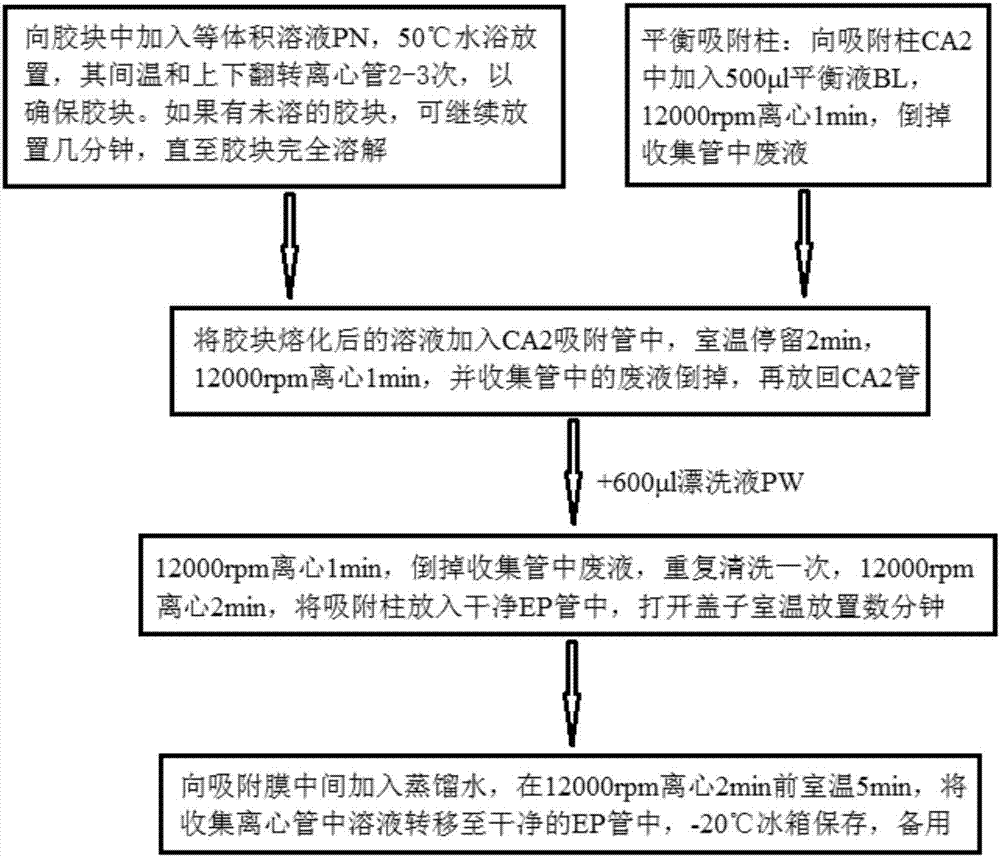
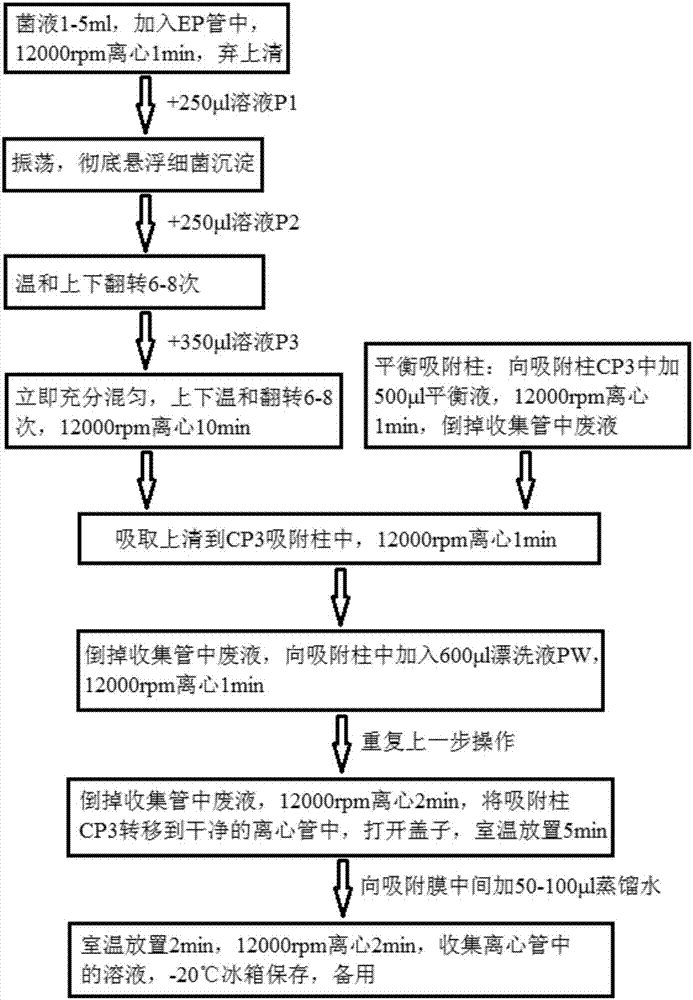
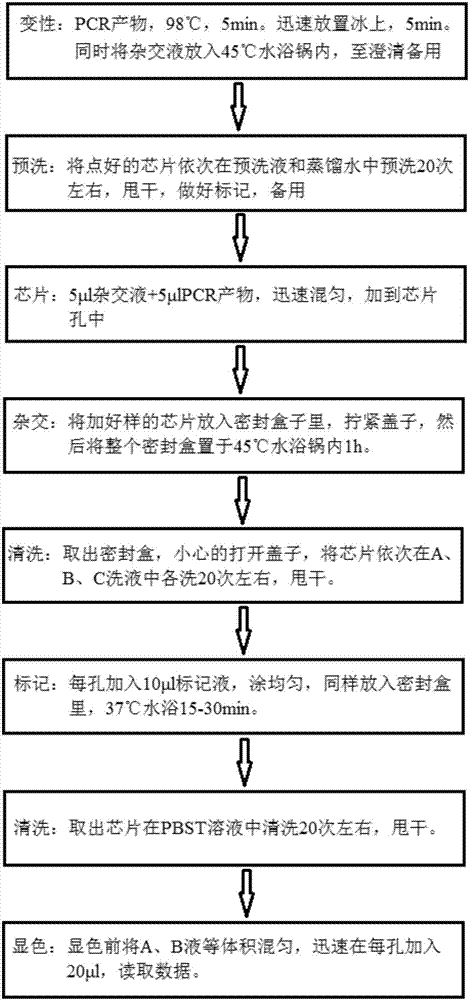
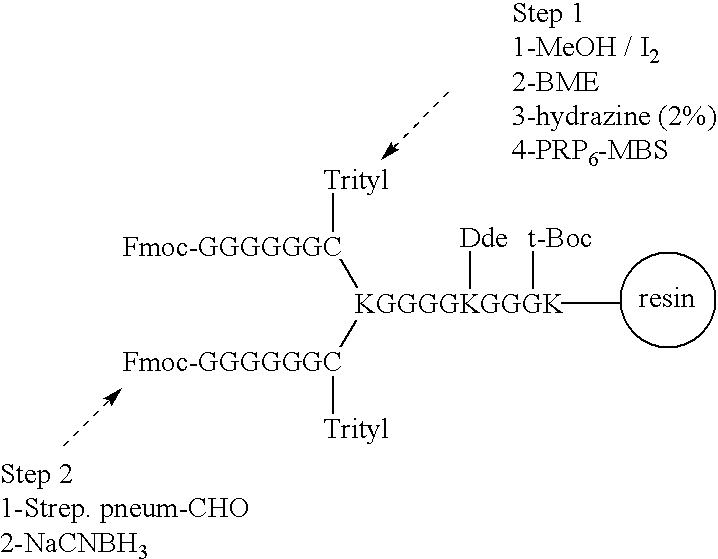
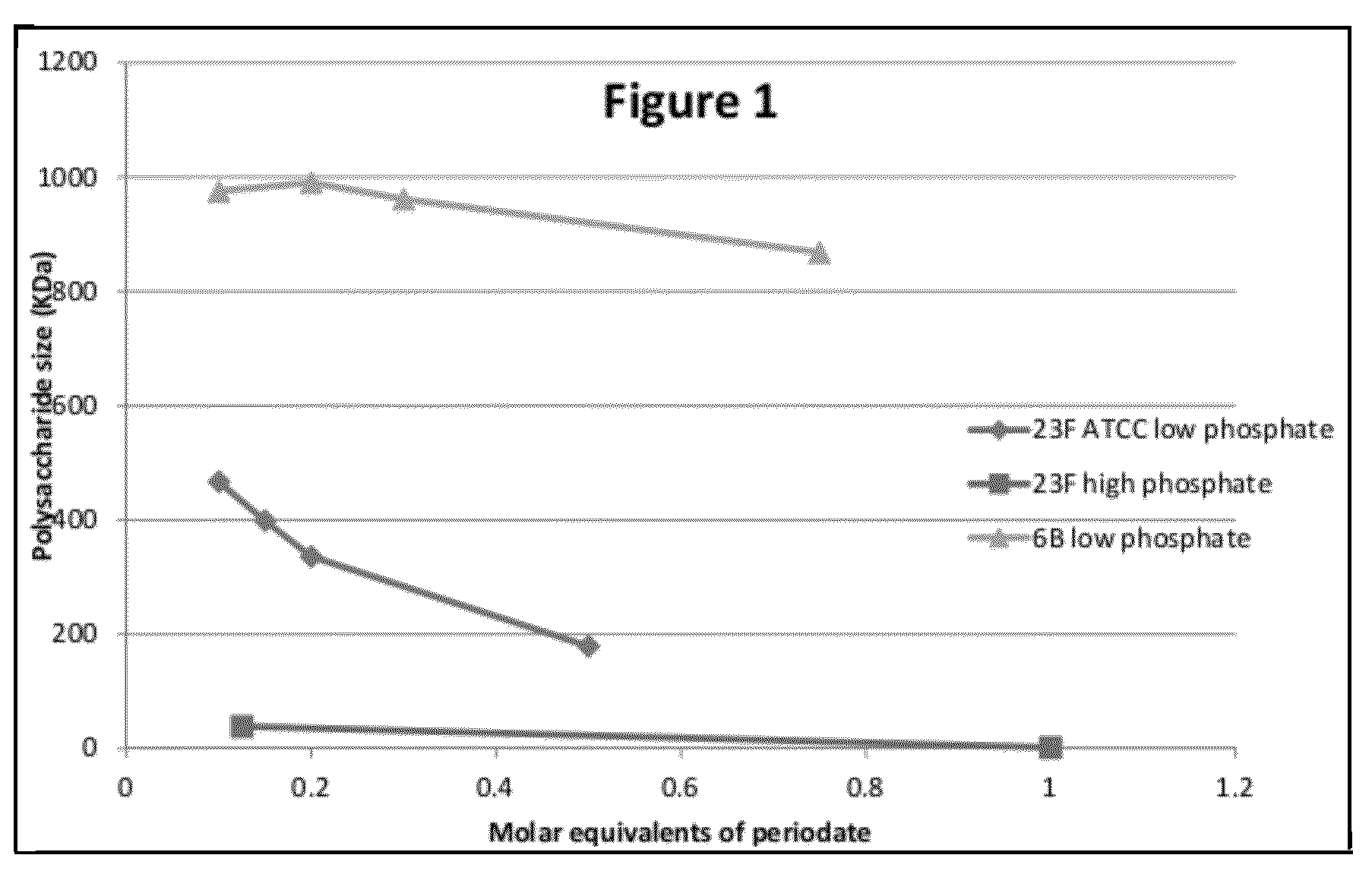
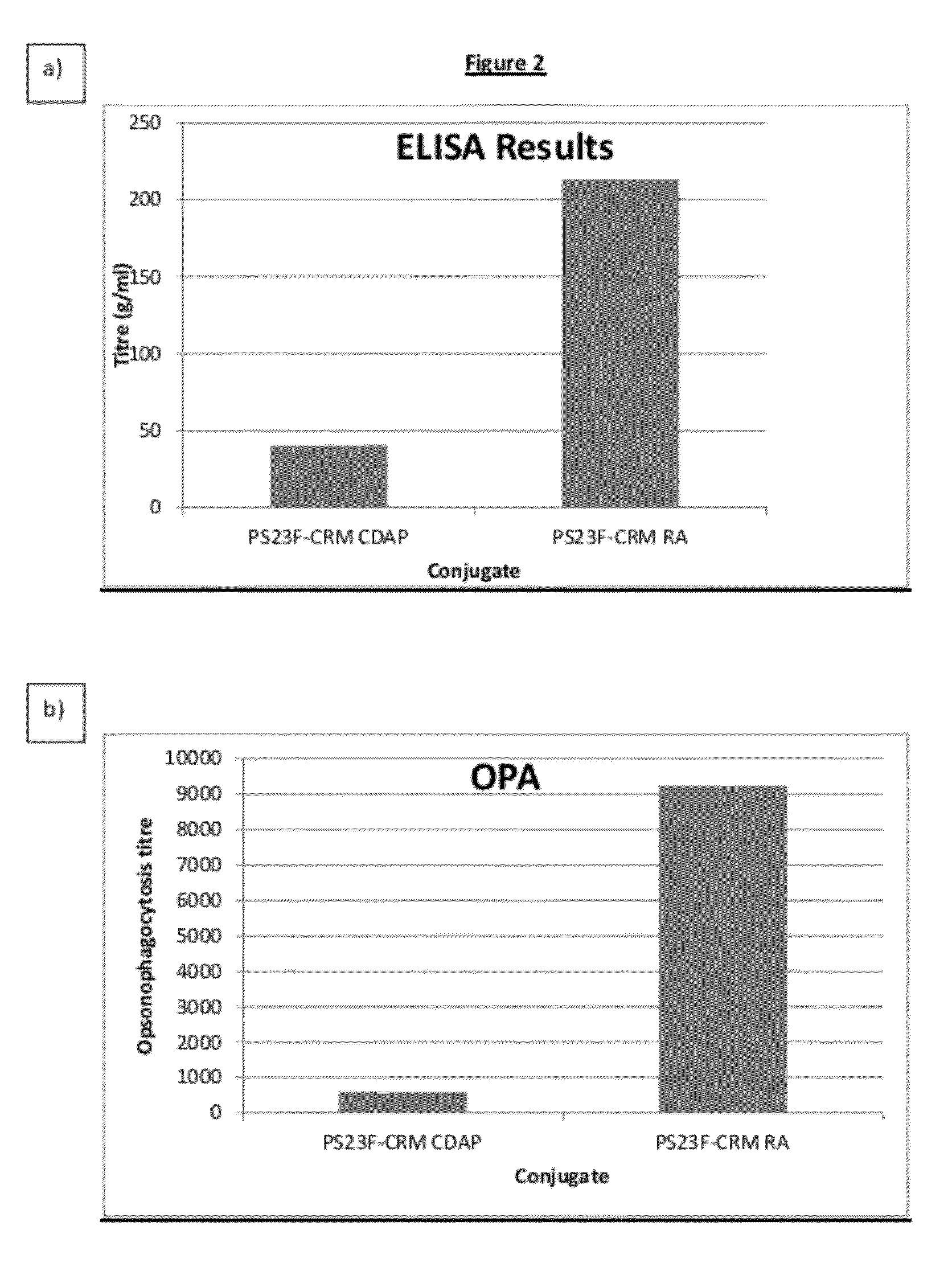
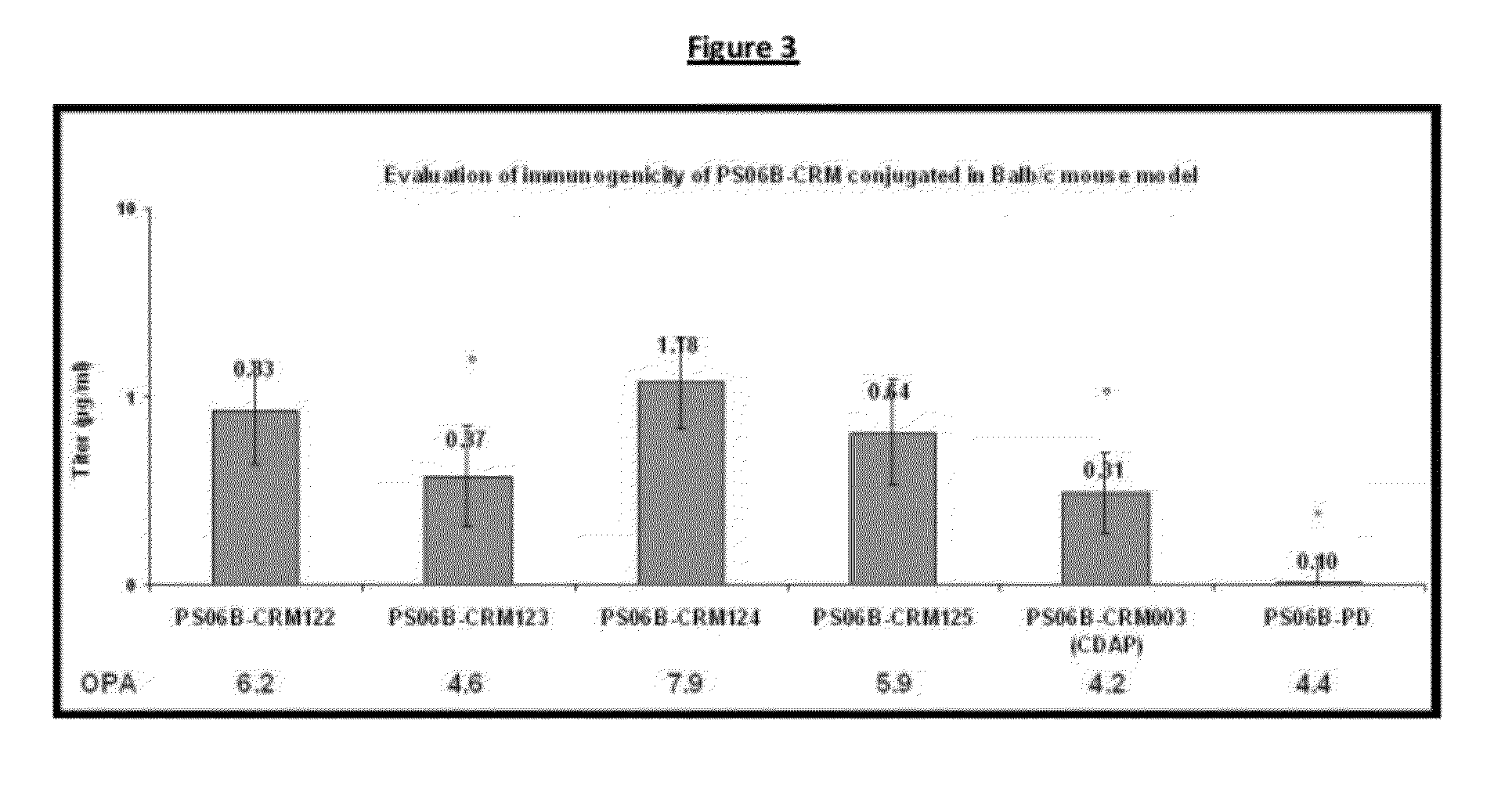
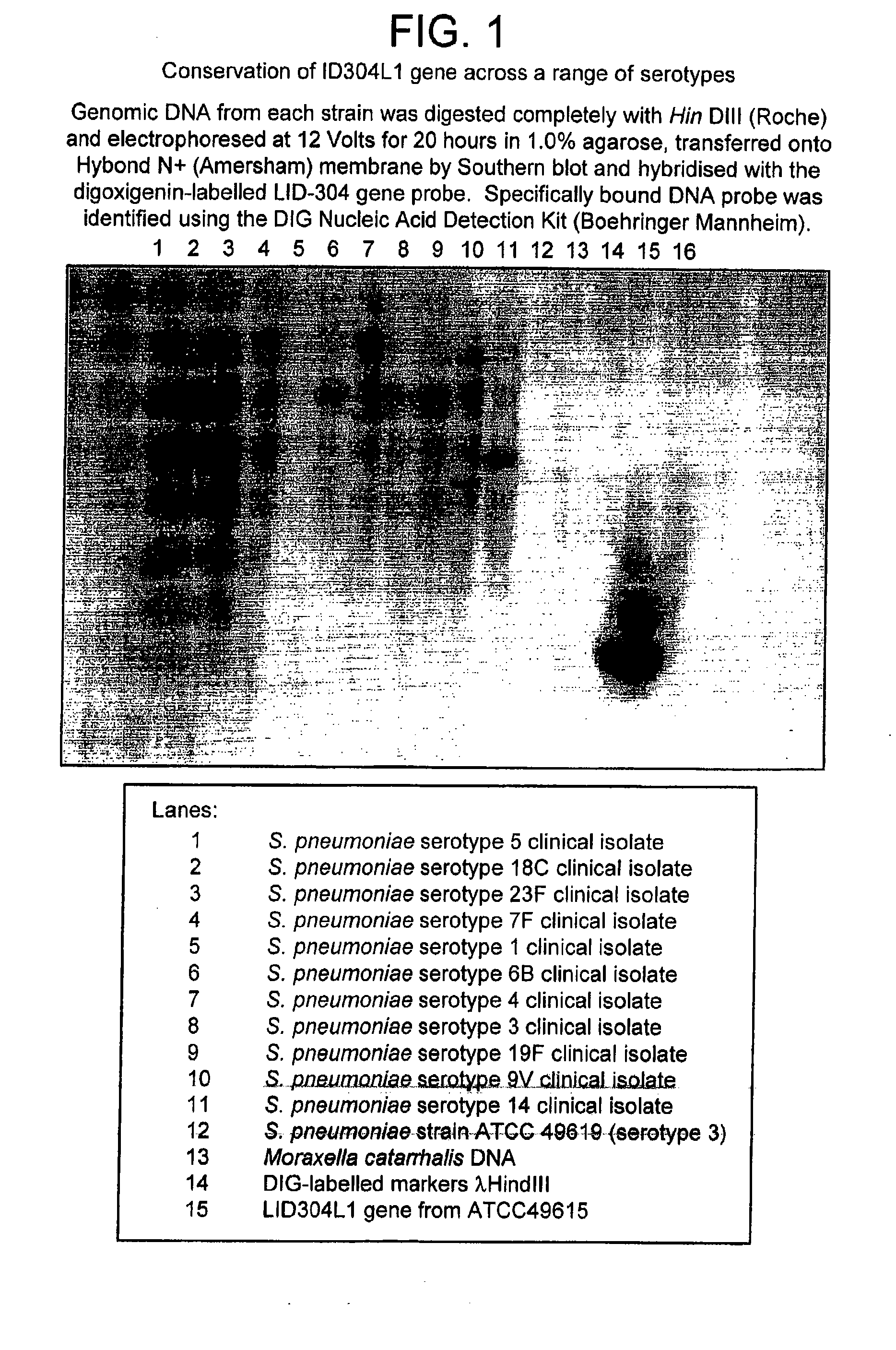
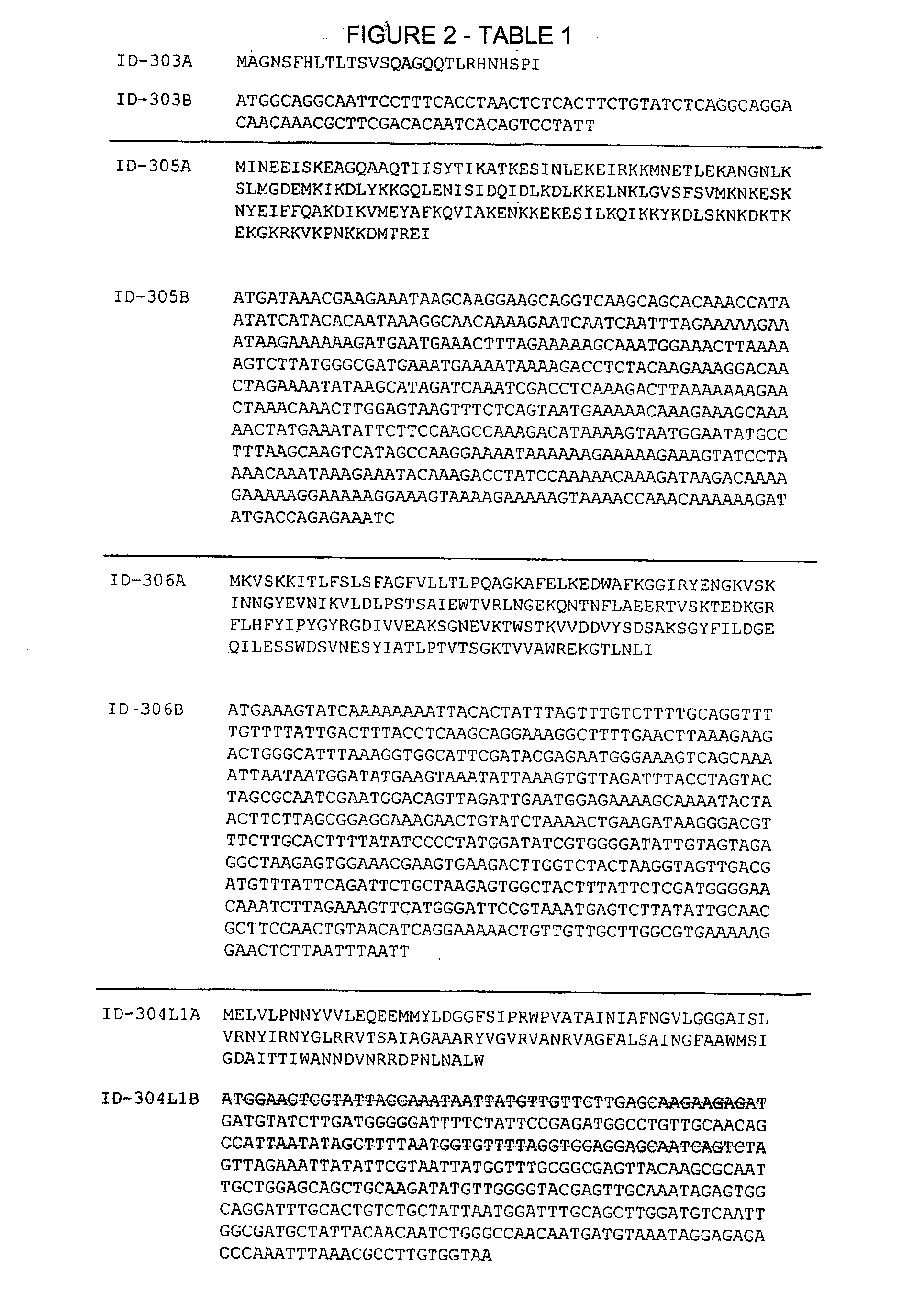
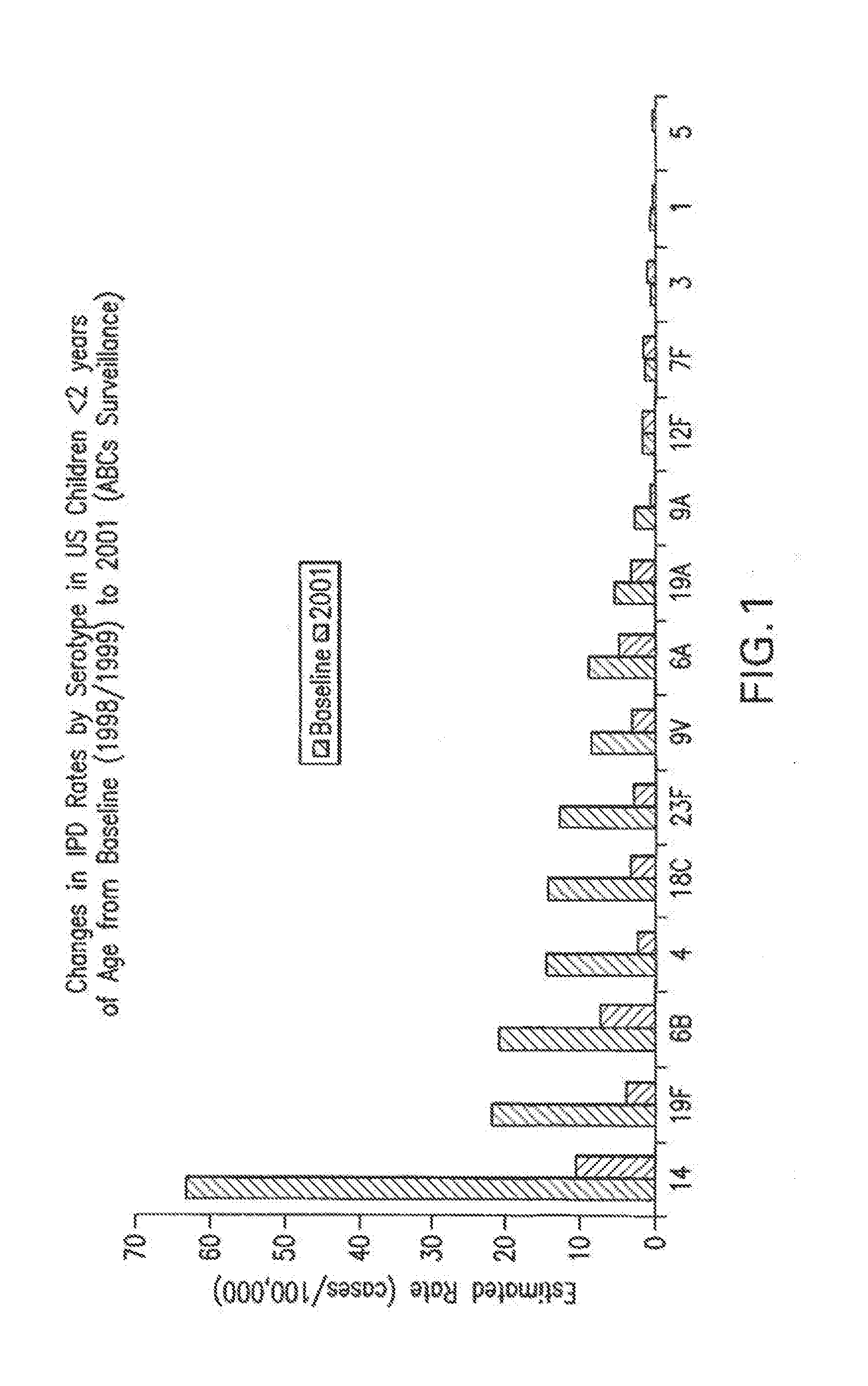
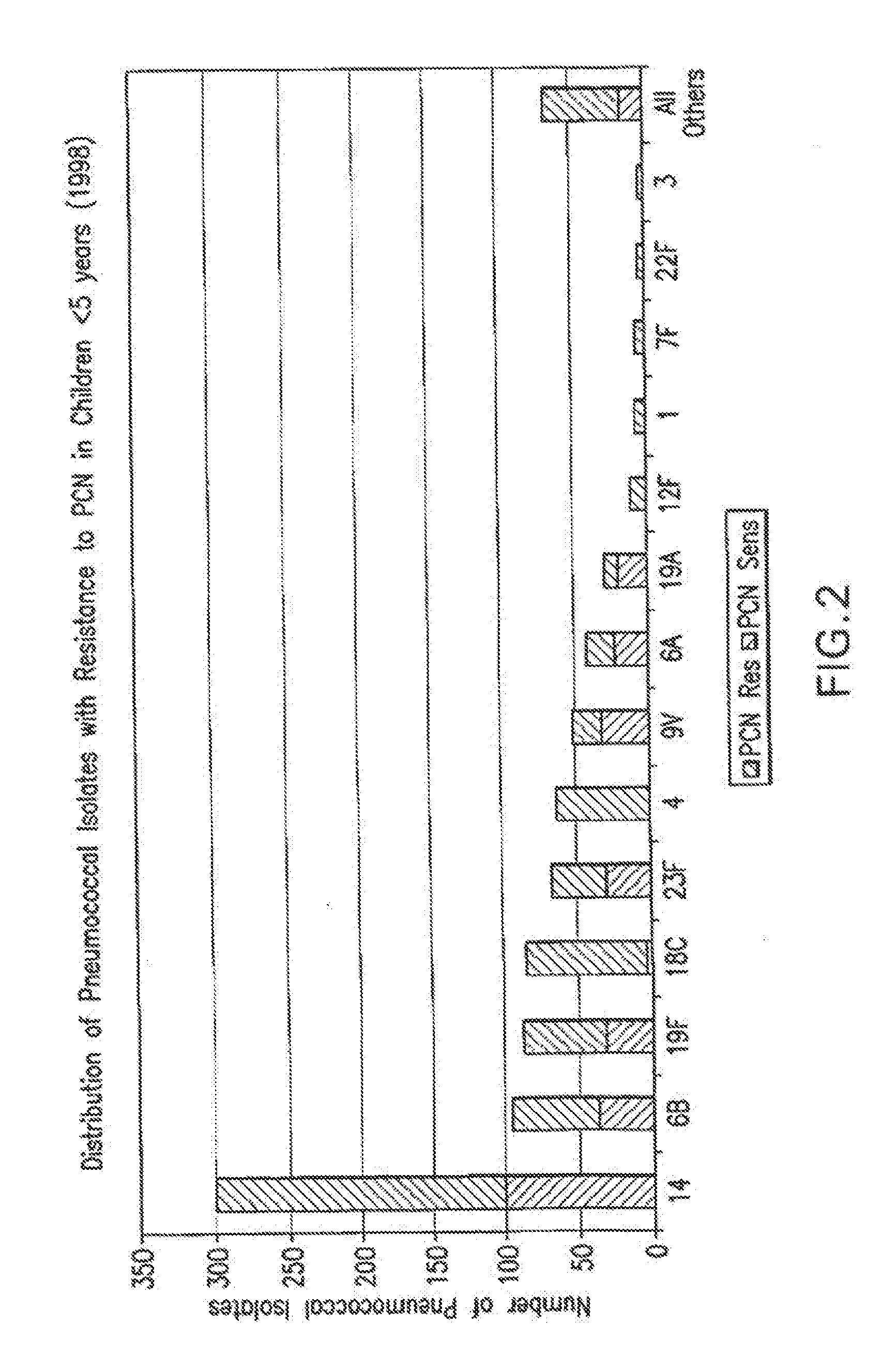
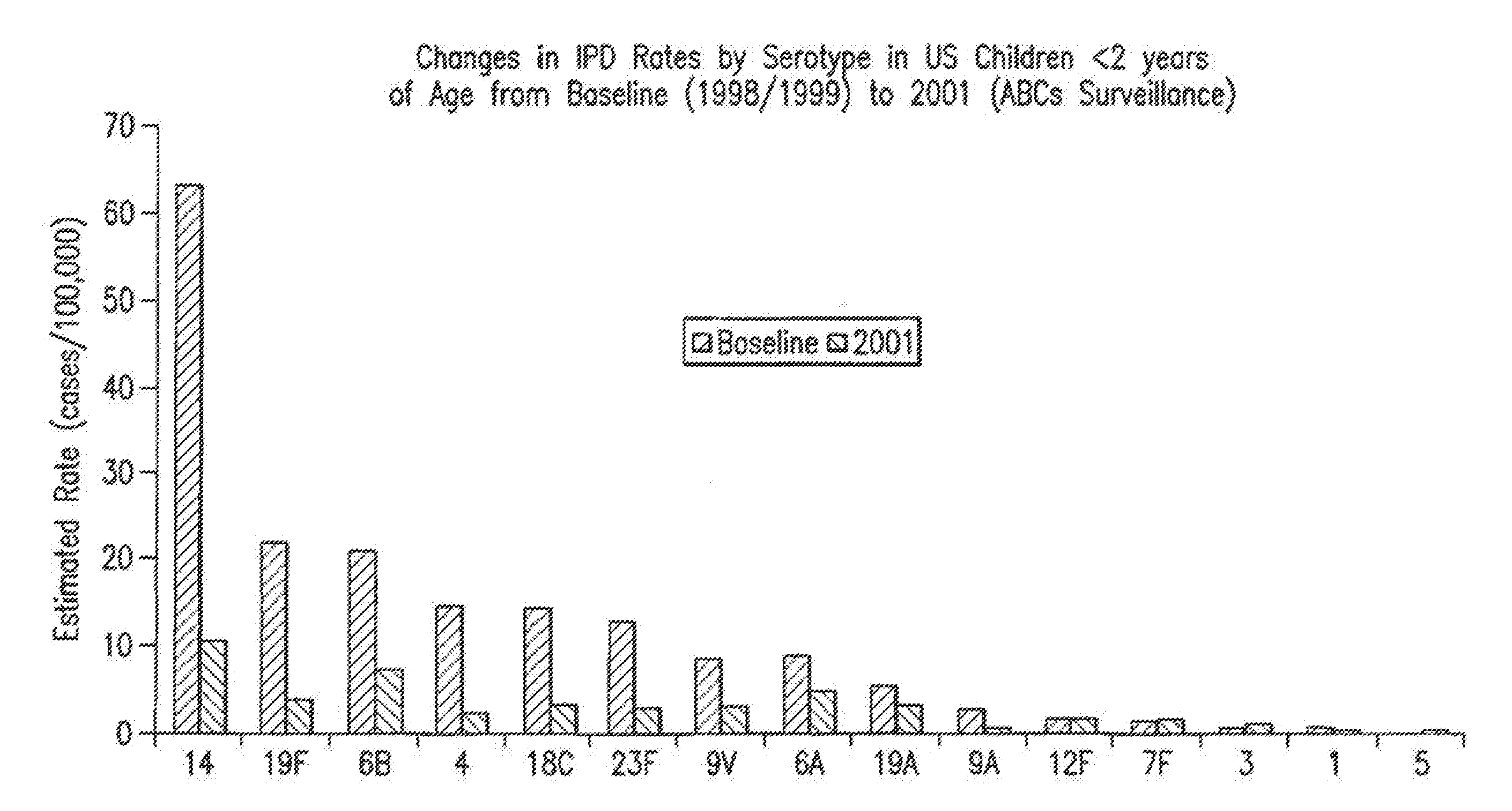Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
294 results about "Streptococcus pneumoniae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Streptococcus pneumoniae, or pneumococcus, is a Gram-positive, alpha-hemolytic (under aerobic conditions) or beta-hemolytic (under anaerobic conditions), facultative anaerobic member of the genus Streptococcus. They are usually found in pairs (diplococci) and do not form spores and are nonmotile. As a