Crisaborole crystal form compound and preparation method thereof
A technology for crisaborol and compounds, which is applied in the field of crisaborol crystal compounds and their preparation, can solve the problems of difficult control of crystal particle size and crystal form changes, etc., and achieve suitable for large-scale industrial production, good stability, The effect of mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
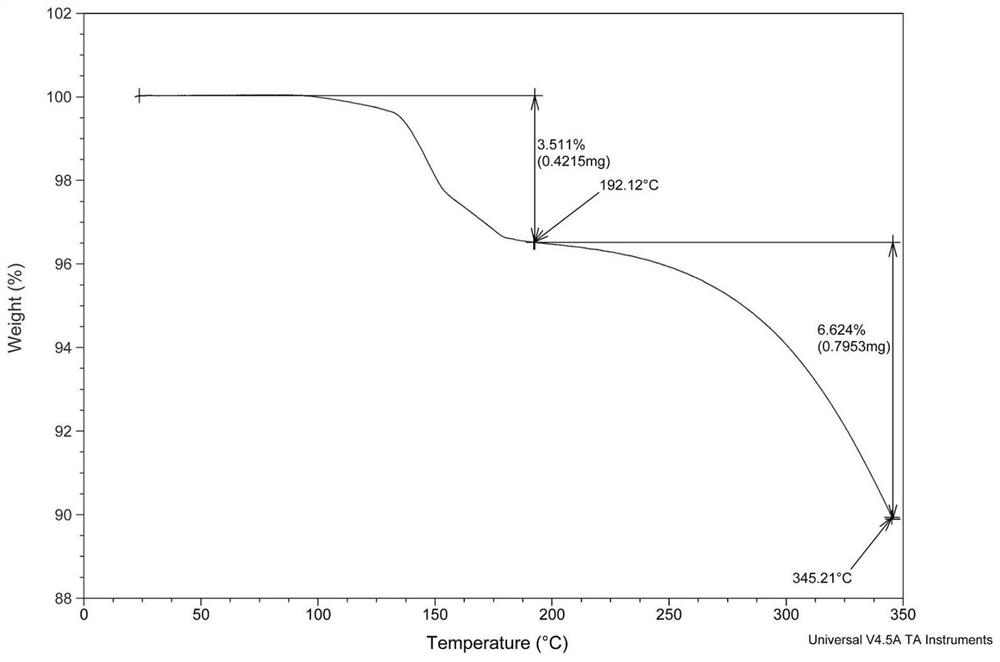
Image
Examples
Embodiment
[0038] Embodiment: Preparation of crisborole crystal compound
[0039] According to the method reported in patent CN10347965, 100 g of crude crisborole was prepared.
[0040] Take 20g of Criborol into a reaction bottle, add 40ml of analytically pure ethyl acetate solvent, heat to 76-82°C, stir to dissolve, then filter while hot, after filtration, the reaction heat is cooled to 20-30°C while stirring (cooling The range is 1-2°C every 10 minutes), after 2 hours of heat preservation, continue to cool down to 0-10°C, and keep the crystal for 1-3 hours. Vacuum filtration, and the filter cake was dried in a blast oven at 60°C for 8 hours to obtain 16.3 g of white crystalline solid powder.
[0041] Further illustrate the present invention by experimental example below:
experiment example 1
[0042] Experimental Example 1: Solubility Determination
[0043] Refer to the Chinese Pharmacopoeia 2015 version to determine its solubility. Method: take an appropriate amount of this product, add ethanol respectively, shake vigorously for 30 seconds every 5 minutes, and observe the dissolution within 30 minutes. The results are shown in Table 1.
[0044] Table 1 Crystal form solubility test result of the present invention
[0045]
experiment example 2
[0046] Experimental Example 2: Stability Test
[0047] In this experimental example, the stability of the crisborole crystals provided by the present invention is investigated through accelerated tests and long-term tests.
[0048] Take the samples prepared in the examples, and place them for 6 months under the conditions of temperature 40±2°C, relative humidity 75±5% and temperature 25±5°C, relative humidity 60±5%, and take samples at the end of 0, 3, and 6 months respectively Determination of properties, related substances and content, the results are shown in Table 2.
[0049] Table 2: Accelerated and long-term stability test results
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com