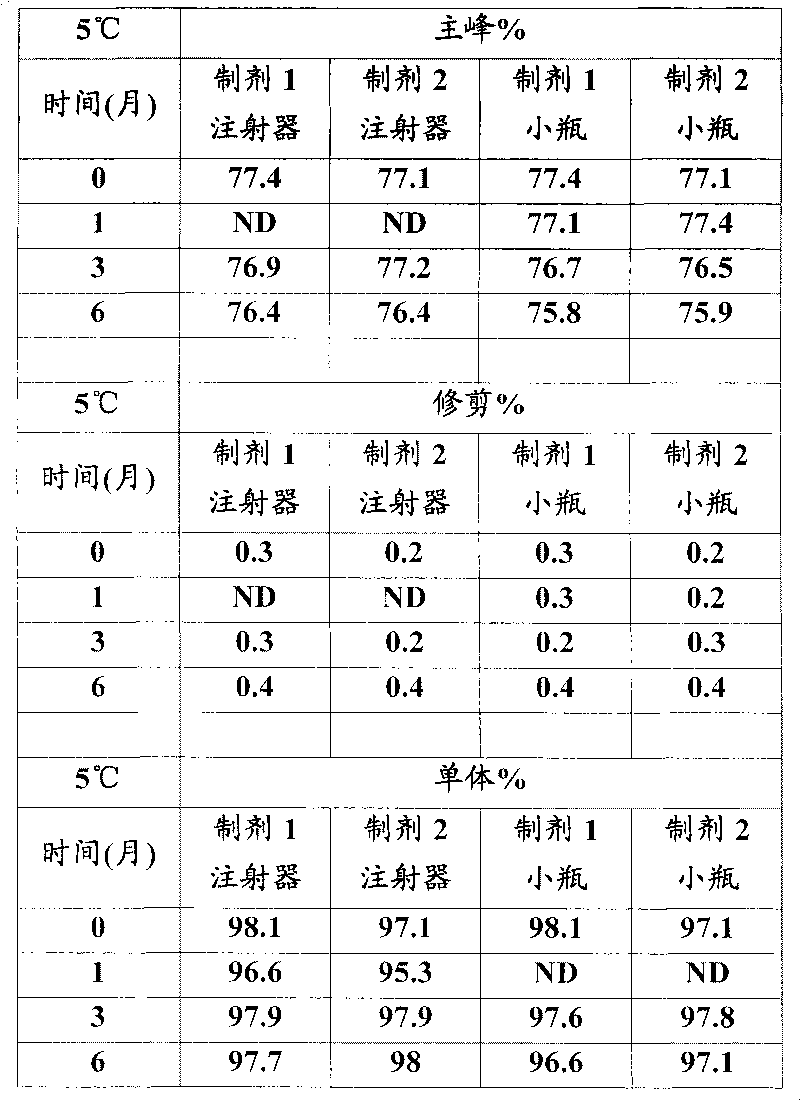
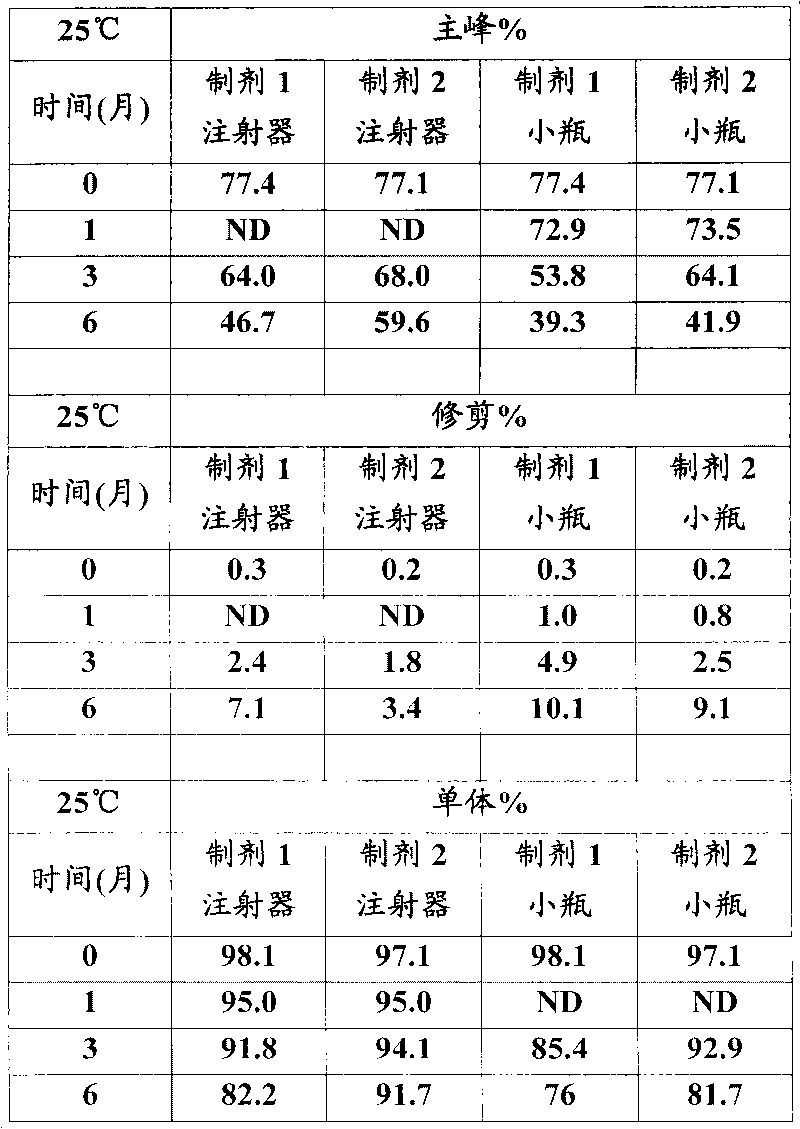
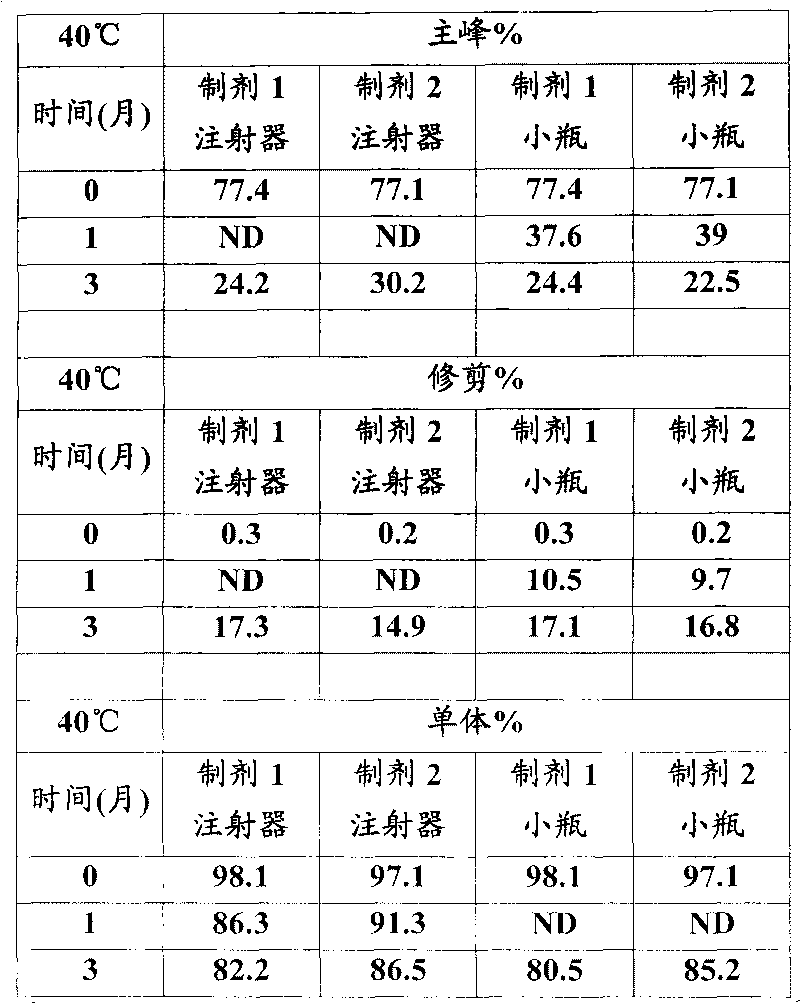
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
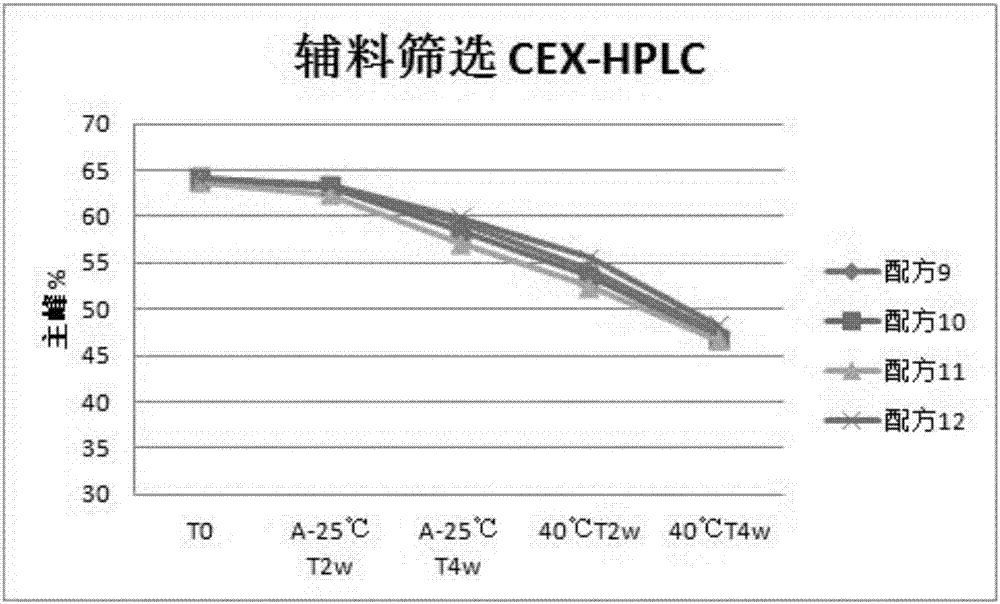
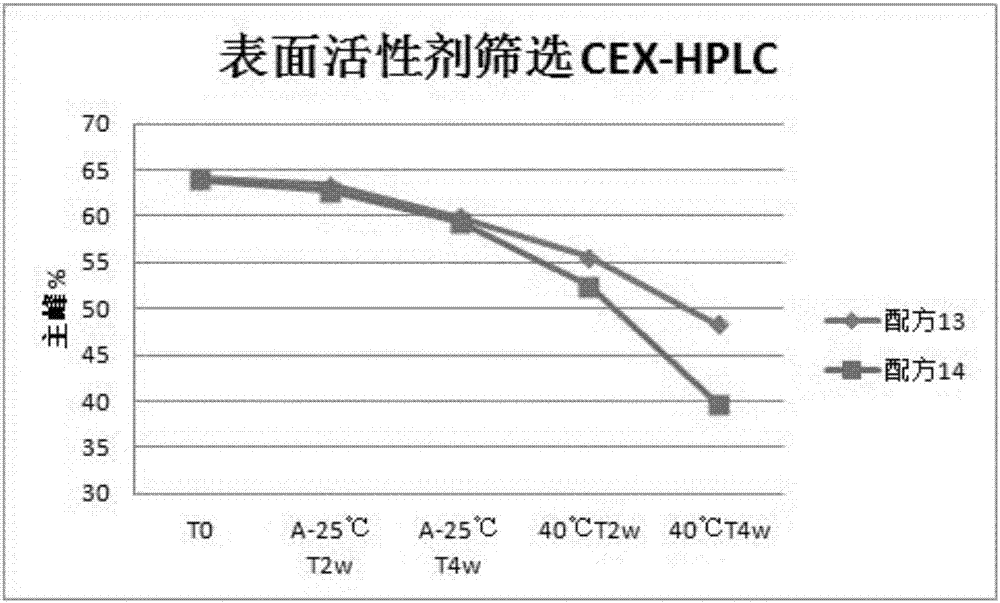
1412 results about "Polysorbate 80" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polysorbate 80 is a nonionic surfactant and emulsifier often used in foods and cosmetics. This synthetic compound is a viscous, water-soluble yellow liquid.
