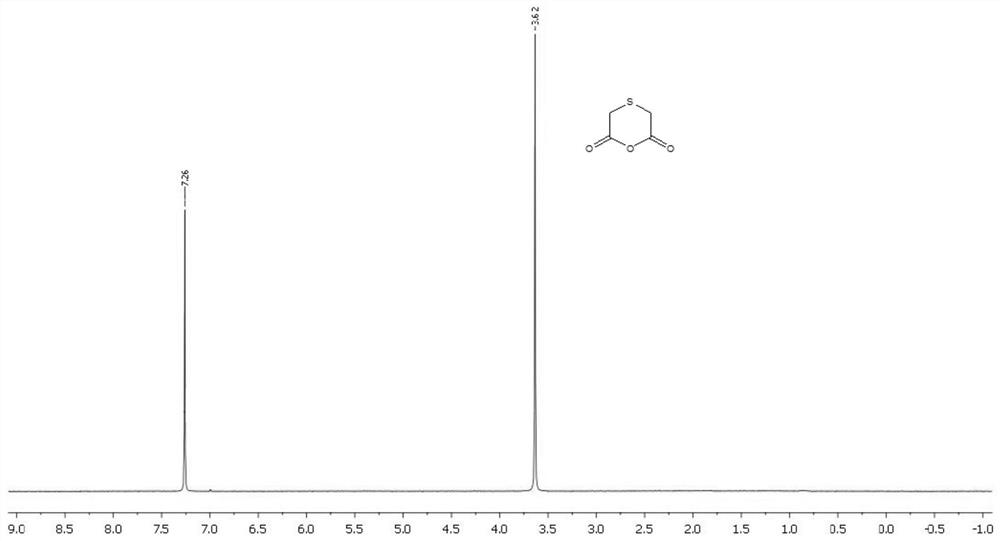
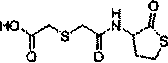Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Erdosteine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
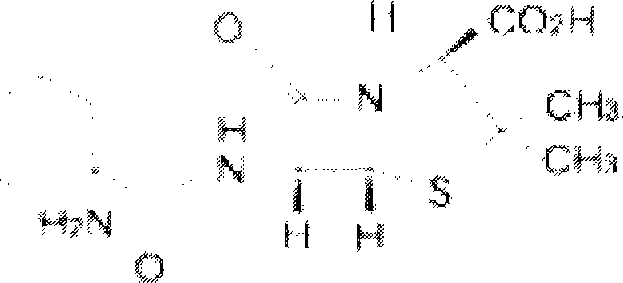
Erdosteine is a mucolytic. Specifically it is a thiol derivative developed for the treatment of chronic obstructive bronchitis, including acute infective exacerbation of chronic bronchitis. Erdosteine contains two blocked sulfhydryl groups which are released following first-pass metabolism. The three active metabolites exhibit mucolytic and free radical scavenging activity. Erdosteine modulates mucus production and viscosity and increases mucociliary transport, thereby improving expectoration. It also exhibits inhibitory activity against the effects of free radicals produced by cigarette smoke.
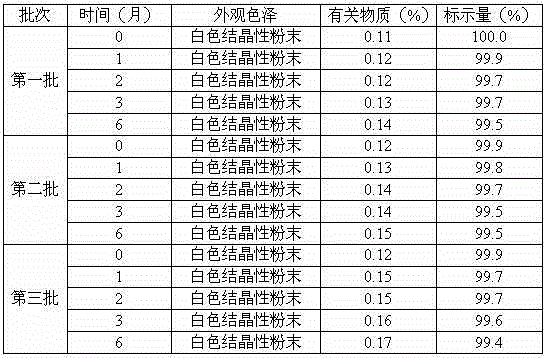
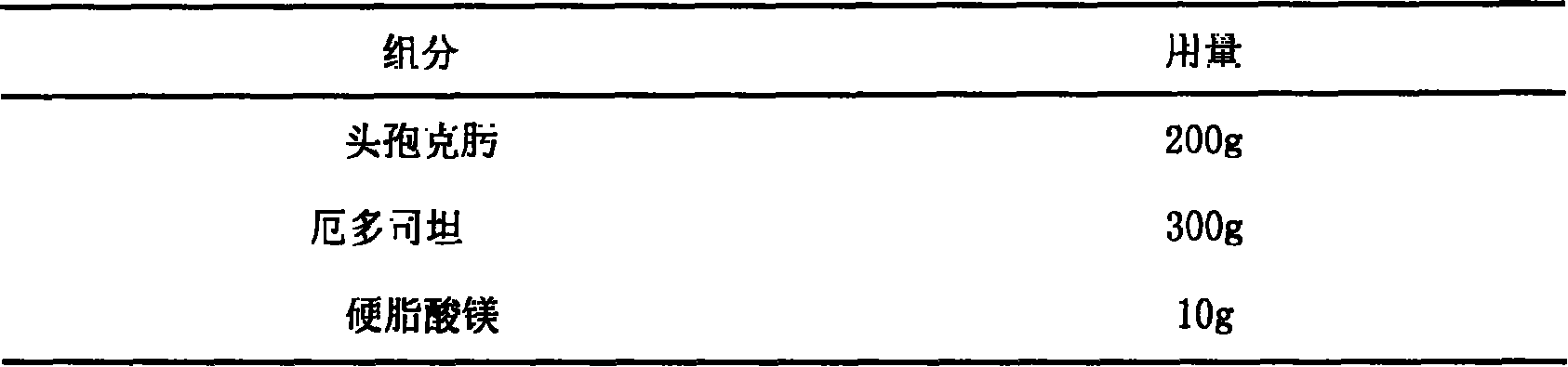
Erdosteine composition and preparation method thereof
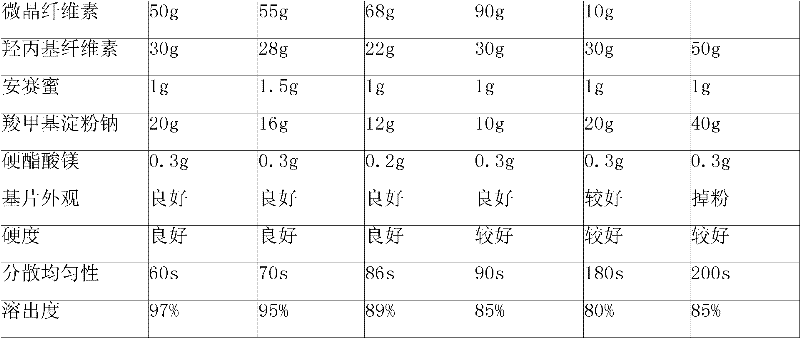
ActiveCN101606931BImprove dispersion uniformityImprove dissolution efficiencyOrganic active ingredientsPharmaceutical product form changeCarboxymethyl starchAlcohol
The invention provides an Erdosteine composition which consists of the following components by weight portions: 130 to 170 portions of Erdosteine, 35 to 65 portions of lactose, 30 to 70 portions of microcrystalline cellulose, 20 to 40 portions of low substituted hydroxypropyl cellulose, 1 to 5 portions of acesulfame, 10 to 30 portions of sodium carboxymethyl starch and 0.1 to 0.5 portions of magnesium stearate; the invention also provides a preparation method of the Erdosteine composition, comprising