CircRNA detection kit for predicting neoadjuvant chemotherapy reactivity of triple-negative breast cancer (TNBC)
A triple-negative breast cancer and kit technology, applied in the field of biomedicine, can solve problems such as poor results, enlarged lesions, and decreased physical fitness of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Predicting the composition of the neoadjuvant chemotherapy response kit for triple-negative breast cancer (50 responses)
[0015] 1. DEPC water or enzyme-free water 10ml, double distilled water 10ml;
[0016] 2. Trizol 50ml;
[0017] 3. Chloroform 100ml;
[0018] 4. Isopropanol 100ml;
[0019] 5. 1ml of 5× reverse transcription buffer;
[0020] 6. 1ml of 25mM magnesium chloride;
[0021] 7. 1ml of 10mM base triphosphate deoxynucleotide;
[0022] 8. 5U / μl RAN enzyme inhibitor 500μl;
[0023] 9. 200U / μl MMLV reverse transcriptase 50μl or 25U / μl AMV enzyme 50μl;
[0024] 10. 2ml real-time quantitative PCR buffer;
[0025] 11. 5U / μl Taq polymerase 50μl;
[0026] 12. 50 μl of 5 μM hsa_circRNA_038632 specific PCR primers;
[0027] (1) The forward primer is 5'-AGAGCTGCATCATCCTTGCA3' (SEQ ID NO: 2),
