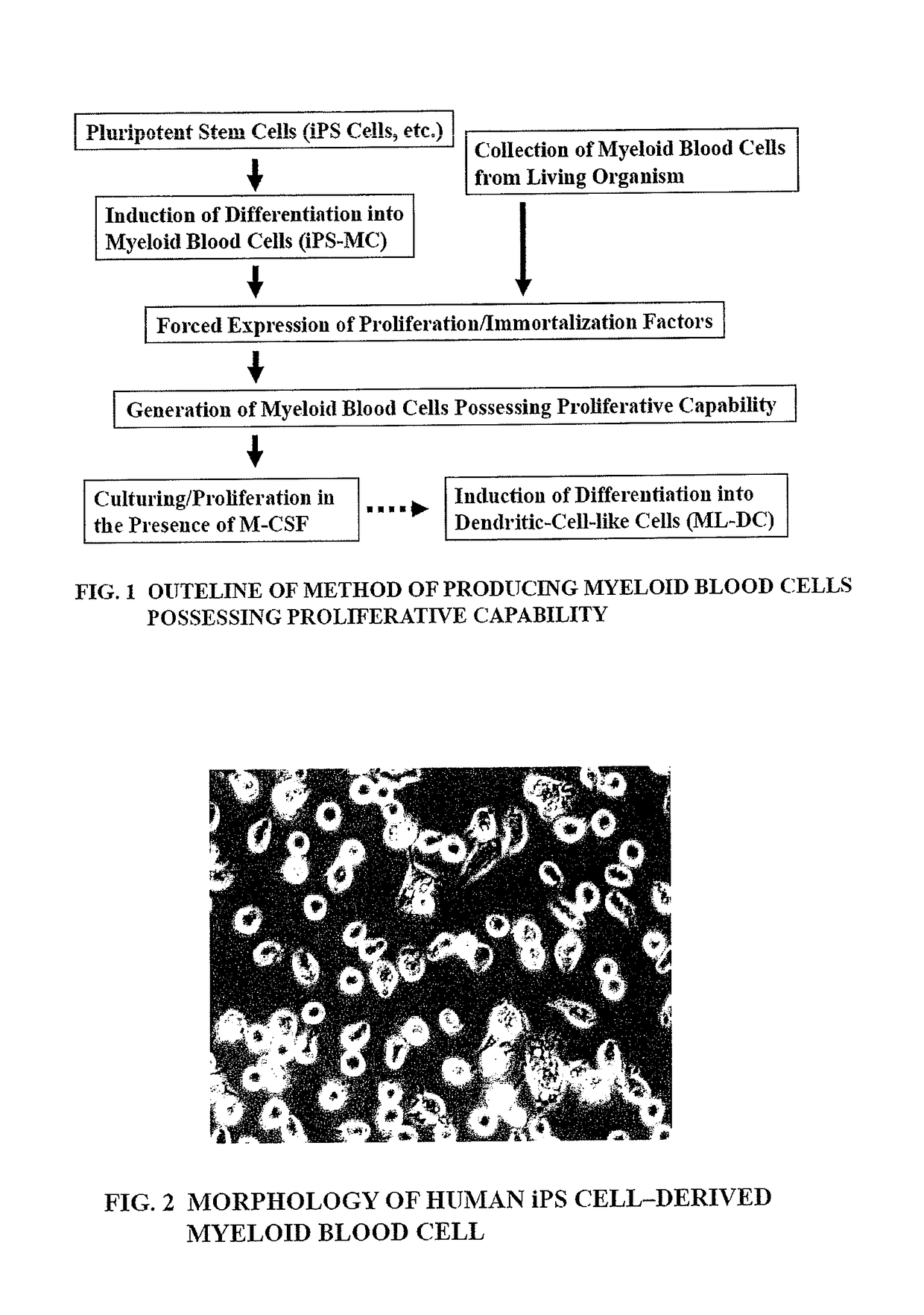
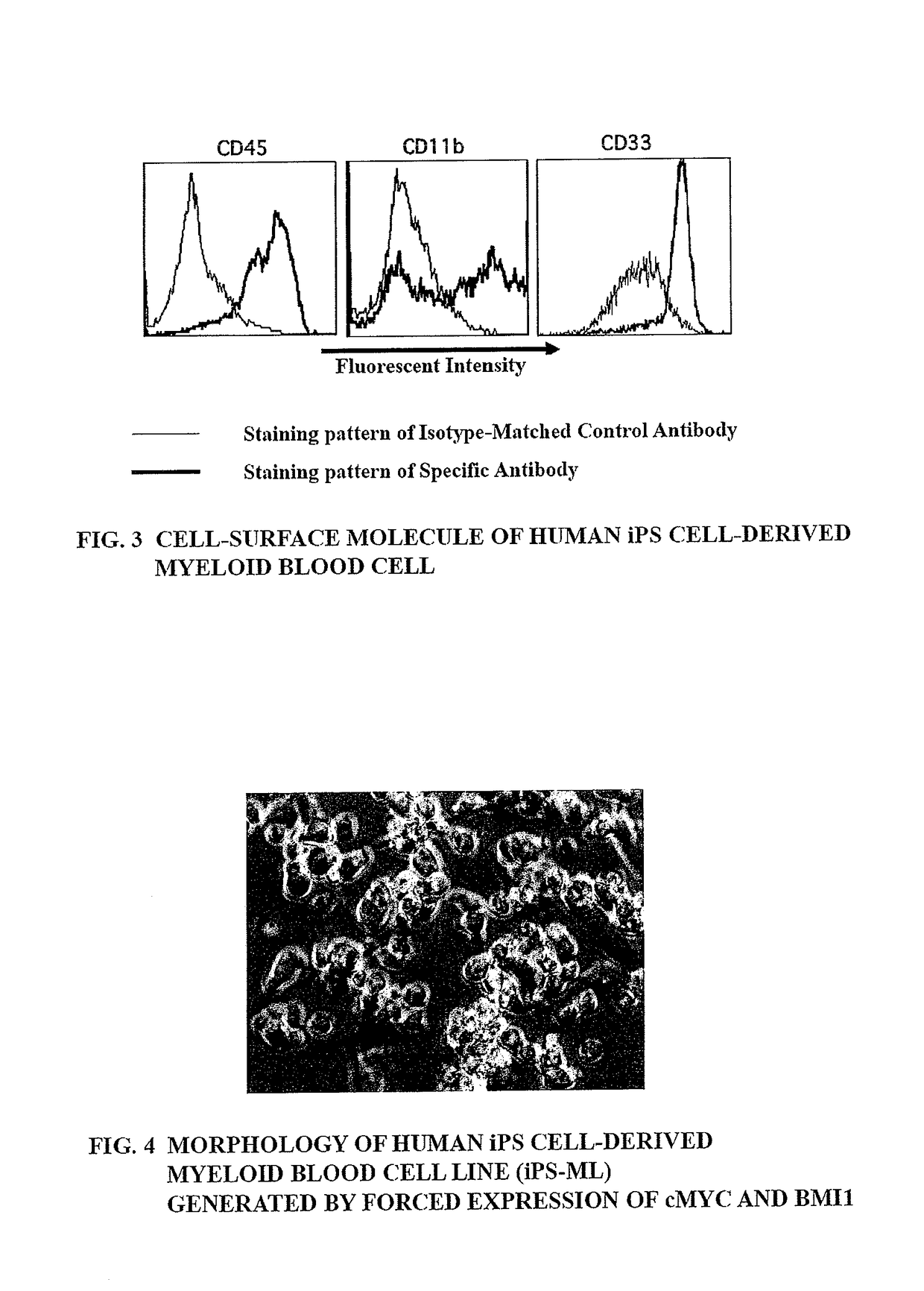
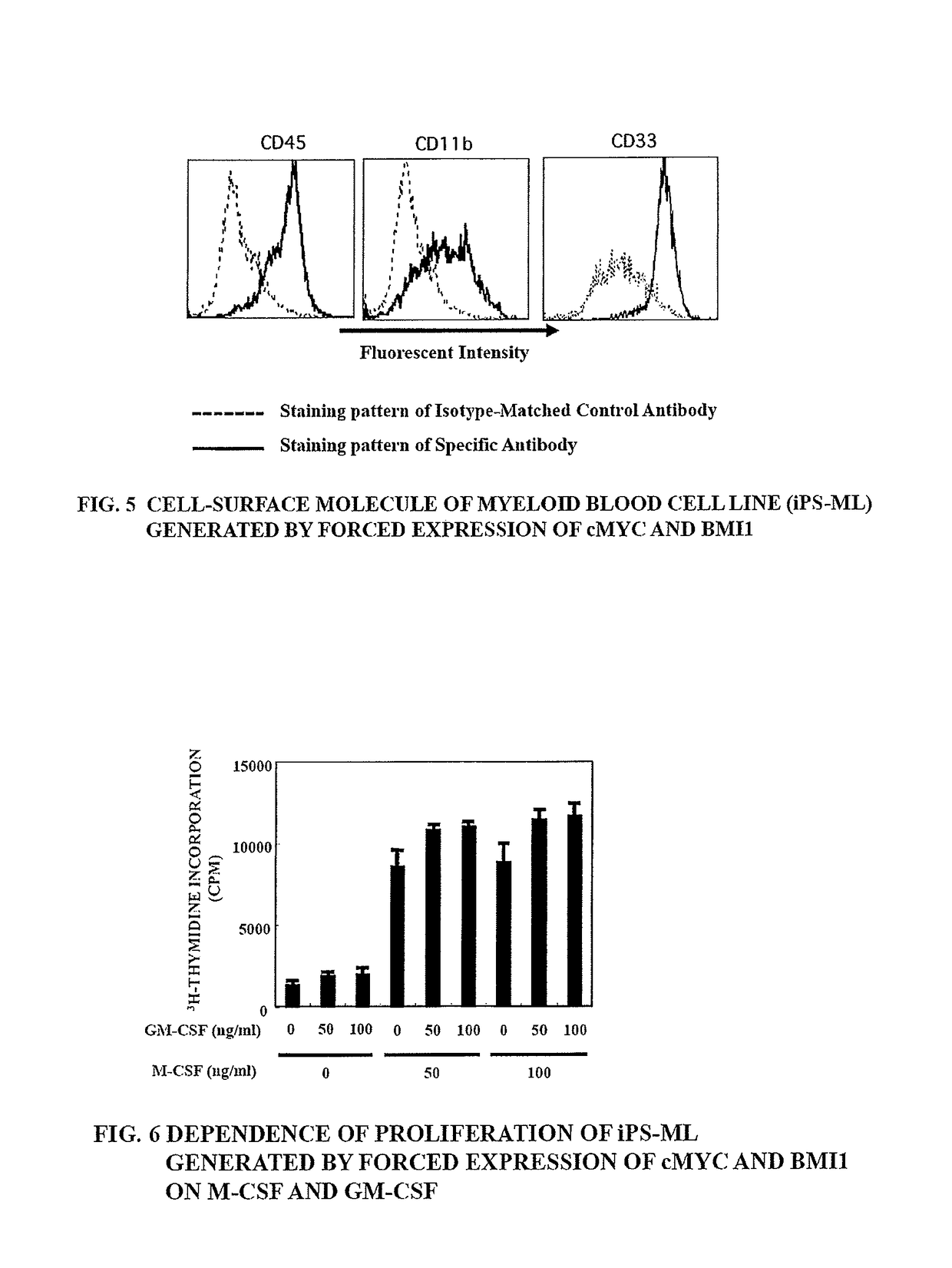
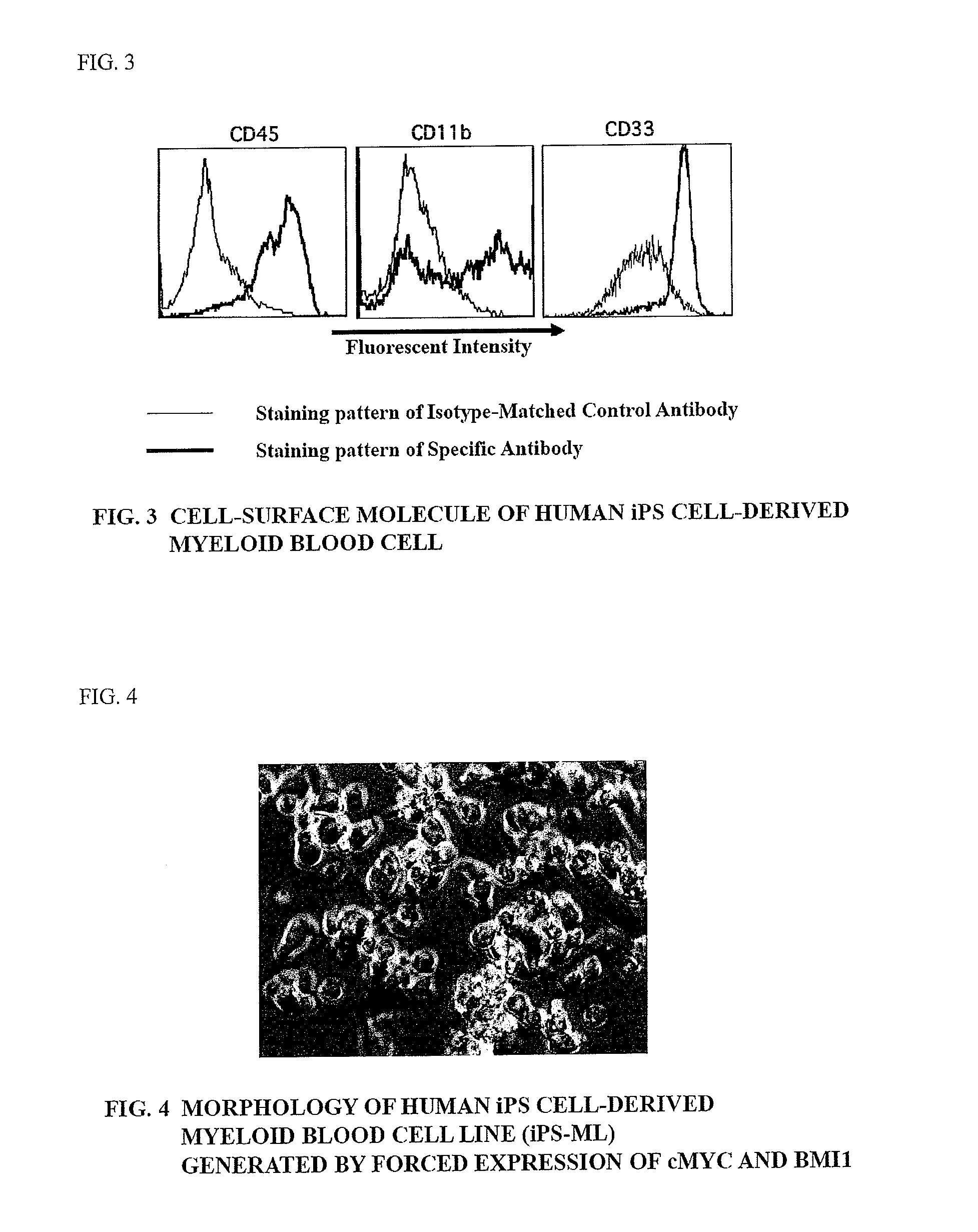
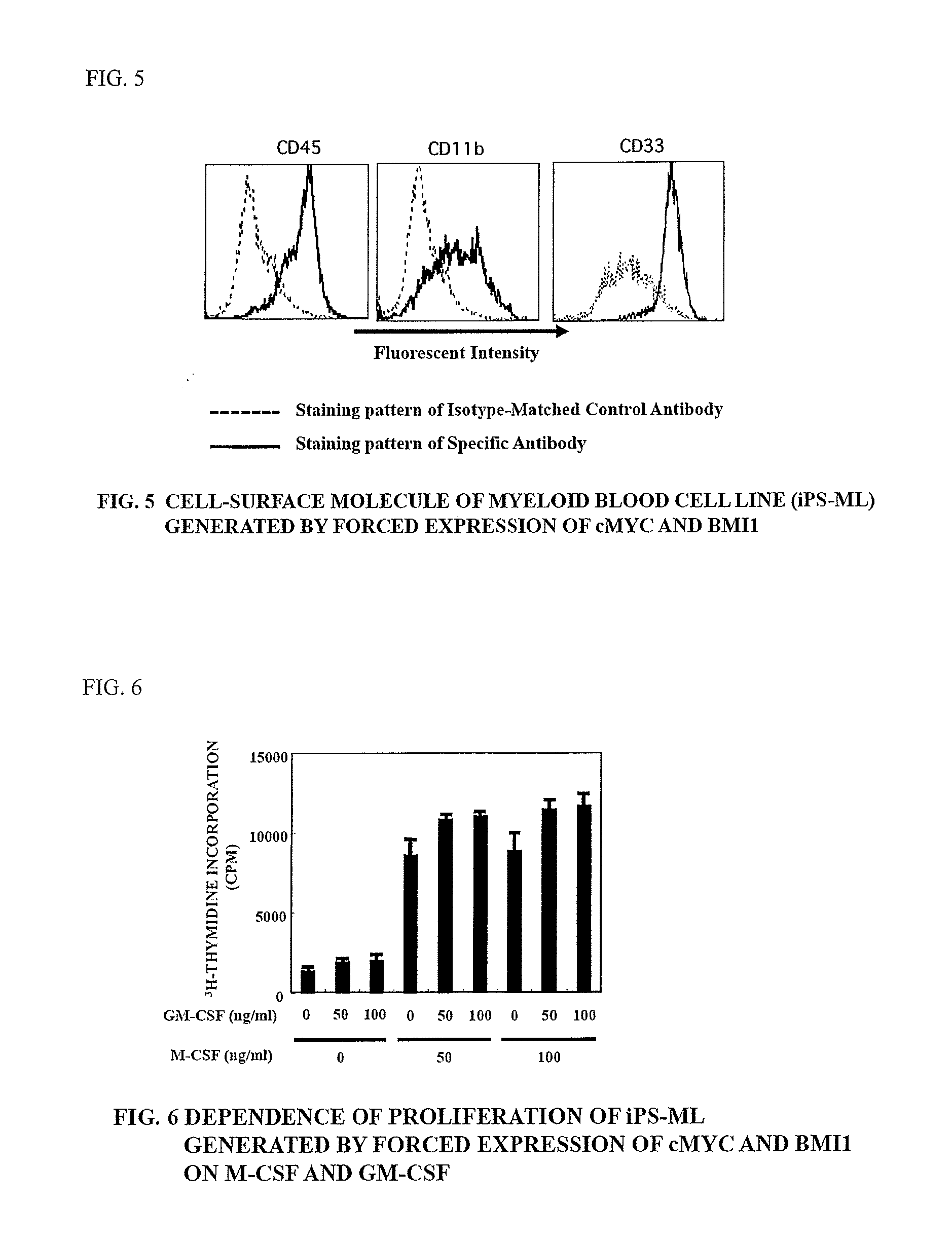
Method of producing myeloid blood cells
An object of the present invention is to provide a method of producing a myeloid blood cell possessing a proliferative capability. According to the present invention, provided is a method of producing a myeloid blood cell possessing a proliferative capability, including forcedly expressing (A) a cMYC gene, and (B) at least one gene selected from the group consisting of a BMI1 gene, an EZH2 gene, an MDM2 gene, an MDM4 gene, and an HIF1A gene in a myeloid blood cell.