the following steps of: preparing materials and pelleting; mixing the Erdosteine and the low substituted hydroxypropyl cellulose evenly, then grinding, adding the sodium carboxymethyl starch, adding absolute ethyl alcohol, wet-mixing, cutting, drying, granulation and tabletting; mixing granules and microcrystalline cellulose evenly, adding the lactose, the acesulfame and the magnesium stearate for pelleting, subpackaging and obtaining the Erdosteine composition. The Erdosteine compositionhas good dispersible uniformity and high drug dissolution efficiency.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Medicine erdosteine composition granules for treating respiratory tract infection
InactiveCN104983693AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistrySucrosePharmaceutical drug
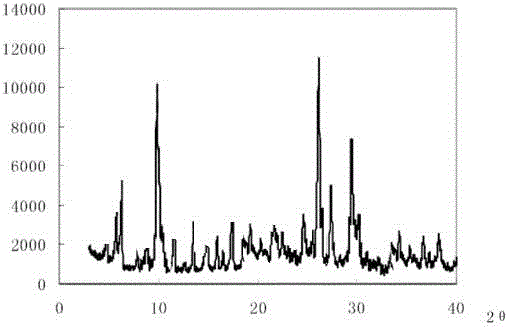
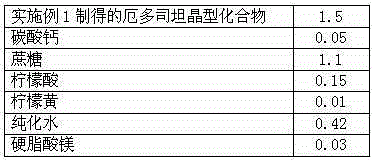
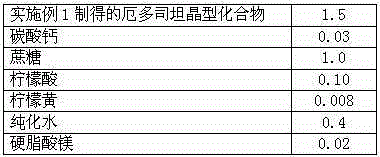
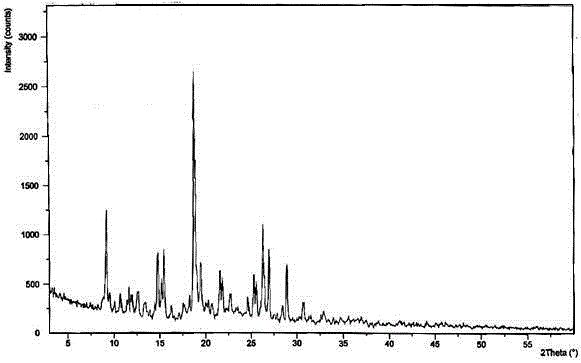
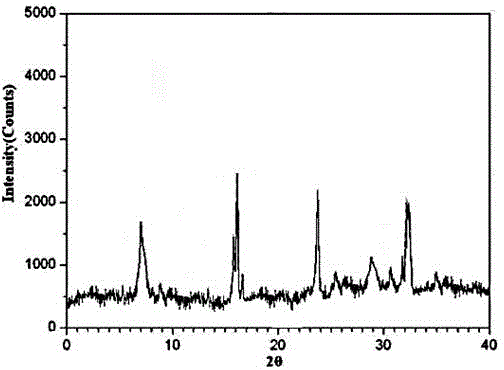
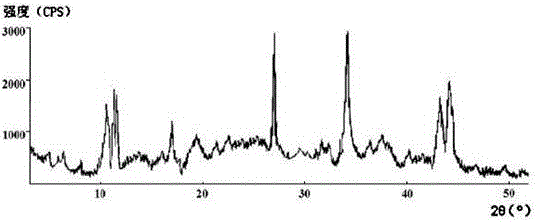
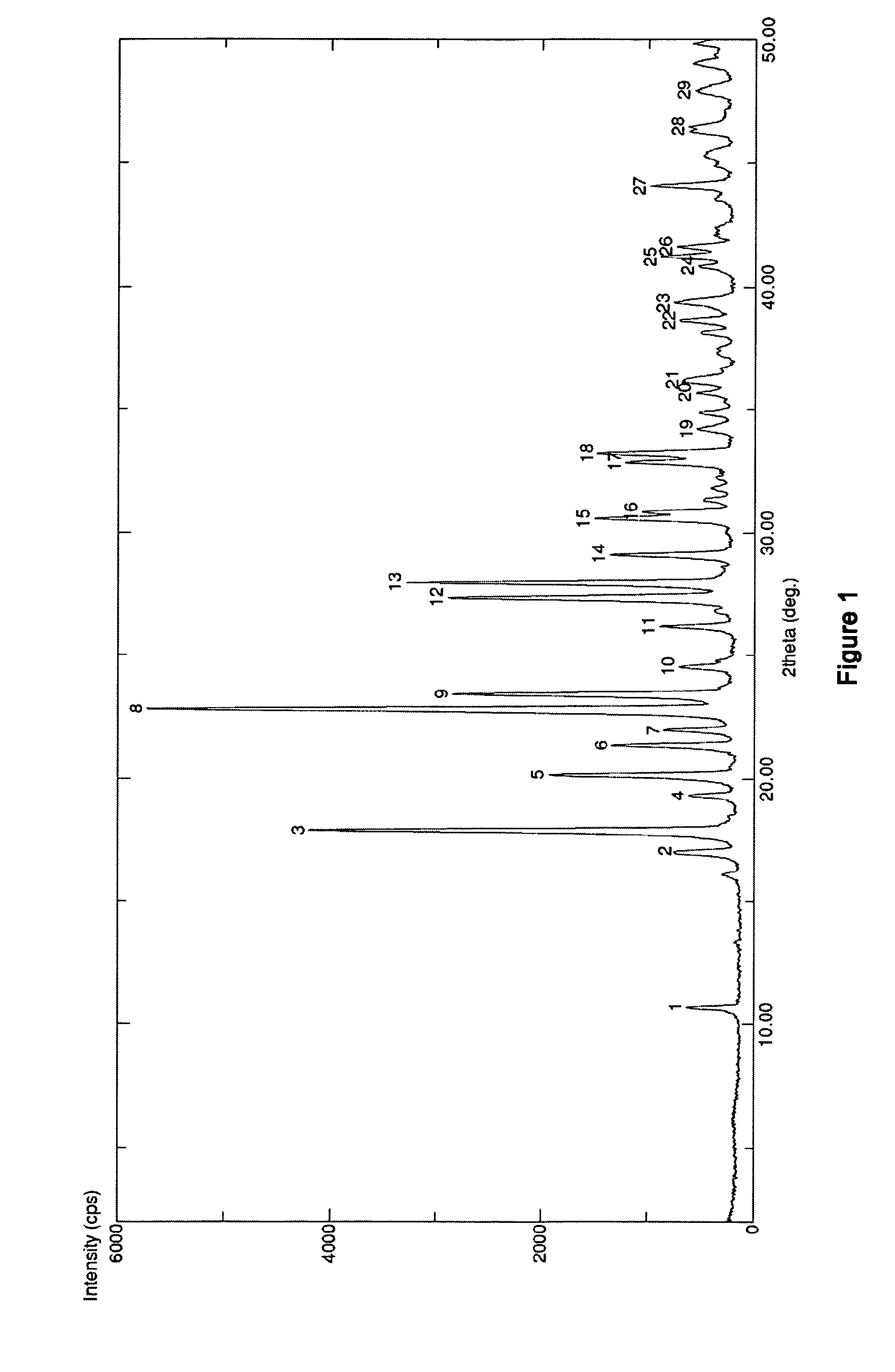
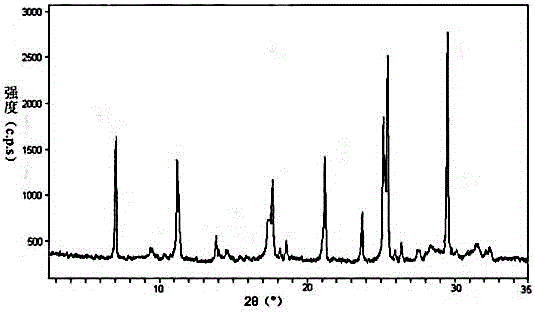
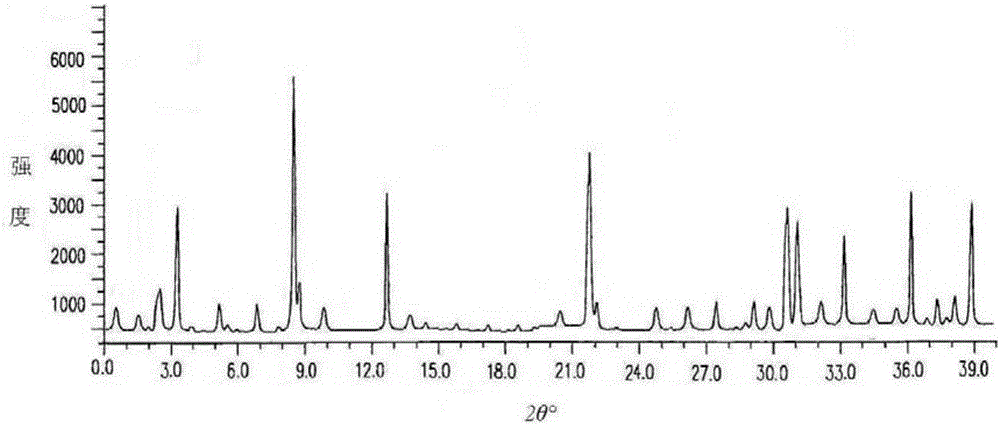
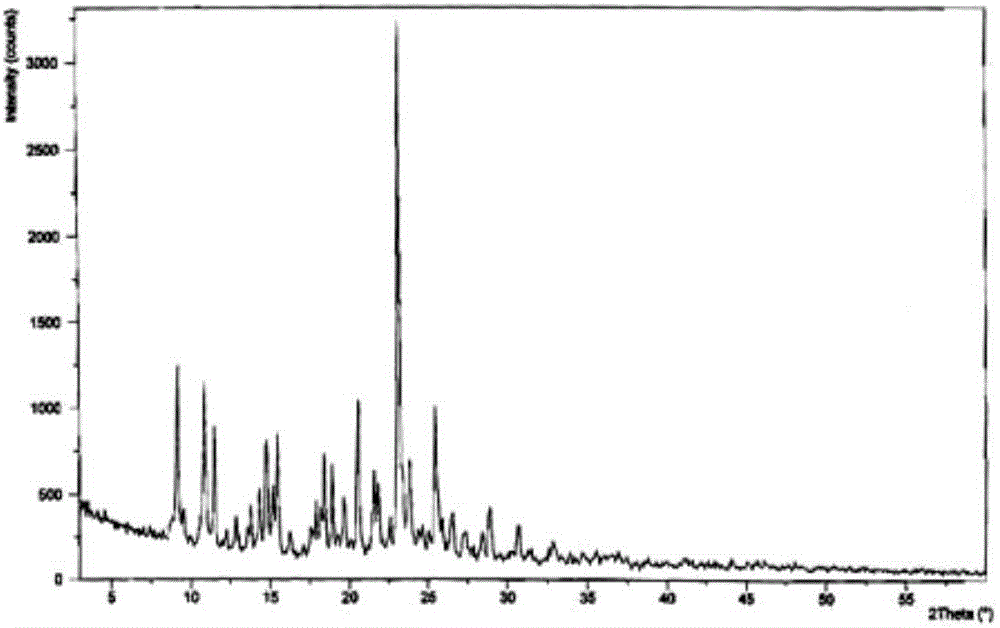
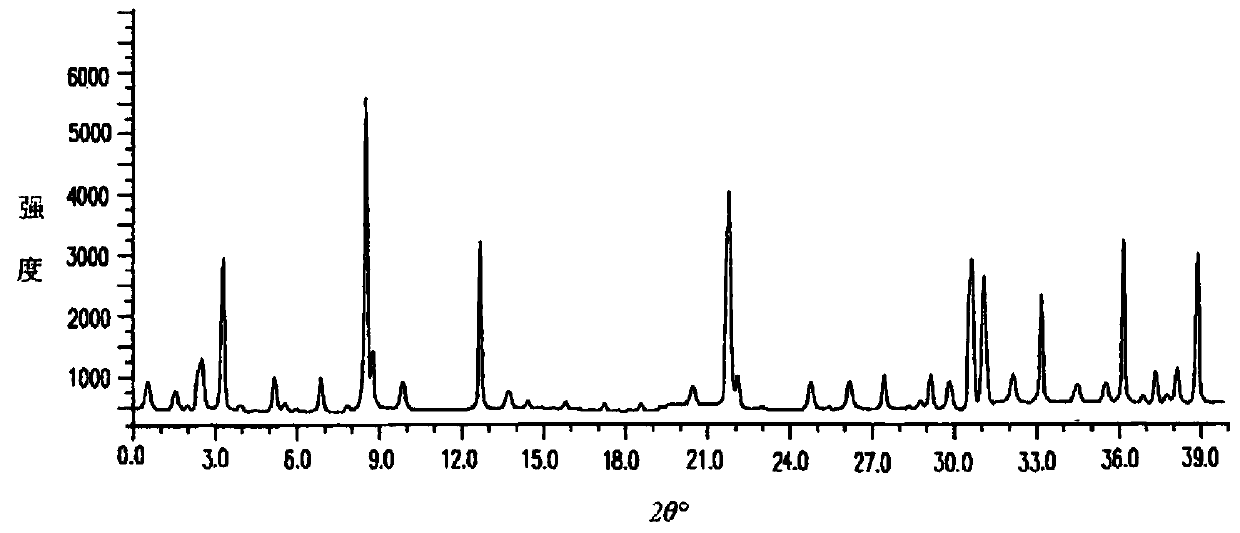
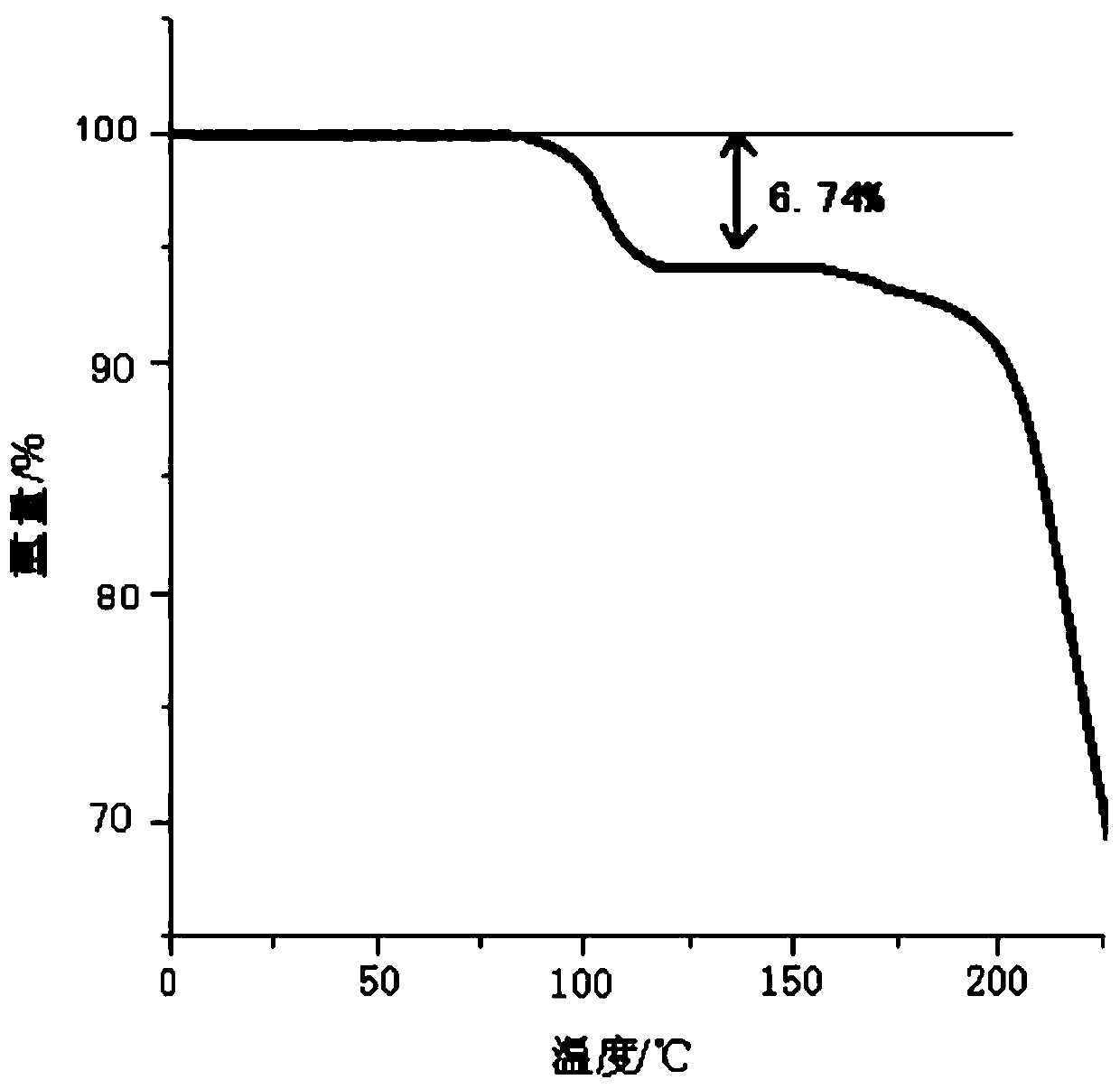
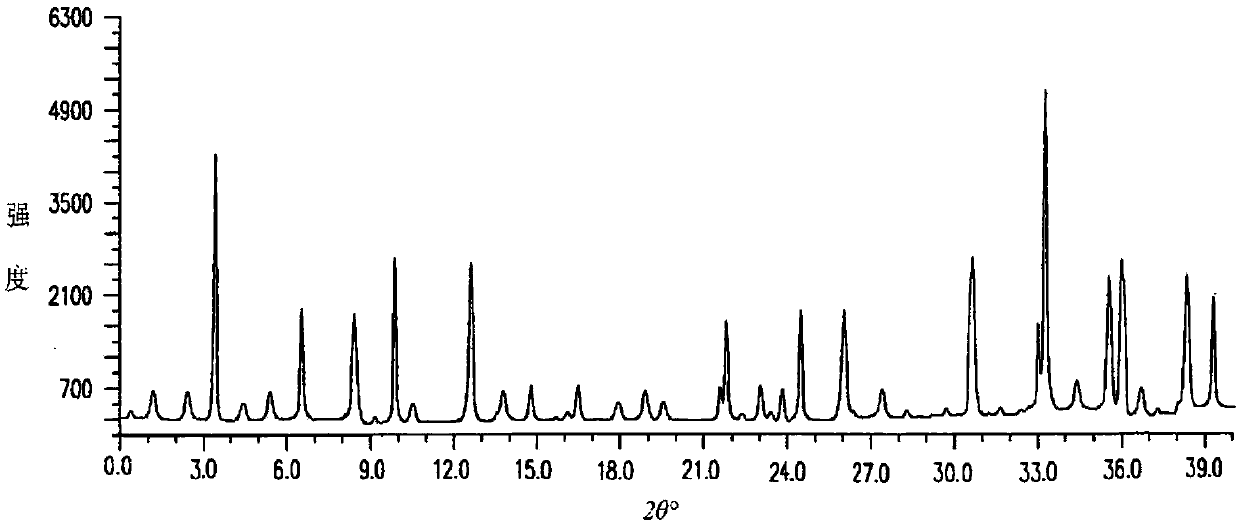
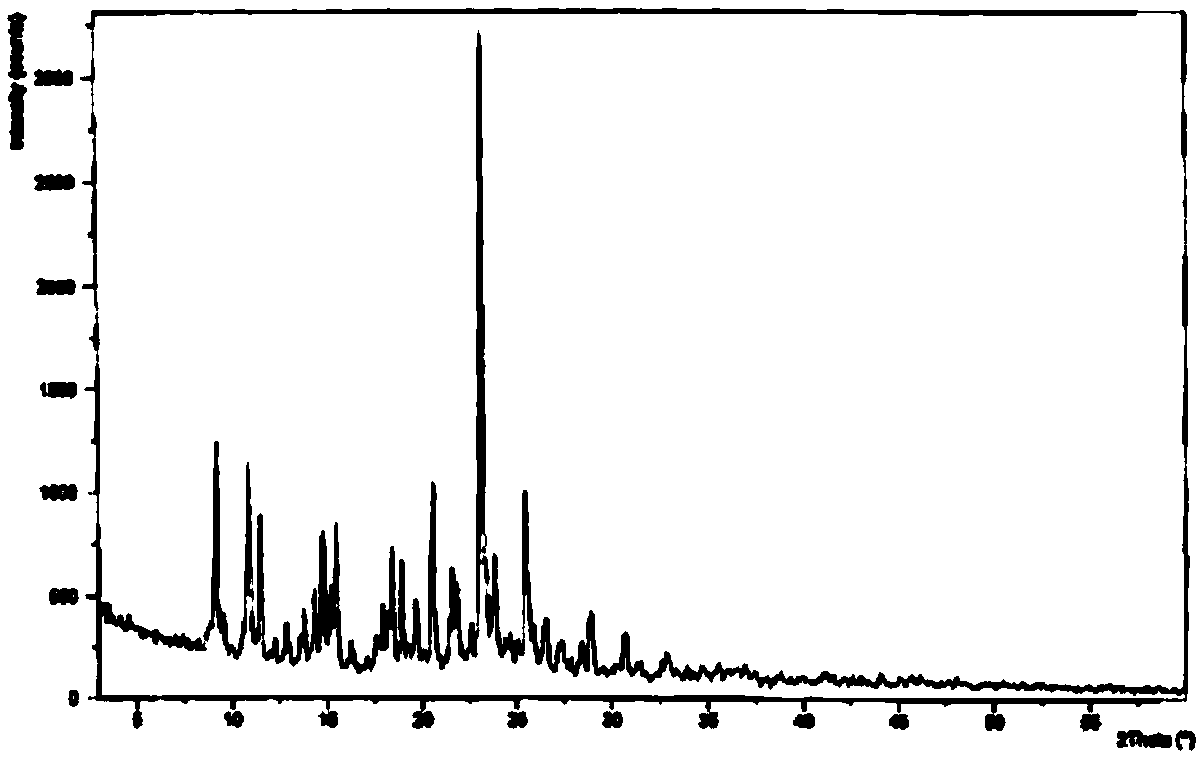
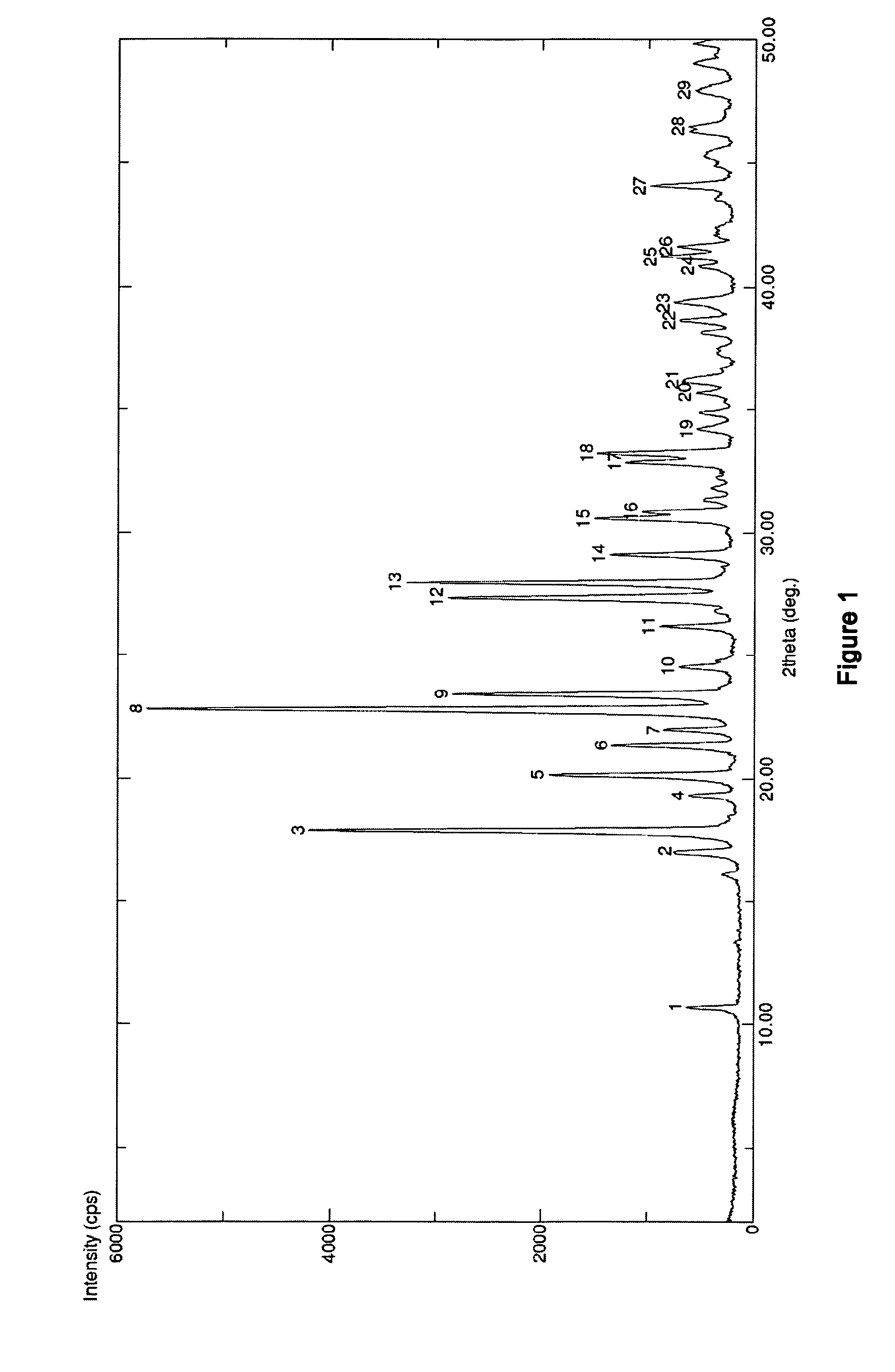
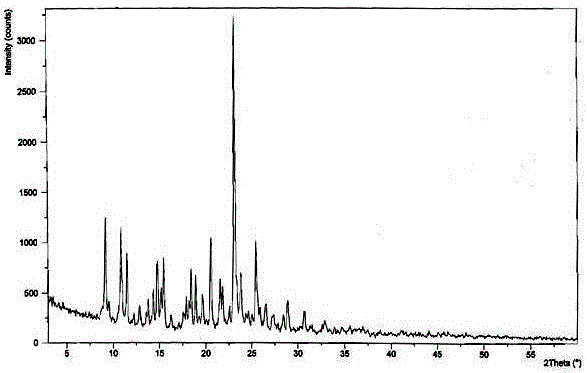