Injectable compositions of nanoparticulate immunosuppressive compounds
InactiveUS20060210638A1Improve complianceImprove efficacyPowder deliveryBiocideDepressantCompound (substance)
The invention is directed to an injectable nanoparticulate immunosuppressant composition for the formation of a subcutaneous or intramuscular depot. The invention is also directed to an injectable composition of nanoparticulate tacrolimus and / or sirolimus which eliminates the need to use polyoxyl 60 hydrogenated castor oil (HCO-60) and / or polysorbate 80 as a solubilizer. This invention further discloses a method of making an injectable nanoparticulate tacrolimus and / or sirolimus composition and is also directed to methods of treatment using the injectable nanoparticulate formulations comprising tacrolimus, sirolimus, or combination thereof for a subcutaneous or intramuscular depot for the prophylaxis of organ rejection and for the treatment of psoriasis or other immune diseases
Owner:ELAN PHRMA INT LTD
Ophthalmic formulations
InactiveUS6953776B2Improve bioavailabilityImprove toleranceOrganic active ingredientsBiocideCyclosporinsAqueous solution
A topical ophthalmic formulation in the form of an aqueous solution comprising a cyclosporin, hyaluronic acid or one of its salts, and polysorbate 80 is described.
Owner:LABE MEDIDOM
Immunoglobulin formulation and method of preparation thereof
InactiveUS20050053598A1Fixed volumeNervous disorderAntipyreticPharmaceutical formulationVariable weight
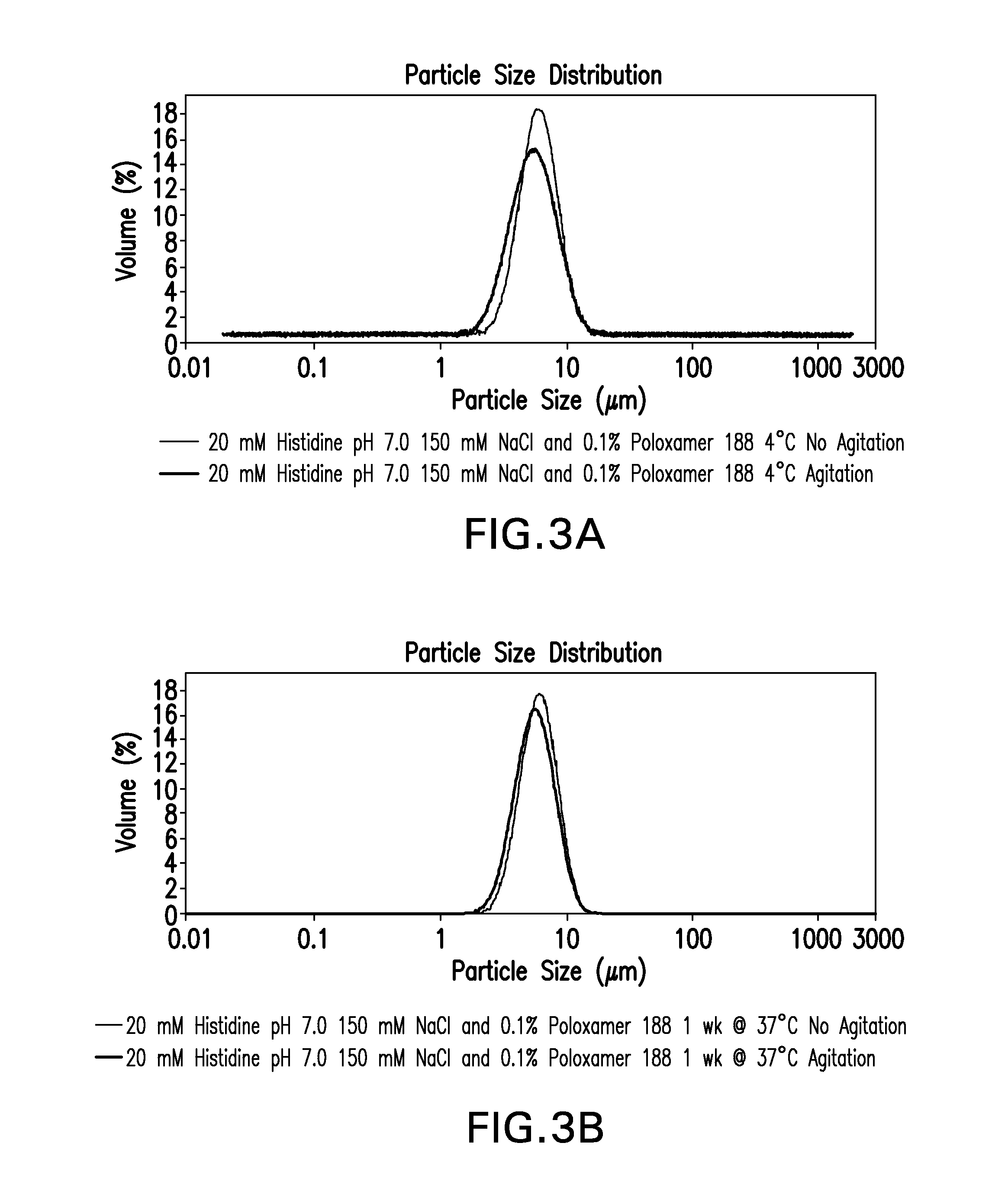
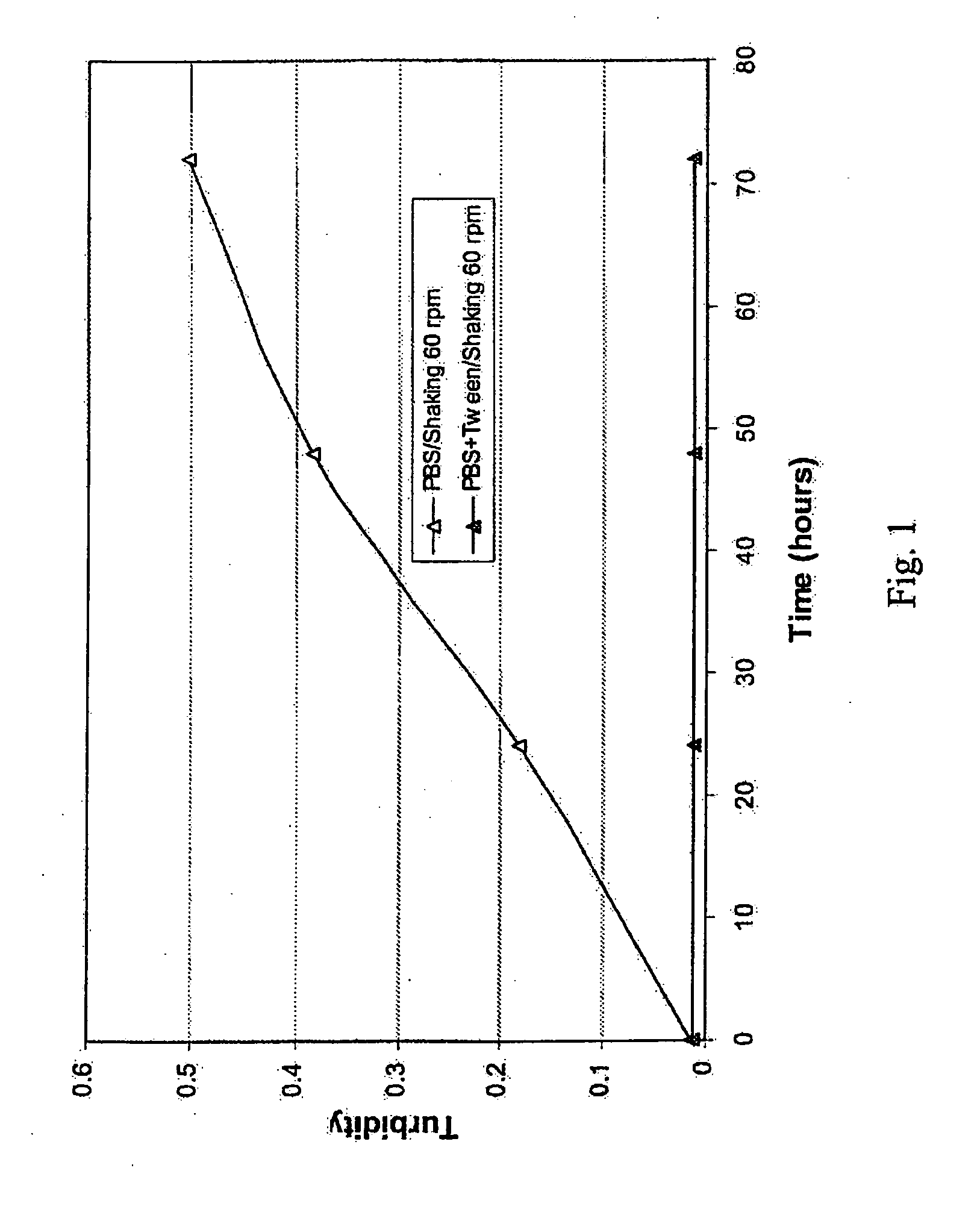
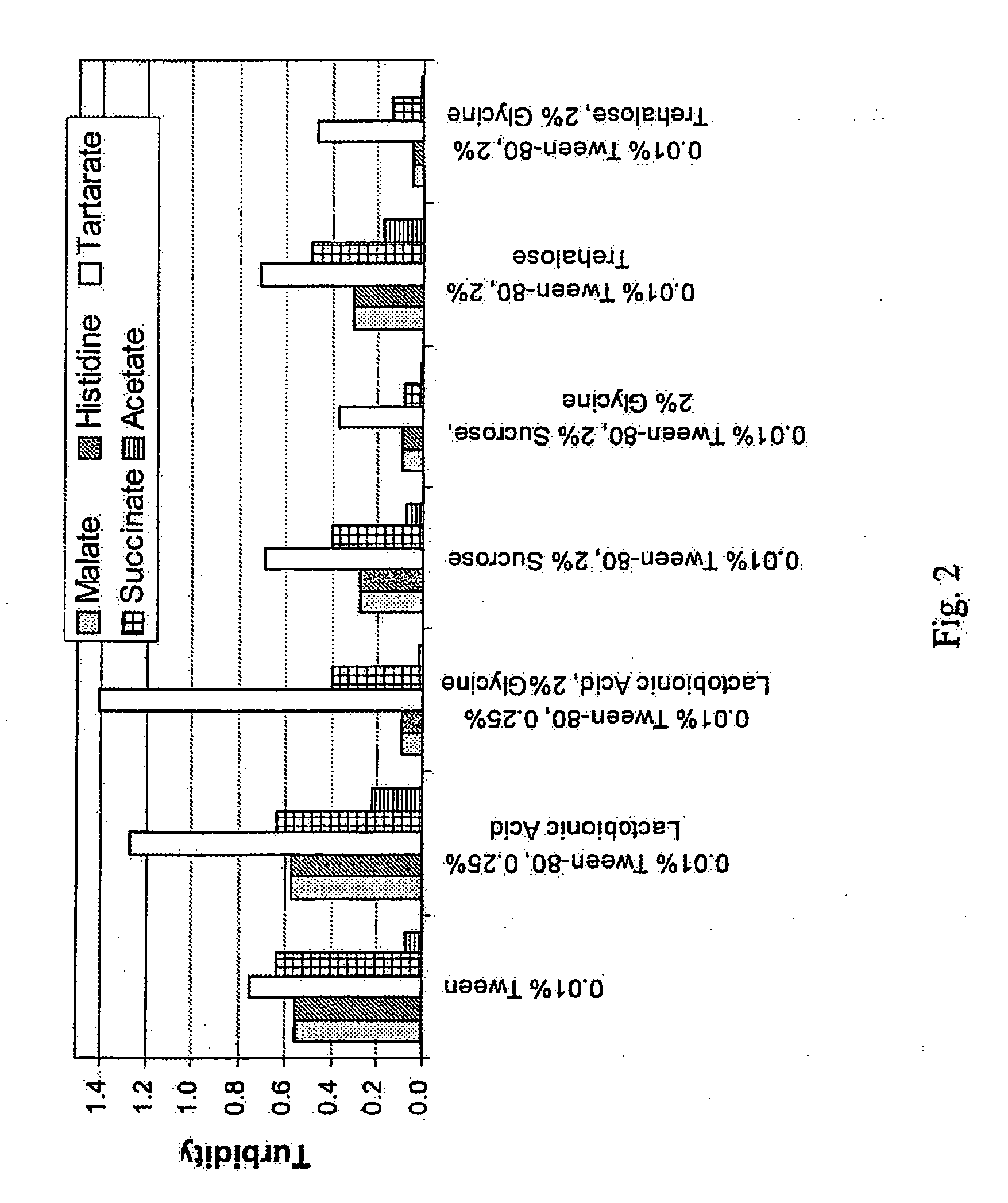
A stable aqueous pharmaceutical formulation comprising a therapeutically effective amount of an antibody, polysorbate 80, a buffer which inhibits polysorbate oxidation is described along with methods of making the preparation. Also described are formulations with high antibody concentrations which maintain fixed volumes and which may be used on patients of variable weight.
