Erdosteine composition for treating respiratory tract inflammation
A composition and respiratory technology, applied in the field of medicine, can solve problems such as poor water solubility of erdosteine, slow drug dissolution rate, and problems with drug absorption rate or degree, and achieve high stability, improved water solubility, and stable chemical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of Erdosteine Crystals
[0035] 1) Take the crude erdosteine and add it to a methanol solution whose volume is 10-15 times the weight of erdosteine at 15-25°C;
[0036] 2) After the crude erdosteine is dissolved, add activated carbon for decolorization and filter;
[0037] 3) Add dropwise a mixed solvent of acetone and dichloromethane whose volume is 8-10 times the weight of erdosteine to the filtrate obtained in step 2) under an ultrasonic field of 0.5-0.7KW, the volume ratio of acetone and dichloromethane is 3:2, until crystallization;
[0038] 4) Turn off the ultrasonic field, cool down to -5°C at a rate of 10-15°C / hour, let stand for 3-5 hours, filter, and dry under reduced pressure at 35-45°C for 3-5 hours to obtain the erdosteine crystals.
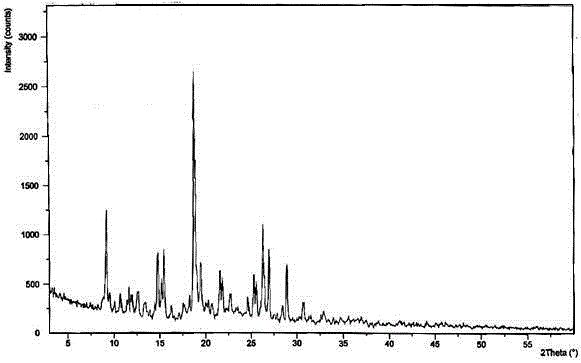
[0039] The X-ray powder diffraction pattern obtained by measuring the obtained erdosteine crystal using Cu-Kα ray is as follows: figure 1 Shown, its purity as determined by high perform...
Embodiment 2
[0040] Example 2: The preparation of erdosteine capsule, step is as follows:
[0041] Prescription such as table 1: by weight
[0042] Table 1 Prescription of Erdosteine Capsules
[0043]
[0044] Preparation:
[0045] 1) Weigh the raw and auxiliary materials according to the prescription quantity;
[0046] 2) Prepare hypromellose solution: put purified water in a stainless steel bucket, add hypromellose and polysorbate 80, and wait for all to dissolve for later use;
[0047] 3) Mixing and granulation: add erdosteine, lactose, and microcrystalline cellulose into the wet mixing granulator, turn on the stirring motor for dry mixing for 10 minutes, add the prepared hypromellose solution, and wet mix 100-150 seconds to make soft materials, choose 14 mesh nylon mesh and install it in a swinging granulator to granulate;
[0048] 4) Drying: Set the inlet air temperature of the fluidized dryer to 60°C, dry until the water content is less than 3.0%, and select a 14-mesh n...
Embodiment 3
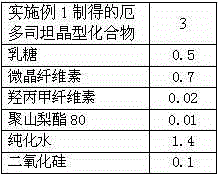
[0052] Example 3: The preparation of erdosteine capsule, step is as follows:
[0053] Prescription such as table 2: by weight
[0054] Table 2 Prescription of Erdosteine Capsules
[0055]
[0056] Preparation:
[0057] 1) Weigh the raw and auxiliary materials according to the prescription quantity;
[0058] 2) Prepare hypromellose solution: put purified water in a stainless steel bucket, add hypromellose and polysorbate 80, and wait for all to dissolve for later use;
[0059] 3) Mixing and granulation: add erdosteine, lactose, and microcrystalline cellulose into the wet mixing granulator, turn on the stirring motor for dry mixing for 10 minutes, add the prepared hypromellose solution, and wet mix 100-150 seconds to make soft materials, choose 14 mesh nylon mesh and install it in a swinging granulator to granulate;
[0060] 4) Drying: Set the inlet air temperature of the fluidized dryer to 60°C, dry until the water content is less than 3.0%, and select a 14-mesh ny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com