Owner:NAT UNIV CORP KUMAMOTO UNIV
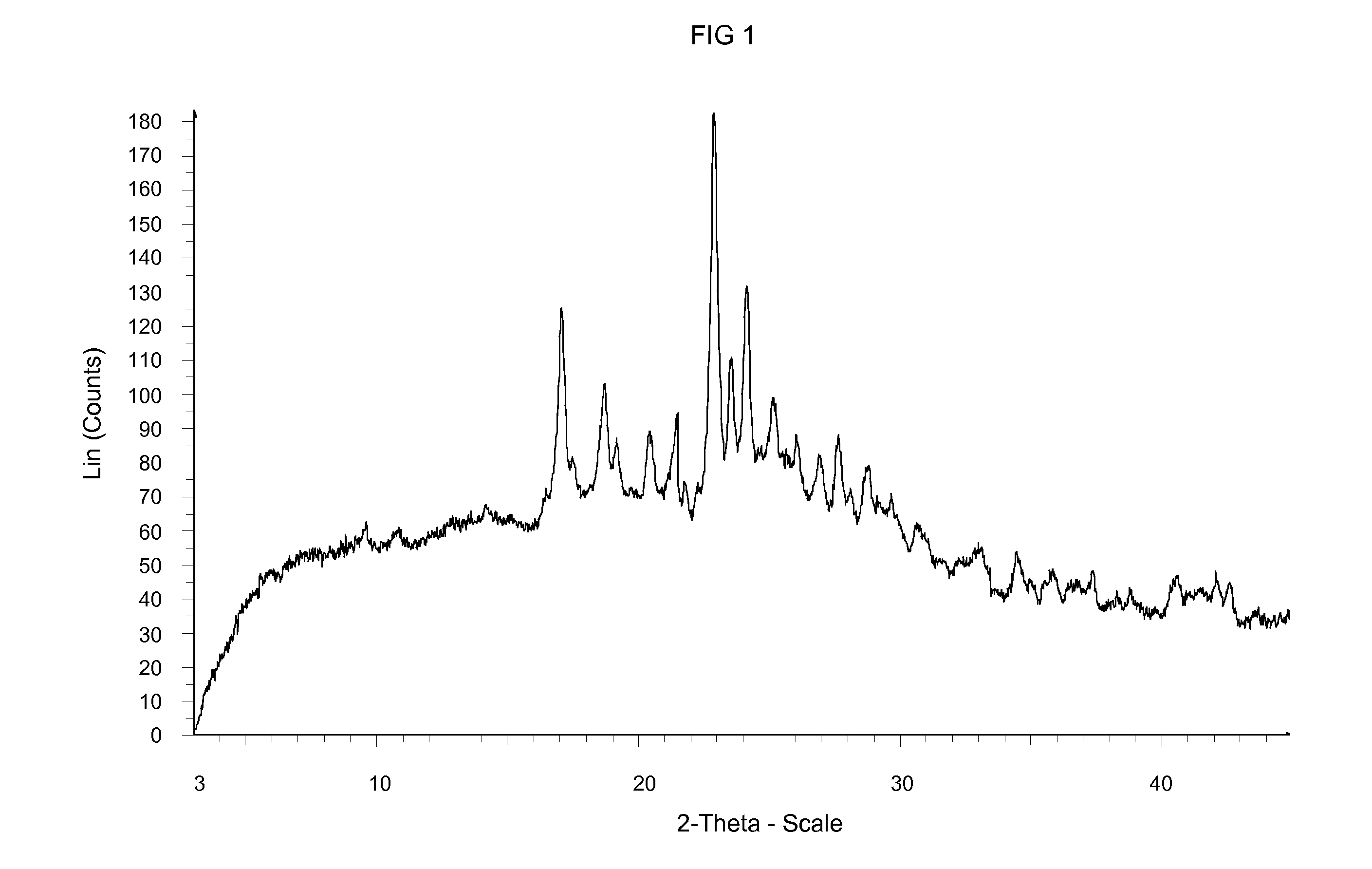
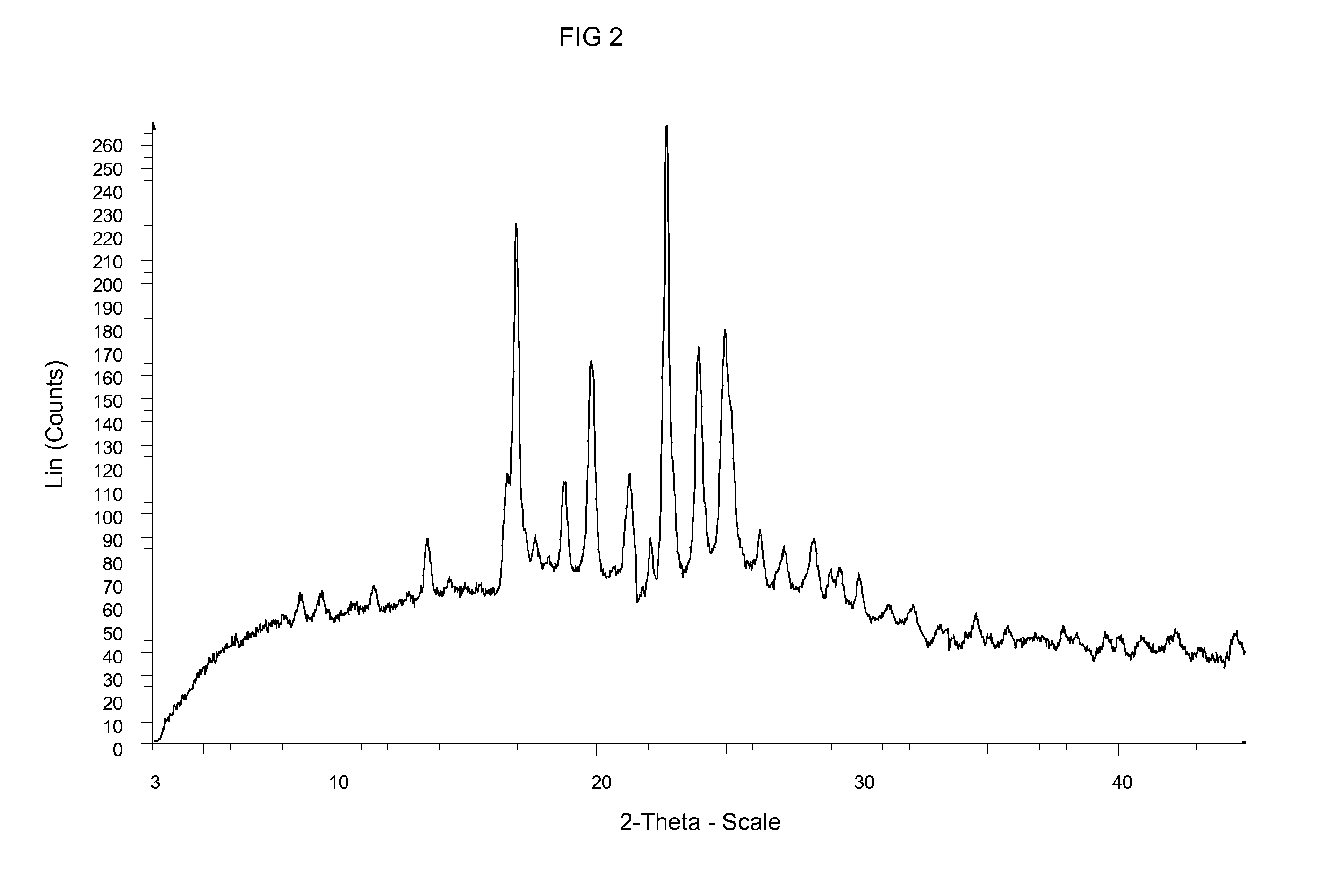
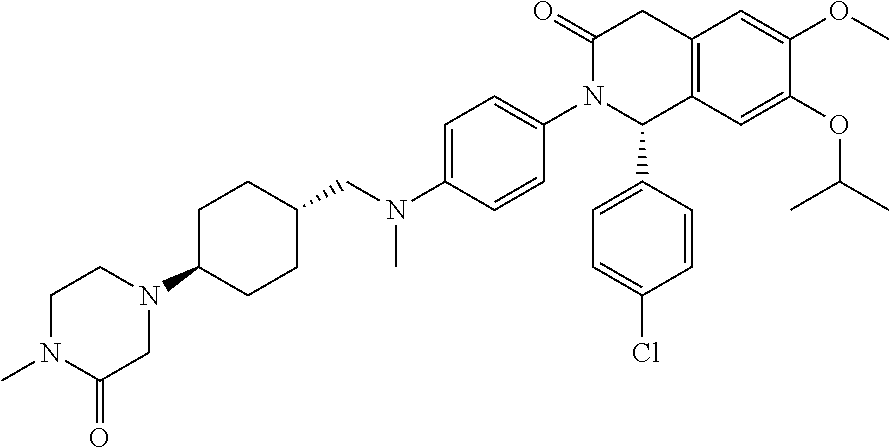
Crystalline form of an inhibitor of MDM2/4 and p53 interaction
InactiveUS20120129871A1Improve solubilityImprove stabilityOrganic active ingredientsOrganic chemistryMedicineKetone
A crystalline form of (S)-1-(4-Chloro-phenyl)-7-isopropoxy-6-methoxy-2-(4-{methyl-[4-(4-methyl-3-oxo-piperazin-1-yl)-trans-cyclohexylmethyl]-amino}-phenyl)-1,4-dihydro-2H-isoquinolin-3-one, which is useful in the treatment of a disease or disorder associated with the interaction between p53, or variants thereof, and MDM2 and / or MDM4, or variants thereof, respectively,
Owner:NOVARTIS AG
A kind of small molecule inhibitor of mdmx/mdm2 and its preparation method and application
ActiveCN103923067BAvoid interactionProduce toxic side effectsOrganic chemistryAntineoplastic agentsCancer cellSide effect
The invention relates to the technical field of medicinal chemistry, particularly discloses a small-molecule inhibitor of Mdm<X> / Mdm<2>, and also relates to a preparation method of the small-molecule inhibitor of Mdm<X> / Mdm<2>. The small-molecule inhibitor compound can inhibit the interaction of Mdm<X> protein and p53 protein and also can inhibit the interaction