Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Carbocisteine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
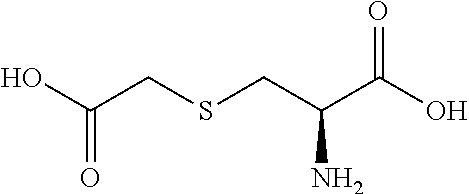
Carbocisteine, also called carbocysteine, is a mucolytic that reduces the viscosity of sputum and so can be used to help relieve the symptoms of chronic obstructive pulmonary disorder (COPD) and bronchiectasis by allowing the sufferer to bring up sputum more easily. Carbocisteine should not be used with antitussives (cough suppressants) or medicines that dry up bronchial secretions.
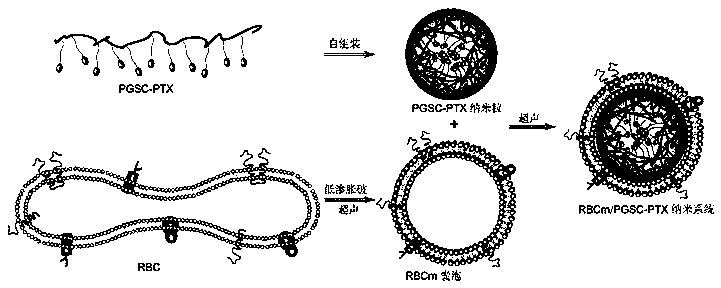
Preparation method and application of erythrocyte membrane-coated acid-sensitive polymer prodrug nano drug delivery system
InactiveCN105497912AEnhanced release propertiesNon-destructiveOrganic active ingredientsPharmaceutical non-active ingredientsErythrocyte membraneCarbocisteine
The invention discloses a preparation method and an application of an erythrocyte membrane-coated acid-sensitive polymer prodrug nano drug delivery system, wherein the polymer is formed by connecting sodium polyglutamate and carbocisteine through a peptide bond and then is bonded with an anti-cancer drug taxol to form the polymer prodrug; and the polymer prodrug is coated by an erythrocyte membrane to obtain an erythrocyte membrane-coated acid-sensitive polymer drug delivery system. The drug delivery system has the following characteristics that the particle size is about 100nm, and the shape is a sphere; the drug delivery system is relatively stable in a phosphate buffer and serum; the drug release character is relatively good in an acid environment; the toxicity is obviously reduced in cell experiments; the ingestion of the system by macrophage is obviously reduced; without destructive effect on erythrocyte, the system can be applied to intravenous injection; the circulating time of the system in blood is remarkably longer than that of polymer; and the system has an obvious inhibiting effect on the tumor growth of lung cancer.
Owner:EAST CHINA NORMAL UNIV
Carbocisteine oral liquid and preparation method thereof
ActiveCN104511025AGood water solubilityEasy to oxidizeOrganic active ingredientsPharmaceutical delivery mechanismCarbocisteineAntioxidant
The invention relates to a carbocisteine oral liquid and a preparation method thereof. The oral liquid comprises carbocisteine accounting for 2% to 10% of the oral liquid, sodium hydroxide, an antioxidant, a solution stabilizer composition, and a flavoring agent, and has a pH value of 6.0 to 7.5. The oral liquid is prepared by the following steps: carrying out salt-forming reactions between carbocisteine and a sodium hydroxide solution, then dissolving the salts into water, adding an antioxidant, a solution stabilizer composition, and a flavoring agent, stirring, adding water to a metered volume, filtering, filling the solution into cans, and finally sterilizing the cans so as to obtain the oral liquid; wherein each can contains 10 to 120 mL of oral liquid. The oral liquid is widely used to treat thick phlegm or dys-expectoration caused by chronic bronchitis, bronchus asthma, and the like, and has the characteristics of stable property, long shelf life, and safer clinical application.
Owner:BEIJING CHENG JI PHARMA
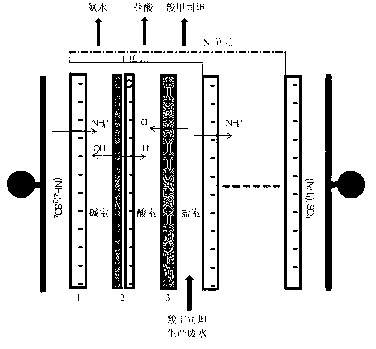
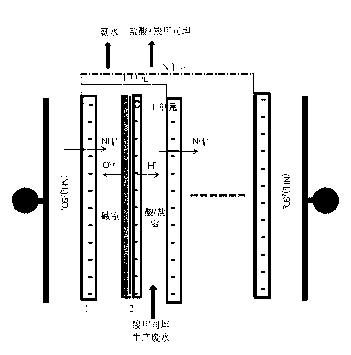
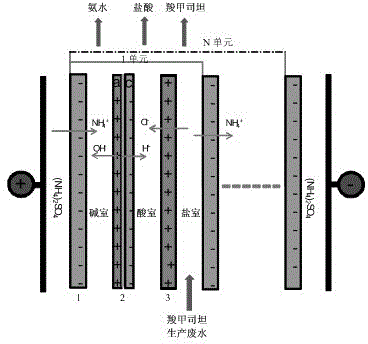
Method and system for recovering waste water generated in carbocisteine production
InactiveCN102838240AHigh yieldEliminate pollutionMultistage water/sewage treatmentNature of treatment waterAmmoniacal nitrogenCarbocisteine
The invention discloses a method and system for recovering waste water generated in carbocisteine production. The system comprises a bipolar membrane electro-dialysis desalination system and a recovering system; the recovering system comprises an acid concentrating and recovering system, a base concentrating and recovering system and a carbocisteine concentrating and recovering system. With the adoption of the system, salts in the waste water generated in carbocisteine production can be converted into corresponding acid and base so as to be removed; the obtained acid and base can be directly used in the carbocisteine production process or be used in the carbocisteine production process after an advanced treatment; the carbocisteine in the waste water is further recovered by using a carbocisteine concentrating crystallizing system. By treating the waste water generated in the carbocisteine production by using the method, organism pollution, salt pollution and ammonia nitrogen pollution are avoided; in addition, the recovered acid and base can be reused in the carbocisteine production process to realize zero emission; by using the recovered carbocisteine, the carbocisteine yield is increased and the cost of the method provided by the invention can be further reduced.