Owner:BIOGEN MA INC
Lyophilized solid taxane composition, a process for preparing said solid composition, a pharmaceutical formulation and a kit for said formulation
InactiveUS20090215882A1High level of chemical degradationImprove solid solubilityOrganic active ingredientsBiocideDocetaxel-PNPDocetaxel
A lyophilized solid composition of taxane (preferably docetaxel and paclitaxel), is suitable to prepare a pharmaceutical formulation to be administered to mammals, particularly humans, comprising a taxane, a tensoactive, a lyophilizing excipient, and acid; also essentially free from organic solvents. The solid composition is free from polysorbate 80 and polyoxyethylated castor oil; it is sterile; it is soluble in aqueous solutions in the absence of organic solvent and it has an apparent density from 0.05 g / ml to 0.45 g / ml. A procedure of double lyophilization obtains a solid composition of taxane. A pharmaceutical formulation of a taxane comprises a solid composition of lyophilized taxane and a solubilizing composition. A kit comprises the compositions and a syringe.
Owner:ERIOCHEM SA
Topical skin care formulations
InactiveUS20110044920A1Improve visual appearanceExtended maintenance periodCosmetic preparationsBiocideCaprylyl GlycolStearic acid
Disclosed is a topical skin care composition that includes water, silymarin, hydrolyzed algin, palmitoyl tripeptide 8, ceramide 2, pomegranate extract comprising pomegranate sterols, glycerin, disodium EDTA, caprylic / capric triglyceride, shea butter, C12-15 alcohols benzoate, dimethicone, glyceryl stearate and PEG 100 stearate, cetyl alcohol, stearyl alcohol, stearic acid, butylene glycol, caprylyl glycol, and (s) a mixture of acrylamide / sodium acryloyldimethyl taurate copolymer, isohexadecane, and polysorbate 80.