of Mdm<2> protein and p53 protein, has antiproliferative activity on cancer cells, and cannot generate toxic and side effects on patients. The small-molecule inhibitor compound can be combined with other therapies for use.
Owner:HUBEI UNIV OF TECH
A serum autoantibody detection kit
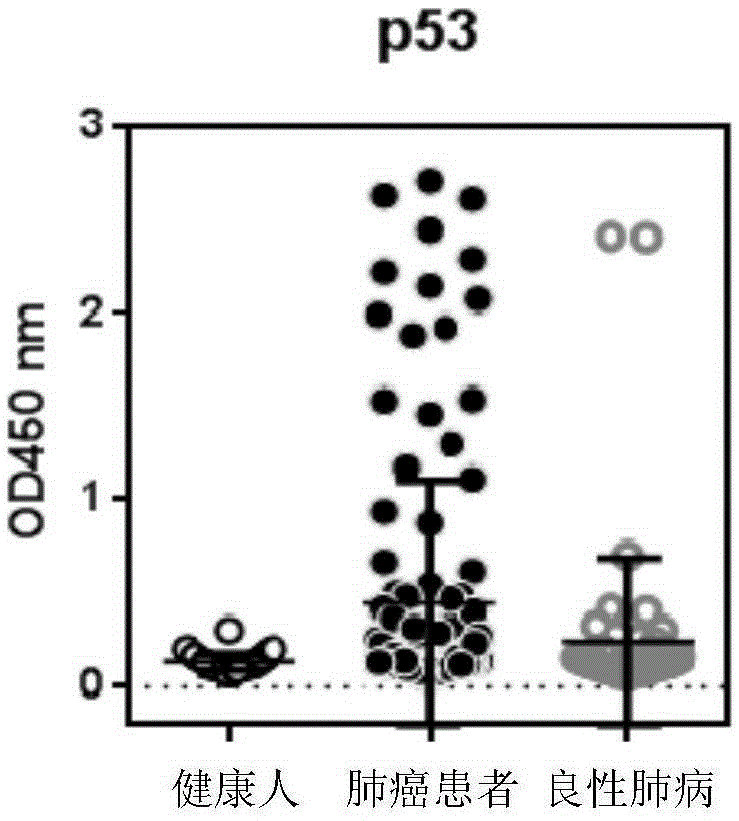
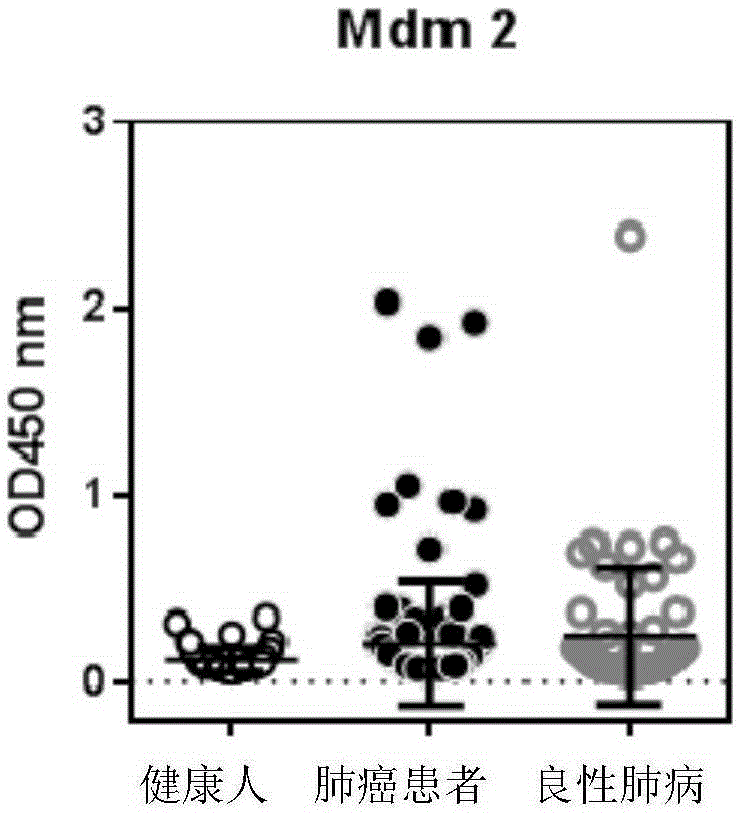
ActiveCN103869086BEfficient identificationIncreased sensitivityDisease diagnosisBiological testingCancers diagnosisSerum autoantibodies
The invention discloses a detection kit used for detecting the serum autoantibody of mammals. The detection kit comprises an antigen protein, wherein the antigen protein is a combination of five or more out of p53, Annexin1, CAGE, NY-ESO-1, HuD, Cyclin D, PGP9.5, GBU4-5, MDM2, GAGE7, XAGE1b, SOX2, MAGE A1 and MAGE A4. The detection kit adopts a group of novel antigen combination corresponding to the autoantibody of a biomarker related to cancers, utilizes biotins to form characteristics of a polymer to envelope the antigen protein, and uses an anti-human label peptide as a standard of quantitative detection, thus increasing the detection sensibility and accuracy of the serum autoantibody, and providing an optimized detection method for using the autoantibody to carry out cancer diagnosis.