Owner:WUHAN UNIV
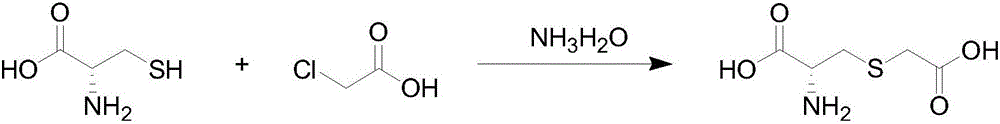
Preparation method of carbocisteine
ActiveCN106565565APrevent oxidationReduced amino acid contentOrganic compound preparationOrganic chemistry methodsCarbocisteineAntioxidant
The invention discloses a preparation method of carbocisteine. The preparation method comprises the three steps of condensation reaction, neutralization and crystallization, and recrystallization. Through adding carbonate and an antioxidant to a reaction system and strictly controlling reaction conditions, the content of impurity amino acid in a finished product is reduced substantially, and the purity and the product quality of carbocisteine are improved. The preparation method has the advantages of simplicity in operation, short production cycle, low cost, and the like.
Owner:WUHAN GRAND HOYO
Synthetic method of carbocisteine
ActiveCN105418471AReduce processEliminate consumptionOrganic compound preparationSulfide preparationCarbocisteinePhysical chemistry
The invention relates to a synthetic method of carbocisteine, wherein a two-pot reaction process in the prior art is changed into a one-pot reaction process, thereby simplifying the process and reducing devices. A low-temperature nitrogen protective process is changed into a normal-temperature nitrogen-free protective process, thereby reducing material and energy consumptions. A two-step crystallization process is changed into a one-step crystallization process, thereby reducing processes and devices and increasing the yield.
Owner:宜昌三峡普诺丁生物制药有限公司
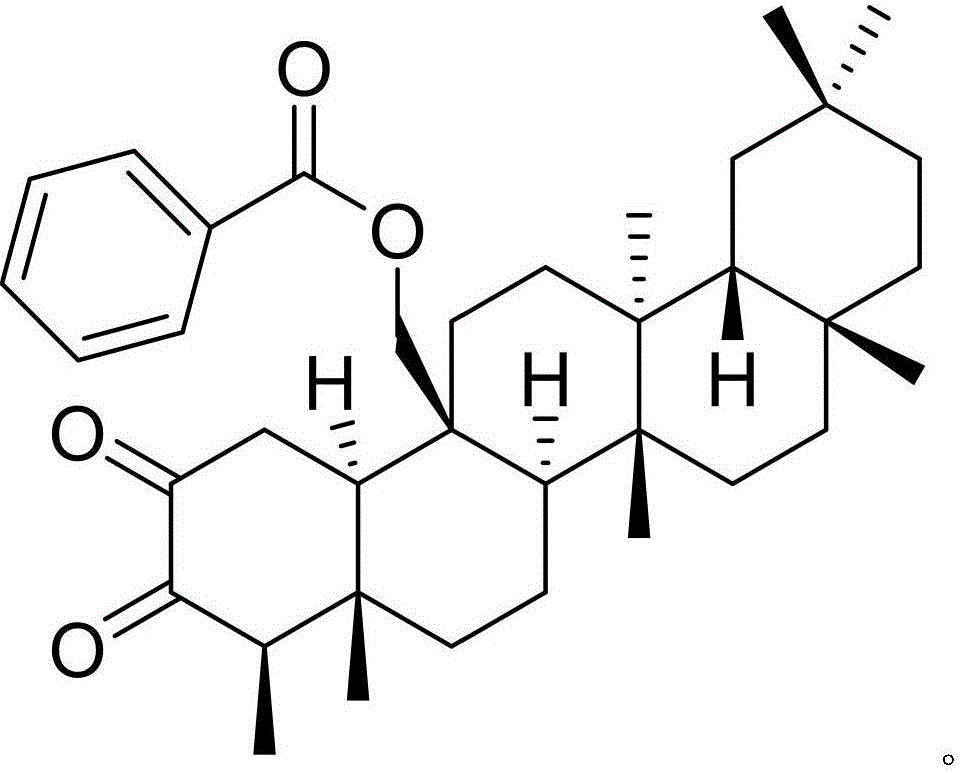
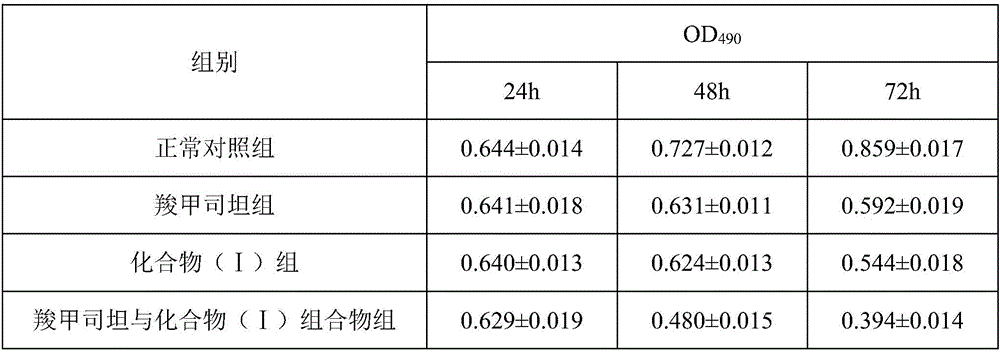
Medicinal composition of carbocisteine and treatment effects of medicinal composition for leukemia
InactiveCN106074561AHas therapeutic effectGood treatment effectOrganic active ingredientsPteridophyta/filicophyta medical ingredientsNatural productCarbocisteine
The invention discloses a medicinal composition of carbocisteine and treatment effects of the medicinal composition for leukemia. The medicinal composition of carbocisteine provided by the invention contains carbocisteine and a natural product compound (I) which is obtained by separating the dried folium pyrrosiae and has the novel structure, when carbocisteine and the natural product act independently, certain treatment effect can be achieved on leukemia, when carbocisteine and the natural product are mixed for use, the treatment effect for leukemia is more obvious, the medicinal composition can be developed into the medicine for treating leukemia, and compared with the prior art, the medicinal composition has outstanding substantive features and obvious progress.
Owner:赵吉永
Biological curing agent for natural rubber latex