The invention discloses medicine erdosteine composition granules for treating respiratory tract infection and belongs to the technical field of medicine. The composition is prepared from erdosteine, calcium carbonate, cane sugar, citric acid, lemon yellow, purified water and magnesium stearate. The erdosteine is a new type crystal-form compound, the X-ray powder diffraction diagram obtained through measurement by the application of Cu-Ka rays is as shown in figure 1, and the erdosteine is different from the erdosteine reported in the prior art, tests find that the new type crystal-form erdosteine has good solubleness and high stability, the prepared granules are good in stability, and the medicine erdosteine composition granules for treating respiratory tract infection is very suitable for clinical application.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Erdosteine composition for treating respiratory tract inflammation
InactiveCN104873495AImprove performanceChemically stableOrganic active ingredientsOrganic chemistryLactoseBiomedical engineering
The invention discloses an erdosteine composition for treating respiratory tract inflammation, relating to the technical field of medicines. The erdosteine composition is prepared from erdosteine, lactose, microcrystalline cellulose, hydroxypropyl methylcellulose, polysorbate 80, purified water and silicon dioxide. The erdosteine is a novel crystal compound; an X-ray powder diffraction figure obtained by measurement by using Cu-Kalpha ray is shown in the figure 1; the erdosteine disclosed by the invention is different from erdosteine reported in the prior art; experiments show that the novel crystal compound has better solubleness and higher stability; and a prepared capsule is very applied to clinical application.
Owner:苗怡文
Pharmaceutical erdosteine composite granules for treating respiratory tract infection