Owner:MARY KAY INC
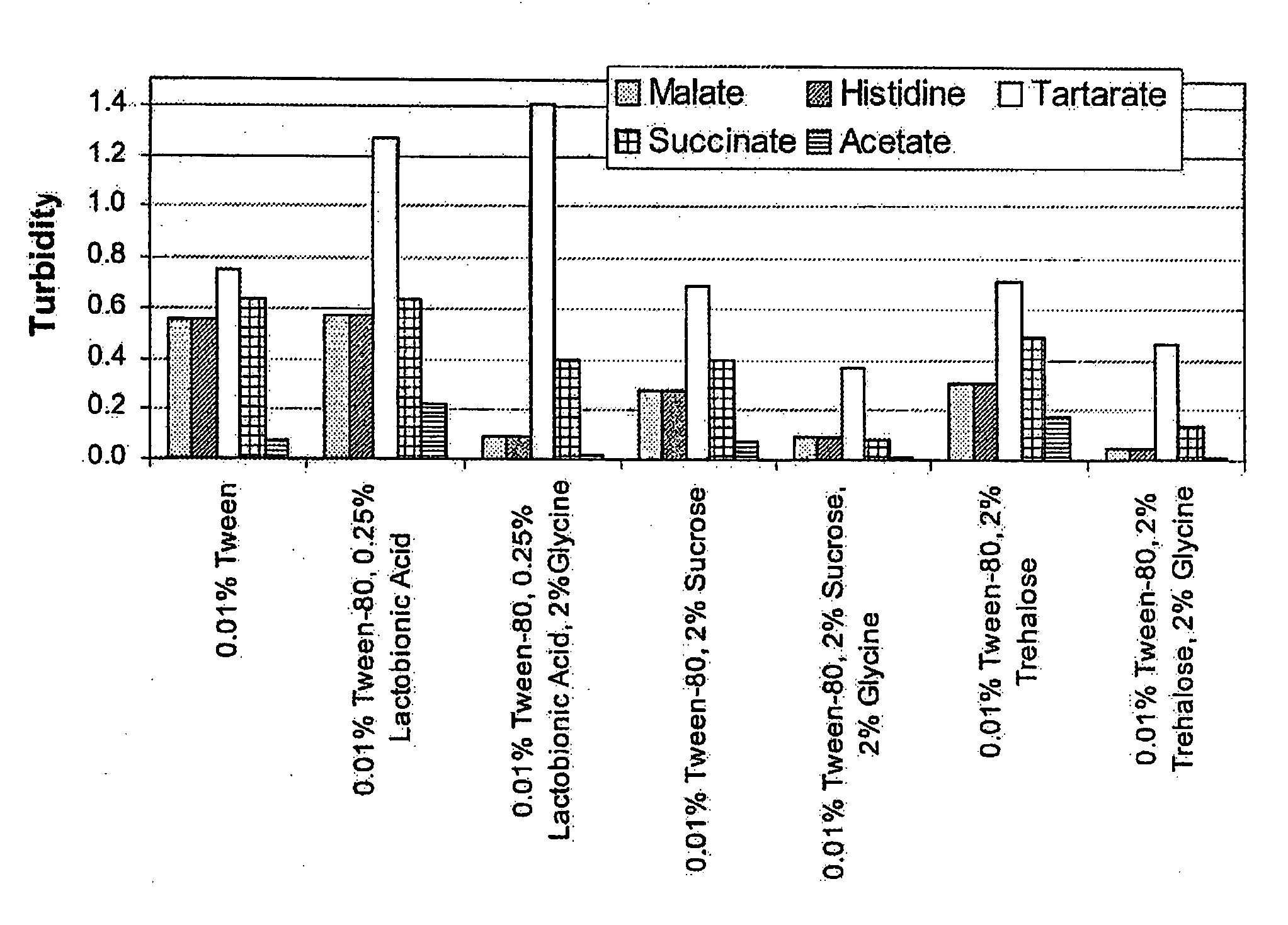
Raman spectroscopy for bioprocess operations
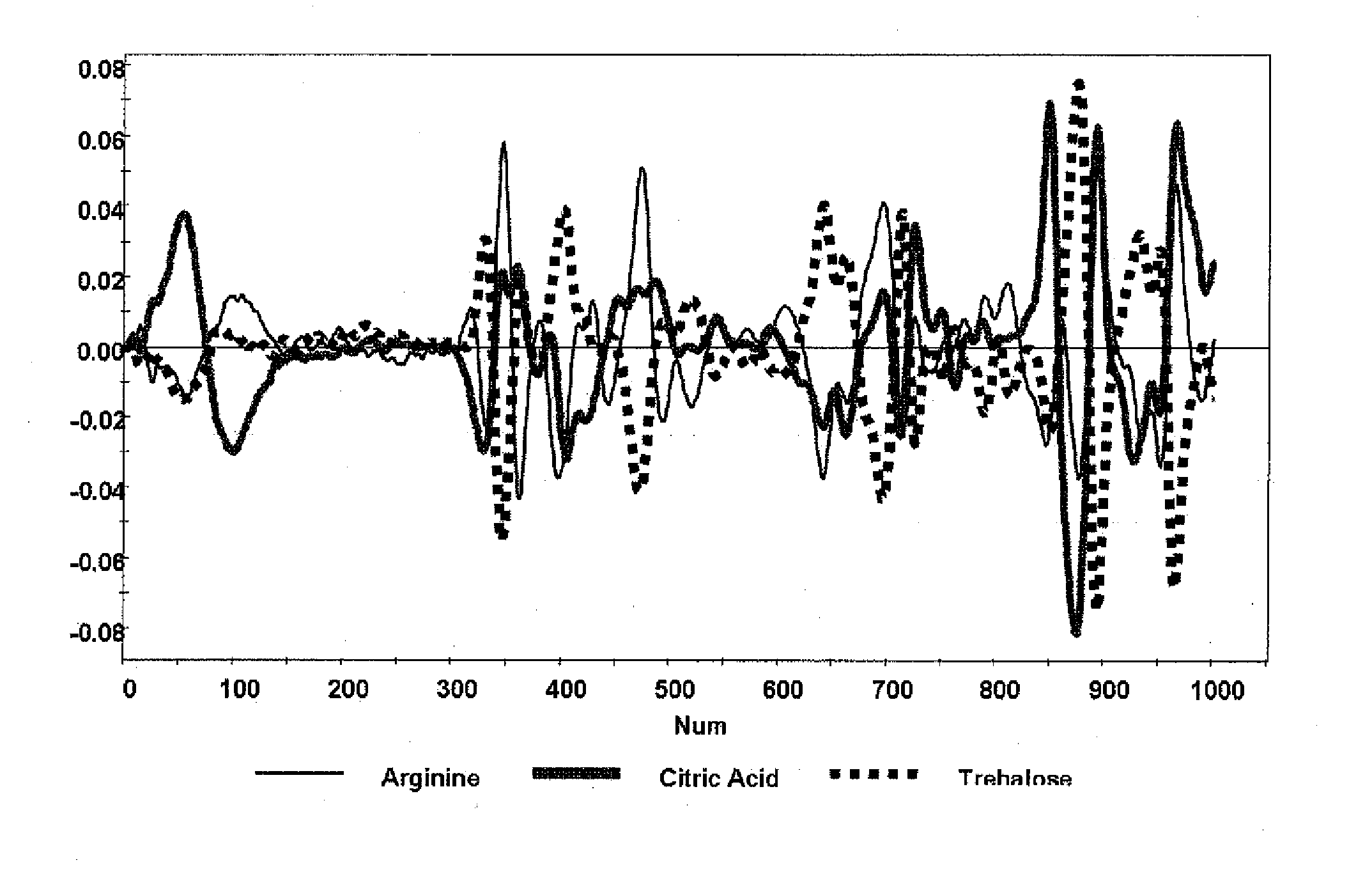
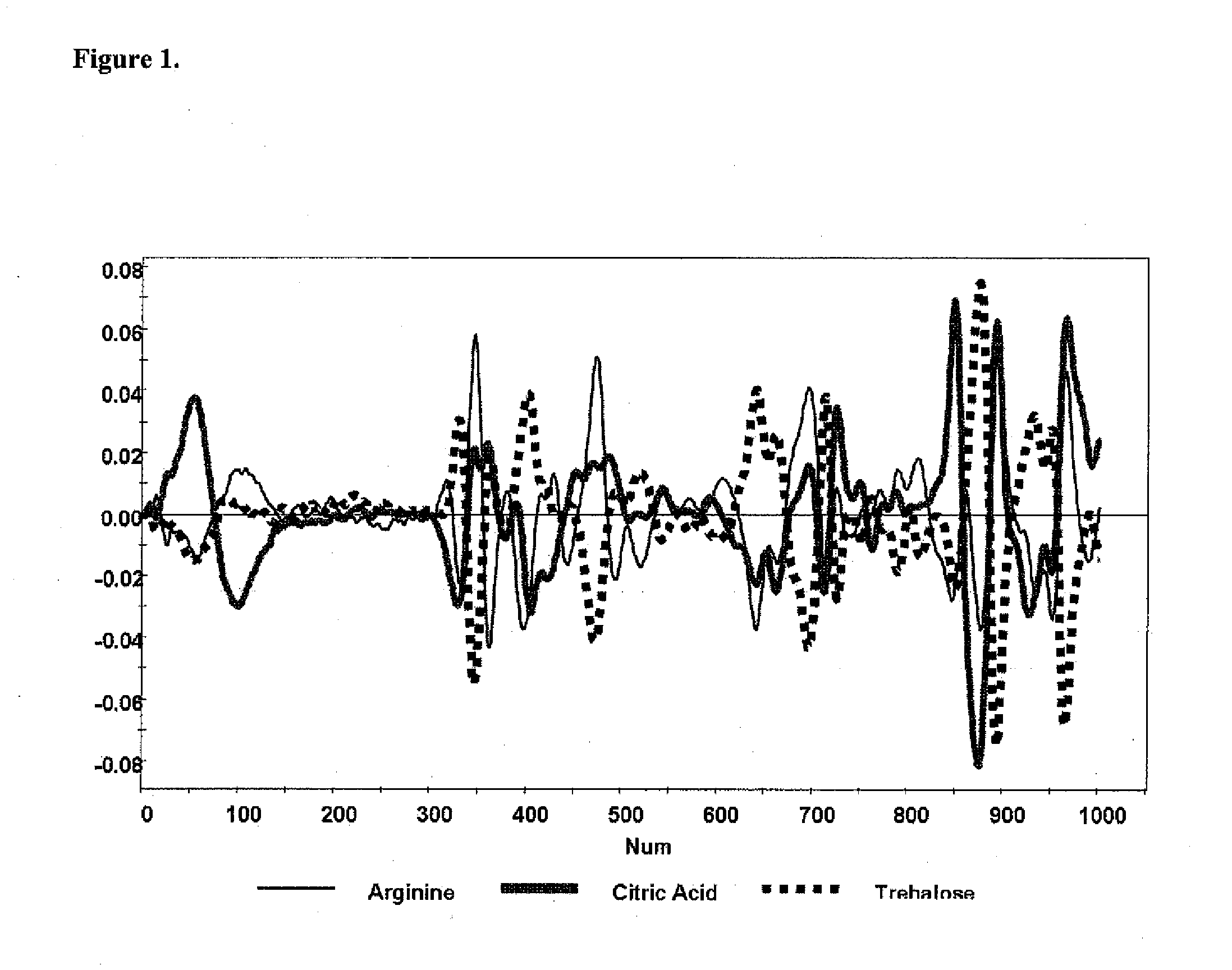
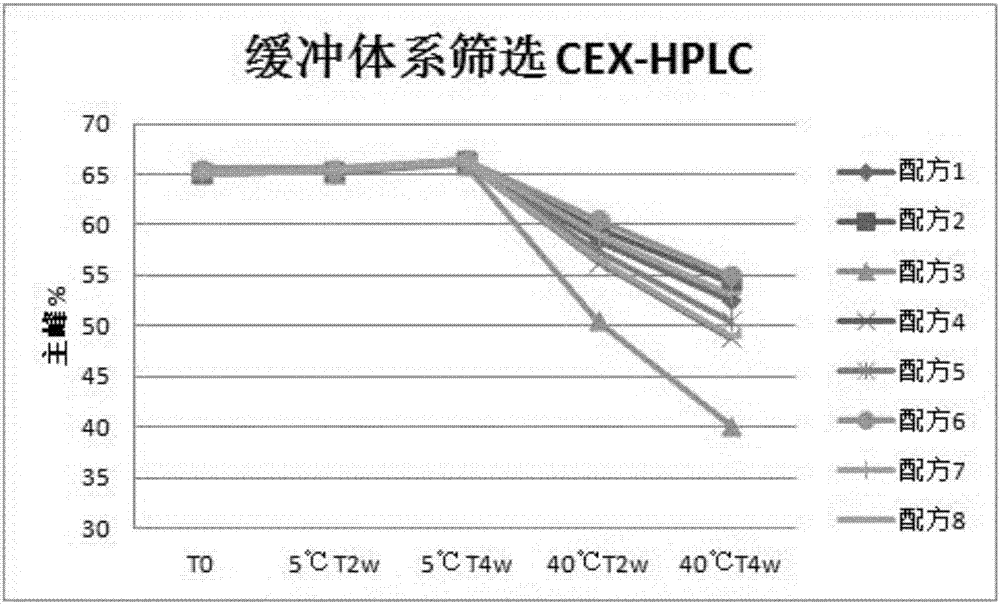
A method of characterizing a multi-component mixture for use in a bioprocess operation that includes providing a multi-component mixture standard with pre-determined amounts of known components; performing a Raman Spectroscopy analysis on the multi-component mixture standard; providing a multi-component test mixture from the bioprocess operation; performing a Raman Spectroscopy analysis on the multi-component test mixture; and comparing the analysis of the multi-component mixture standard and the multi-component test mixture to characterize the multi-component test mixture. In one embodiment, the multi-component mixture standard and the multi-component test mixture both comprise one or more of, at least two, at least three of, or each of, a polysaccharide (e.g. sucrose or mannitol), an amino acid (e.g., L-arginine, L-histidine or L-ornithine), a surfactant (e.g. polysorbate 80) and a pH buffer (e.g., a citrate formulation).
Owner:ABBVIE INC
Novel formulations which mitigate agitation-induced aggregation of immunogenic compositions
InactiveUS20130273098A1Stabilize immunogenic compositionInhibit aggregationAntibacterial agentsBiocideStreptococcus pneumoniaeCarrier protein
The present invention provides novel formulations which mitigate agitation-induced aggregation of immunogenic compositions particularly those having polysaccharide-protein conjugates. Specifically, the novel formulations comprise a poloxamer within a molecular weight range of 1100 to 17,400 which provides significant advantages over previously used surfactants including polysorbate 80. In one embodiment, the present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates and a poloxamer. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197.
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical formulation comprising taxane, a solid composition of taxane, a process for preparing said solid composition of taxane, a solubilizing composition of said solid composition of taxane, and a kit for the injectable formulation of taxane
InactiveUS20090215883A1Improve solubilityReduce usageBiocideOrganic active ingredientsSolubilityOrganic solvent
A pharmaceutical formulation of taxane intended to be administered to mammals, preferably humans, comprises two compositions combined prior to being administered, forming a transparent solution free from precipitates, in which the compositions comprise a solid composition of lyophilized taxane, free from tensoactives, oils, polymers, solubility enhancers, preservatives and excipients; and a solubilizing composition of the lyophilized taxane solid composition that comprises at least one tensoactive. This formulation is free from polysorbate 80 and polyoxyethylated castor oil. A procedure is provided for the preparation of the solid composition by means of the lyophilization of taxane in a lyophilizing organic solvent. A kit for the injectable formulation of taxane comprises a prefilled syringe. Also a pharmaceutical taxane solution for perfusion, free from organic solvent, is provided.
Owner:ERIOCHEM SA
Oxiracetam liposome injection
InactiveCN101732251AUnexpected effectNo change in colorOrganic active ingredientsNervous disorderSide effectMedicine
The invention discloses an oxiracetam liposome injection which is characterized by comprising the following components in parts by weight: 1 part of oxiracetam, 3-18 parts of phospholipid, 1-12 parts of cholesterol, 0.5-7 parts of tween 80 and an appropriate amount of osmotic-pressure regulating agent and buffering solution. The oxiracetam liposome injection has the advantages of good stability, high entrapment rate, small toxic and side effects and simple preparation, and is suitable for clinical requirements.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Gelled immunomodulating topical compositions and a method of treating warts and other human papilloma virus skin infections