The invention relates to a biological curing agent for natural rubber latex. A formula of the biological curing agent comprises the following components in parts by weight: 0.5-2.0 parts of water, 8-10 parts of glacial acetic acid, 0.3-0.7 part of citric acid and 0.009-0.02 part of carbocisteine. The biological curing agent is prepared by the following steps of: after the components are compounded into the formula, adding 500-800 parts of natural rubber latex, fully stirring to be uniform and curing. The biological curing agent has the beneficial effects that not only are no toxicity and harm caused, but also the environment is not polluted, the molecular structure of the rubber is not damaged, and the quality and the dry content of the natural rubber latex are improved; and the use is convenient and the cost is reduced.
Owner:深圳正洋生物科技有限公司
Production process for carbocisteine buccal tablet
InactiveCN105012254AControl granularityArrive quicklyOrganic active ingredientsPill deliveryGranularityCarbocisteine
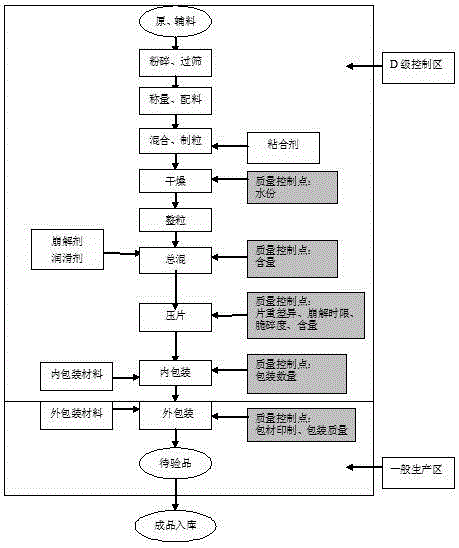
The invention relates to a production process for a carbocisteine buccal tablet. The production process comprises the following steps: crushing and sieving of raw and accessory materials; weighing and batching; mixing and granulating; drying; size stabilization; total blending; tableting; packaging; and warehousing. The carbocisteine buccal tablet produced in the invention is used for cough and difficult expectoration caused by chronic bronchitis and bronchial asthma mucus; in the production process of the carbocisteine buccal tablet, crushing granularity of the main material--carbocisteine is strictly controlled so as to ensure that all the main material is crushed and sieved with a 100-mesh sieve, and control of raw material granularity provides prerequisites for rapid absorption of the carbocisteine buccal tablet; and a high-grade material of the industry is a binder, so the appearance of the tablet surface is beautiful and rapid arrival of a drug at a designated position can be promoted.
Owner:临汾奇林药业有限公司
Carbocisteine buccal tablet
InactiveCN105012351AConvenient self-healing solutionsDoes not inhibit secretionHydroxy compound active ingredientsAlgae medical ingredientsSide effectSucrose
The invention relates to a carbocisteine buccal tablet. The carbocisteine buccal tablet is prepared from the following raw materials by weight: 15.014 to 15.563 parts of carbocisteine, 0.319 to 0.449 part of borneol, 0.0018 to 0.003 part of menthol, 0.004 to 0.005 part of aspartame, 54.470 to 56.544 parts of sucrose, 25.738 to 26.196 parts of mannitol, 0.479 to 0.598 part of PVPK 30, 0.958 to 2.394 parts of magnesium stearate, 0.006 to 0.021 part of lake and 0.035 to 0.048 of essence. The buccal tablet provided by the invention is used for cough and difficult expectoration caused by chronic bronchitis and bronchial asthma mucus, takes effect rapidly and has occasional or slight side effects (like dizziness, belching, nausea; and compared with a general dosage form, the carbocisteine buccal tablet provided by the invention does not need to be taken with water and is placed in an oral cavity, and the drug in the tablet is slowly dissolved and produces long-lasting and local effect.