significant human pathogenic bacterium S. pneumoniae was recognized as a major cause of pneumonia in the late 19th century, and is the subject of many humoral immunity studies.
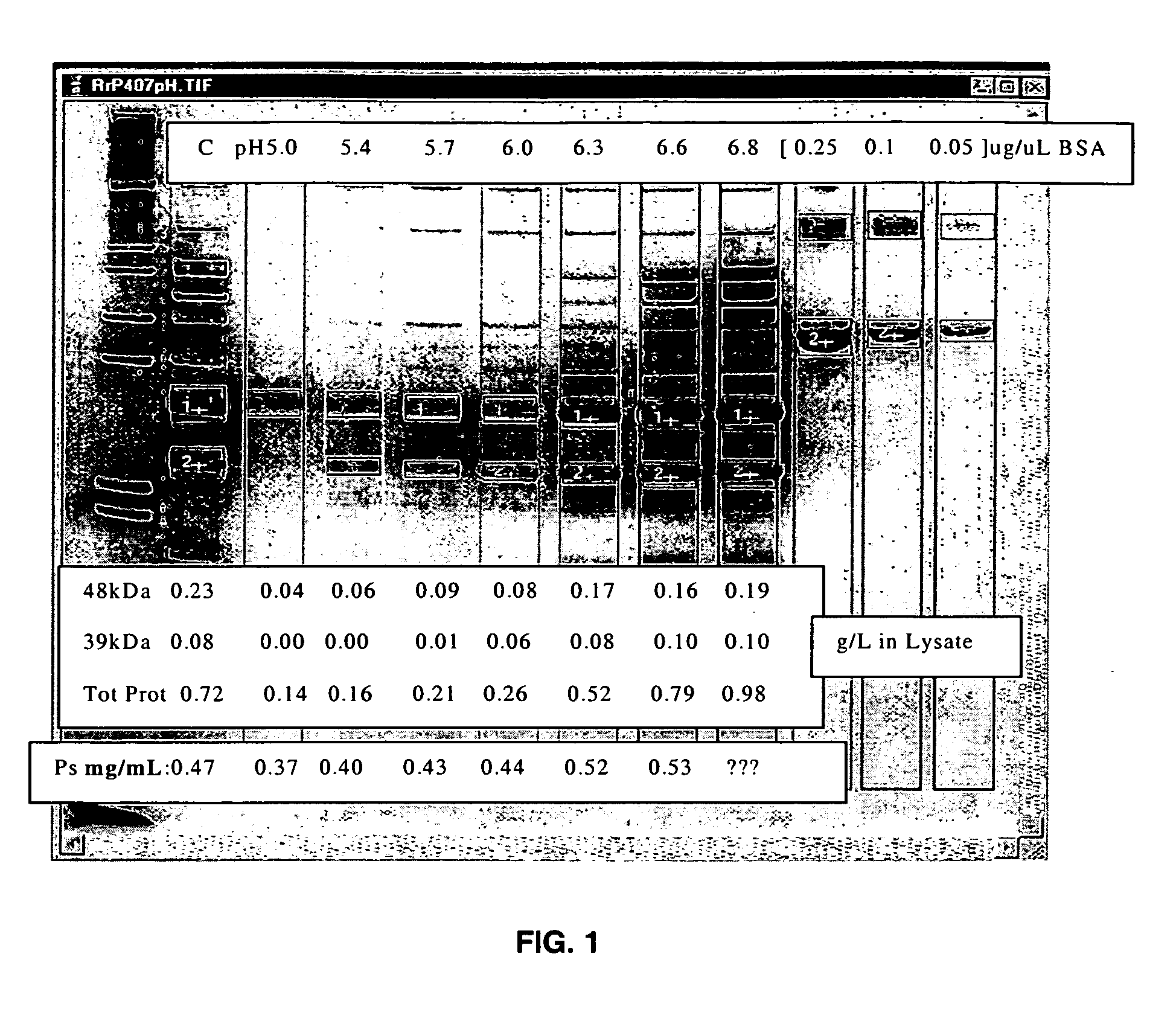
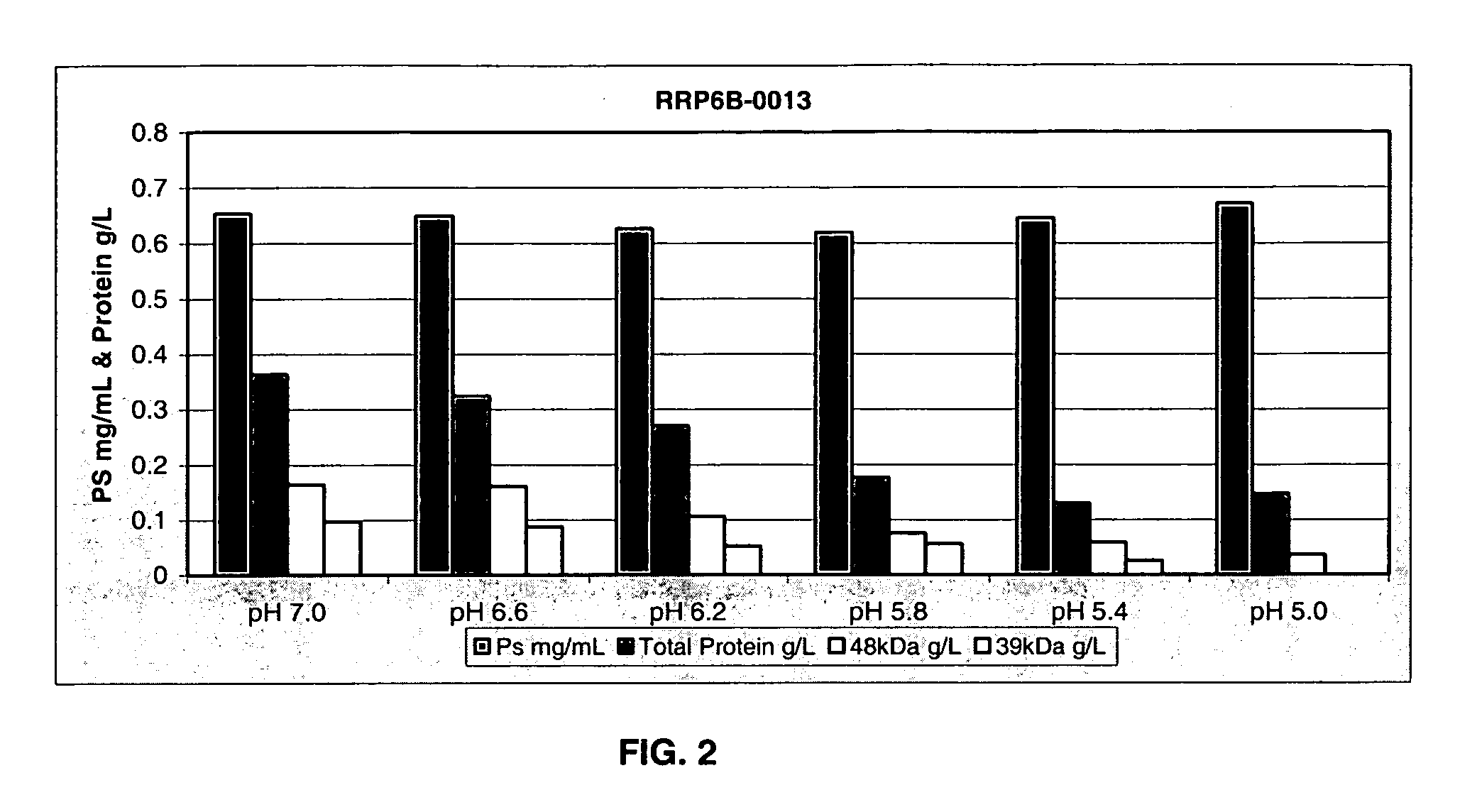
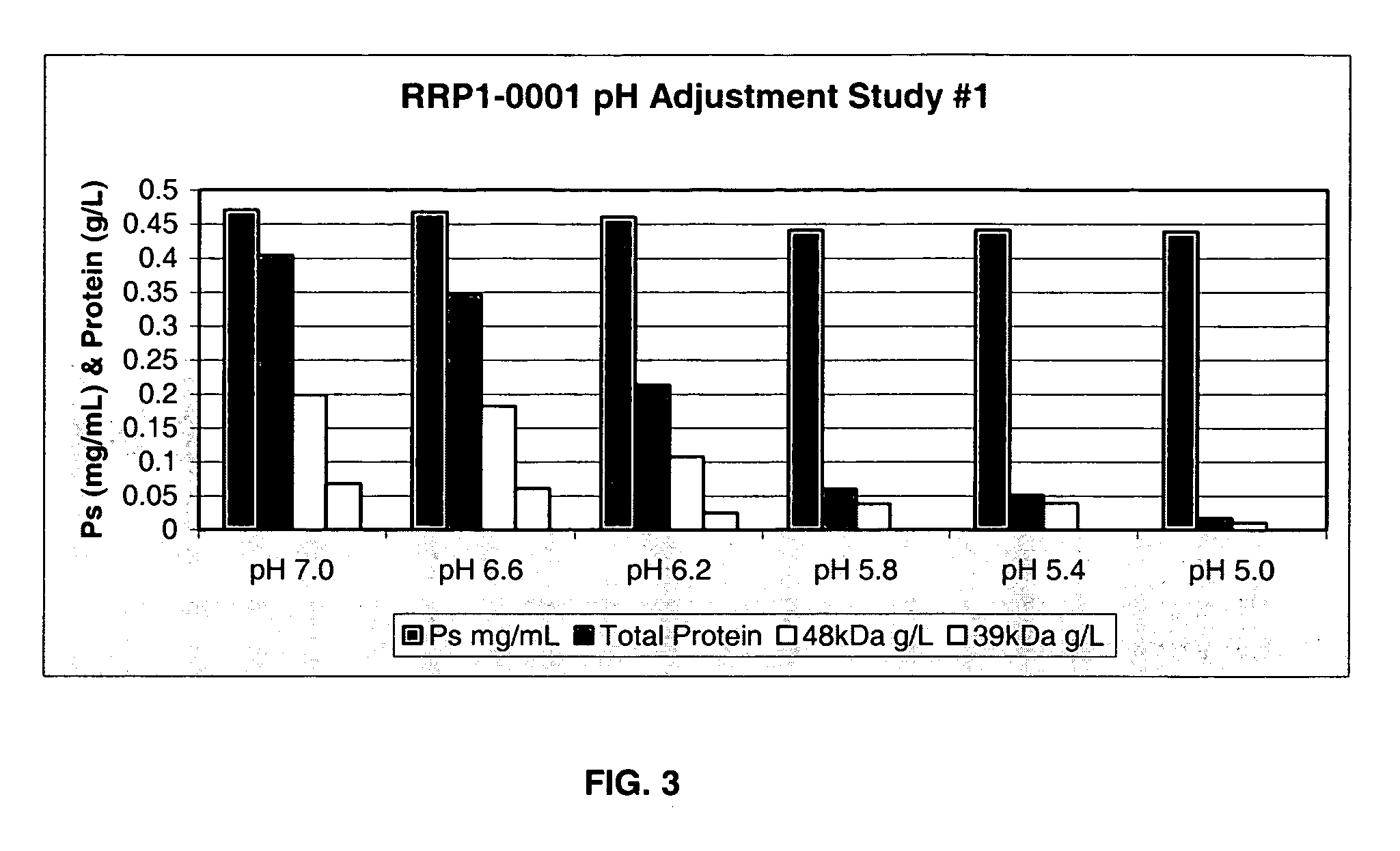
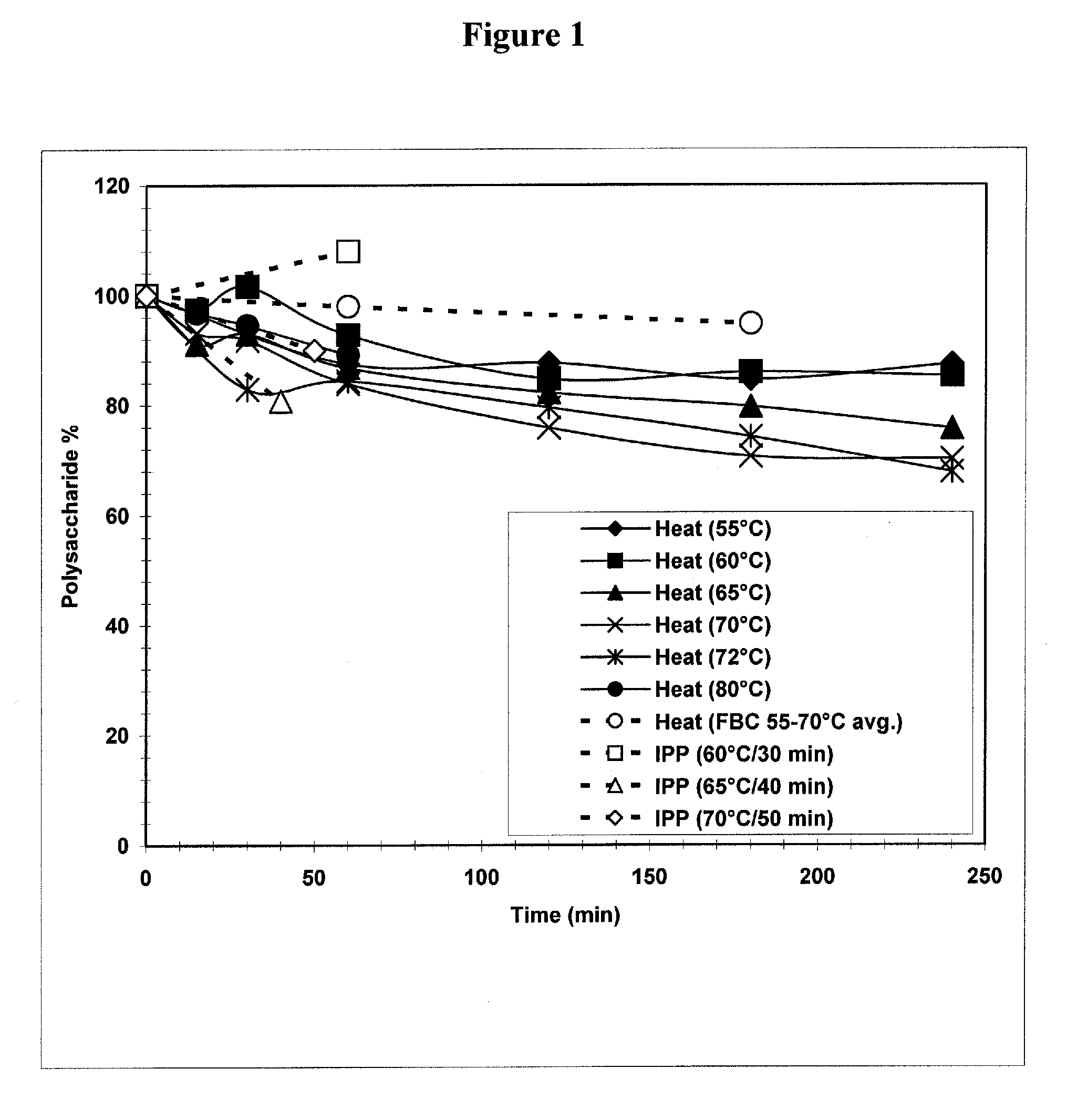
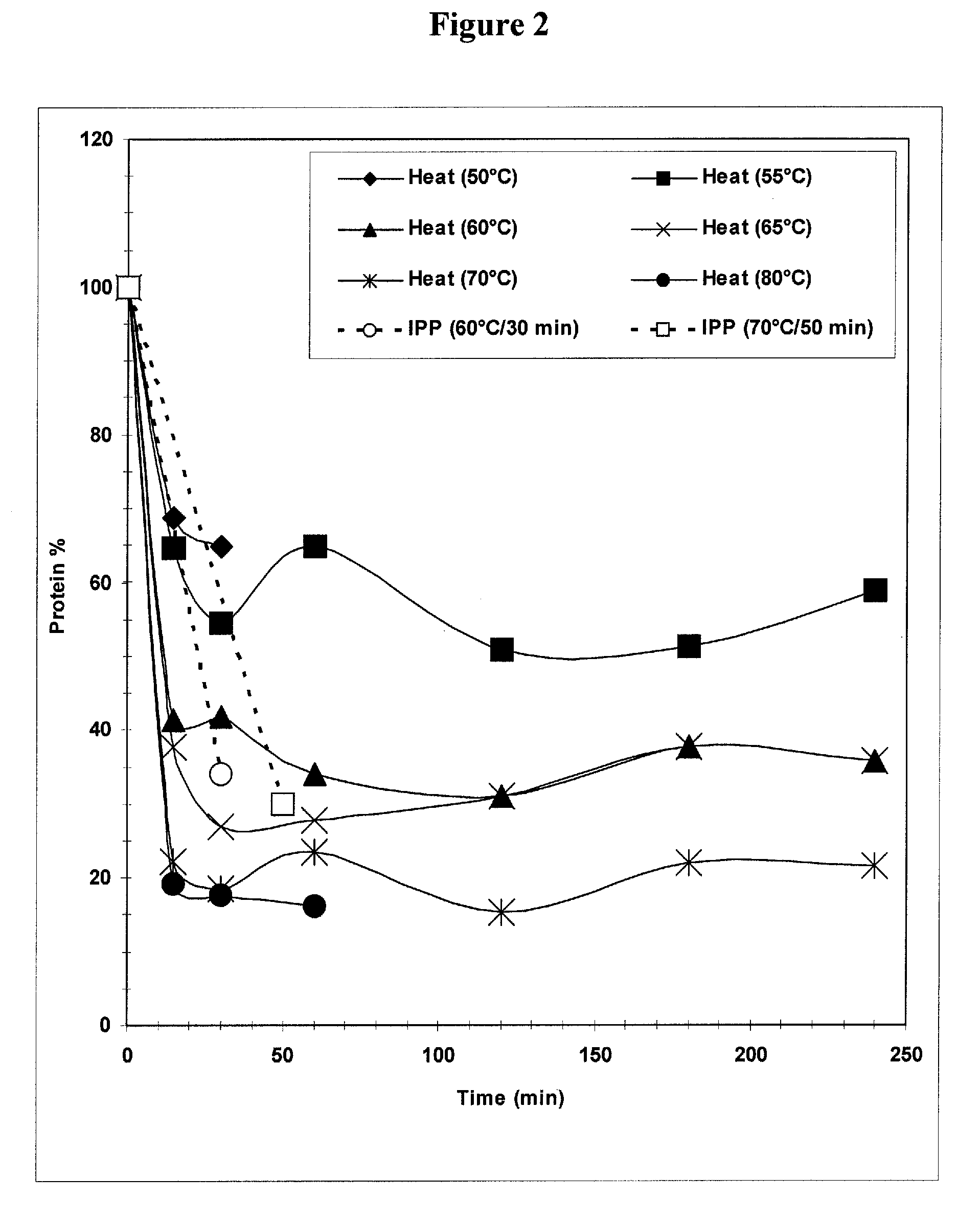
Separation of contaminants from Streptococcus pneumoniae polysaccharide by pH manipulation
ActiveUS20060228381A1Soluble protein is effectively reducedBacterial antigen ingredientsSugar derivativesStreptococcus pneumoniaeLysis
A process for reducing the protein content and preserving the capsular polysaccharide content in a complex cellular Streptococcus pneumoniae lysate broth prior to purification is described. Utilizing pH reduction after cellular lysis has resulted in a purified polysaccharide that consistently meets the protein specification, and higher recovery yields of polysaccharide during the purification process.
