Compound amino acid injection and preparation method thereof
A compound amino acid and injection technology, applied in the field of medicine, can solve problems such as affecting calcium ion levels and inappropriateness, and achieve the effects of eliminating toxic and side effects, appropriate nitrogen filling level, and reasonable production equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
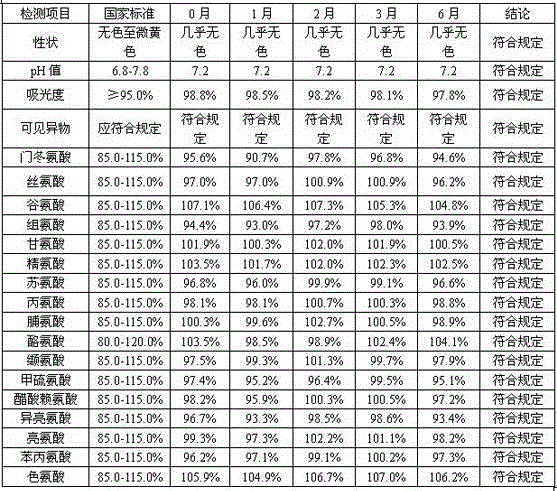
Image
Examples
Embodiment 1
[0034] Prescription: Leucine 12.9kg, Threonine 7.5kg, Isoleucine 9.1kg, Glycine 7.0kg, Valine 14.0kg, Phenylalanine 7.0kg, Tryptophan 1.3kg, Methionine 4.4kg , glutamic acid 0.5kg, alanine 7.1kg, serine 1.7kg, proline 5.0kg, tyrosine 0.4kg, lysine acetate 10.0kg, arginine 9.0kg, histidine 5.0kg, door Aspartic acid 1.0kg, cysteine 0.35kg, edetate calcium sodium 0.05kg, glacial acetic acid amount, add water to 1000000mL.
[0035] The preparation method of this compound amino acid injection comprises the following steps:
[0036] 1) Add 600,000 mL of water for injection into the concentrated tank, fill it with nitrogen for 15 minutes while heating, add calcium and sodium edetate at 100°C to dissolve, then add tyrosine, leucine, isoleucine, valine Acid and methionine were stirred and dissolved, and the heating was stopped;
[0037] 2) Under the condition of nitrogen protection, cool down the solution obtained in step 1) to 65-75°C, add phenylalanine, glutamic acid, aspartic ac...
Embodiment 2
[0042] Prescription: Leucine 11.6kg, Threonine 6.8kg, Isoleucine 8.2kg, Glycine 6.3kg, Valine 12.6kg, Phenylalanine 6.3kg, Tryptophan 1.2kg, Methionine 4.0kg , glutamic acid 0.4kg, alanine 6.4kg, serine 1.5kg, proline 4.5kg, tyrosine 0.35kg, lysine acetate 9.0kg, arginine 8.1kg, histidine 4.5kg, aspart Amino acid 0.9kg, cysteine 0.3kg, edetate calcium sodium 0.04kg, glacial acetic acid amount, add water amount to 1000000ml.
[0043] The preparation method of this compound amino acid injection comprises the following steps:
[0044] 1) Add 600,000 mL of water for injection into the concentrated tank, fill it with nitrogen for 15 minutes while heating, add calcium and sodium edetate at 100°C to dissolve, then add tyrosine, leucine, isoleucine, valine Acid and methionine were stirred and dissolved, and the heating was stopped;
[0045] 2) Under the condition of nitrogen protection, cool down the solution obtained in step 1) to 65-75°C, add phenylalanine, glutamic acid, aspart...
Embodiment 3
[0050] Prescription: Leucine 14.2kg, Threonine 8.3kg, Isoleucine 10.0kg, Glycine 7.7kg, Valine 15.4kg, Phenylalanine 7.7kg, Tryptophan 1.4kg, Methionine 4.8kg , glutamic acid 0.6kg, alanine 7.8kg, serine 1.9kg, proline 5.5kg, tyrosine 0.45kg, lysine acetate 11.0kg, arginine 9.9kg, histidine 5.5kg, asparagine Amino acid 1.1kg, cysteine 0.4kg, edetate calcium sodium 0.06kg, glacial acetic acid amount, add water amount to 1000000ml.
[0051] The preparation method of this compound amino acid injection comprises the following steps:
[0052] 1) Add 600,000 mL of water for injection into the concentrated preparation tank, fill with nitrogen for 15 minutes while heating, add calcium and sodium edetate at 100°C to dissolve, then add tyrosine, leucine, isoleucine, valine Acid and methionine were stirred and dissolved, and the heating was stopped;
[0053] 2) Under the condition of nitrogen protection, cool down the solution obtained in step 1) to 65-75°C, add phenylalanine, glutam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com