Owner:HANGZHOU KAIBAOLUO BIOLOGICAL SCI & TECH
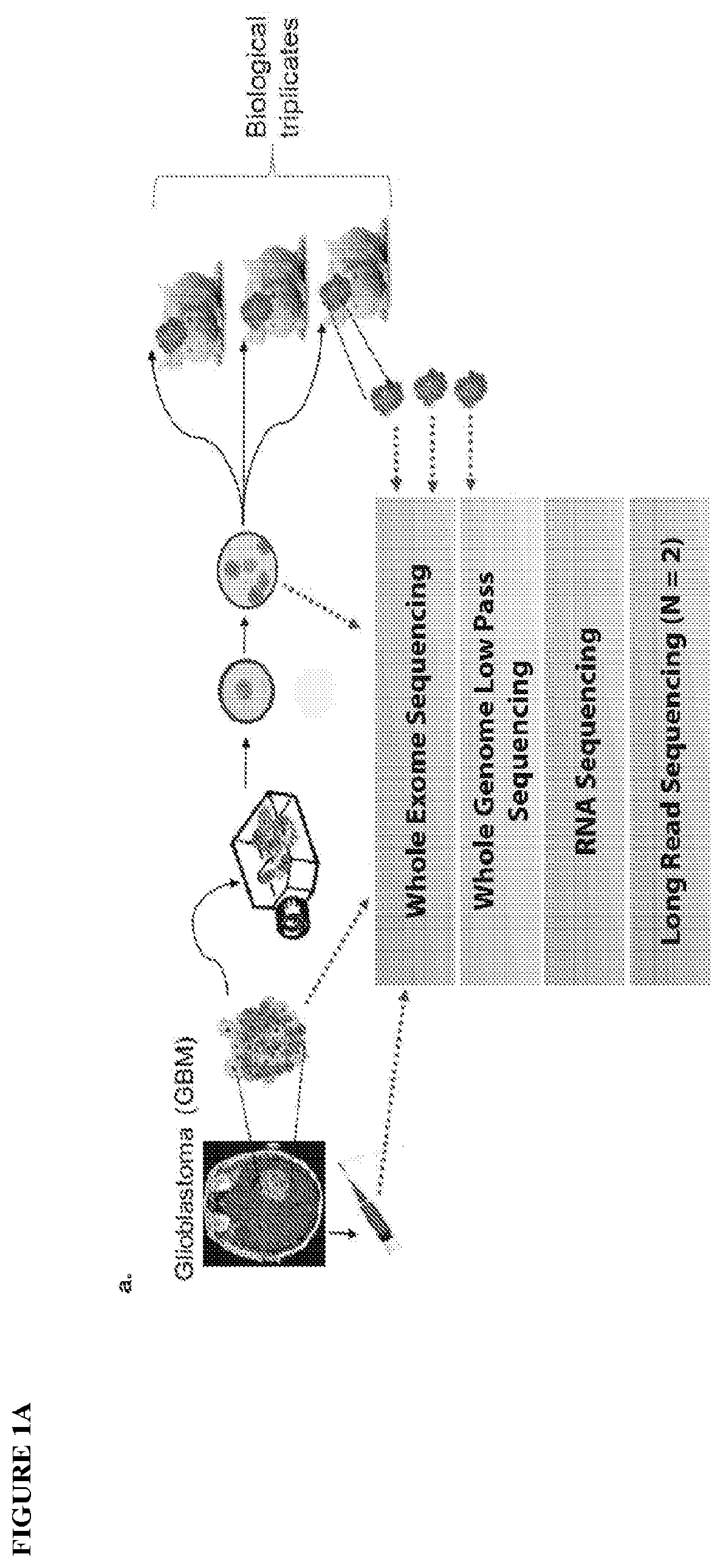
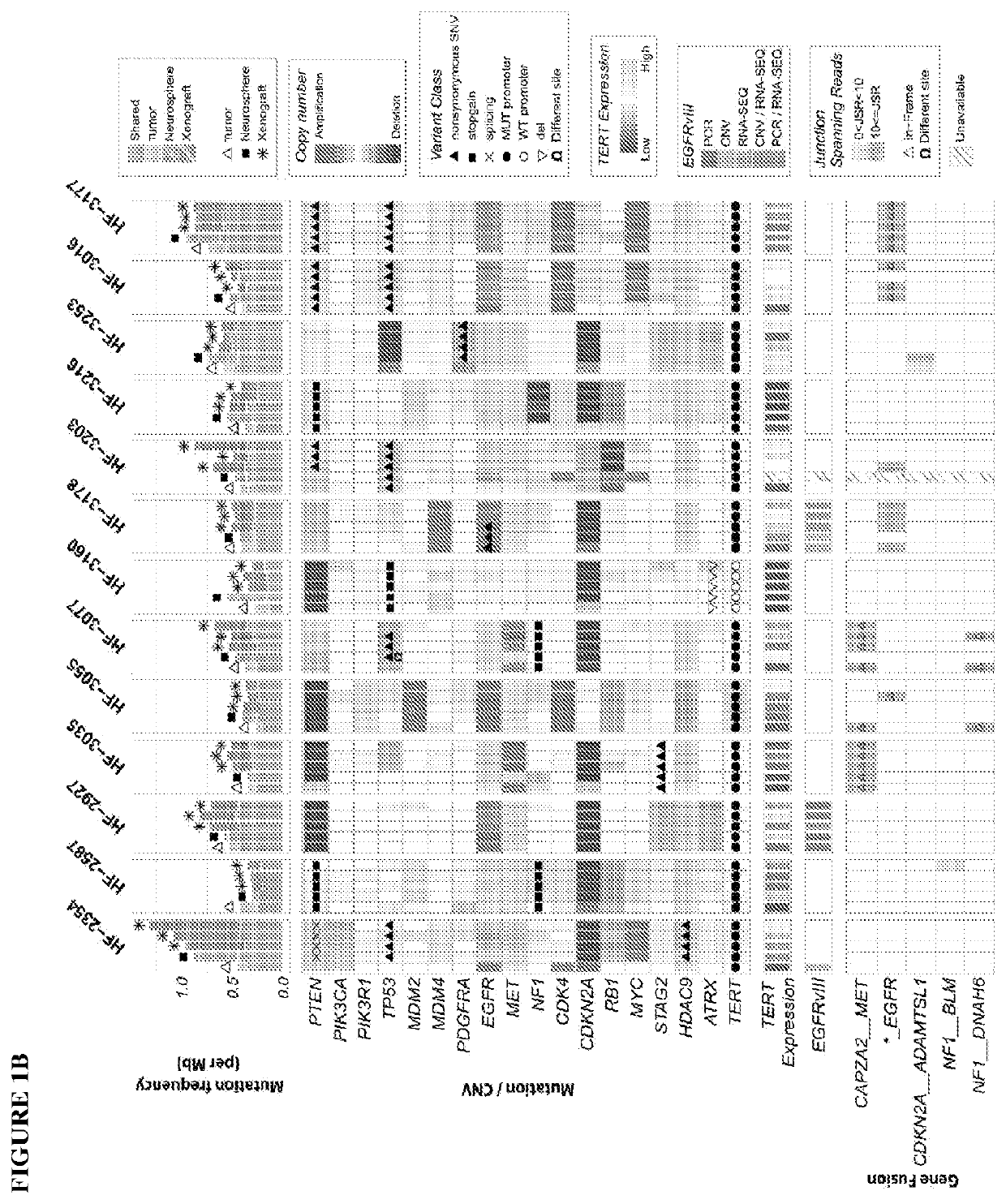
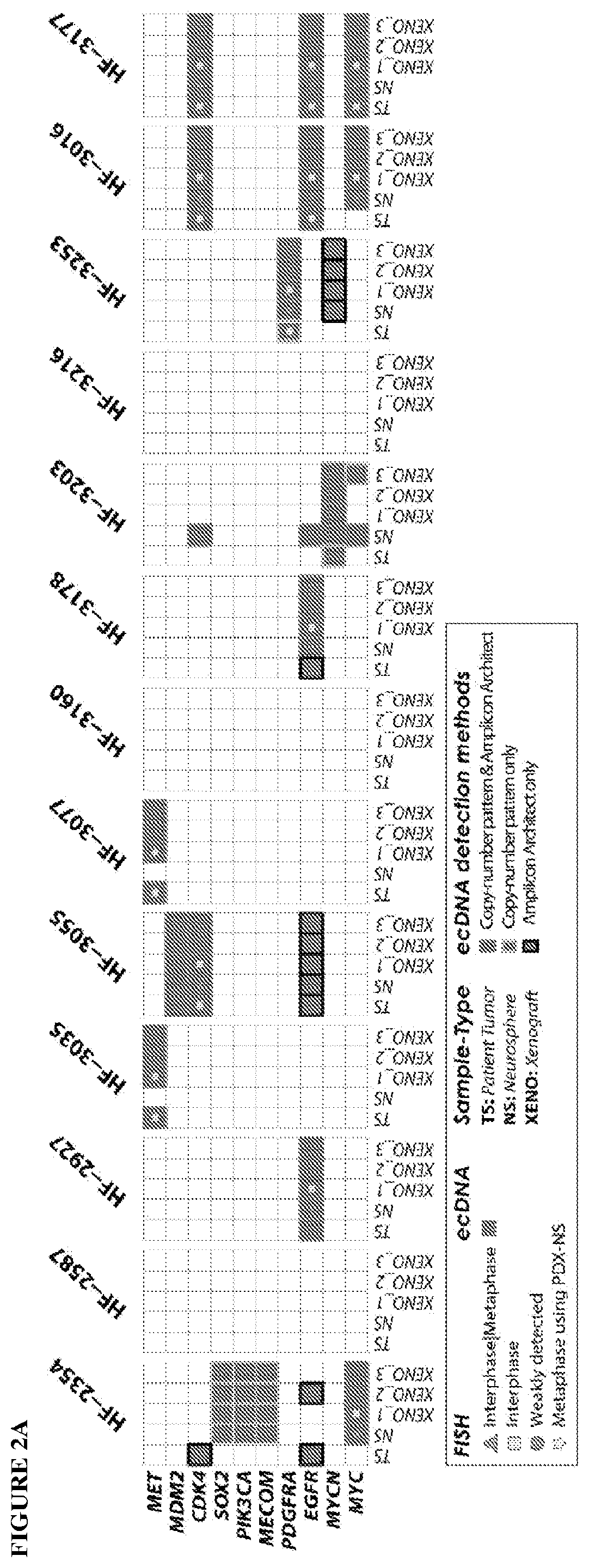
A method of targeting patient-specific oncogenes in extrachromosomal DNA to treat glioblastoma
PendingUS20190360029A1Inhibits tumor glioma growthCompounds screening/testingMicrobiological testing/measurementBAP1Present method
Provided are methods of targeting patient-specific oncogenes in extrachromosomal DNA (ecDNA) to treat glioma in a human. The present methods include identifying a drug that targets against an oncogene present in ecDNA of a human suffering from glioma, such as glioblastoma. The identified oncogenes present in ecDNA include MET, MET / CAPZA2, MDM2, CDK4, SOX2, PIK3CA, MECOM, PDGFRA, EGFR, MYCN, MYC, TERT, SMARCA4, RP56, FBXW7, CDK6, CCND2, ERBB2, BRCA1, and BAP1. The present methods include identifying a drug targeted against the ecDNA oncogene, which drug inhibits the function of the identified oncogene, so as to inhibit tumor growth or progression of the glioma in the human. Also provided are PDX mouse models to further identify and / or confirm patient-specific drugs that target the identified oncogene(s) present in ecDNA. Also provided are methods of diagnosing gliomas or recurrent gliomas and methods of screening or monitoring for recurrence of gliomas. Further provided are methods of validating a predicted presence of ecDNA in a brain tumor using fluorescence in situ hybridization (FISH). Also provided are methods of screening drug candidates for a patient by implanting different identified drugs that target an identified oncogene into PDX mouse models.
