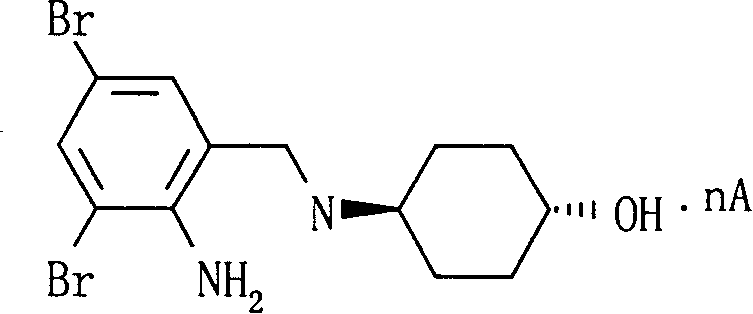
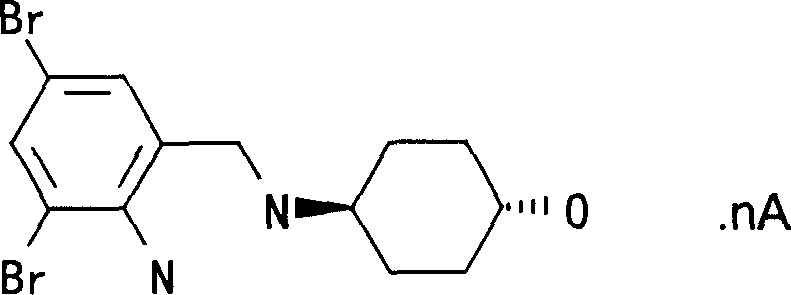
Ambroxol cysteine analogs and their preparation process and use thereof
A technology of cysteine and acetylcysteine, which is applied in the field of ambroxol cysteine analogue salt and its preparation and application, to achieve good curative effect and tolerance, improve therapeutic curative effect and reduce dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of ambroxol N-acetyl cysteine salt
[0028] In a 2000ml reaction flask, put 37.8g (0.1mol) of ambroxol, 28.2g (0.1mol) of N-acetylcysteine, add 200ml of ethanol, stir to dissolve, concentrate under reduced pressure to dryness to obtain an oily substance, add dichloro Heat 1000ml of methane in a water bath to dissolve, concentrate under reduced pressure until cloudy and precipitate oily precipitate, add 400ml of diethyl ether, stir until the precipitate turns into white crystals, then concentrate and evaporate most of the solvent, then add 200ml of diethyl ether dropwise, then stir at 40°C for 2 hours , placed at room temperature for 2 hours, filtered, washed with ether, and dried under vacuum at 50°C to obtain 46.3 g of white crystals with a yield of 85.6%. Melting point: 75-78°C. ( 1 HNMR (D 2O)δ: 1.18(m, 4H), 1.95(m, 4H), 2.08(s, 3H), 2.72(m, 1H), 2.95(d, 2H), 3.35(m, 1H), 3.95(s, 2H), 4.60(m, 1H), 7.25(d, 1H), 7.50(d, 1H))
Embodiment 2
[0029] Embodiment 2: Ambroxol erdosteine salt
[0030] In a 500ml reaction flask, Ambroxol (37.8g, 0.1mol) and Erdosteine (24.9g, 0.1mol) were dissolved in 150ml absolute ethanol respectively, filtered, the two filtrates were combined, heated to reflux, Allow to cool, filter, wash with ethanol, and dry to obtain 58 g of white solid, with a yield of 92.5% and a melting point of 170-172°C. ( 1 HNMR (D 2 O), 1.20(m, 4H), δ: 1.20(m, 4H), 1.90(m, 4H), 2.13(m, 1H), 2.40(m, 1H), 2.70(m, 1H), 3.20(s , 2H), 3.25(s, 2H), 3.30(m, 1H), 3.40(m, 2H), 3.90(s, 2H), 4.50(m, 1H), 7.31(d, 1H), 7.54(d, 1H))
Embodiment 3
[0031] Embodiment 3: the preparation of ambroxol double N-acetyl cysteine salt lyophilized powder
[0032] In the 250ml reaction bottle, drop into Ambroxol 4.00g (10.6mmol), N-acetyl cysteine 3.454g (21.2mmol), add deionized water 60ml, stir and dissolve, filter with microporous membrane, freeze-dry, 7.34 g of freeze-dried powder was obtained, with a yield of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com