Pharmaceutical composition for improving safety of Shenmai injection and method for preparing same
A technology of Shenmai injection and composition, applied in the field of medicine, can solve the problems of pH value drop of solution, easy rancidity of polysorbate 80, accelerated pH value of liquid medicine, etc. The effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Red Ginseng 1000g
[0016] Ophiopogon japonicus 1000g
[0017] Solutol 20g
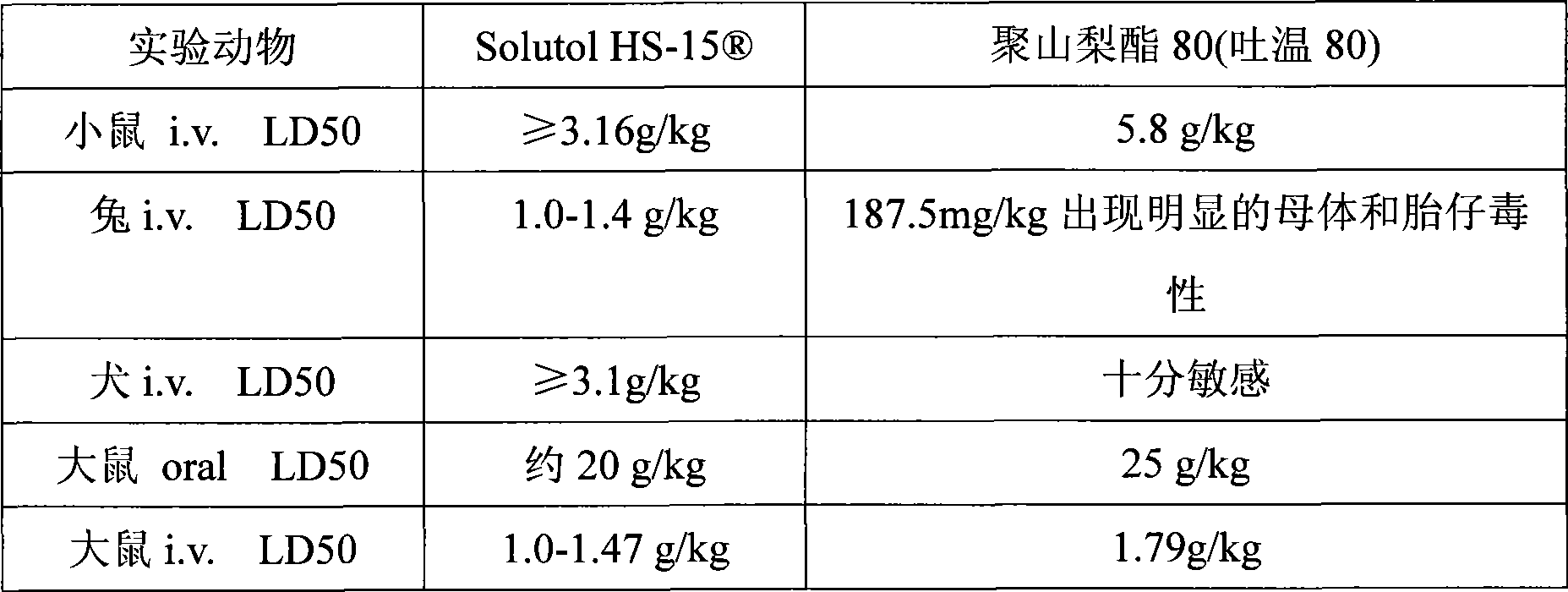
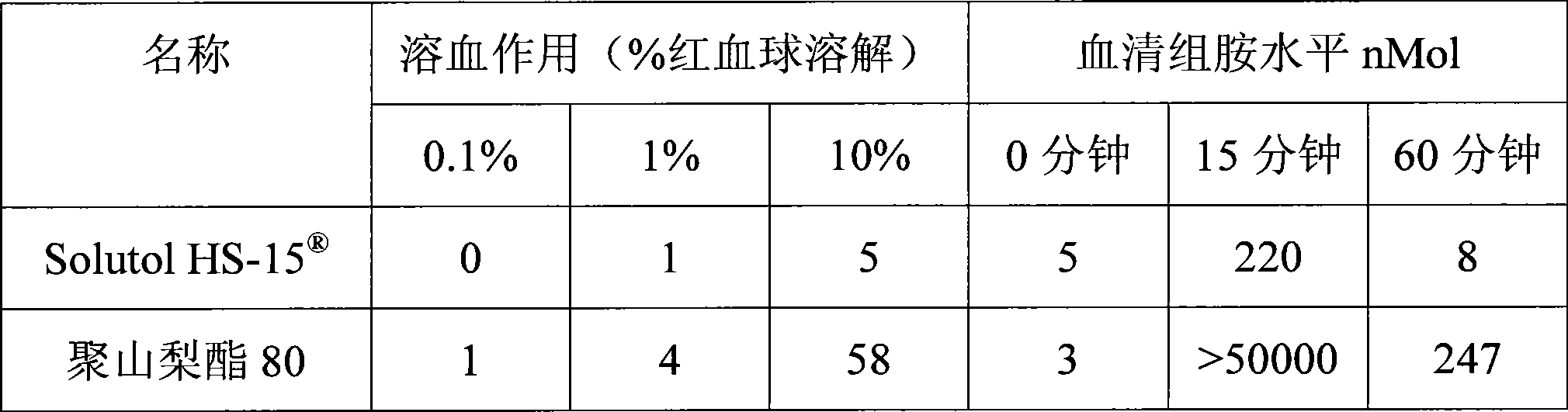
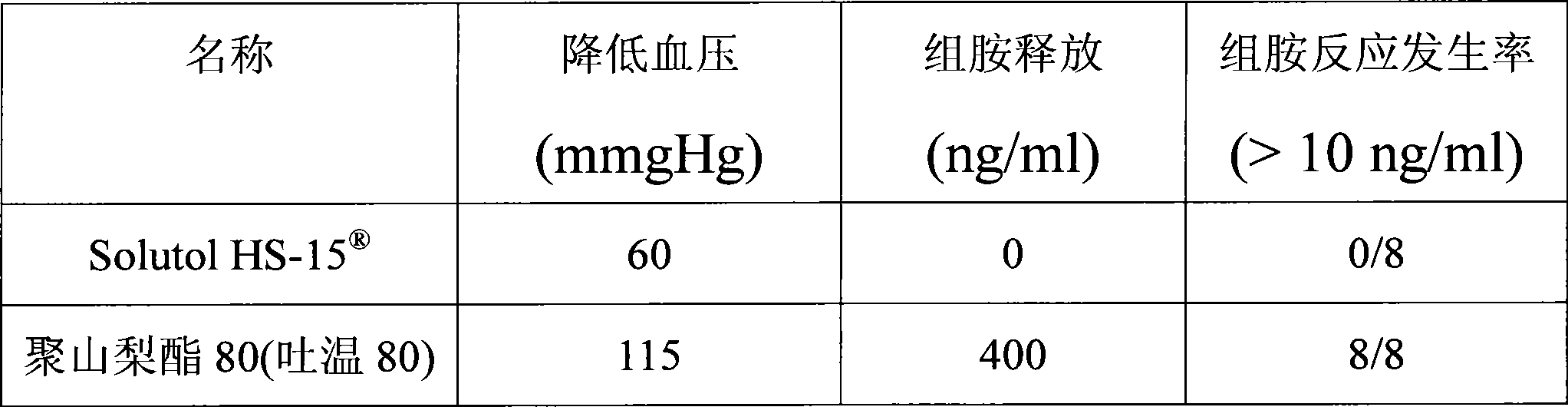
[0018] Extracts of red ginseng and Ophiopogon japonicus after extraction and refining process, adding polyethylene glycol (PEG) lauryl hydroxystearate (Solutol ) as cosolvent and water for injection to be prepared into 10000ml solution. Adjust the pH of the solution to 6.0-8.5. The above solution was filtered through a microporous membrane. Filling, sterilization, that is.
Embodiment 2
[0020] Red Ginseng 1000g
[0021] Ophiopogon japonicus 1000g
[0022] Solutol 10g
[0023] Polysorbate 80 (Tween 80) 10g
[0024] Red ginseng and Ophiopogon japonicus extract after extraction and refining process, adding polyethylene glycol (PEG) lauryl hydroxystearate (Solutol HS-15 ) and polysorbate 80 (Tween 80) as cosolvent and water for injection are prepared into 10000ml solution. Adjust the pH of the solution to 6.0-8.5. The above solution was filtered through a microporous membrane. Filling, sterilization, that is.
Embodiment 3
[0026] Red Ginseng 1000g
[0027] Ophiopogon japonicus 1000g
[0028] Solutol 20g
[0029] Red ginseng and Ophiopogon japonicus extract after extraction and refining process, adding polyethylene glycol (PEG) lauryl hydroxystearate (Solutol HS-15 ) as co-solvent and water for injection to prepare a solution. Adjust the pH value of the solution to 6.0-8.5. The above solution was filtered through a microporous membrane. Subpackaged, freeze-dried, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com