Owner:HENRY FORD HEALTH SYST
Novel N-substituted-pyrrolidines as inhibitors of MDM2-p53 interactions
Owner:F HOFFMANN LA ROCHE & CO AG
Prokaryotic expression method of oncoprotein
InactiveCN104830885AImprove experimental efficiencyReduce experiment costPeptide preparation methodsFermentationBiotechnologyEscherichia coli
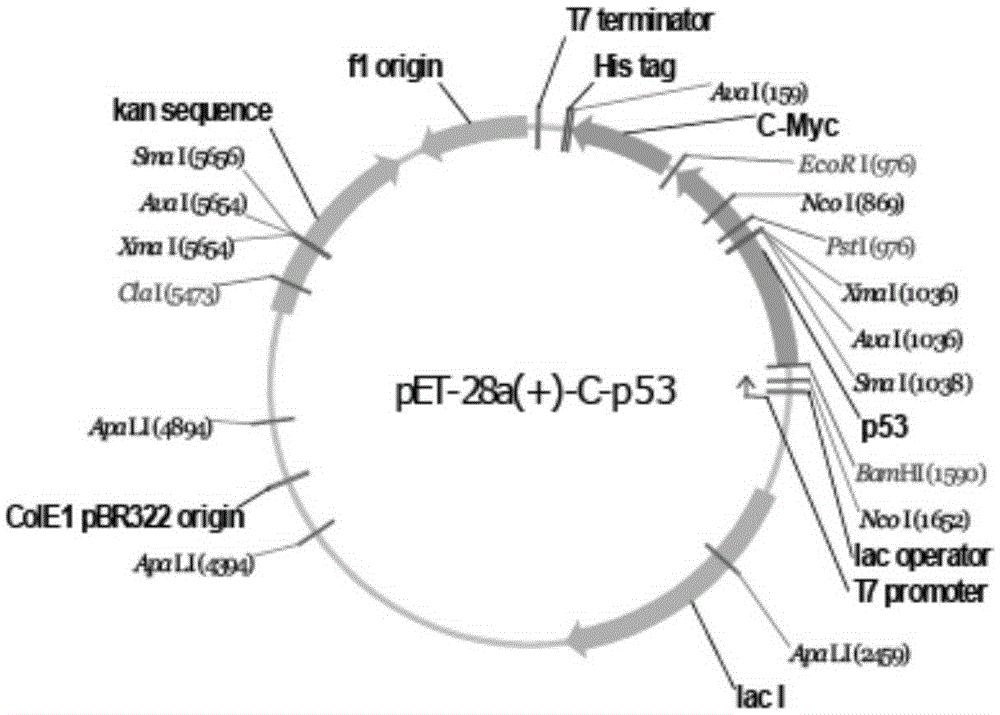
The present invention discloses a prokaryotic expression method of oncoprotein. The method comprises the steps of: extracting total RNA in liver cancer cells HepG2 and conducting reverse transcription to obtain cDNA pool; designing specific oligonucleotide primers; conducting PCR amplification by using the cDNA pool as a template and the designed primers, and then by enzyme digestion and connection to clone part of the MDM2 gene into a pET28a (+) expression vector to obtain a recombinant expression vector; introducing the recombinant expression vector into E. coli expression strains to construct genetically engineered bacteria containing the recombinant expression vector; culturing the genetically engineered bacteria at 37 DEG C to OD600 at about 0.6-0.8, and inducing the expression of the recombinant MDM2 gene in the genetically engineered bacteria; centrifuging, collecting thalli, washing twice, centrifuging, discarding the supernatant and collecting thalli. The invention greatly increases the experiment efficiency of laboratory research based on MDM2 protein, and greatly reduces experiment cost.
Owner:SHENYANG PHARMA UNIVERSITY
Method of producing myeloid blood cells
An object of the present invention is to provide a method of producing a myeloid blood cell possessing a proliferative capability. According to the present invention, provided is a method of producing a myeloid blood cell possessing a proliferative capability, including forcedly expressing (A) a cMYC gene, and (B) at least one gene selected from the group consisting of a BMI1 gene, an EZH2 gene, an MDM2 gene, an MDM4 gene, and an HIF1A gene in a myeloid blood cell.
Owner:NAT UNIV CORP KUMAMOTO UNIV
ARF-BP1 as mediator of p53-dependent and independent tumor suppression and uses thereof
The present invention relates to the mechanism of ARF-mediated cell growth suppression. ARF-BP1 is identified as a novel ubiquitin ligase, and a major component of ARF-containing nuclear complexes in human cells. The present invention discloses a novel mechanism of ARF-mediated p53 activation and that ARF-BP1 is a critical mediator of both p53-independent and p53-dependent tumor suppression functions of ARF. Inactivation of ARF-BP1 in normal cells stabilizes p53 and induces p53-dependent apoptosis. Inactivation of ARF-BP1, but not Mdm2, in p53-wildtype cells promotes cell growth inhibition in a manner reminiscent of ARF induction. ARF-BP1 directly binds and ubiquitinates p53 and inactivation of endogenous ARF-BP1 is crucial for ARF-mediated p53 stabilization in Mdm2-null cells. ARF-BP1 is advantageous over Mdm2 as a target for suppressing tumor cell growth regardless of p53 status.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Inhibitor by virtue of interaction of P53-MDM2 and applications of inhibitor
InactiveCN104188976AInhibitory activityPromote apoptosisOrganic active ingredientsAntineoplastic agentsGanoderic acid TPharmacometrics
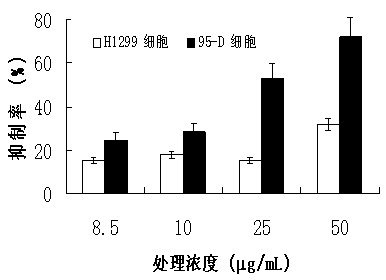
The invention discloses an inhibitor by virtue of interaction of P53-MDM2 and applications of the inhibitor. The inhibitor by virtue of interaction of P53-MDM2 is ganoderic acid X, ganoderic acid Me, ganoderic acid Y, ganoderic acid T or ganoderic acid F or a composition formed by combining one of ganoderic acid X, ganoderic acid Me, ganoderic acid Y, ganoderic acid T and ganoderic acid F with pharmacologically acceptable additive components. The inhibitor by virtue of interaction of P53-MDM2, disclosed by the invention, is applied to promotion of the apoptosis of tumor cells and particularly applied to promotion of the apoptosis of the 95-D lung cancer cells expressing P53 proteins or H1299 lung cancer cells not expressing the P53 proteins; the inhibitor by virtue of interaction of P53-MDM2, disclosed by the invention, has the effects of remarkably inhibiting the activity of MDM2 in human malignant lung cancer cells, thereby inhibiting the degradation of the P53 proteins and further promoting the apoptosis of the tumor cells containing P53.