InactiveCN105232473AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistrySucrosePharmacology
The invention discloses pharmaceutical erdosteine composite granules for treating respiratory tract infection and belongs to the technical field of medicine. A composite is made from erdosteine, cane sugar, lactitol, vanillin and purified water. The erdosteine is a novel-crystal form compound, an X-ray powder diffraction pattern is obtained by measurement using Cu-KAlpha ray is shown in 1, and this erdosteine is different from that reported in the prior art, and testing shows that the erdosteine novel-crystal form compound has better solubility and higher stability, and the prepared granules are good in stability and very suitable for clinical application.
Owner:杨献美
Medicine of erdosteine composition capsule for treating airway inflammation
InactiveCN105106172AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryXerulinic acidPharmaceutical drug
The invention discloses medicine of an erdosteine composition capsule for treating airway inflammation, and belongs to the technical field of medicine. The erdosteine composition capsule is prepared from erdosteine, starch, nordihydroguaiaretic acid, 95-percent ethanol, polyvidone K30 and talcum. The erdosteine is a novel crystal form compound; an X-ray powder diffraction pattern obtained through Cu-Ka ray measurement is shown as a Figure 1; the medicine belongs to the erdosteine different from that reported in the prior art. Experiments show that the erdosteine novel crystal form compound has better solubility and higher stability; the prepared capsule has high dissolution rate and good stability, and is very suitable for clinic application.