[0028] (2) The reverse primer is 5'-TCTTGTGCAGCTCCAGGAGA-3' (SEQ ID NO: 3).
[0029] 13. 5μM β-actin-specific PCR primers 30μl
[0030](1) The forward primer...
Embodiment 2
[0034] Example 2 Detection of hsa_circRNA_038632 in tissue samples
[0035] 1. Extract tissue RNA
[0036] Take the tissue sample and add liquid nitrogen to the mortar to grind the sample; add 0.6ml Trizol to the mortar to grind the sample, grind it into a homogenous slurry and add it to the tube with a medicine spoon; add 0.4ml Trizol to the tube; add chloroform 200μl / ml Trizol In the tube, shake by hand for 15-30s, place on ice for 5min, centrifuge at 12000g at 4°C for 15min; carefully take the upper aqueous phase into a new tube, add pre-cooled isopropanol 0.5ml / ml Trizol and mix well, store in -20°C refrigerator Stand still for 20min, centrifuge at 12000g at 4°C for 10min; discard the supernatant, add 1-2ml of ethanol diluted with 75% DEPC water and mix well, centrifuge at 7500g at 4°C for 5min, discard the supernatant as much as possible, dry at room temperature for 5-10min, add DEPC water for 10- 20 μl of dissolved RNA. The concentration and quality of RNA were measure...
Embodiment 3