Owner:WYETH LLC
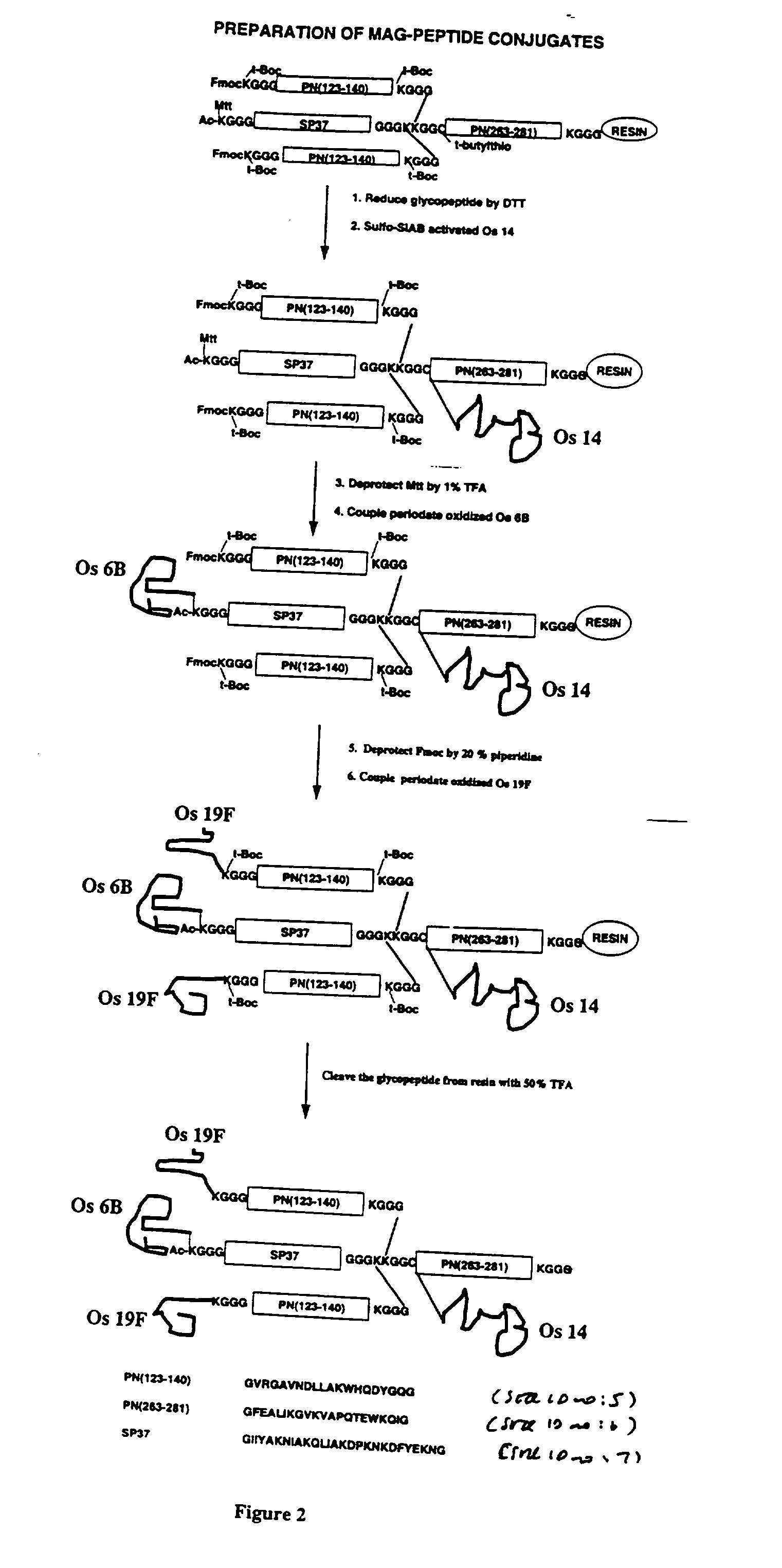
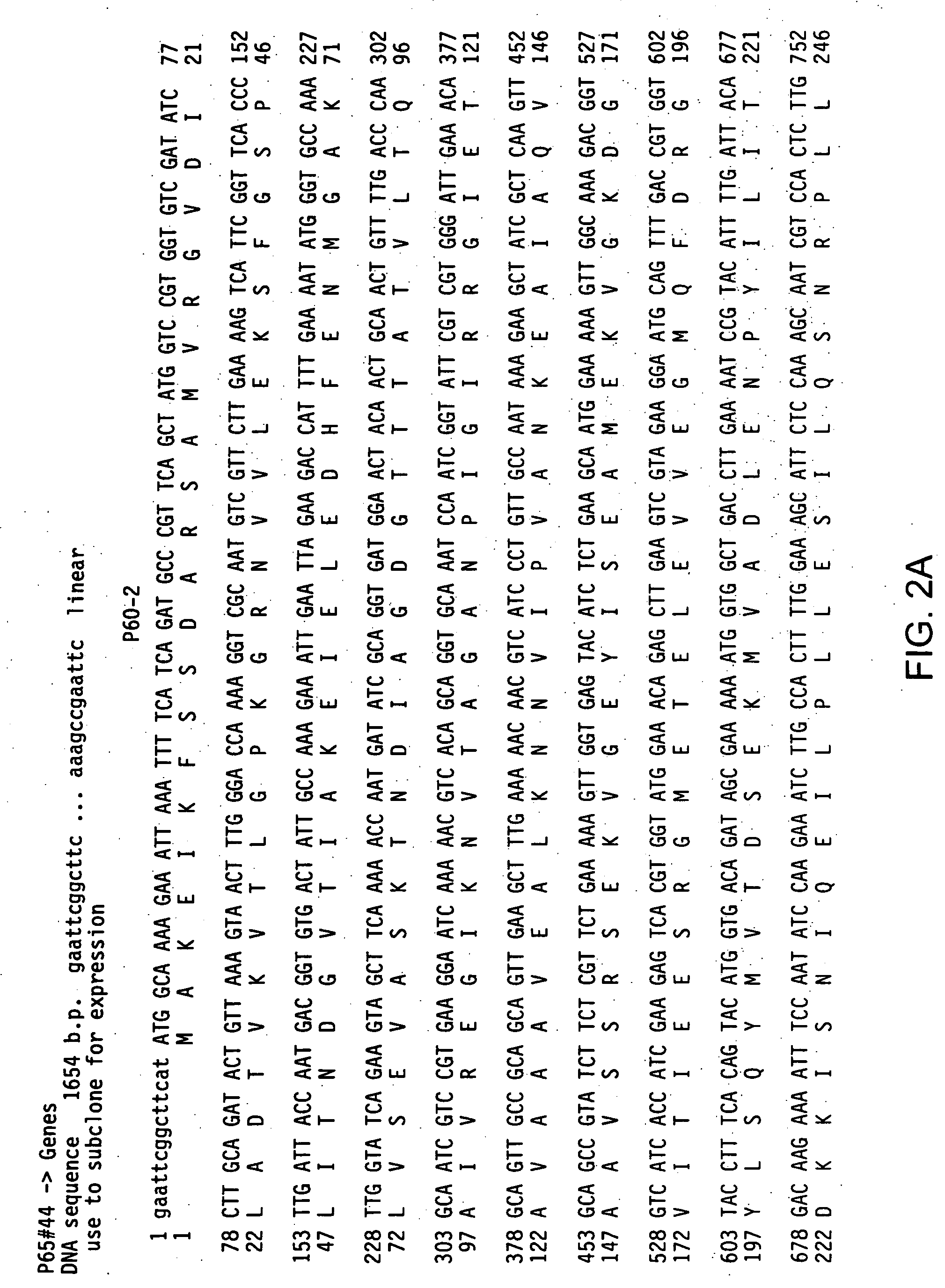
Streptococcus pneumoniae 37-kDa surface adhesin a protein
The invention provides a nucleic acid encoding the 37-kDa protein from Streptococcus pneumoniae. Also provided are isolated nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. The invention also provides purified polypeptides encoded by the nucleic acid encoding the 37-kDa protein from and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are antibodies which selectively binds the polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are vaccines comprising immunogenic polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Further provided is a method of detecting the presence of Streptococcus pneumoniae in a sample comprising the steps of contacting a sample suspected of containing Streptococcus pneumoniae