Topical drug compositions of this invention contain delayed type contact sensitizing haptens in a unique non-flowable, non-toxic, non-volatile, anhydrous gel composition to achieve retained site application on warts and other human papilloma virus (HPV) skin infections. The preferred gelled compositions contain, but are not limited to, the sensitizing haptens, squaric acid dibutylester and diphenylcyclopropenone in optimized blends of Polysorbate 80, Isopropyl myristate uniquely gelled with Polyoxyl 40 stearate to form a penetrant of keratinized epitheliiuim of warts for direct application wherein virucidal pharlacologic action is induced by Th-1 cell mediated immune responses with resultant releases of CD4 helper T cells, CD8 killer T cells and cytokines to attack the human papilloma viruses. The commonly used vehicles with these contact sensitizers are acetone, petrolatum, or water containing emulsion creams which do not have the capacity to penetrate the keratinized wart surfaces and are therefore minimally effective in treating warts.
Owner:HAPTEN PHARMA
Liquid preparation of recombinant anti-PD-L1 whole-human monoclonal antibody
InactiveCN107198773AImprove stabilityEasy to administerAntibody ingredientsImmunoglobulinsMonoclonal antibodyPD-L1
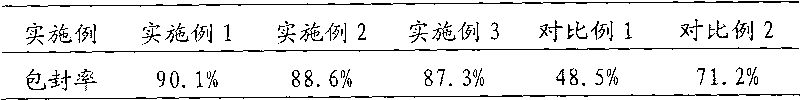
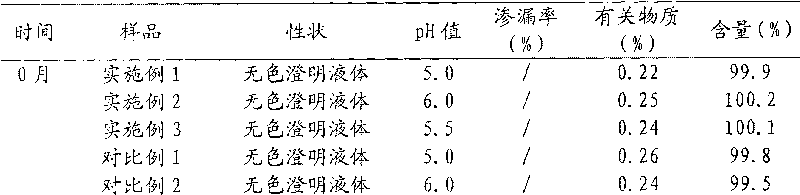
The invention discloses a stable liquid preparation of a recombinant anti-PD-L1 whole-human monoclonal antibody. The liquid preparation is prepared from a recombinant anti-PD-L1 whole-human monoclonal antibody, a buffer solution, a stabilizing agent, an osmotic pressure regulator and a surfactant, wherein the buffer solution is a histidine-histidine hydrochloride buffer solution; the stabilizing agent is mannitol; the osmotic pressure regulator is sodium chloride; the surfactant is polysorbate 80. The liquid preparation of the recombinant anti-PD-L1 whole-human monoclonal antibody has superior stability, and can be stored stably at the temperature of 5+ / -3 DEG C for at least 24 months.
Owner:CSTONE PHARM (SUZHOU) CO LTD +1
Instant lyophilized fibrinogen and fibrin ferment formulation composition, preparation method and use thereof
ActiveCN101371921ASolve the shortcoming of short storage timeImprove solubilityPeptide/protein ingredientsBlood disorderArginineIrritation
The invention provides a composition of instant freeze-dried fibrinogen and thrombin preparation, a preparation method and a use thereof, and the composition comprises composition 1 composed of 35-70mg / ml of fibrinogen, 9-15mg / ml of sodium citrate, 7-12mg / ml of sodium chloride, 0.3-0.6mg / ml of polysorbate-80, 10-15mg / ml of mannitol, 4 -8mg / ml of arginine and 3.5-5.5mg / ml of glutamic acid by mg / ml and composition 2 composed of 700-1,200 mg / ml of thrombin, 3-6mg / ml of dextran 20, 15-25mg / ml of glycine, 5-7mg / ml of sodium chloride and 4-7mg / ml of calcium chloride by mg / ml. A sealant avoids the risk of spreading of AIDS virus, and the like, increases the drug stability, reduces the degeneration during the virus inactivation process by dry-heat method, protects biological activity, avoids local liquid storage of the using part caused by high permeability and the irritation of a large amount of inorganic salts to tissues, is conductive to the healing of trauma sites and ensures product safety and independent package to the maximum extent, thereby increasing storage time, facilitating use and meeting the clinical and field first-aid needs.
