Method for synthesizing high-purity polysorbate-80
A technology of polysorbate and synthesis method, applied in chemical instruments and methods, transportation and packaging, dissolution, etc., can solve the problem of poor color of polysorbate-80 (I), low purity of polysorbate-80, and product quality Difficult to control and other problems, to achieve the effect of perfect emulsification and solubilization performance, good clinical safety, and less harmful impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 sorbitan
[0045] Example 1.1 Put 3500g of 70% sorbitol into the kettle, dehydrate at 100-120°C for 3h, when the temperature is lowered to 80°C, add 120ml of 20% sulfuric acid aqueous solution, and stir the reaction for 5 hours at 750mmHg vacuum and 105-110°C; The aqueous solution of sodium hydroxide was neutralized to neutral, decolorized with activated carbon, filtered, evaporated to dryness under reduced pressure, and dried in vacuo to obtain white solid sorbitan with a hydroxyl value of 1100 mgKOH / g.
[0046] Example 1.2 is basically the same as Example 1.1, but with the following changes, 120ml of 20% aqueous sulfuric acid is replaced with 24.5g of phosphorous acid, and the hydroxyl value of the obtained sorbitan is 1092mgKOH / g.
[0047] Example 1.3 is basically the same as Example 1.1, but with the following changes, 120ml of 20% aqueous sulfuric acid is replaced with 24.5g of p-toluenesulfonic acid, and the hydroxyl value of the obta...
Embodiment 2
[0051] The synthesis of embodiment 2 sorbitan polyoxyethylene ethers
[0052] Example 2.1 Add 102g of sorbitan and 1.0g of NaOH (or KOH) solid in a pressure-resistant reactor, replace it with nitrogen and raise the temperature to 90°C, slowly add 618g of ethylene oxide, and react at 110°C and 0.4MPa , until the pressure no longer decreases, the temperature is lowered and the material is discharged, neutralized with 10g 600NS, vacuum dehydrated, filtered to obtain polyoxyethylene sorbitan ether, the measured hydroxyl value is 151.5mgKOH / g, and the chromaticity APHA<30.
[0053] Embodiment 2.2 is substantially the same as embodiment 2.1, but has following changes, replaces 1.0g NaOH (or KOH) solid with the mineral oil solution of 3.3g30% KH (or NaH), obtains sorbitan polyoxyethylene ether, measures The hydroxyl value is 149.2mgKOH / g, and the chroma APHA<20.
[0054] Embodiment 2.3 is substantially the same as embodiment 2.1, but following changes are arranged: the addition of a...
Embodiment 3
[0058] The synthesis of embodiment 3 polysorbate-80
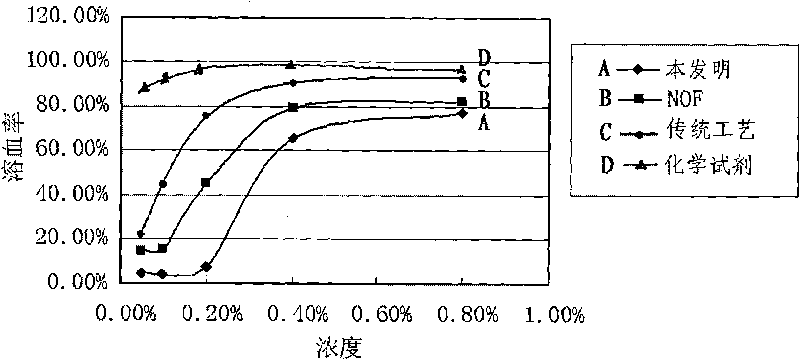
[0059] The main physicochemical data of the polysorbate-80 obtained by the following different methods are listed in Table 1.
[0060] Embodiment 3.1 adds the sorbitan polyoxyethylene ether that 412g embodiment 2 makes in reactor, 132g oleic acid (90.5%) and 5gNaH 2 PO 3 , stirred and reacted at 220° C. for 5 h under nitrogen blowing, cooled to room temperature, treated with bleaching earth and diatomaceous earth, and filtered to obtain high-purity polysorbate-80.
[0061] Example 3.2 is basically the same as Example 3.1, but with the following changes, replacing 5g NaH with 5g phosphorous acid 2 PO 3 .
[0062] Example 3.3 is basically the same as Example 3.1, but with the following changes, replacing 5g NaH with 5g p-toluenesulfonic acid monohydrate 2 PO 3 .
[0063] Embodiment 3.4 is substantially the same as embodiment 3.1, but with the following changes, the purity of oleic acid used is 95.6%.
[0064] Embodime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com