Owner:临汾奇林药业有限公司
Carbocisteine syrup and preparation method thereof
InactiveCN106109396ASolve not easily soluble in waterSolve the problem of low bioavailabilityOrganic active ingredientsInorganic non-active ingredientsSolubilityCarbocisteine
The invention relates to carbocisteine syrup with improved water solubility and a preparation method of the carbocisteine syrup. The method comprises the following steps: weighing syrup substrate, adding an appropriate amount of purified water into the syrup substrate, carrying out complete boiling so that the syrup substrate is dissolved, carrying out filtering, carrying out standing for cooling till the temperature is 40-60 DEG C, and carrying out shaking till the product is uniform, thus obtaining simple syrup; weighing an active pharmaceutical ingredient, namely, carbocisteine, adding carbocisteine into an appropriate amount of water, slowly adding with an appropriate amount of sodium hydroxide solution under stirring, so that carbocisteine is completely dissolved (alkali is added according to the molar ratio of 1 to 1 for forming salt), adding with an appropriate amount of a preservative and an appropriate amount of a corrigent, and carrying out sufficient stirring; adding the carbocisteine solution into the simple syrup; enabling the relative density of the liquid medicine to be 1.08-1.25; adding with an appropriate amount of boiling water, carrying out stirring till the uniform state is reached, and carrying out filtering after the assay was approved after the liquid medicine is cooled to be 50 DEG C or below, and thus obtaining the filtrate, which is the syrup capable of relieving cough and eliminating phlegm. The method provided by the invention is simple in process and low in cost, the obtained carbocisteine syrup is good in water solubility, the method is suitable for industrial production, and thus the difficulties that carbocisteine has difficulty in dissolving in water, and the bioavailability is low are solved.
Owner:BEIJING VENTUREPHARM BIOTECH
Carbocisteine tablet preparation process and equipment
PendingCN111632034AImprove product qualityHigh yieldOrganic active ingredientsShaking/oscillating/vibrating mixersIcing sugarCarboxymethyl starch
The invention discloses a carbocisteine tablet. The carbocisteine tablet is prepared from the following components in parts by weight: 1000 parts of carbocisteine, 120 parts of corn starch, 200 partsof pregelatinized starch, 2800 parts of powdered sugar, 200 parts of low-substituted hydroxypropyl cellulose, 500 parts of 30% ethanol, 16.5 parts of magnesium stearate, 120 parts of carboxymethyl starch sodium, 120 parts of microcrystalline cellulose, 15 parts of steviosin and 3 parts of pineapple essence. Preparation equipment comprises a mixing tank, reciprocating motion mechanisms are installed on the two sides of the mixing tank respectively, a protection box is installed on each reciprocating motion mechanism, a cavity is formed in the protection box, an automatic lifting mechanism is arranged in the cavity, a supporting column is installed on the automatic lifting mechanism, and a transverse plate is fixed to the upper end of the supporting column. According to the invention, not only can the total mixing processing capacity of the carbocisteine tablet be enlarged, but also the mixing effect during total mixing can be ensured, the processing time is greatly shortened, the processing efficiency is enhanced, and the production cost is reduced.
Owner:HEBEI TIANCHENG PHARMA
Solution preparation containing carbocisteine for atomizing inhalation and preparation method thereof
InactiveCN109745301AAvoid first pass effectAvoid destructionPowder deliveryOrganic active ingredientsLiver and kidneyInhalation
The invention discloses a solution preparation containing carbocisteine for atomizing inhalation and a preparation method thereof. The solution preparation containing one dose of carbocisteine for atomizing inhalation is prepared from 20-120 mg of carbocisteine or salts and / or hydrates thereof (calculated as free carbocisteine), 0.1-5 mg of a metal complexing agent and a proper volume of a pH modifier and water for injection. The prepared solution preparation containing carbocisteine for atomizing inhalation has the advantages of high efficiency, low toxicity, good stability and high safety, and particularly reduces the medicinal toxicity in the liver and kidney of a patient greatly.
Owner:BEIJING INCREASE INNOVATIVE DRUG RESEARCH CO LTD
Compound Chemical Medicine Acting on Respiratory Disease, Preparation Process and Use Thereof
InactiveUS20140163044A1Good effectReduce incidenceOrganic active ingredientsDispersion deliveryDiseaseCarbocisteine
A pharmaceutical composition for respiratory system disease and the preparation process therefor, consisting of levodropropizine and carbocisteine as active ingredients with a pharmaceutically acceptable excipient. The present pharmaceutical composition has a marked cough-relieving effect and fewer negative reactions.