Owner:HANBANG MEDICAL SCI & TECH HARBIN CITY
Racecadotril liposome solid preparation
InactiveCN102133186AImprove solubilityFast absorbingOrganic active ingredientsDigestive systemSolubilitySide effect
The invention discloses a racecadotril liposome solid preparation. The racecadotril liposome is prepared from racecadotril, soy lecithin, hydrogenated egg lecithin, cholesterol and tween 80 in the weight ratio of 1:2-8: 2-8:1.5-6:0.5-3; and the solid preparation is prepared from the racecadotril liposome and other pharmaceutically common auxiliary materials. The other pharmaceutically common auxiliary materials comprise filler, a disintegrating agent, a sweetener, an adhesive and a lubricant. The racecadotril liposome solid preparation improves the dissolubility of the racecadotril so as to improve bioavailability; a medicament is absorbed and distributed in a body fast; a curative effect is improved obviously; and the product quality of the preparation is improved and the toxic and side effects are reduced.
Owner:HAINAN MEIDA PHARMA
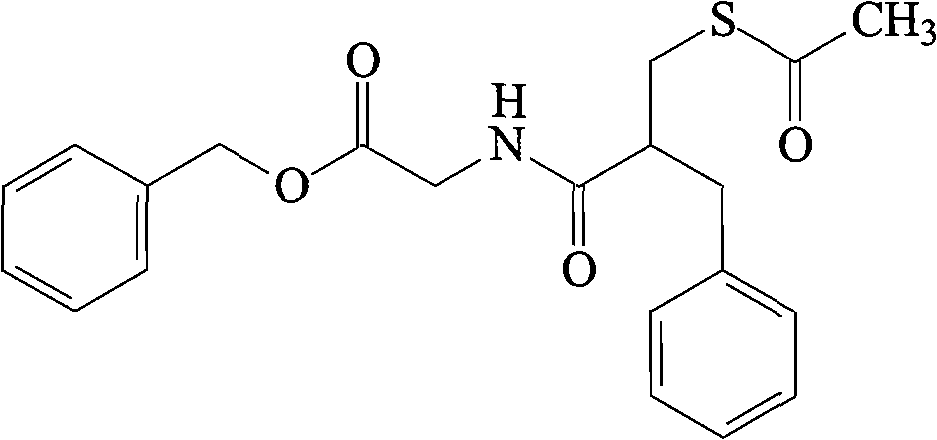
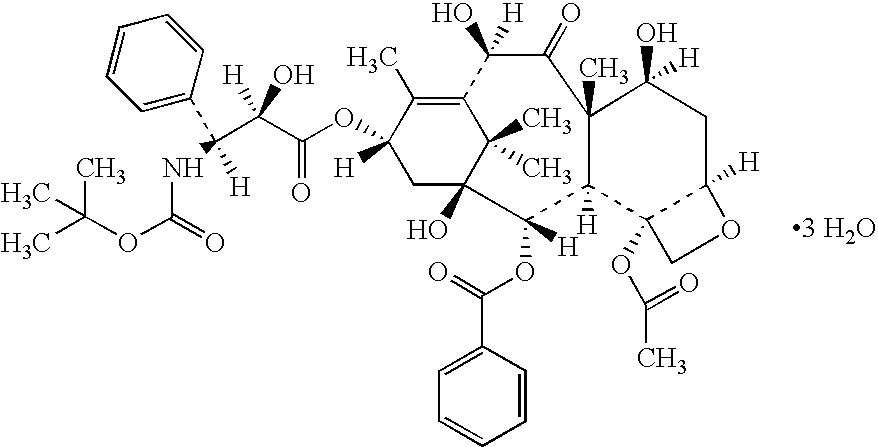
Lyophilized formulations of anti-egfr antibodies
In one embodiment, the present invention provides a stable lyophilized formulation comprising an anti-EGFR antibody, preferably cetuximab; lactobionic acid; and a buffer, preferably histidine. In one preferred embodiment, the present invention provides a stable lyophilized formulation comprising about 50 mg / mL to about 140 mg / mL of ERBITUX?, about 0.125% lactobionic acid, about 25 mM histidine buffer at a pH of about 6.0, about 0.005% Tween 80, and about 1.875% glycine.
Owner:IMCLONE SYSTEMS
Crease-resistant dressing liquid for cellulose fabric and its preparing method and use
The invention relates to anti-crease finishing liquid of cellulose fiber fabrics, the method for preparing the finishing liquid and the use of the finishing liquid. A formula of the finishing liquid comprises: 2D derivant no-ironing resin 60-160g / l, catalyst 4-8g / l, nanometer titanium dioxide 0.05-2.0g / g, dispersant 0.05-2.0g / l and penetrating agent 1.0-2.0g / l, wherein the 2D derivant no-ironing resin is modified 2D or derivant no-ironing resin, the catalyst is one of magnesium chloride, zinc nitrate, ammonium chloride and aluminum sulfate, the average grain diameter of the nanometer titanium dioxide is 20-100nm, the dispersant is one of sodium polyphosphate, PEG-1000, sodium dodecyl benzene sulfonate, sodium dodecyl sulfonate and tween 80, the penetrating agent is one of penetrating agent JFC, penetrating agent M and penetrating agent T. The method for preparing is realized through mixing with high speed according to the formula of the finishing liquid. The use of the finishing liquid adopts a twice dipping twice rolling fishing technique to carry out anti-crease finishing for the cellulose fiber fabrics.
Owner:TIANJIN POLYTECHNIC UNIV
Solubilized formulation of docetaxel without tween 80
InactiveUS20080319048A1Avoid side effectsAvoid hypersensitivityBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
Lyophilizates containing docetaxel and the use thereof in preparing concentrated liquid formulations, and ready to use formulations for injection, as well as such concentrates and ready to use formulations themselves are disclosed in which Tween surfactants are avoided so that hypersensitivity reactions to Tween surfactants can be avoided and docetaxel can be administered at higher doses and / or for longer periods of time and / or for additional treatment cycles.
Owner:SCIDOSE
Organic and inorganic composite flushing liquid as well as preparation method and applications thereof
ActiveCN102732239AImprove the interface bonding strengthImprove flushing efficiencyDrilling compositionWater basedPolyethylene glycol
The invention discloses a preparation method of an organic and inorganic composite flushing liquid, and belongs to the technique of oil well cement well cementation. The flushing liquid comprises the following components: polysorbate-80, alkyl phenol polyethylene glycol ether, dodecyl benzene sulfonic acid sodium, sodium silicate, sodium pyrophosphate and water, wherein the mass ratio of each component is as follows: 20%-30% of polysorbate-80, 10%-15% of alkyl phenol polyethylene glycol ether, 5%-10% of dodecyl benzene sulfonic acid sodium, 10%-20% of sodium silicate, 5%-10% of sodium pyrophosphate and the balance of water. The flushing liquid is suitable for both the oil-based mud and water-based mud, has high flushing efficiency, improves the replacement efficiency of a well drilling liquid and cement sheath interfacial cementation quality, and can be used under the condition of 50 DEG C-200 DEG C.
Owner:天津科力奥尔工程材料技术有限公司
Preparation method of high-purity 1,4-sorbitan
Owner:NANJING WELL BIOCHEM
Glp-1-fc fusion protein formulation
InactiveCN101730523APeptide/protein ingredientsMetabolism disorderDiabetes mellitusMANNITOL/SORBITOL
The invention provides a stable solution formulation comprising a therapeutically effective amount of a GLP-1-Fc fusion protein at about pH 6.5 in citrate buffer with polysorbate-80 and mannitol. The formulation is useful in treating diabetes and obesity as well as a variety of other conditions or disorders.