Owner:SHANGHAI INST OF TECH
Combination therapy of an afucosylated cd20 antibody with a mdm2 inhibitor
InactiveUS20140140988A1Enhanced antiproliferative effectGood effectOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsFucosylationMdm2 Protein
The present invention is directed to the combination therapy of an afucosylated anti-CD20 antibody with a MDM2 inhibitor for the treatment of cancer, especially to the combination therapy of CD20 expressing cancers with an afucosylated humanized B-Ly1 antibody and a MDM2 inhibitor.
Owner:ROCHE GLYCART AG
Peptides and methods for treating cancer
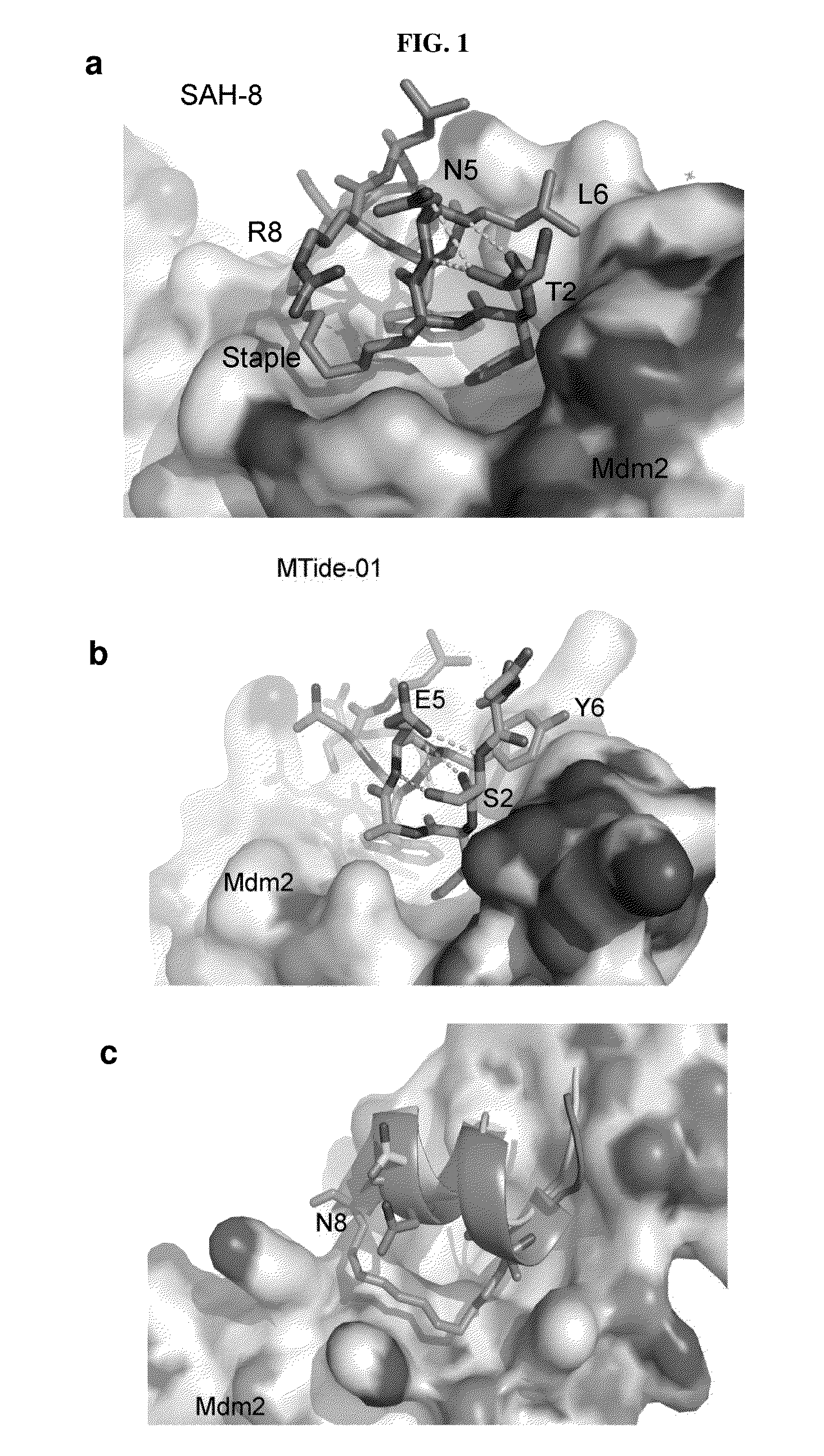
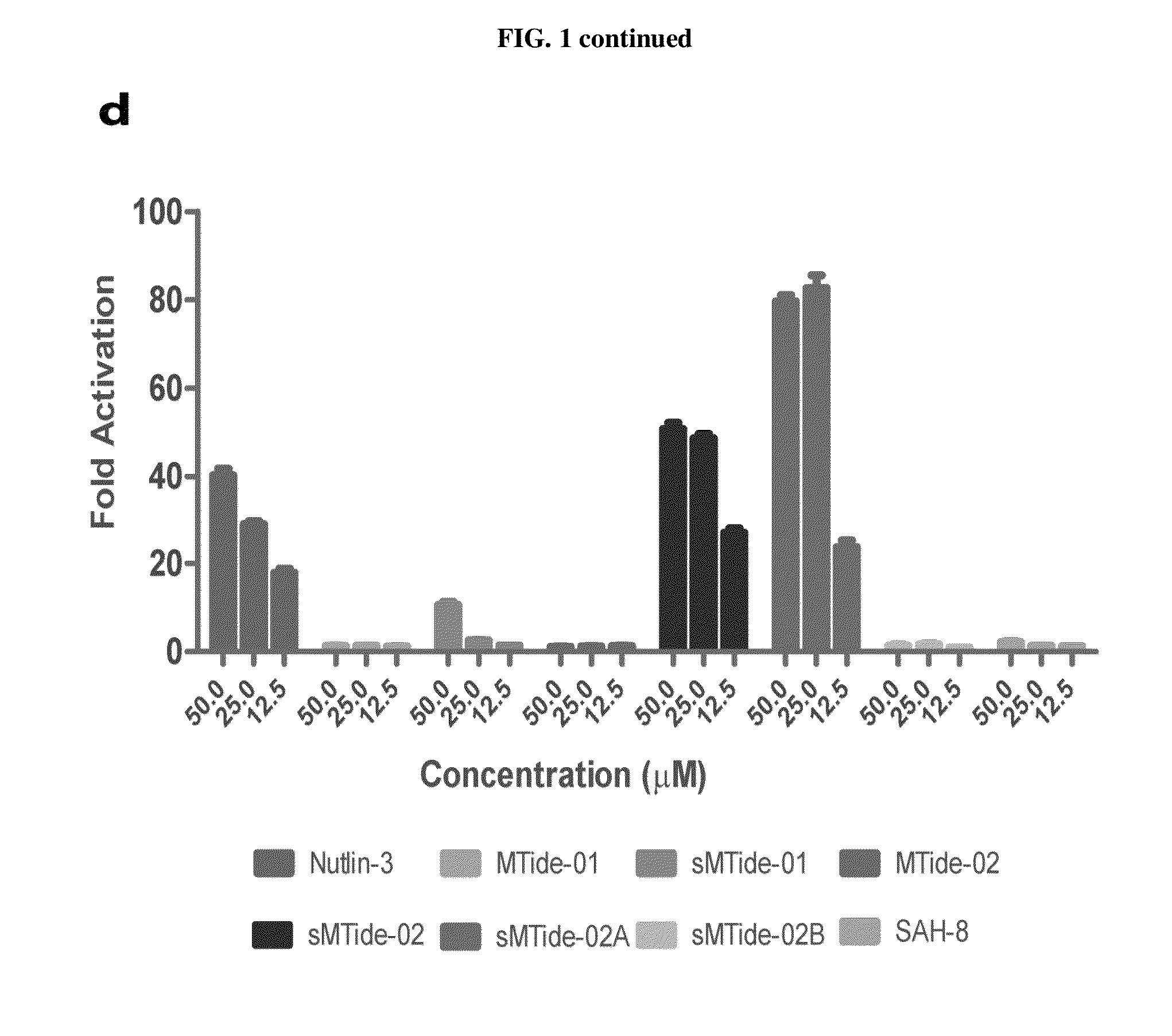
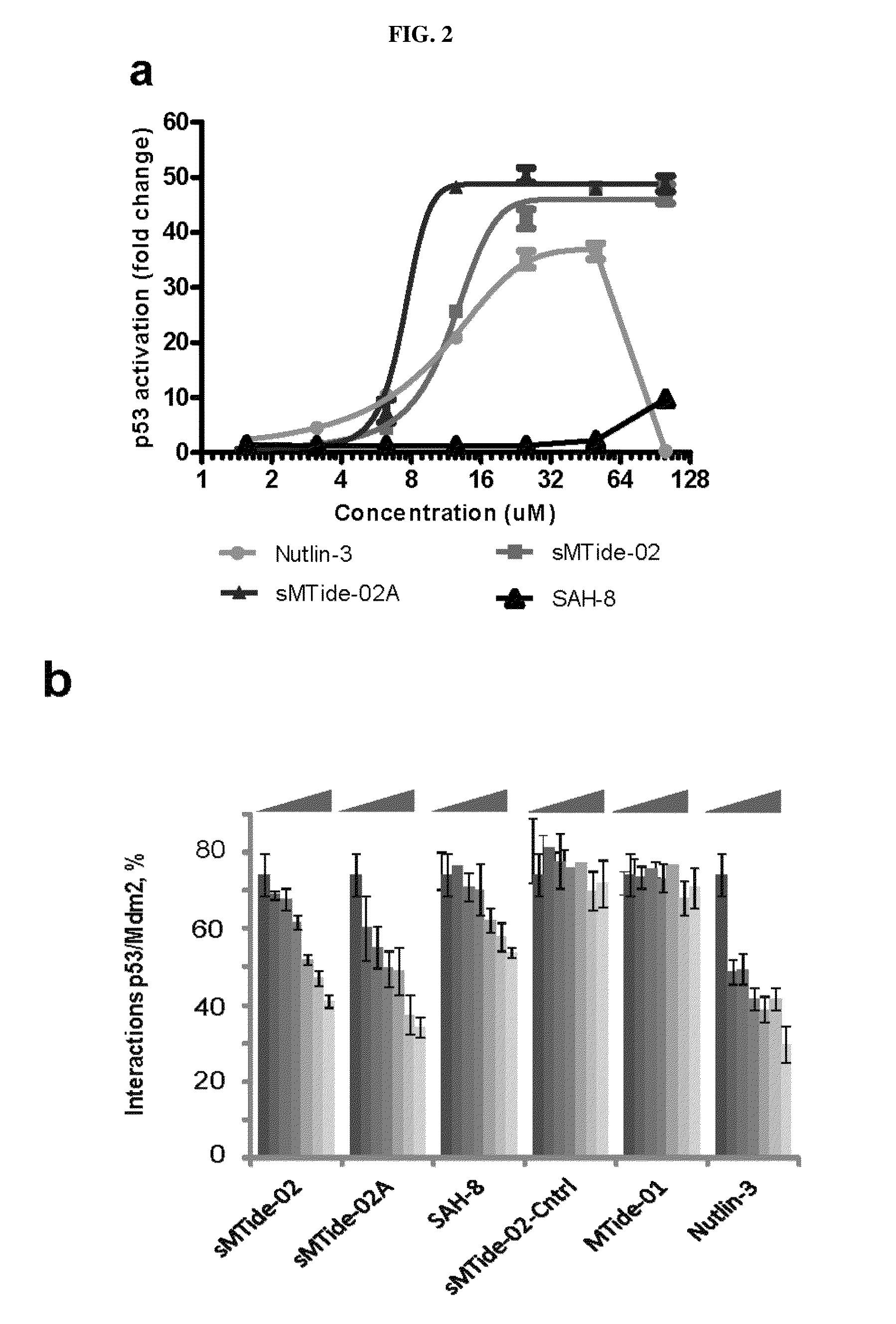
By using a phage display derived peptide as an initial template, compounds have been developed that are highly specific against Mdm2 / Mdm4. These compounds exhibit greater potency in p53 activation and protein-protein interaction assays than a compound derived from the p53 wild-type sequence. Unlike nutlin, a small molecule inhibitor of Mdm2 / Mdm4, the phage derived compounds can arrest cells resistant to p53 induced apoptosis over a wide concentration range without cellular toxicity, suggesting they are highly suitable for cyclotherapy.