Owner:HUNAN JIUDIAN PHARMA
Preparation method of lysine carbocisteine
InactiveCN108715582AConsistent purityHigh yieldOrganic compound preparationOrganic chemistry methodsProduct systemCarbocisteine
The invention provides a preparation method of lysine carbocisteine. The preparation method comprises the following steps: mixing L-lysine with a carbocisteine water solution to generate salt formingreaction, so as to obtain a salt forming product system; and carrying out spray drying on the salt forming product system, so as to obtain lysine carbocisteine. According to the preparation method, afinished product can be obtained without separation and purification through a solvent-out crystallization method, prepared lysine carbocisteine does not contain residues of organic solvents such as methanol, ethanol and acetone, the product does not have solvent residue and is uniform in particles, the impurity is not generated during the spray drying, the purity of lysine carbocisteine is accordant with that of raw material carbocisteine, and the yield is high. According to the records of an embodiment, the yield of lysine carbocisteine is 98%, and meanwhile, a large number of organic solvents are not used in the preparation method, so that the production cost can be remarkably lowered, and the environment pressure can be reduced.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Carbocisteine oral solution and preparation method thereof
InactiveCN107714643ATaste DiscomfortSolve the problem of low bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityMedicine
The invention provides a carbocisteine oral solution and a preparation method thereof, relating to the technical field of medicine, comprising carbocisteine, glycerin, lemon juice, loquat, antioxidant, solution stabilizer, flavoring agent, and pH regulator; The method comprises the following steps: (1) taking the glycerin, lemon juice and loquat dew of the formula quantity, adding ultrapure water, and ultrasonic treatment for 10-12min; (2) adding the carbocisteine of the formula quantity into the solution in step (1) , heated to boiling, then stirred, and mixed evenly; (3) adding antioxidant, solution stabilizer, flavoring agent in boiling state, keeping boiling for 5‑8min, standing, and cooling to room temperature; (4) adding pH adjustment medicament, stir evenly, make the pH value of solution be 6.5-7.0, make carbocisteine oral solution. The method of the invention has simple process and low cost, and the obtained carbocisteine oral solution has good water solubility and is easy to industrialized production, and solves the problems of carbocisteine taste discomfort and low bioavailability.
Owner:HUAYI PHARMA ANHUI CO LTD
Hair straightening with carbocisteine
ActiveUS20160263000A1Preventing electrostatic charging and hydrophilizationCosmetic preparationsHair cosmeticsFiberHydrophilization
Methods for styling, in particular for straightening, keratin fibers, in particular human hair, in which (i) a styling agent comprising carbocisteine and / or a salt thereof is applied to the keratin fibers and left there, (ii) the fibers, after a leave-in time, —are not rinsed, —are optionally dried, (iii) the fibers are mechanically deformed from exposure to heat, significantly minimize the negative consequences of styling and reduce in particular electrostatic charging and hydrophilization of the hair.
Owner:HENKEL KGAA
Oil-controlling, anti-blackening and skin-whitening cream
InactiveCN101185622BResist irritationPrevent photoagingCosmetic preparationsToilet preparationsTherapeutic effectHyperpigmentation
The invention discloses a cosmetic which can balance oil secreting, resist sunlight and skin aging and essentially consists of rennin, honey, titanium dioxide, carbocisteine, niacin amide, vitamin E, vitamin F, cod liver oil, spironolactone and substrate cream, which are prepared according to certain weight proportion and function coordinately to achieve the effect of whitening and skincare. The invention has the triple effects including skincare, sun block and aging resistance. Firstly, the cosmetics regulates the metabolism of skin oil secretion to balance the secretion of the oil secretion, smoothen pores and reduce the occurrence rate of acne; secondly, the product effectively prevents people from being hurt by ultraviolet and reduces the settlement of melanin, thereby having good sunblock and whitening effects; finally, the product slows down the aging of skin, smoothens the skin and provides delicate and fair skin to users. The invention has scientific components, simple fabrication and unique treatment effect and is suitable for large-batch production.
Owner:孙奕
Method for separating and determining carbocisteine and impurities thereof by liquid chromatography
ActiveCN112782327AAchieve complete separation at the same timeAccurate quantitative analysisComponent separationFluid phaseCarbocisteine
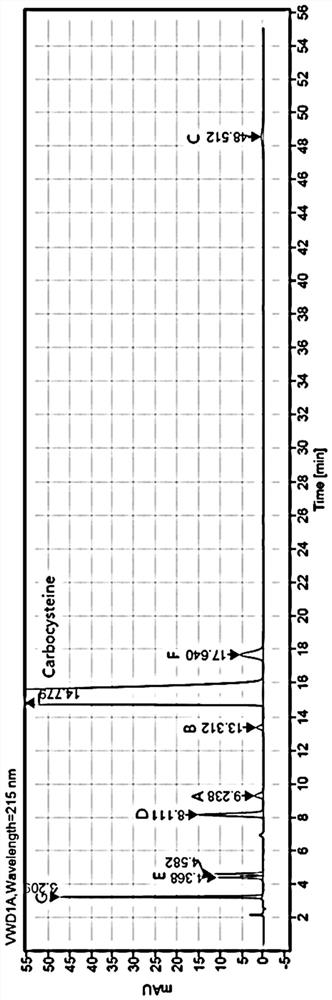
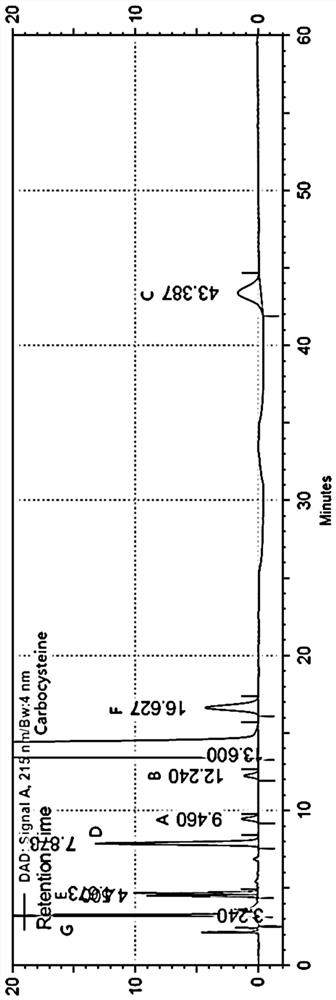