Owner:ELI LILLY & CO
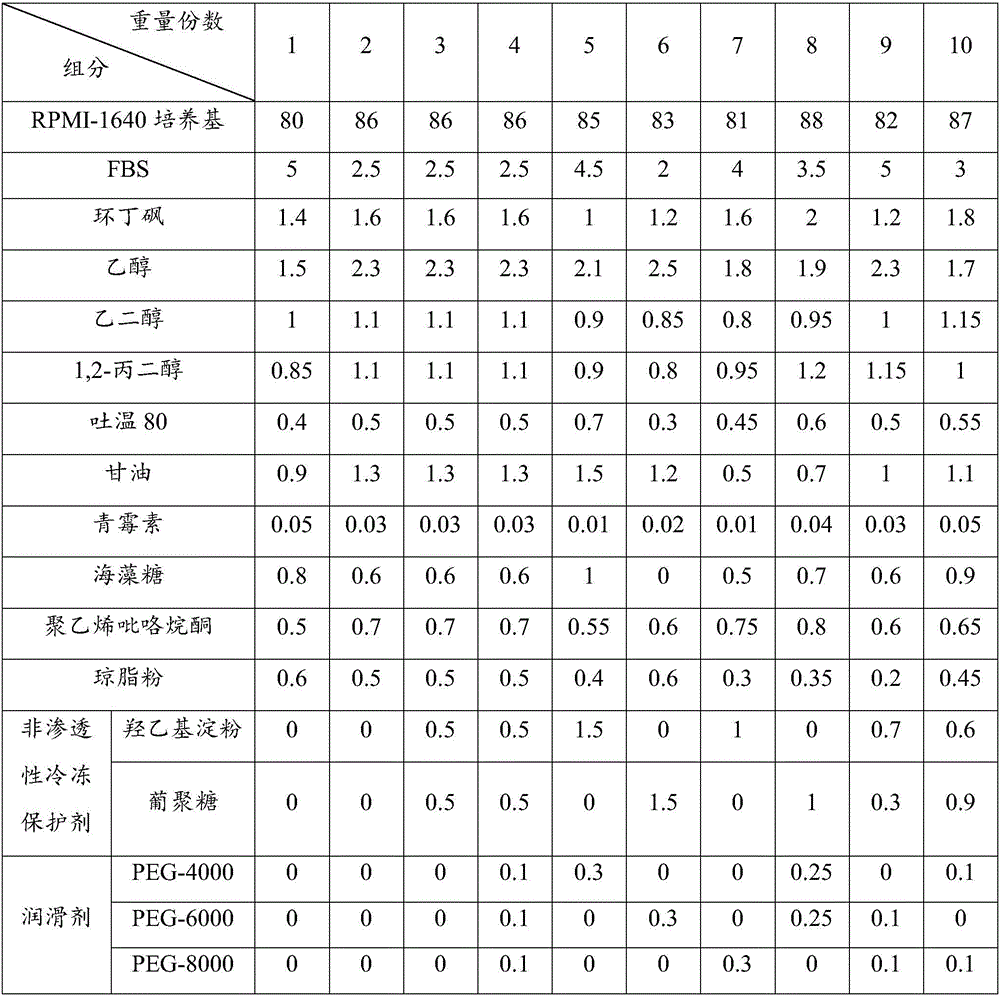
Adipose mesenchymal stem cell cryoprotectant and method for cryopreservation of adipose mesenchymal stem cells
The invention discloses an adipose mesenchymal stem cell cryoprotectant and a method for cryopreservation of adipose mesenchymal stem cells. The adipose mesenchymal stem cell cryoprotectant comprises, by weight parts, 80-88 parts of RPMI-1640 culture medium, 2-5 parts of FBS, 1-2 parts of sulfolane, 1.5-2.5 parts of 5 wt% of ethanol, 0.8-1.2 parts of 10 wt% of ethylene glycol, 0.8-1.2 parts of 10 wt% of 1,2-propylene glycol, 0.3-0.7 part of Tween 80, 0.5-1.5 parts of glycerin, 0.01-0.05 part of 50 U / mL of penicillin, 0.5-1 part of trehalose, 0.5-0.8 part of polyvinylpyrrolidone and 0.2-0.6 part of agar powder. The adipose mesenchymal stem cell cryoprotectant has the advantages of having no toxicity to the adipose mesenchymal stem cells and being good in cryopreservation effect.
Owner:浙江译美生物科技有限公司
Composition with efficacies of whitening, moisturizing, repairing and caring skin, as well as preparation method and applications of composition
InactiveCN107411982AIncrease brightnessFirming and Lifting ContourCosmetic preparationsToilet preparationsArginineCuticle
The invention relates to a composition with the efficacies of whitening, moisturizing, repairing and caring the skin, as well as a preparation method and applications of the composition. The skin care composition is prepared from the following raw materials: water, sodium hyaluronate, sodium dihydrogen phosphate dihydrate, potassium chloride, sodium hydroxide, calcium chloride, glutamine, benzalkonium chloride, magnesium sulfate, aminobutyric acid, sodium ascorbate, alanine, arginine, lysine hydrochloride, valine, histidine, leucine, taurine, coenzyme A, alcohol, polysorbate 80, thiamine, disodium diphosphate, recombinant human epidermal cell growth factors and the like. The skin care composition disclosed by the invention can permeate the muscle bottom for replenishing water for the skin from the deep layer, so that the skin is full of elasticity, the color of the skin is brightened, the fine wrinkles are reduced, the skin is clean and watery, the balance of water and oil is regulated, the pores are shrunk, meanwhile, the brightness of the face can be increased, the injured epidermal layer is effectively repaired, the activity of tyrosinase is inhibited, the generation of skin melanin is reduced, so that the skin is healthy, natural and white.
Owner:刘毅
Extraction method for astragalus polysacharin
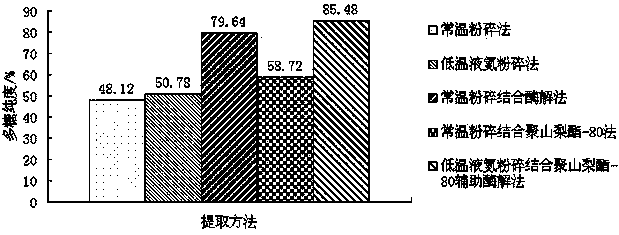
The invention relates to the health care food processing technology field, and concretely relates to an extraction method for astragalus polysacharin. In the method, polysacharin is extracted by utilization of low temperature liquid nitrogen crushing, non-ionic surface active agent auxiliary and plant compound enzyme enzymolysis. The method is advantaged by easily available raw materials, simple technology, and convenient operation and is suitable for large-scale industrial production. On the one side, the method decreases extraction times, raises yield and purity of the polysacharin, and lowers the production cost, on the other side, the method maintains the stabilization of structures and properties of the polysacharin, and raises trophism, safety and acceptability of the astragalus polysacharin greatly. In addition, the added non-ionic surface active agent is preferably polysorbate-80, polysorbate-80 has promotion effects on cellulase and pectase in hydrolytic process, and has solubilizing effects on the polysacharin at the same time. The pretreatment time and the extraction time are shortened, the time cost is lowered, and economic benefits of enterprises are raised.
Owner:越好生物科技(广州)股份有限公司
Carboxymethyl chitosan fruit preserving coating agent and preparation method thereof
InactiveCN101120697AReduce respirationSmall water absorptionFruits/vegetable preservation by coatingWater bathsGlycerol
A carboxymethyl chitosan fruit fresh-keeping coating agent in the technical field of food engineering and a preparation method thereof. The fresh-keeping coating agent is: 0.5%-4% of carboxymethyl chitosan, 0.1-1% of glycerin, 0.3-2.4% of Tween 80, 0.1-1% of DL-α-tocopheryl acetate, 92% of water -99%. Heat water in a water bath to constant temperature, add carboxymethyl chitosan, stir until completely dissolved, then add glycerin, stir well with Tween, then add DL-α-tocopheryl acetate and stir until completely dissolved, let it sit at room temperature Cooling, followed by filtering to remove undissolved substances, degassing under vacuum, standing overnight at room temperature, degassing again under vacuum, standing at room temperature, and obtaining carboxymethyl chitosan fruit fresh-keeping coating agent. The fresh-keeping coating agent of the present invention is coated on the surface of fruits by soaking or spraying to form a fresh-keeping film and maintain the quality of the fruits during storage.
Owner:上海伊禾农产品科技发展股份有限公司
Injectable compositions of nanoparticulate immunosuppressive compounds
The present invention relates to an injectable nanoparticulate immunosuppressant composition for the formation of a subcutaneous or intramuscular depot. The invention is also directed to an injectable composition of nanoparticulate tacrolimus and / or sirolimus which eliminates the need to use polyoxyl 60 hydrogenated castor oil (HCO-60) and / or polysorbate 80 as a solubilizer. This invention further discloses a method of making an injectable nanoparticulate tacrolimus and / or sirolimus composition and is also directed to methods of treatment using the injectable nanoparticulate formulations comprising tacrolimus, sirolimus, or combination thereof for a subcutaneous or intramuscular depot for the prophylaxis of organ rejection and for the treatment of psoriasis or other immune diseases.
Owner:ELAN PHRMA INT LTD
Preparation method of starch grafted acrylamide flocculating agent
InactiveCN101704928AHigh solid contentHigh molecular weightWater/sewage treatment by flocculation/precipitationParaffin waxEmulsion
The invention provides a preparation method of a starch grafted acrylamide flocculating agent, which comprises the following concrete step of polymerizing starch and acrylamide in a reversed emulsion formed by liquid paraffin, water and a compounded emulsifying agent to form a graft copolymer of the starch and the acrylamide, wherein potassium permanganate is used as an initiator, and the compounded emulsifying agent comprises span 80, Tween 80 and alkylphenol polyoxyethylene (4) in a mass ratio of 7-9:0.1-0.5:0.9-2.5. The method adopts the compounded emulsifying agent comprising the span 80, the Tween 80 and the alkylphenol polyoxyethylene (4) for preparing the reversed emulsion, thereby stabilizing the system of the reversed emulsion, providing a stable reaction environment for polyreaction, and being helpful for improving the stability of products. In addition, in the method, the potassium permanganate is also selected as the initiator, thereby effectively improving the efficiency of initiating the polyreaction.