Owner:杨献美
Erdosteine composition dry suspension for treating respiratory system disease
InactiveCN105232445AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryDiseasePharmaceutical drug
The invention discloses an erdosteine composition dry suspension for treating a respiratory system disease and belongs to the technical field of medicine. A composition is prepared from erdosteine, sorbitol, potassium metaphosphate, acacia gum and glycyrrhizin. The erdosteine is a novel crystalline compound and is different from erdosteine in the prior art, and an X-ray powder diffraction pattern shown as figure 1 is obtained by means of measurement with Cu-Kalpha rays. Through experiments, the novel erdosteine crystalline compound has good solubility and high stability, and the prepared dry suspension is high in stability and bioavailability and extremely suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Polymorphs of enantiopure erdosteine
ActiveUS20100249435A1Advantageous chemical-physical propertyEasy to handleOrganic chemistryMedicineErdosteine
The present invention provides novel crystalline polymorphs of enantiopure Erdosteine, referred to as Form I and Form II, and processes for the preparation thereof.
Owner:EDMOND PHARMA SRL
Erdosteine pharmaceutical composition dry suspension for treating diseases of respiratory system
InactiveCN105078901AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryDiseaseSucrose
The invention relates to an erdosteine pharmaceutical composition dry suspension for treating diseases of respiratory system, and belongs to the technical field of medicine. The composition dry suspension is prepared from erdosteine, sucrose, xylitol, disodium hydrogen phosphate, propylene glycol alginate, sucralose and purified water. The erdosteine, different from that reported by the prior art, is a novel crystalline compound, and the X-ray powder diffraction pattern of the erdosteine measured by virtue of Cu-K alpha ray is as shown in Figure 1; tests discover that the erdosteine crystalline compound is relatively good in solubility and relatively high in stability; and the prepared dry suspension is good in stability, high in bioavailability, and the dry suspension is quite suitable for clinical application.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Medicine erdosteine composition tablet for treating airway inflammation
InactiveCN105193750AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryPolyethylene glycolMagnesium stearate
The invention discloses a medicine erdosteine composition tablet for treating airway inflammation, and belongs to the technical field of medicine. The composition is prepared from erdosteine, lactose, crospovidone, zinc oxide, 95% ethyl alcohol, polyethylene glycol 6000 and magnesium stearate. Erdosteine is a new crystal form compound, the X-ray powder diffraction pattern obtained by conducting measurement through Cu-K alpha rays is shown in the figure 1, erdosteine is different from erodstein reported in the prior art, tests show that the erdosteine new crystal form compound has high solubility and high stability, and the prepared table is high in dissolution rate and stability, and is quite suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Medicinal erdosteine composition tablet for treating respiratory tract inflammation and preparation method of tablet
InactiveCN105055348AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryPharmaceutical drugLactose
The invention relates to a medicinal erdosteine composition tablet for treating respiratory tract inflammation and a preparation method of the composition tablet, belonging to the technical field of medicine. The composition tablet is prepared from erdosteine, sodium carboxymethyl starch, lactose, L-HPC21, 95% of ethanol, magnesium stearate and peppermint essence. The erdosteine is a novel crystalline compound; as shown in X-ray powder diffraction pattern 1 obtained from Cu-K alpha-ray measurement, the erdosteine is different from that reported in the prior art; tests discover that the erdosteine novel crystalline compound is relatively good in dissolution and relatively high in stability; and the prepared tablet is high in dissolution degree, good in stability and quite suitable for clinical application.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Composition containing ciclacillin or its derivatives and preparation thereof
InactiveCN101214245AHigh protection rateGood curative effectAntiinfectivesRespiratory disorderFUDOSTEINEActive component
The present invention provides a series of combination which contains ciclacillin or the derivative of the ciclacillin and is the combination essentially consisting of the ciclacillin or the derivative of the ciclacillin and an expectorant drug, wherein, the derivative of the ciclacillin is the officinal salt or officinal ester of the ciclacillin. The expectorant drug is any one of ambroxol, acetylcysteine, carbocisteine, erdosteine, fudosteine and lignum vitae glycerol ether. The unit medication dosage of the ciclacillin is 0.25g to 0.5g which is preferential (counted by active component), and the unit medication dosage of the expectorant drug is 0.01g to 2.0g. The combination is mainly used for remedying the respiratory tract infection with the symptom of expectoration.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
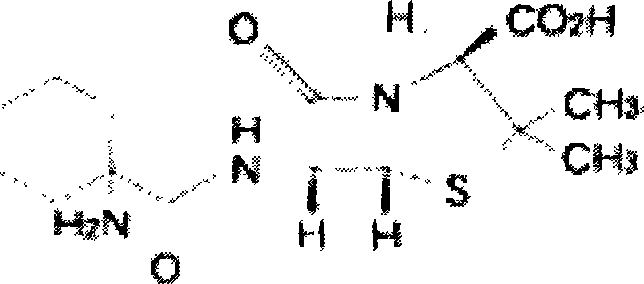
Treating Bronchiectasis With Doxofylline and Erdosteine
A method and pharmaceutical composition for the treatment of bronchiectasis comprising doxofylline and erdosteine.
Owner:ALITAIR PHARMA
Pharmaceutical composition containing ambroxol and erdosteine or acetylcysteine and application thereof
The invention relates to ambroxol, erdosteine or the medicinal composition of the pharmaceutically usable salts of the two and their use, ambroxol and acetylcysteine or the medicinal composition of the pharmaceutically usable salts of the two and their use, the kit containing these compositions, and the use of these pharmaceutical compositions in preparing expectorant medicaments. The invention also relates to the cooperative compositions of ambroxol and erdosterne, ambroxol and acetylcysteine, and the use of these cooperative compositions in treating viscous phlegm and phlegm difficulty caused by chronic bronchitis and bronchial asthma.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Dispersing tablet for treating diseases of respiratory systems and method for preparing dispersing tablet
ActiveCN106176642AImprove stabilityHigh dissolution rateOrganic active ingredientsDispersion deliveryDiseaseSolubility
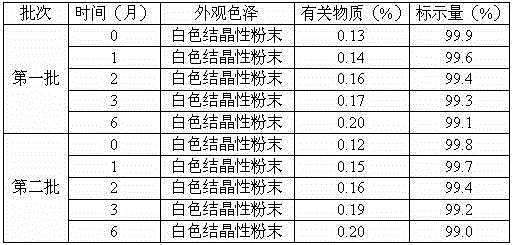
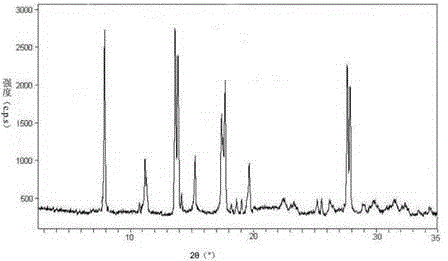
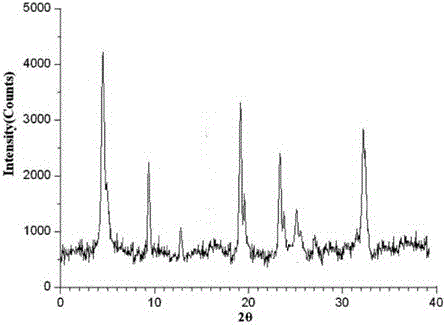
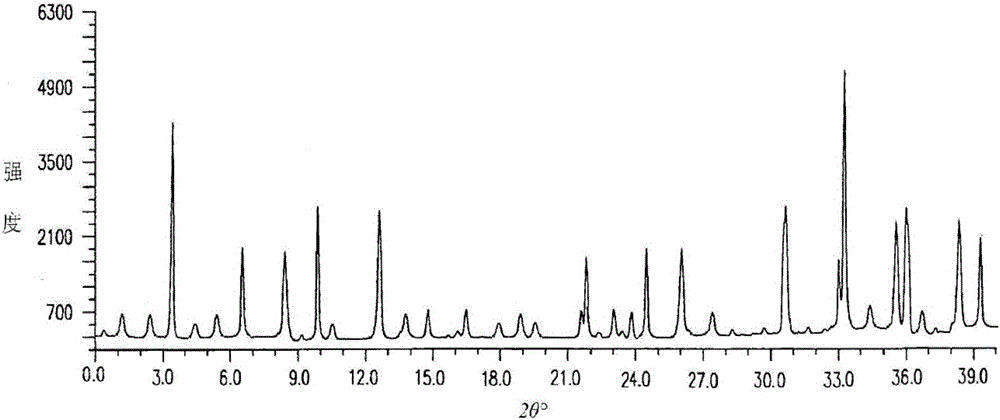
The invention relates to an erdosteine dispersing tablet for treating diseases of respiratory systems and a method for preparing the dispersing tablets. The erdosteine dispersing tablet comprises erdosteine, hydroxy propyl celluloses, sodium carboxymethyl starch, polyethylene glycol 4000-6000, microcrystalline celluloses 102, crospovidone, magnesium stearate and povidone K30. The erdosteine is erdosteine monohydrate, a molecular formula of the erdosteine is C8H11NO4S2.H2O, and characteristic diffraction peaks are displayed at 3.323, 8.484, 12.661, 21.823, 30.645, 31.161, 33.209, 36.161 and 38.935 positions of X-ray powder diffraction spectra expressed at diffraction angles of 2 theta+ / -0.2 degrees. As discovered in tests, the erdosteine dispersing tablet and the method have the advantages that the erdosteine dispersing tablet is good in stability and high in bioavailability, and problems of poor solubility and compressibility, sticking easiness in production procedures and the like of erdosteine dispersing tablets in the prior art can be solved.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
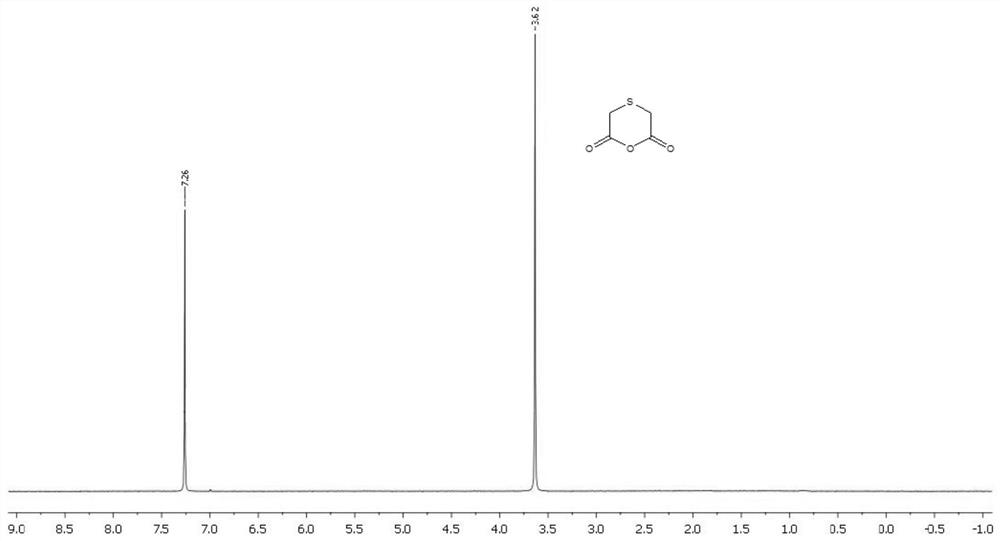
Preparation method of thioglycolic anhydride
The invention discloses a preparation method of thioglycolic anhydride. The method comprises the following steps of: vaporization of trifluoroacetic anhydride: vaporizing trifluoroacetic anhydride ina vaporizing device for later use; synthesis of thioglycolic anhydride: preheating the interior of a suspended gas-solid reaction device to 45-65 DEG C, introducing the vaporized trifluoroacetic anhydride into the suspended-state gas-solid reaction device, intermittently adding thionyl diacetic acid from the top of the suspended-state gas-solid reaction device, reacting for 2 hours, stopping introducing the vaporized trifluoroacetic anhydride from a gas inlet, stopping heating, and leading out an obtained solid product from a discharging port; and purification of thioglycolic anhydride: washing the solid product with refrigerated anhydrous petroleum ether and anhydrous ether respectively to obtain pure thioglycolic anhydride. The thioglycolic anhydride synthesized by the method is high inpurity and high in yield; and the yield of erdosteine prepared by reacting the thioglycolic anhydride serving as a raw material with homocysteine thiolactone hydrochloride is high.