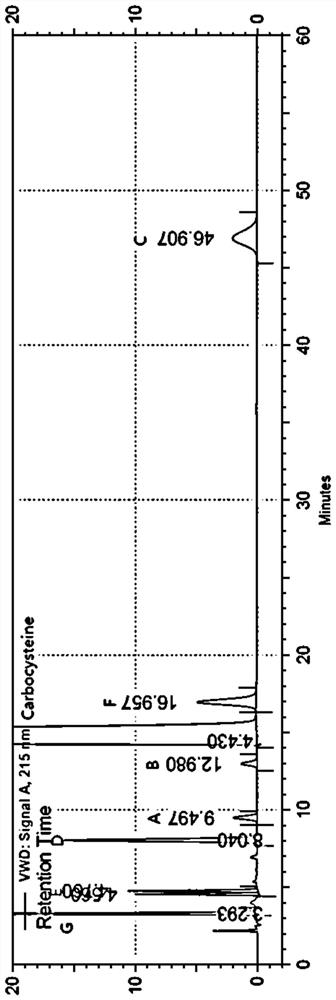
The invention discloses a method for separating and determining carbocisteine and impurities thereof by high performance liquid chromatography. The determination method adopts a chromatographic column taking octadecylsilane chemically bonded silica as a filler, and the detection conditions are as follows: chromatographic conditions: the chromatographic column is an octadecylsilane chemically bonded silica chromatographic column, a mobile phase is a phosphate-ion pair buffer solution, the pH value of the mobile phase is 1.6-2.0, and the detection wavelength is 215 nm. The method disclosed by the invention is high in precision, good in repeatability and high in recovery rate, and can be widely applied to quality detection of carbocisteine raw material medicines from different sources and corresponding preparations thereof.
Owner:广东逸舒制药股份有限公司 +3
Carbocisteine composition freeze-drying tablet and preparation method thereof
InactiveCN104546683AGood molding effectHigh dissolution rateOrganic active ingredientsPharmaceutical delivery mechanismCarbocisteineFreeze-drying
The invention provides a carbocisteine composition freeze-drying tablet and a preparation method thereof, and relates to technical fields of medicines and medicine production. The carbocisteine composition freeze-drying tablet comprises carbocisteine, starch and cane sugar, wherein the starch and cane sugar are taken as auxiliary materials; by performing heating technology processing on the common corn starch, the binding effect and disintegration effect of the starch in the tablet can be improved, the formability of the tablet can be improved, and the carbocisteine composition freeze-drying tablet only requires two auxiliary materials, namely starch and cane sugar. The carbocisteine composition freeze-drying tablet adopts a freeze-drying technology of twice cooling and twice heating up which can ensure that the formability of the tablet is better, and improves the dissolution rate of the tablet, so that the bioavailability of the tablet is improved; the tablet overcomes the disadvantage of the common carbocisteine tablet, reduces the variety and the use level of the auxiliary materials in the carbocisteine tablet, is high in dissolution rate and high in bioavailability, and guarantees the curative effect and safety of clinical medication.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Oil-controlling, anti-blackening and skin-whitening cream
InactiveCN101185622AImprove nutritional statusImprove the bactericidal effectCosmetic preparationsToilet preparationsAdditive ingredientUltraviolet A Radiation
The invention discloses a cosmetic which can balance oil secreting, resist sunlight and skin aging and essentially consists of rennin, honey, titanium dioxide, carbocisteine, niacin amide, vitamin E, vitamin F, cod liver oil, spironolactone and substrate cream, which are prepared according to certain weight proportion and function coordinately to achieve the effect of whitening and skincare. The invention has the triple effects including skincare, sun block and aging resistance. Firstly, the cosmetics regulates the metabolism of skin oil secretion to balance the secretion of the oil secretion, smoothen pores and reduce the occurrence rate of acne; secondly, the product effectively prevents people from being hurt by ultraviolet and reduces the settlement of melanin, thereby having good sun block and whitening effects; finally, the product slows down the aging of skin, smoothens the skin and provides delicate and fair skin to users. The invention has scientific components, simple fabrication and unique treatment effect and is suitable for large-batch production.
Owner:孙奕
A kind of preparation technology of carbocisteine
ActiveCN106083673BHigh purityHigh yieldOrganic compound preparationSulfide preparationAcetic acidOrganic synthesis
The invention discloses a preparation process of a compound, in particular to a preparation process of carbocisteine, and belongs to the technical field of organic synthesis. The preparation method includes following steps: (a), taking L-cysteine hydrochloride monohydrate and solid chloroacetic acid, wherein chloroacetic acid accounts for 60-70% of mass of L-cysteine hydrochloride monohydrate; (b), adding water into L-cysteine hydrochloride monohydrate and chloroacetic acid, stirring for dissolution, and controlling temperature of a system to 15-25 DEG C in the process of dissolution; (c), adjusting PH of the system to 7.0-7.5, controlling temperature of the system to 45-50 DEG C, and stirring and allowing reaction; (d), after reaction is completed, adjusting PH of the system to 6.0-6.4, and performing decoloring and impurity adsorption treatment; (e), filtering, adjusting PH of filtrate to 2.8-3.0, and cooling to obtain carbocisteine. Carbocisteine prepared by the preparation process has the advantages of high purity, high yield and high stability, and the preparation process is simple and easy to operate and control.