Owner:GUANGZHOU UNIVERSITY
Cabazitaxel injection and preparation method thereof
InactiveCN102068407AEasy to makeReduce adverse outcomesOrganic active ingredientsPharmaceutical delivery mechanismMedicineCabazitaxel
The invention provides cabazitaxel injection. The injection of each milliliter comprises the following components by weight: 10 to 100 milligrams of cabazitaxel, 250 to 800 milligrams of polysorbate 80, 200 to 600 milligrams of absolute ethanol, and 0.05 to 20 milligrams of citric acid. The cabazitaxel injection is a single preparation, does not need to be previously dissolved and prepared by using a special solvent, and can be directly diluted for administration; compared with the conventional preparation technology, the preparation has simple preparation process, low cost and good stability; meanwhile, the preparation shortens the time for preparing the medicinal liquor, improves the stability of clinical medicinal liquor, enhances the accuracy of administration dose, and reduces toxic or side reaction; and the preparation simplifies the preparation process of medicinal personnel, and reduces the risks of the preparation process on harm to the medicinal personnel and environmental pollution.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
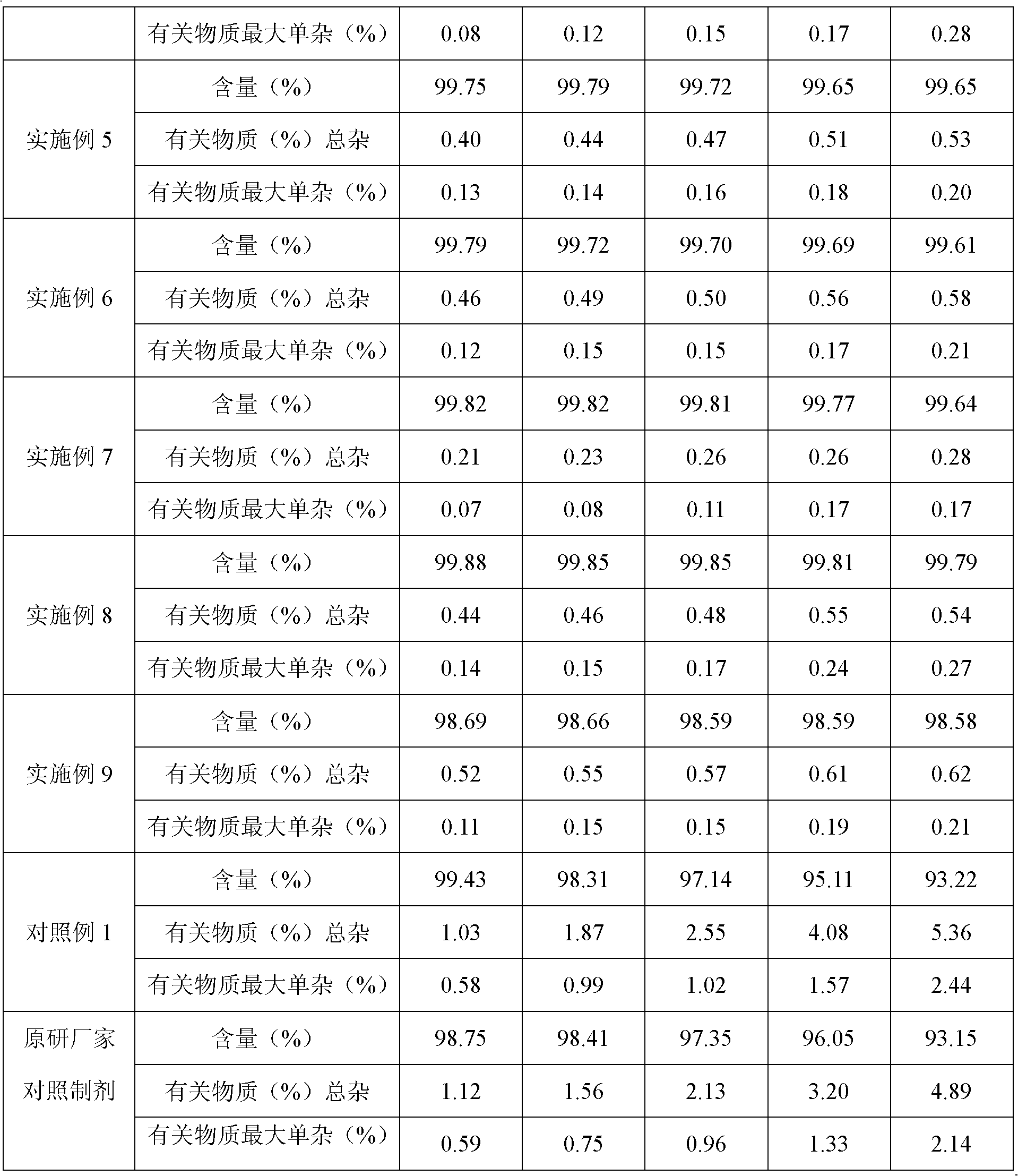
Hydrochloric acid Fasudil liposome injection and new application thereof
InactiveCN101601654AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsSkeletal disorderLeft vertebral arteryCervical spondylosis
The invention discloses hydrochloric acid Fasudil liposome injection and new application thereof. The injection mainly comprises the following components according to parts by weight: 1 part of hydrochloric acid Fasudil, 2 to 20 parts of phospholipid, 0.5 to 10 parts of cholesterol and 1 to 8 parts of polysorbate 80. Simultaneously, the hydrochloric acid Fasudil liposome injection also can be used for treating vertebral artery type cervical spondylosis.
Owner:HAINAN LINGKANG PHARMA CO LTD
Makeup-free face cream containing enriched emulsion and preparation method of makeup-free face cream
InactiveCN104825371ANatural makeupIncrease moisture contentCosmetic preparationsToilet preparationsAluminum silicateButanediol
The invention provides a makeup-free face cream containing enriched emulsion and a preparation method of makeup-free face cream. The makeup-free face cream comprises, by weight percentage, 10.0-30.0% of enriched emulsion and 70.0-90.0% of liquid foundation, wherein the enriched emulsion comprises butanediol, polyglutamic acid, trehalose, sodium acrylate / acrylyl dimethyl sodium taurate polymer, isohexadecane, polysorbate-80, cyclopentyl siloxane (and) dimethiconol, moringa pterygosperma extracts and the like; the liquid foundation comprises physical skin whitening agent, colorant, cyclopentyl siloxane (and) trimethyl siloxy silicate ester, sorbitan sesquioleate, acrylic acid (ester) / C10-30 alkanol acrylic ester cross-linked polymer, magnesium aluminum silicate, xanthan gum, propylene glycol and the like. The makeup-free face cream is good in water and sweat resistance, natural in makeup feeling and easy to remove and has certain skin care effect.
Owner:广州智媛生物科技有限公司
Method for synthesizing high-purity polysorbate-80
ActiveCN101701065AHigh purityLight colorTransportation and packagingMixingSorbitanOleic Acid Triglyceride
The invention relates to a method for synthesizing high-purity polysorbate-80. The polysorbate-80 (I) is a partial esterified product of a sorbitan ethyoxyl compound and oleic acid. The method comprises the following steps of: (1) partially dehydrating sorbitol as a raw material under the action of an acid catalyst in a state of vacuum to obtain sorbitan (II); (2) carrying out addition polymerization on the sorbitan (II) and oxirane under the action of a base catalyst to obtain sorbitan polyethenoxy ether (III), wherein the addition number of the oxirane is 20; (3) reacting the sorbitan polyethenoxy ether (III) with the high-purity oleic acid under the action of an esterifying catalyst and refining to obtain the high-purity polysorbate-80. The invention leads the emulsifying and solubilizing performance of products to be more perfect and the quality of the products to easily meet the requirement of an injection class and has easily controlled quality and good stability; and the high-purity polysorbate-80 has lower blood dissolving rate in same concentration, and predictable and safer clinical use by being used as an auxiliary material for injection.
Owner:NANJING WELL BIOCHEM
Pharmaceutical composition for improving safety of Shenmai injection and method for preparing same
ActiveCN101518617ADelays the problem of significant drops in pHImprove stabilityPowder deliveryPharmaceutical non-active ingredientsMedicineHydroxystearic Acid
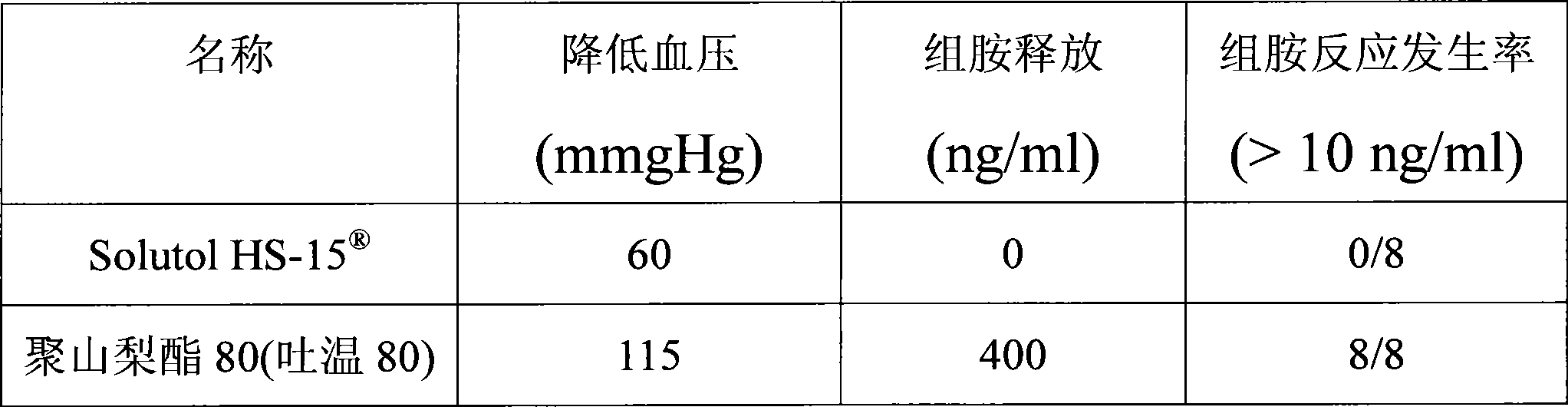
The invention discloses a pharmaceutical composition for improving the safety of Shenmai injection and a method for preparing the same. The pharmaceutical composition is a pharmaceutical composition for injection and is mainly prepared from red ginseng extract, dwarf lilyturf root extract and polyethylene glycol 12-hydroxy stearic acid ester. The pharmaceutical composition adopts a latent solvent with better safety and more obvious hydrotropy to replace the latent solvent polysorbate-80 which has potential safety hazard and influences the product quality, thus the reduction of the pH value of the Shenmai injection in the processes of storing and high temperature sterilization is obviously delayed, and the stability of the pharmaceutical composition is increased; besides, the safety of the polyethylene glycol 12-hydroxy stearic acid ester is higher than that of the polysorbate-80 and the dosage thereof is lower, thus the probability and the risk of untoward reactions of the pharmaceutical composition is reduced, and the safety for clinical application is improved.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com