Owner:武汉本杰明医药股份有限公司
Pharmaceutical composition taking cefixime and erdosteine as active component as well as preparation method and usage thereof
InactiveCN101181274AOrganic active ingredientsPill deliveryAdditive ingredientObstructive chronic bronchitis
The invention relates to a pharmaceutical composition which takes cefixime and erdosteine as active ingredients, the preparation method and the usage. The invention takes cefixime and erdosteine as the pharmaceutical active ingredients, pharmaceutically acceptable excipients are mixed to form the pharmaceutical composition, and the invention can be used for the treatment of chronic bronchitis with cough, sputum and other clinical symptoms. The contents of the invention take cefixime and erdosteine as raw materials, certain specific type and proportion of excipients are added, the invention is prepared and developed into tablets, capsules, granules, dispersion tablets, chew tablets, buccal tablets, effervescent tablets, effervescent granules and other various oral preparations according to the technical means which is illustrated by the invention.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Combination containing clacillin or derivative and preparing method thereof
The invention relates to a series of combined substances which contain cyclacillin or derivative of the cyclacillin, in particular to a combined substance where the cyclacillin or the derivative of the cyclacillin and an expectorant are combined. Wherein, the derivative of the cyclacillin is medicine salt or medicine ester of the cyclacillin; and the expectorant is any of ambroxol, acetylcysteine, carbocisteine, erdosteine, fudosteine and guaiacum glycerol ether. The drug dosage per unit of the cyclacillin is in preference to 0.25g to 0.5g ( the content is calculated according to active components). The drug dosage per unit of the expectorant is 0.01 to 2.0g. The combined substance is mainly used to treat respiratory tract infection with expectoration symptom.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
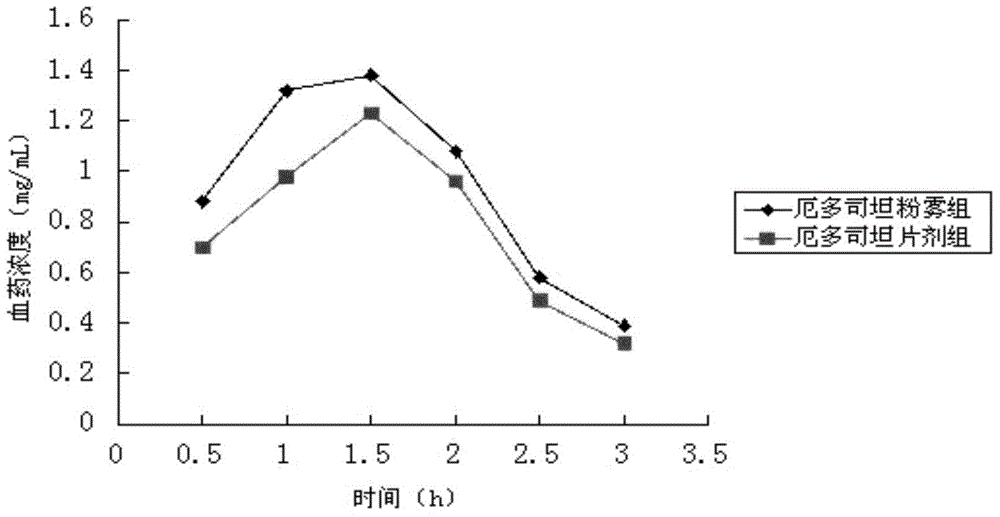
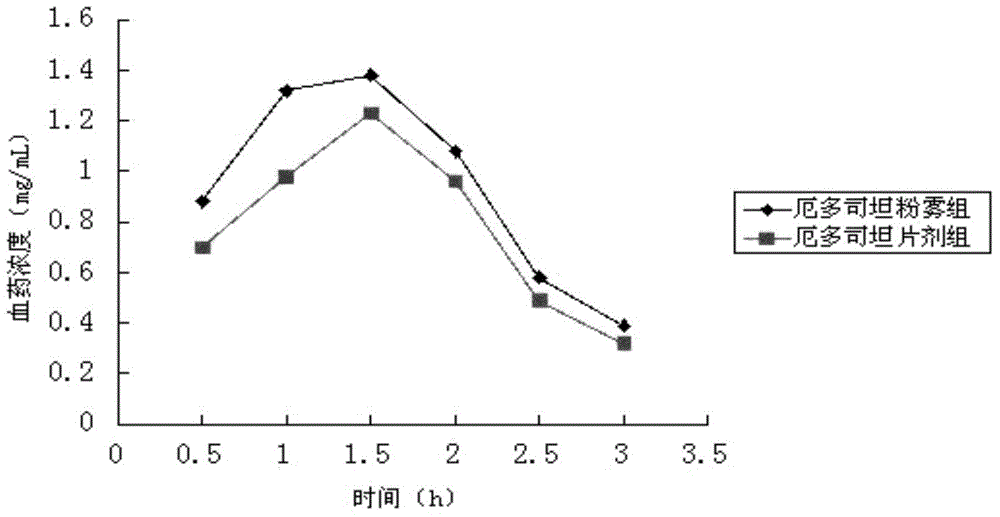
A kind of erdosteine inhalation powder mist and preparation method thereof
ActiveCN104523658BNo degradationNo first pass effectOrganic active ingredientsPharmaceutical delivery mechanismMedicineMicrometer
The invention belongs to the field of medicines, and particularly relates to an erdosteine powder inhalation which is actively inhaled in a superfine powder mode and directly delivers a medicine to the lung to exert effect, solving the problem of low degradation effect and liver first pass elimination effect bioavailability of a medicine in gastrointestinal tracts. The erdosteine powder inhalation is prepared by mixing superfine erdosteine powder and superfine poloxamer 188 powder, wherein the average particle size of the superfine erdosteine powder at the weight ratio of 20:3-2:1 is 1-5 micrometers, and the maximum particle size is not more than 10 micrometers; the average particle size of the superfine poloxamer 188 powder is 60-80 micrometers, and the maximum particle size is not more than 100 micrometers. A preparation method comprises the following steps: grinding erdosteine, carrying out superfine crushing by using a superfine crusher to obtain the superfine powder of which the average particle size is 1-5 micrometers; carrying out superfine crushing on poloxamer 188 by using the superfine crusher to obtain the superfine powder of which the average particle size is 60-80 micrometers; and uniformly mixing the crushed superfine powder according to a proportion of 20:3-2:1, and then subpackaging.
Owner:海南伊顺药业有限公司
Combination therapy of erdosteine and beta-2 agonists for treating respiratory pathologies characterized by non reversible or partially reversible airway obstruction
InactiveUS20080033010A1Good reversibilityBiocideRespiratory disorderDiseaseObstructive Pulmonary Diseases
The invention relates to a method for improving the reversibility of bronchial obstruction in a subject affected by chronic obstructive pulmonary disease comprising the administration to said subject of erdosteine in combination with a beta agonist.