Owner:AGENCY FOR SCI TECH & RES
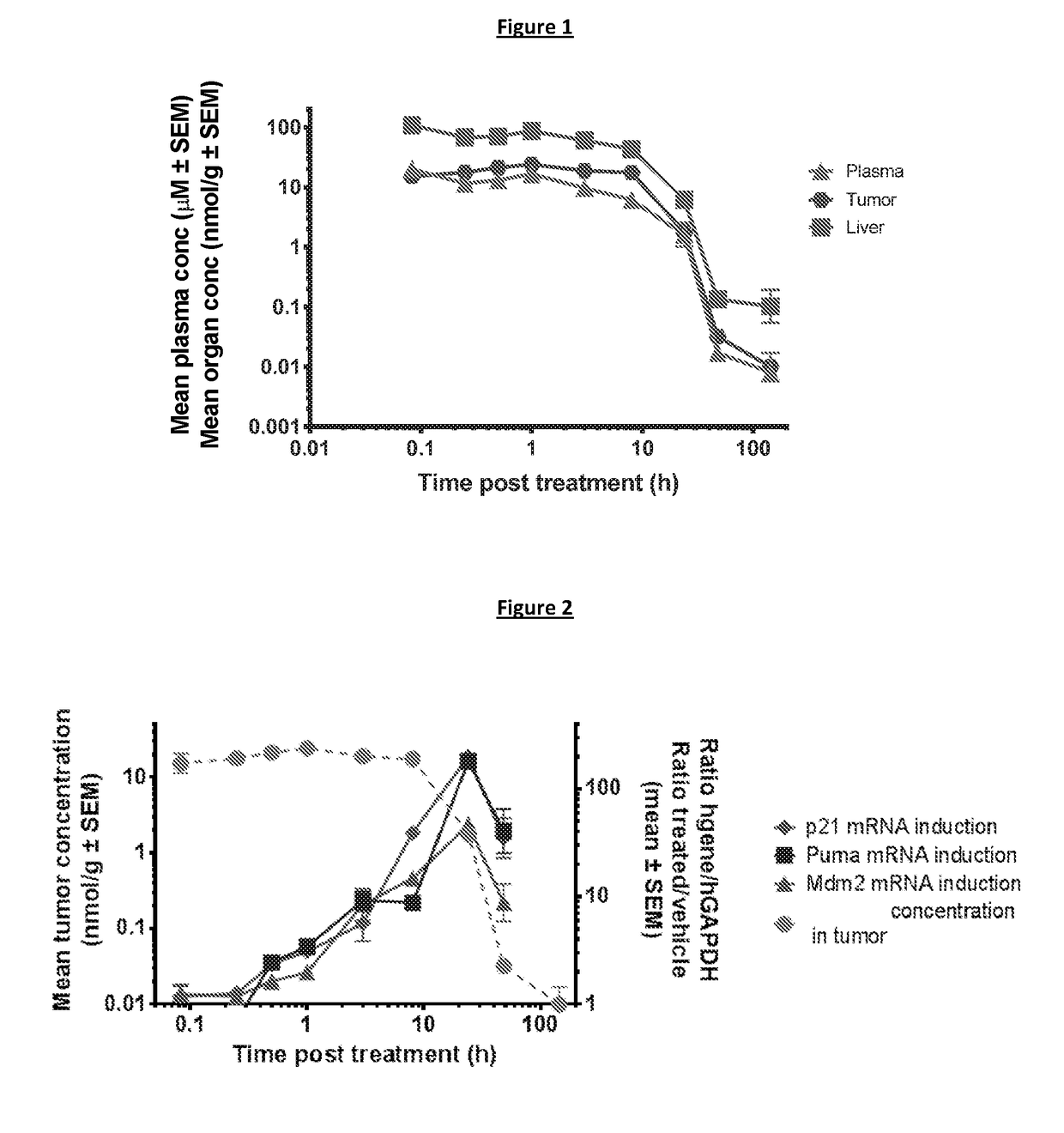
Intermittent dosing of mdm2 inhibitor
InactiveUS20170196866A1Good curative effectImprove tolerancePharmaceutical delivery mechanismAntineoplastic agentsMedicineHigh doses
The present disclosure relates to mdm2 inhibitors for use in specific dosing schedules. It was found that if sufficiently potent or, in alternative, sufficiently high dose of a Mdm2 inhibitor is used, it can cause antineoplastic effect by triggering much longer lasting antiproliferative mechanism in cells. The long lasting effect can sustain for several weeks after a single dose, which eliminates the need for daily treatment and allows administering the Mdm2i intermittently. A treatment with the intermittent dosing schedule of a Mdm2 inhibitor can be combined with a daily treatment of the Mdm2i or with another pharmaceutically acceptable ingredient.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com