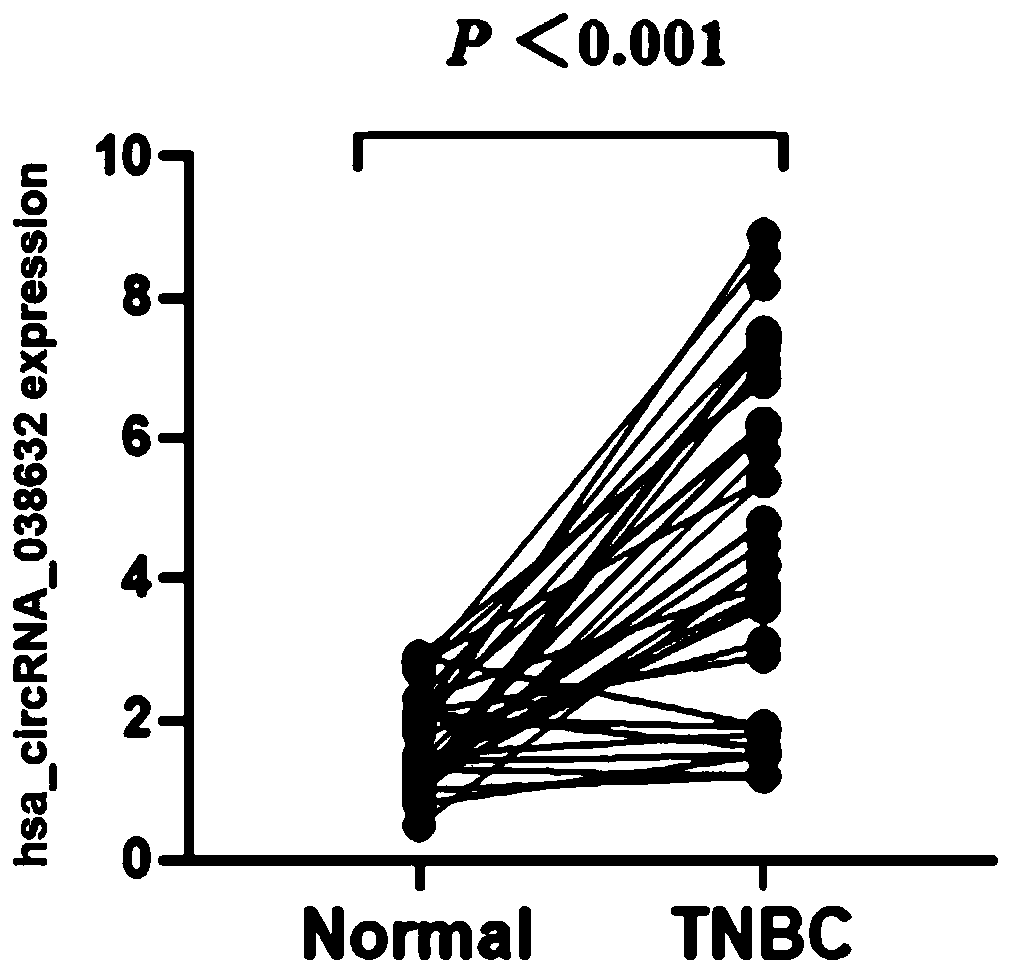
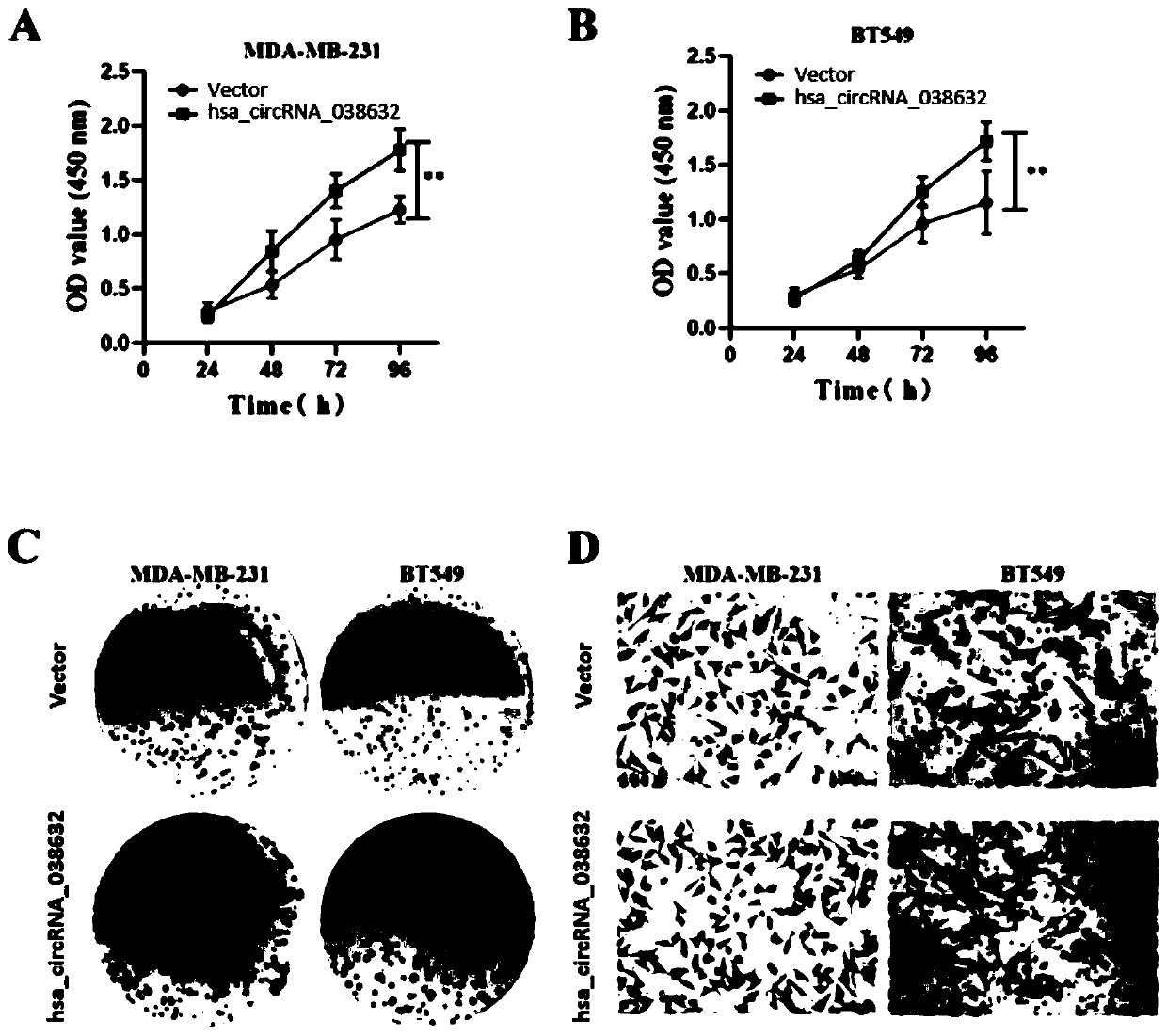
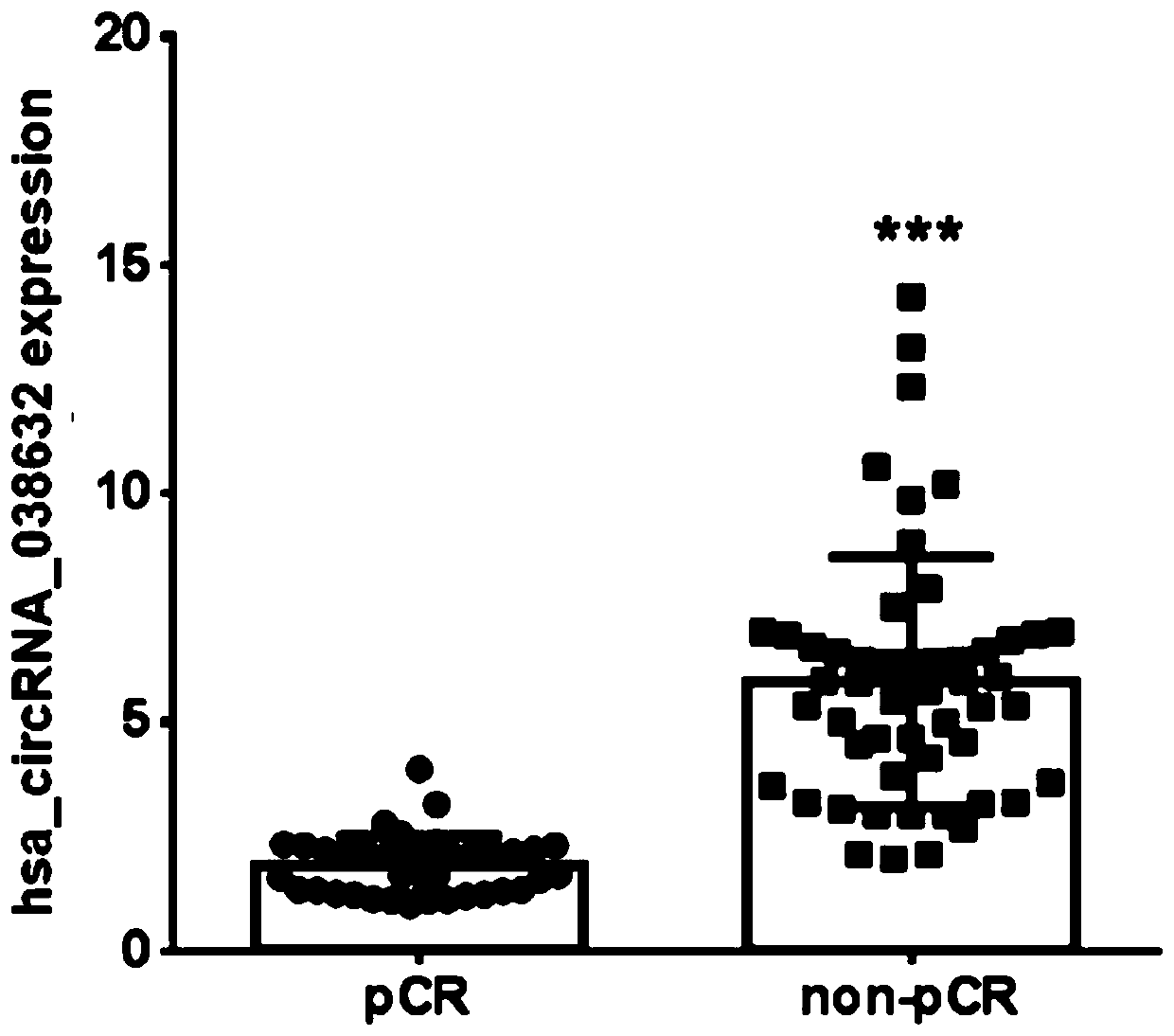
[0049] Example 3 Relationship between hsa_circRNA_038632 and responsiveness to neoadjuvant chemotherapy in patients with triple-negative breast cancer
[0050] The inventor detected the expression of hsa_circRNA_038632 in tumor tissues of 89 triple-negative breast cancer patients receiving neoadjuvant chemotherapy before treatment, and then performed postoperative pathological evaluation on each detected triple-negative breast cancer patient. According to the International Union Against Cancer (UICC) general efficacy evaluation criteria for solid tumors. Patients with curative effect pCR were defined as chemotherapy effective, and non-pCR patients were defined as chemotherapy ineffective / less sensitive. The included patients were all female, all of them were diagnosed as invasive breast cancer by hollow needle aspiration, and the pathological and immunohistochemical results showed triple negative type. Imaging tests confirmed no distant metastasis. All patients received 6 cy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com