with nucleic acid primers capable of hybridizing to a nucleic acid comprising a portion of the nucleic acid encoding the 37-kDa protein, amplifying the nucleic acid and detecting the presence of an amplification product, the presence of the amplification product indicating the presence of Streptococcus pneumoniae in the sample. Further provided are methods of detecting the presence of Streptococcus pneumoniae in a sample using antibodies or antigens, methods of preventing and treating Streptococcus pneumoniae infection in a subject.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
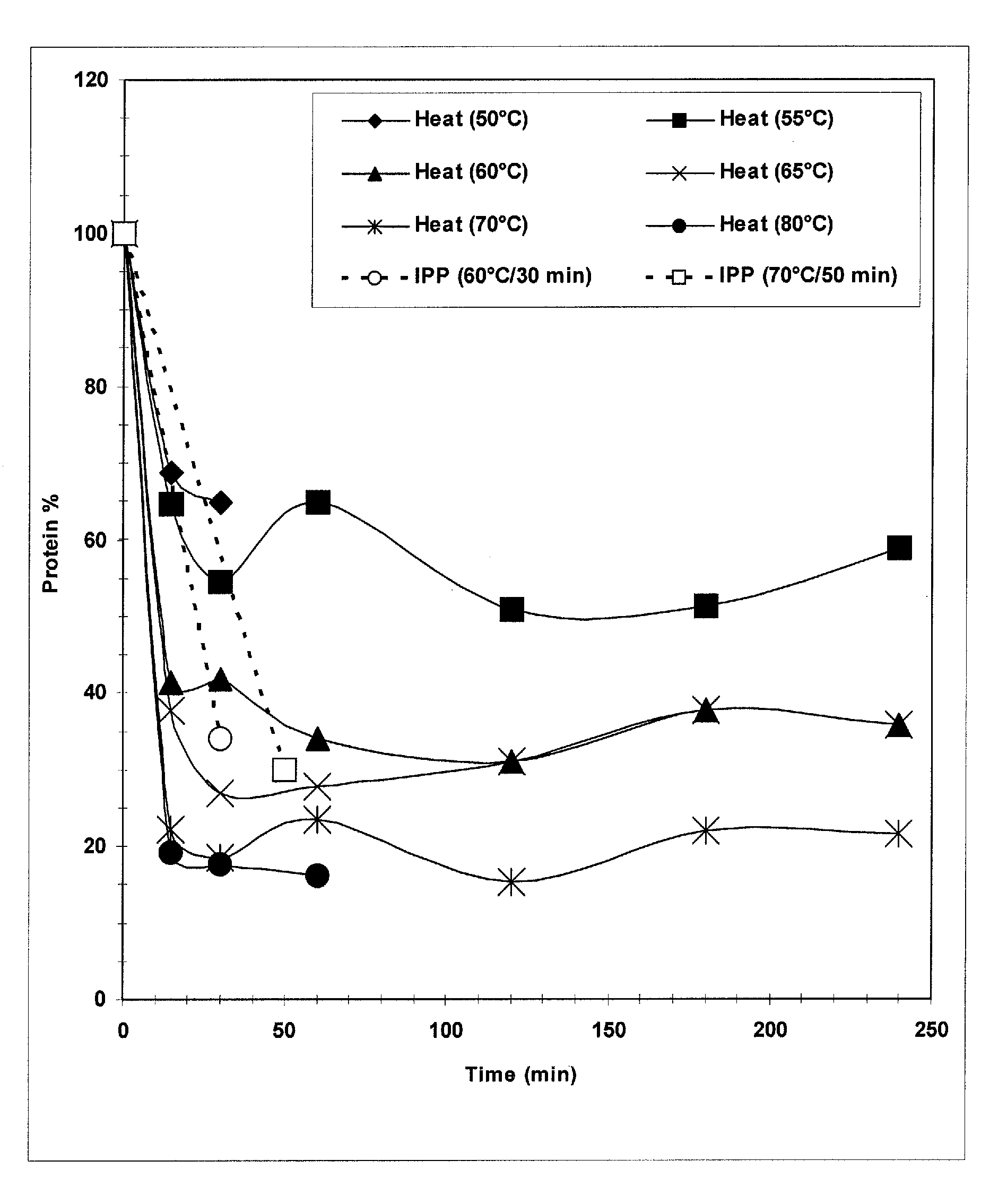
Methods for the separation of streptococcus pneumoniae type 3 polysaccharides
ActiveUS20080102498A1Reducing and removing impurityImprove filtering effectAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeLysis
The present invention provides improved methods for the reduction or removal of protein impurities from a complex cellular Streptococcus pneumoniae lysate or centrate comprising serotype 3 polysaccharides involving steps relating to post-lysis heating or pH adjustment. In certain methods, the lysate is heated for a time and at a temperature sufficient to denature proteins present in the lysate and cause their aggregation and precipitation. In one embodiment, the lysate is heated to at least 60° C. for at least 30 minutes to cause protein aggregation and precipitation, more particularly about 60° C. to about 70° C. for about 30 to about 50 minutes, and even more particularly about 65° C. for about 40 minutes. In other methods, the pH of the lysate or centrate is increased to at least 8.0 to improve filterability, more particularly about 8.0 to 8.4, and even more particularly about 8.2. In further methods, heating and pH adjustment steps are combined to cause the aggregation and precipitation of proteins as well as to improve filterability of the lysates or centrates. In other methods, the pH of the lysate or centrate is lowered to about 3.0 to about 5.0 to cause protein aggregation and precipitation. Such methods allow for the production of substantially purified serotype 3 polysaccharide-containing lysates or centrates.
Owner:WYETH LLC
Streptococcus pneumoniae SP042 polynucleotides
The present invention relates to novel vaccines for the prevention or attenuation of infection by Streptococcus pneumoniae. The invention further relates to isolated nucleic acid molecules encoding antigenic polypeptides of Streptococcus pneumoniae. Antigenic polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention additionally relates to diagnostic methods for detecting Streptococcus nucleic acids, polypeptides and antibodies in a biological sample.
Owner:HUMAN GENOME SCI INC
Streptococcus pneumoniae SP036 polynucleotides
The present invention relates to novel vaccines for the prevention or attenuation of infection by Streptococcus pneumoniae. The invention further relates to isolated nucleic acid molecules encoding antigenic polypeptides of Streptococcus pneumoniae. Antigenic polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention additionally relates to diagnostic methods for detecting Streptococcus nucleic acids, polypeptides and antibodies in a biological sample.
Owner:HUMAN GENOME SCI INC
Streptococcus pneumoniae antigens and vaccines
The present invention relates to novel vaccines for the prevention or attenuation of infection by Streptococcus pneumoniae. The invention further relates to isolated nucleic acid molecules encoding antigenic polypeptides of Streptococcus pneumoniae. Antigenic polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention additionally relates to diagnostic methods for detecting Streptococcus nucleic acids, polypeptides and antibodies in a biological sample.