Owner:LUOJIANG CHENMING BIOLOGICAL PROD
A kind of synthetic method of carbocisteine
ActiveCN105418471BReduce processEliminate consumptionOrganic compound preparationSulfide preparationCarbocisteineNitrogen
The invention relates to a synthetic method of carbocisteine, wherein a two-pot reaction process in the prior art is changed into a one-pot reaction process, thereby simplifying the process and reducing devices. A low-temperature nitrogen protective process is changed into a normal-temperature nitrogen-free protective process, thereby reducing material and energy consumptions. A two-step crystallization process is changed into a one-step crystallization process, thereby reducing processes and devices and increasing the yield.
Owner:宜昌三峡普诺丁生物制药有限公司
Biological curing agent for natural rubber latex
The invention relates to a biological curing agent for natural rubber latex. A formula of the biological curing agent comprises the following components in parts by weight: 0.5-2.0 parts of water, 8-10 parts of glacial acetic acid, 0.3-0.7 part of citric acid and 0.009-0.02 part of carbocisteine. The biological curing agent is prepared by the following steps of: after the components are compounded into the formula, adding 500-800 parts of natural rubber latex, fully stirring to be uniform and curing. The biological curing agent has the beneficial effects that not only are no toxicity and harm caused, but also the environment is not polluted, the molecular structure of the rubber is not damaged, and the quality and the dry content of the natural rubber latex are improved; and the use is convenient and the cost is reduced.
Owner:深圳正洋生物科技有限公司
Stable carbocisteine pharmaceutical composition
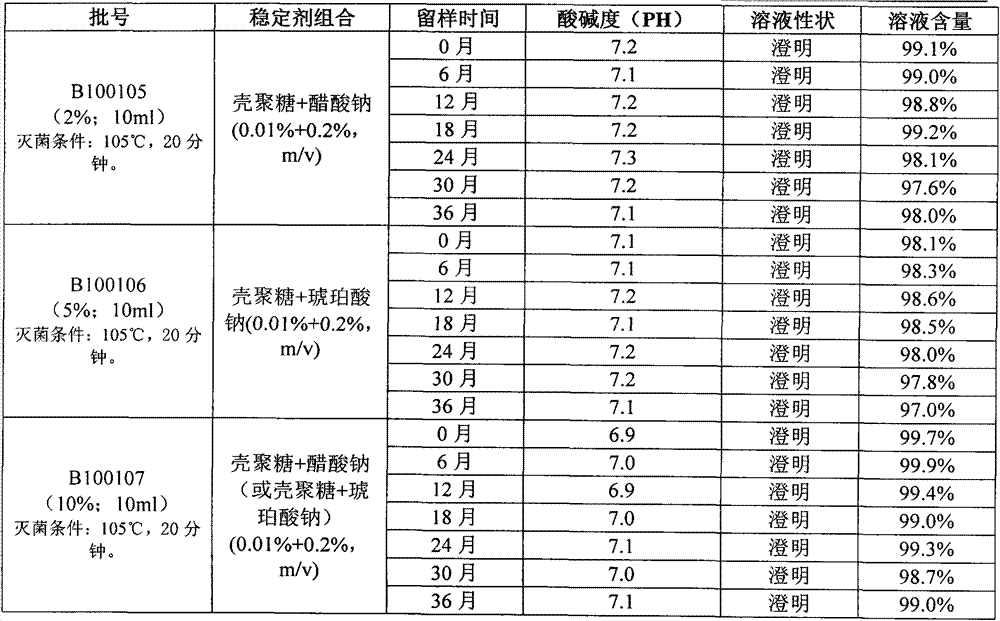
ActiveCN109045008BImprove long-term stabilityLow content of active ingredientsOrganic active ingredientsPharmaceutical delivery mechanismCelluloseMedicine
The invention belongs to the technical field of medical science, and particularly relates to a stable carbocisteine drug composition and preparation thereof. The stable carbocisteine drug compositionis prepared from, in percentage by weight: 3-10% of carbocisteine, 3-10% of sodium hydroxide and 0.05-0.2% of stabilizer, wherein the stabilizer is composed of hydroxy propyl cellulose and D-sodium gluconate at the weight ratio of 1 to (0.1-1). The stable carbocisteine drug composition has the excellent long-term stability, and can still be clear after being stored for 36 months at the temperatureof 20 DEG C in dark, the content of the effective component just decreases within 0.3-0.4%, and outstanding advancement is made compared with the prior art.
Owner:GUANGZHOU LIXIN PHARM CO LTD
Hair straightening with carbocisteine
ActiveUS10328007B2Preventing electrostatic charging and hydrophilizationCosmetic preparationsHair cosmeticsFiberHydrophilization
Methods for styling, in particular for straightening, keratin fibers, in particular human hair, in which (i) a styling agent comprising carbocisteine and / or a salt thereof is applied to the keratin fibers and left there, (ii) the fibers, after a leave-in time, —are not rinsed, —are optionally dried, (iii) the fibers are mechanically deformed from exposure to heat, significantly minimize the negative consequences of styling and reduce in particular electrostatic charging and hydrophilization of the hair.