Owner:EDMOND PHARMA SRL
Phlegm specimen treatment liquid containing composite decomposition agent and preparation method of phlegm specimen treatment liquid
InactiveCN108240927AImprove decomposition efficiencyImprove decomposition speedPreparing sample for investigationDispersityPhosphate
The invention relates to phlegm specimen treatment liquid containing a composite decomposition agent. The phlegm specimen treatment liquid is characterized by containing the following raw materials inpercentage by weight: 1wt%-6wt% of phosphate, 2wt% of phenol, 1wt%-3wt% of a composite decomposition agent, 10wt%-30wt% of an alcohol solvent and 59wt%-86wt% of distilled water. Acetylcysteine and erdosteine compounded for use, so that the viscosity is reduced during the decomposition of phlegm, the fluidity and dispersity of phlegm are improved, the decomposition efficiency and speed are relatively excellent, and more phlegm specimens can be treated by the same mass of the treatment liquid; and a prepared thin layer cell smear has a clear and transparent background, and cells are uniformly distributed, so that the accuracy and readability of a diagnosis result are greatly improved.
Owner:孝感市森茂激光数控设备有限公司
The preparation method of thioglycolic anhydride
The invention discloses a preparation method of thioglycolic anhydride, which comprises the following steps: vaporization of trifluoroacetic anhydride: vaporizing trifluoroacetic anhydride in a vaporization device for use; synthesis of thioglycolic anhydride: After the internal temperature of the suspended gas-solid reaction device is preheated to 45-65°C, the vaporized trifluoroacetic anhydride is passed into the suspended gas-solid reaction device, and sulfide disulfide is intermittently added from the top of the suspended gas-solid reaction device. Acetic acid, after reacting for 2 hours, stop feeding vaporized trifluoroacetic anhydride from the air inlet, stop heating, and the solid product obtained is derived from the discharge port; the purification of thioglycolic anhydride: use the solid product respectively with refrigerated Wash with anhydrous petroleum ether and anhydrous ether to obtain pure thioglycolic anhydride. The thioglycolic anhydride synthesized by the invention has high purity and high yield, and the erdosteine produced by reacting the thioglycolic anhydride as a raw material with homocysteine thiolactone hydrochloride has a high yield.
Owner:武汉本杰明医药股份有限公司
Compound for treatment of respiratory system diseases and preparation method thereof
ActiveCN106187991AImprove stabilityImprove solubilityOrganic chemistry methodsRespiratory disorderSolubilityErdosteine
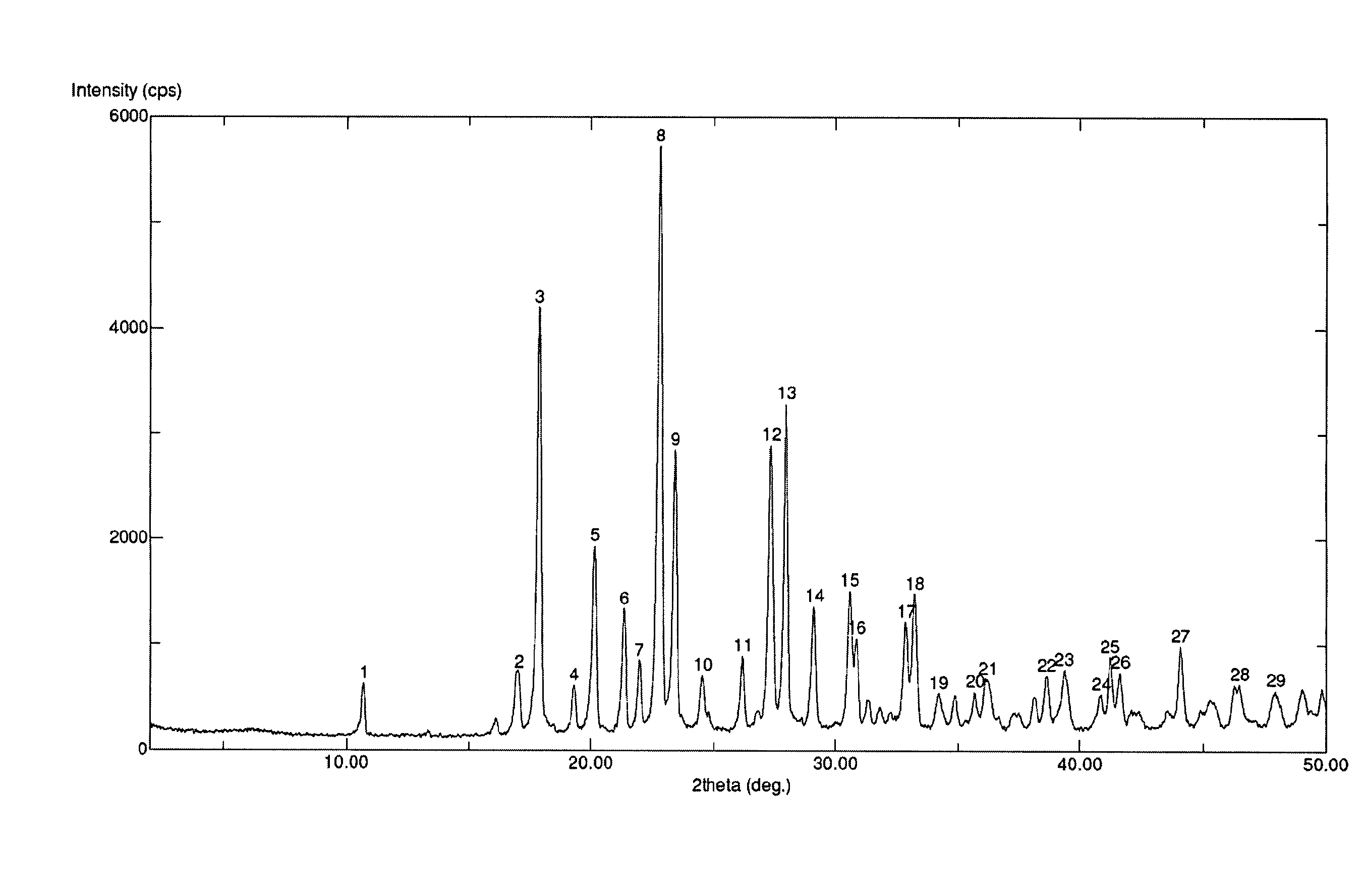
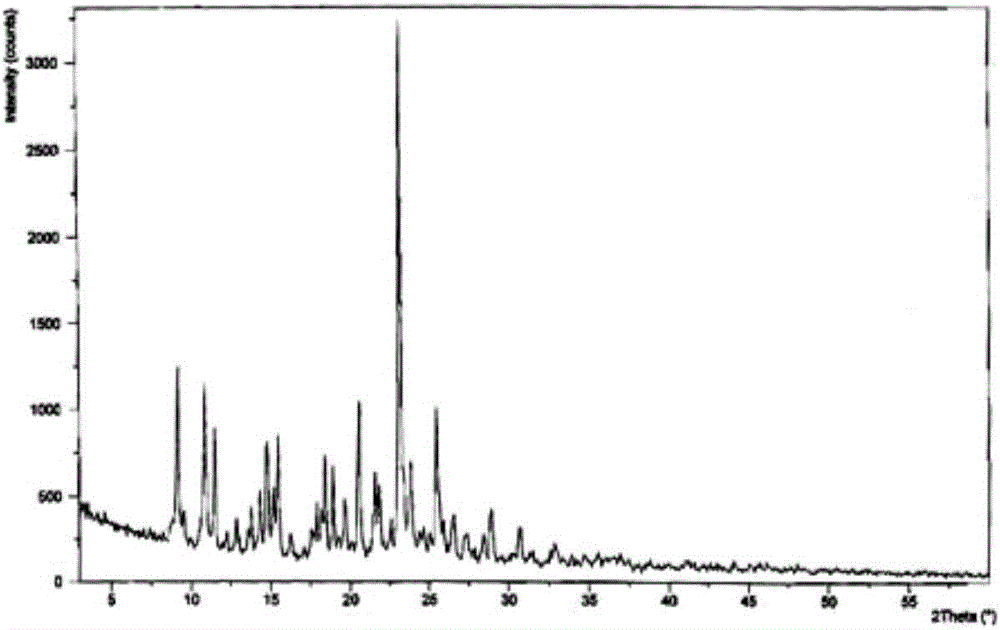
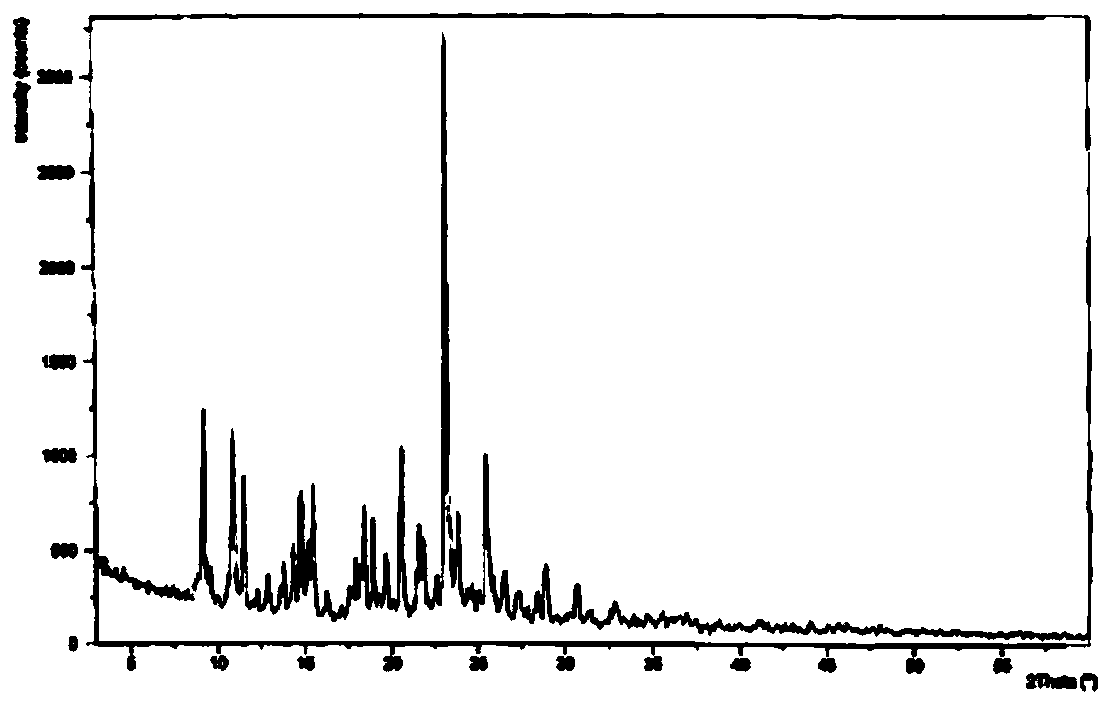
Belonging to the field of medical technology, the invention discloses a compound for treatment of respiratory system diseases and a preparation method thereof, and specifically discloses a new erdosteine compound and a preparation method thereof. The erdosteine compound crystal's X-ray powder diffraction spectrum represented by a diffraction angle of 2theta+ / -0.2 degrees shows characteristic diffraction peaks at the positions of 3.41 degrees, 6.51 degrees, 8.48 degrees, 9.91 degrees, 12.65 degrees, 21.89 degrees, 24.48 degrees, 26.15 degrees, 30.69 degrees, 33.24 degrees, 35.56 degrees, 36.09 degrees, 38.35 degrees and 39.40 degrees, and the X-ray powder diffraction spectrum obtained by Cu-Kalpha ray measurement is shown as figure 1. The erdosteine crystal provided by the invention is different from the existing technology, has good stability, good solubility, good flowability and compressibility, and can be mixed evenly with other auxiliary materials.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Dispersible tablet for treating respiratory diseases and preparation method thereof
ActiveCN106176642BImprove stabilityHigh dissolution rateOrganic active ingredientsDispersion deliveryDiseaseSolubility
The invention relates to an erdosteine dispersing tablet for treating diseases of respiratory systems and a method for preparing the dispersing tablets. The erdosteine dispersing tablet comprises erdosteine, hydroxy propyl celluloses, sodium carboxymethyl starch, polyethylene glycol 4000-6000, microcrystalline celluloses 102, crospovidone, magnesium stearate and povidone K30. The erdosteine is erdosteine monohydrate, a molecular formula of the erdosteine is C8H11NO4S2.H2O, and characteristic diffraction peaks are displayed at 3.323, 8.484, 12.661, 21.823, 30.645, 31.161, 33.209, 36.161 and 38.935 positions of X-ray powder diffraction spectra expressed at diffraction angles of 2 theta+ / -0.2 degrees. As discovered in tests, the erdosteine dispersing tablet and the method have the advantages that the erdosteine dispersing tablet is good in stability and high in bioavailability, and problems of poor solubility and compressibility, sticking easiness in production procedures and the like of erdosteine dispersing tablets in the prior art can be solved.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Antigen diluent and immunodeficiency virus detection kit
PendingCN114113594AAvoid risk of transmissionImprove stabilityBiological material analysisBiological testingAntigenImmunodeficiency virus
The invention provides an antigen diluent and an immunodeficiency virus detection kit, the antigen diluent is mainly composed of a PBS buffer solution, calf serum and a protective agent, the protective agent comprises dimethyl diacetoxysilane, Ectoin and a synergistic protective agent, the synergistic protective agent is prepared from any one of erdosteine, thiomersalate, S-allyl mercaptocysteine and mercaptopyridoxine or a mixture of more of erdosteine, thiomersalate, S-allyl mercaptocysteine and mercaptopyridoxine. According to the antigen diluent, the protective agent capable of enabling the antigen and the antibody to have relatively high activity is added, and the types of the protective agent are screened and reasonably proportioned and mixed, so that after the antigen diluent is adopted, the stability and the accuracy of an immunological detection device are remarkably improved, and the antigen diluent is worthy of wide popularization and application.
