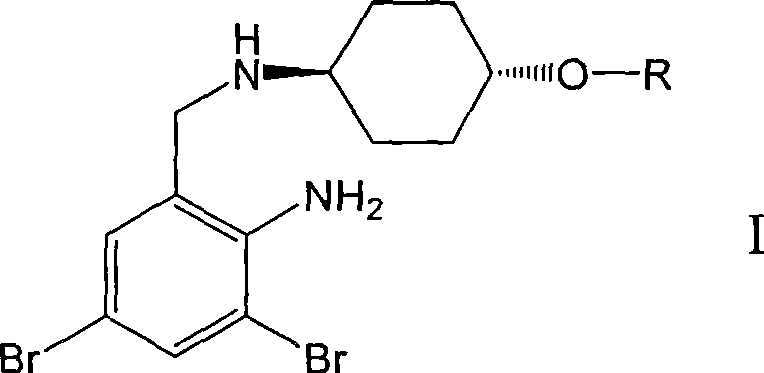
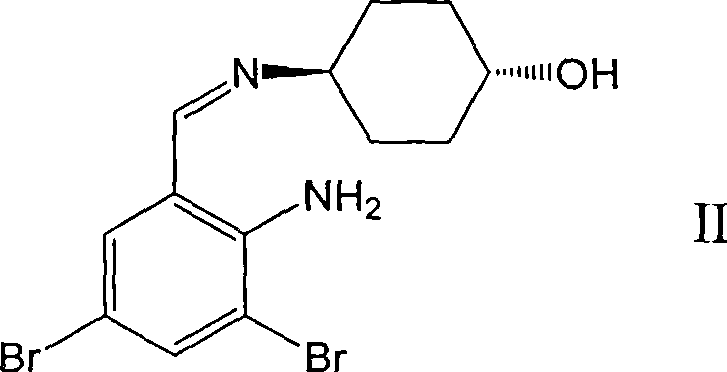
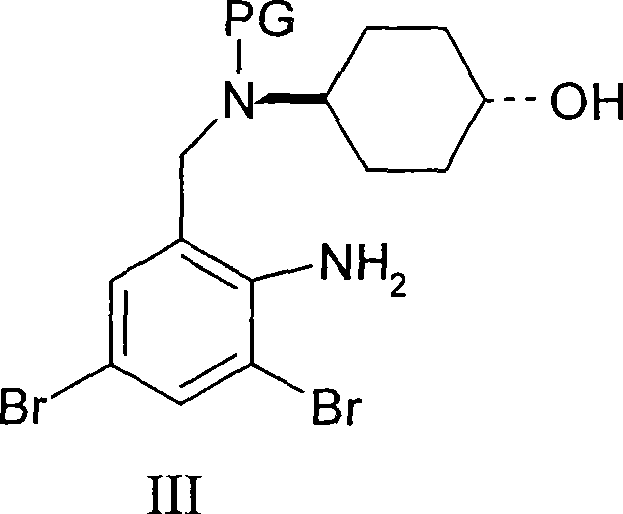
Ambroxol derivative and method for preparing same
A compound and amino acid technology, applied in the field of ambroxol derivatives and its preparation, can solve the problems of unsatisfactory water solubility of ambroxol hydrochloride, limitation of preparation application and clinical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] L-Alanine [trans-4-[(2-amino-3,5-dibromobenzyl)amino]cyclohexanol] ester dimesylate
[0023] The specific implementation is as follows:
[0024] The first step: trans-4-[(2-amino-3,5-dibromobenzyl)-N-tert-butoxycarbonylamino]cyclohexanol
[0025] Dissolve 0.16g (4mmol) of sodium hydroxide in 10ml of water, add 10ml of THF, add 1.134g (3mmol) of ambroxol, cool to 0-5°C, add 0.87g (4mmol) of di-tert-butyl dicarbonate dropwise, and keep warm after dropping Stir for 6 hours, add 30 ml of ethyl acetate to the reaction solution, separate the organic layer, wash with water, wash with brine, dry over magnesium sulfate, concentrate to remove the solvent, and obtain 1.3 g of oil, ESI-MS (m / z): 501.01 ([M +Na]).
[0026] The second step: N-tert-butoxycarbonyl-L-alanine [trans-4-[(2-amino-3,5-dibromobenzyl)-N-tert-butoxycarbonylamino]cyclohexanol ]ester
[0027] Trans-4-[(2-amino-3,5-dibromobenzyl)-N-tert-butoxycarbonylamino]cyclohexanol 3.0g (6.28mmol), N-tert-butoxycarbonyl-L...
Embodiment 2
[0031] The specific implementation is as follows:
[0032] The first step: N-tert-butoxycarbonyl-L-alanine [trans-4-[(2-amino-3,5-dibromobenzylidene) amino] cyclohexanol] ester
[0033] Trans-4-[(2-amino-3,5-dibromobenzylidene) amino]cyclohexanol 3g (8mmol), N-tert-butoxycarbonyl-L-alanine 1.89g (10mmol) In 20ml of dry dichloromethane, cool to 0-5°C, add 2.06g (10mmol) of N,N-dicyclohexylcarboimide and 0.36g (3mmol) of 4-dimethylaminopyridine, stir for 0.5 hours, and react Liquid filtration, the filtrate was washed with water, washed with brine, dried over magnesium sulfate, concentrated to remove the solvent to obtain a colorless oil, added 40ml of methanol, stirred at 0-5°C, a solid precipitated, filtered to obtain 3.5g of a white solid, mp: 116-118°C.
[0034] The second step: N-tert-butoxycarbonyl-L-alanine [trans-4-[(2-amino-3,5-dibromobenzyl) amino] cyclohexanol] ester
[0035] 4g of the above product was dissolved in a mixture of 20ml of methanol and 10ml of tetrahydr...
Embodiment 3
[0039] L-Valine [trans-4-[(2-amino-3,5-dibromobenzyl)amino]cyclohexanol] ester dimesylate
[0040] The specific implementation is as follows:
[0041] The first step: N-tert-butoxycarbonyl-L-valine [trans-4-[(2-amino-3,5-dibromobenzylidene) amino] cyclohexanol] ester
[0042] Trans-4-[(2-amino-3,5-dibromobenzylidene)amino]cyclohexanol 3g (8mmol), N-tert-butoxycarbonyl-L-valine 2.17g (10mmol) In 20ml of dry dichloromethane, cool to 0-5°C, add 2.06g (10mmol) of N,N-dicyclohexylcarboimide and 0.36g (3mmol) of 4-dimethylaminopyridine, and stir for 0.5 hours , the reaction solution was filtered, the filtrate was washed with water and brine, dried over magnesium sulfate, concentrated to remove the solvent, and 4.0 g of a colorless oil was obtained.
[0043] The second step: N-tert-butoxycarbonyl-L-valine [trans-4-[(2-amino-3,5-dibromobenzyl) amino] cyclohexanol] ester
[0044] The above oil was dissolved in a mixture of 20ml of methanol and 10ml of tetrahydrofuran, 0.8g of potassiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com