Owner:HENKEL KGAA
Dry powder inhalation preparation of expectorant and preparation method of dry powder inhalation preparation
ActiveCN113925849AIncrease secretionReduced bioavailabilityPowder deliveryOrganic active ingredientsCarbocisteinePhospholipid
The invention discloses a dry powder inhalation preparation of an expectorant and a preparation method of the dry powder inhalation preparation, and belongs to the technical field of dry powder inhalation preparations. The dry powder inhalation preparation comprises the following raw materials of an active drug component, a carrier and an auxiliary material, wherein the mass percentage of the active drug component in the total amount is 0.1%-99.9%, the mass percentage of the carrier in the total amount is 0%-99.9%, and the mass percentage of the auxiliary material in the total amount of the raw materials is 0%-10%; the active drug component is one or two of ammonium chloride, potassium iodide, bromhexine, ambroxol, acetylcysteine, carbocisteine and tiloxpol; the carrier is lactose; and the auxiliary material is one or more of magnesium stearate, phospholipid, leucine and lysine. The prepared dry powder inhalation preparation of the expectorant can effectively improve the secretion amount of phenol red in the trachea of mice.
Owner:苏州易合医药有限公司
Granules for eliminating phlegm and stopping cough and preparation method thereof
InactiveCN108159144APromote secretionIncrease viscosityOrganic active ingredientsGranular deliveryDiseaseThick sputum
The invention relates to the technical field of soluble granules and particularly relates to granules for eliminating phlegm and stopping cough and a preparation method thereof. The granules for eliminating phlegm and stopping cough comprise 40-50 parts by weight of carbocisteine, 80 to 160 parts by weight of loquat leaf dry extract, 100 to 120 parts by weight of saccharose, 1 to 5 parts by weightof citric acid, 0.5 to 1 part by weight of saccharin sodium salt and 0 to 10 parts by weight of additive aids. Through combination of the traditional Chinese medicine loquat leaf dry paste and the western medicine carbocisteine, the acute exacerbation of chronic obstructive pulmonary disease is effectively reduced. The granules have good effects of treating thick sputum, expectoration or dyspneacaused by bronchitis and bronchial asthma, can reduce the acute exacerbation of chronic obstructive pulmonary disease and are suitable for people with high incidence of respiratory infection.
Owner:广东广发制药有限公司
Carbocisteine oral solution and preparation method thereof
ActiveCN104511025BGood water solubilityEasy to oxidizeOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantCarbocisteine
Owner:BEIJING CHENG JI PHARMA
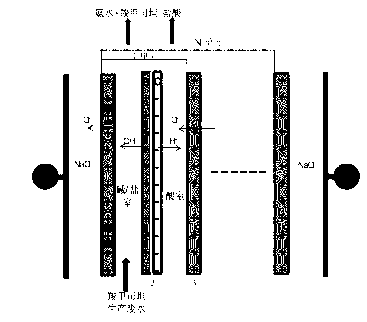
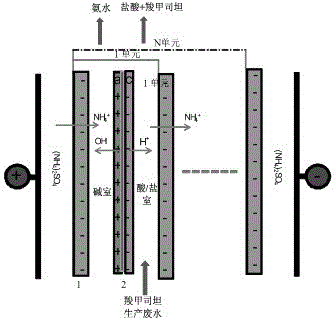
Method and system for recovering waste water generated in carbocisteine production
InactiveCN102838240BHigh yieldEliminate pollutionMultistage water/sewage treatmentNature of treatment waterCarbocisteineDesalination
The invention discloses a method and system for recovering waste water generated in carbocisteine production. The system comprises a bipolar membrane electro-dialysis desalination system and a recovering system; the recovering system comprises an acid concentrating and recovering system, a base concentrating and recovering system and a carbocisteine concentrating and recovering system. With the adoption of the system, salts in the waste water generated in carbocisteine production can be converted into corresponding acid and base so as to be removed; the obtained acid and base can be directly used in the carbocisteine production process or be used in the carbocisteine production process after an advanced treatment; the carbocisteine in the waste water is further recovered by using a carbocisteine concentrating crystallizing system. By treating the waste water generated in the carbocisteine production by using the method, organism pollution, salt pollution and ammonia nitrogen pollution are avoided; in addition, the recovered acid and base can be reused in the carbocisteine production process to realize zero emission; by using the recovered carbocisteine, the carbocisteine yield is increased and the cost of the method provided by the invention can be further reduced.
Owner:WUHAN UNIV
Carbocisteine oral solution
PendingCN114617840AImprove long-term stabilityGreat tasteOrganic active ingredientsPharmaceutical delivery mechanismOral medicationCarbocisteine
The invention belongs to the technical field of medicine, and particularly relates to a carbocisteine oral solution and preparation thereof, the carbocisteine oral solution comprises 2-3% of carbocisteine, 5-10% of pregelatinized starch, 5-20% of saccharin sodium salt, 1-3% of sodium hydroxide and 0.2-0.3% of preservative, and the preservative is sodium methyl p-hydroxybenzoate. The carbocisteine oral solution provided by the invention has better long-term stability and good taste, the pregelatinized starch increases the taste and the solubility of the carbocisteine oral solution to prevent insoluble raw materials from being separated out, the oral solution is still stable and clear after being stored for 36 months at room temperature, the content of effective components is reduced by only 0.1-0.3%, and the oral solution is suitable for oral administration. Compared with the prior art, remarkable progress is achieved.
Owner:BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com