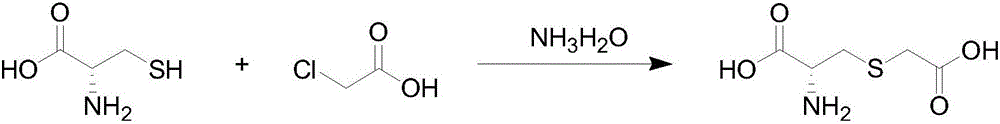
Preparation method of carbocisteine
A technology of carbocisteine and condensation reaction, which is applied in the field of preparation of carbocisteine, can solve the problems of ammonium salt or residue on ignition exceeding the standard, difficult to remove, amino acid easily exceeding the standard, etc., and achieve the reduction of impurity amino acid content, low cost, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Condensation reaction: L-cysteine hydrochloride and chloroacetic acid are mixed in a molar ratio of 1: 1.1 and dissolved in water accounting for 1.5 times of the total weight of the mixture, then continuously and slowly dripping ammonia water for condensation reaction, while Slowly add sodium carbonate powder, when the pH of the reaction system rises to 3, add 0.3% Na 2 SO 3 , when the pH of the reaction system continues to rise to 5, stop adding sodium carbonate powder, when the pH of the reaction system continues to rise to 7.3, stop dripping ammonia water to end the reaction, the temperature of the entire reaction is 55 ° C, and the reaction time is 30min. The total weight of the added sodium carbonate powder accounts for 20% of the L-cysteine hydrochloride weight.
[0024] (2) Neutralization and crystallization: add water accounting for 10 times the weight of L-cysteine hydrochloride to the system after the condensation reaction, heat to 55°C and adjust t...
Embodiment 2
[0030] (1) Condensation reaction: L-cysteine hydrochloride and chloroacetic acid are mixed at a molar ratio of 1: 1.05 and dissolved in water accounting for 2 times the total weight of the mixture, and then continuously and slowly dripping ammonia water for condensation reaction, while Slowly add sodium bicarbonate powder, and when the pH of the reaction system rises to 2.5, add 0.5% of the Na bicarbonate that accounts for the weight of L-cysteine hydrochloride in one go. 2 SO 3 , when the pH of the reaction system continues to rise to 4.5, stop adding sodium bicarbonate powder, when the pH of the reaction system continues to rise to 7, stop dripping ammonia water to end the reaction, the temperature of the entire reaction is 45 ° C, and the reaction time is 40min. The total weight of the added sodium carbonate powder accounts for 10% of the L-cysteine hydrochloride weight.
[0031] (2) Neutralization and crystallization: add water accounting for 7 times the weight of L...
Embodiment 3
[0034] (1) Condensation reaction: L-cysteine hydrochloride and chloroacetic acid are mixed at a molar ratio of 1: 1.08 and dissolved in water accounting for 1 time of the total weight of the mixture, and then continuously and slowly dripping ammonia water for condensation reaction, while Slowly add potassium carbonate powder, and when the pH of the reaction system rises to 3.5, add 0.1% Na 2 S 2 o 5 , when the pH of the reaction system continues to rise to 5.5, stop adding potassium carbonate powder, when the pH of the reaction system continues to rise to 7.5, stop dripping ammonia water to end the reaction, the temperature of the entire reaction is 60 ° C, and the reaction time is 20min. The total weight of the added sodium carbonate powder accounts for 30% of the L-cysteine hydrochloride weight.
[0035] (2) Neutralization and crystallization: add water accounting for 15 times the weight of L-cysteine hydrochloride to the system after the condensation reaction, heat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com