Owner:SHANGHAI CHEMTRON BIOTECH
A compound for treating respiratory diseases and its preparation method
ActiveCN106187991BImprove stabilityImprove solubilityOrganic chemistry methodsRespiratory disorderDiseaseSolubility
Belonging to the field of medical technology, the invention discloses a compound for treatment of respiratory system diseases and a preparation method thereof, and specifically discloses a new erdosteine compound and a preparation method thereof. The erdosteine compound crystal's X-ray powder diffraction spectrum represented by a diffraction angle of 2theta+ / -0.2 degrees shows characteristic diffraction peaks at the positions of 3.41 degrees, 6.51 degrees, 8.48 degrees, 9.91 degrees, 12.65 degrees, 21.89 degrees, 24.48 degrees, 26.15 degrees, 30.69 degrees, 33.24 degrees, 35.56 degrees, 36.09 degrees, 38.35 degrees and 39.40 degrees, and the X-ray powder diffraction spectrum obtained by Cu-Kalpha ray measurement is shown as figure 1. The erdosteine crystal provided by the invention is different from the existing technology, has good stability, good solubility, good flowability and compressibility, and can be mixed evenly with other auxiliary materials.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Polymorphs of enantiopure erdosteine
ActiveUS8269022B2Advantageous chemical-physical propertyEasy to handleOrganic chemistryMedicineErdosteine
The present invention provides novel crystalline polymorphs of enantiopure Erdosteine, referred to as Form I and Form II, and processes for the preparation thereof.
Owner:EDMOND PHARMA SRL
Erdosteine compound for treating respiratory inflammation and preparation method thereof
InactiveCN104788421BChemically stableGood water solubilityOrganic active ingredientsAntipyreticBiochemistryAqueous solubility
The invention discloses an erdosteine compound for treating respiratory inflammation and a preparation method thereof, belonging to the technical field of medicine. The X-ray powder diffraction pattern obtained by measuring the erdosteine compound using Cu-Kα rays is shown in Figure 1. The erdosteine crystal provided by the invention has stable chemical properties, good water solubility and high stability, brings convenience to the preparation of various preparations, and is very suitable for clinical application.
Owner:NANTONG QIWU AGRI PROD CO LTD
Pharmaceutical composition containing ambroxol and erdosteine or acetylcysteine and application thereof
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Erdosteine powder inhalation and preparation method thereof
ActiveCN104523658ANo degradationNo first pass effectOrganic active ingredientsPharmaceutical delivery mechanismInhalationMedicine
The invention belongs to the field of medicines, and particularly relates to an erdosteine powder inhalation which is actively inhaled in a superfine powder mode and directly delivers a medicine to the lung to exert effect, solving the problem of low degradation effect and liver first pass elimination effect bioavailability of a medicine in gastrointestinal tracts. The erdosteine powder inhalation is prepared by mixing superfine erdosteine powder and superfine poloxamer 188 powder, wherein the average particle size of the superfine erdosteine powder at the weight ratio of 20:3-2:1 is 1-5 micrometers, and the maximum particle size is not more than 10 micrometers; the average particle size of the superfine poloxamer 188 powder is 60-80 micrometers, and the maximum particle size is not more than 100 micrometers. A preparation method comprises the following steps: grinding erdosteine, carrying out superfine crushing by using a superfine crusher to obtain the superfine powder of which the average particle size is 1-5 micrometers; carrying out superfine crushing on poloxamer 188 by using the superfine crusher to obtain the superfine powder of which the average particle size is 60-80 micrometers; and uniformly mixing the crushed superfine powder according to a proportion of 20:3-2:1, and then subpackaging.
Owner:海南伊顺药业有限公司
Erdosteine purifying method
InactiveCN108640900AEfficient removalSimple and fast operationOrganic chemistryOrganic solventAqueous solution
The invention provides an erdosteine purifying method. The method comprises the following steps: 1) preparing an aqueous solution of crude erdosteine: dissolving crude erdosteine in an aqueous solution which is near-neutral to alkaline, adding an organic solvent capable of being layered with water for extraction to obtain the aqueous solution of crude erdosteine, wherein the volume ratio of the organic solvent to the alkaline aqueous solution is 0.05-20:1; 2) preparing fine erdosteine: regulating the aqueous solution of the crude erdosteine obtained in step 1) to be acidic by acid, separatingout solids, and performing filtering and drying to obtain the fine erdosteine. On the basis of the specificity that the erdosteine structure contains carboxyl groups, column chromatography which is high in operation difficulty and not suitable for industrial production is not needed for purification, only an alkali-solution and acid-isolation method is adopted for purification, operation is convenient in the purifying process, impurities in the crude erdosteine can be removed effectively, finally, purity of the fine erdosteine can reach 99.4% or higher, any individual impurity is lower than 0.1%, yield is high, and the method is applicable to industrial production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com