Owner:HUMAN GENOME SCI INC
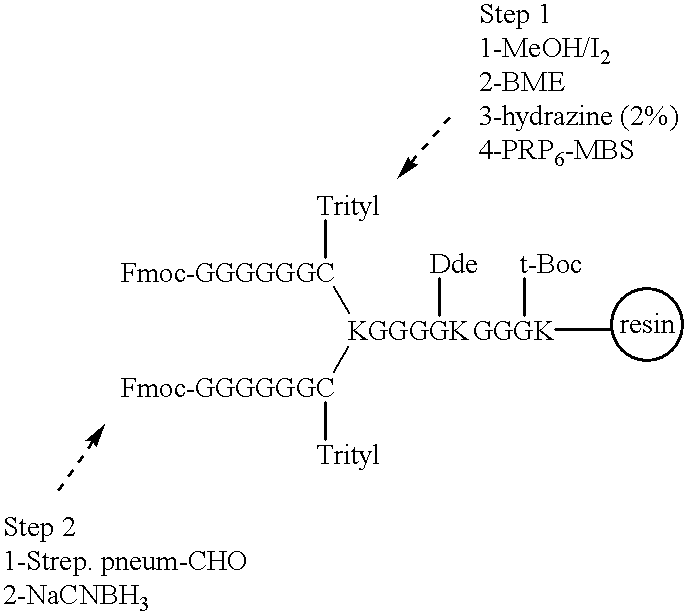
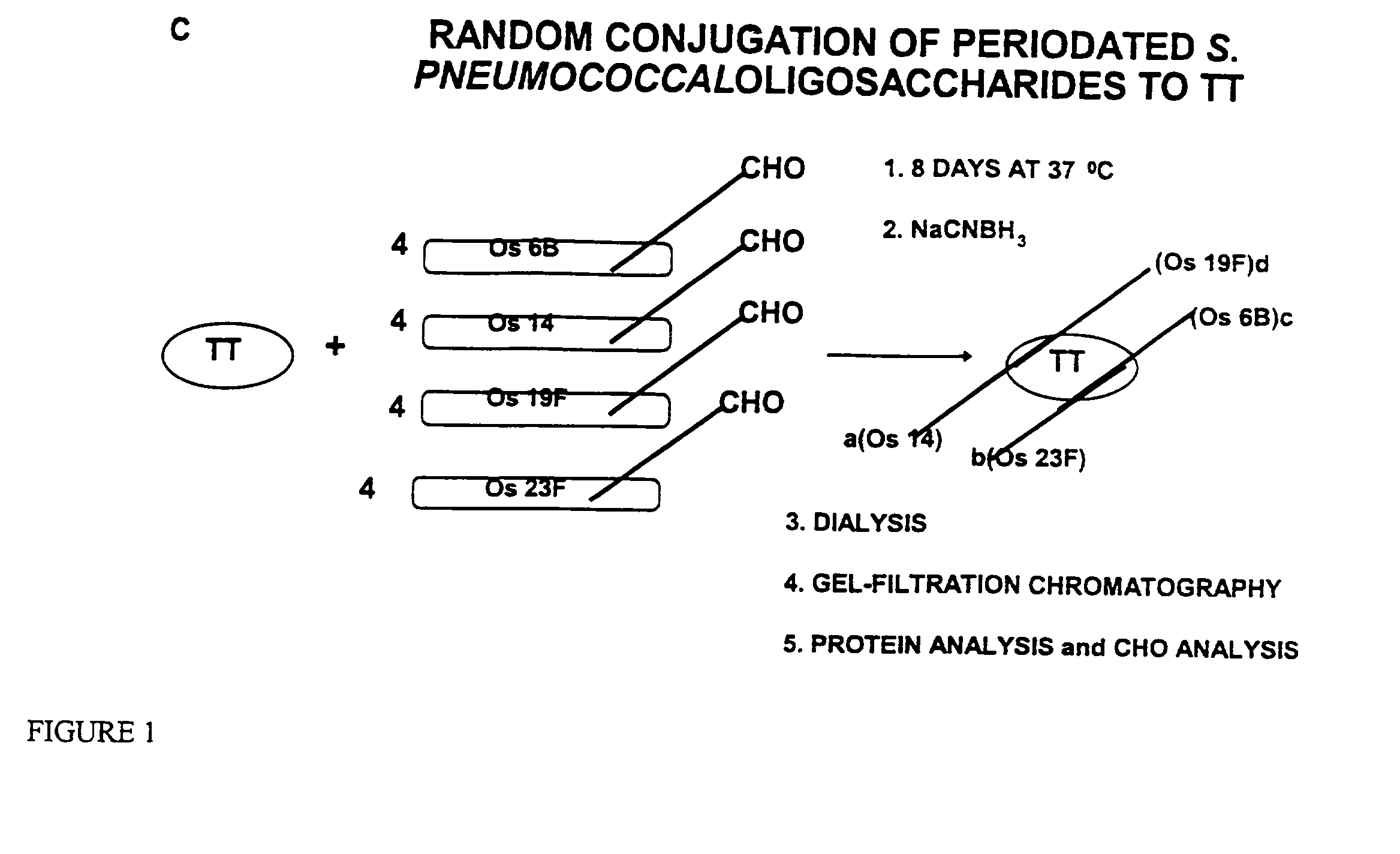
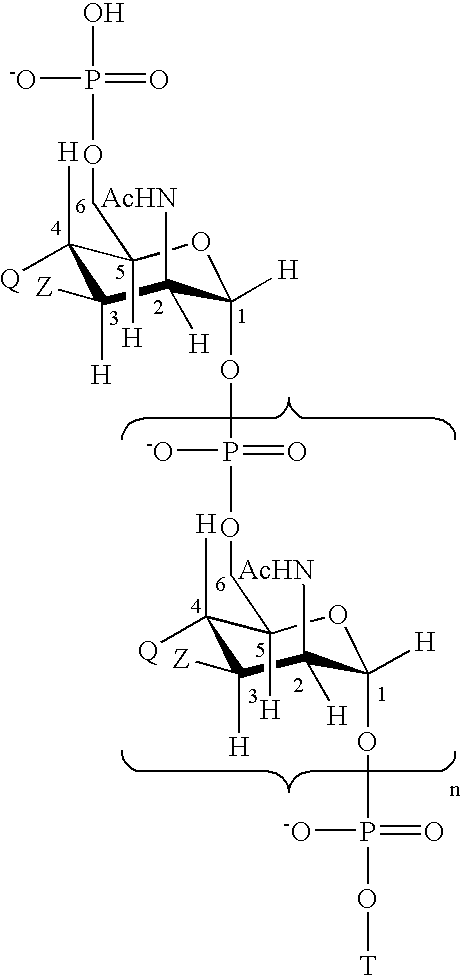
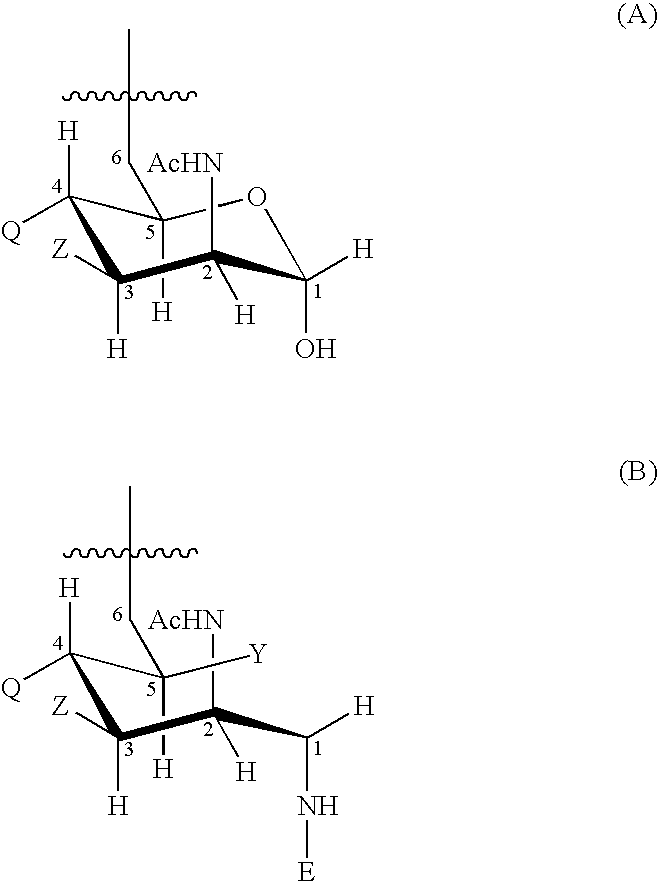
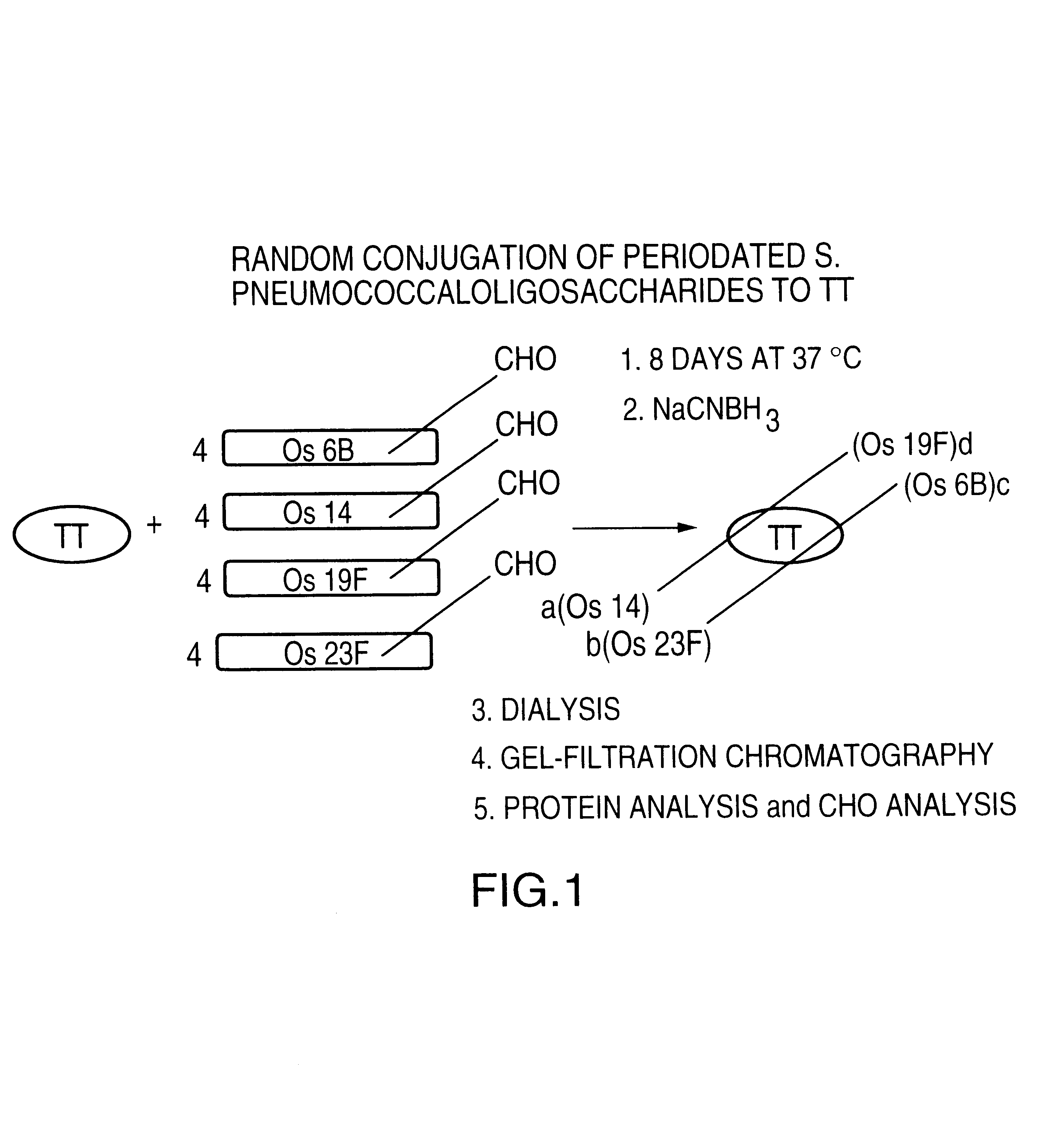
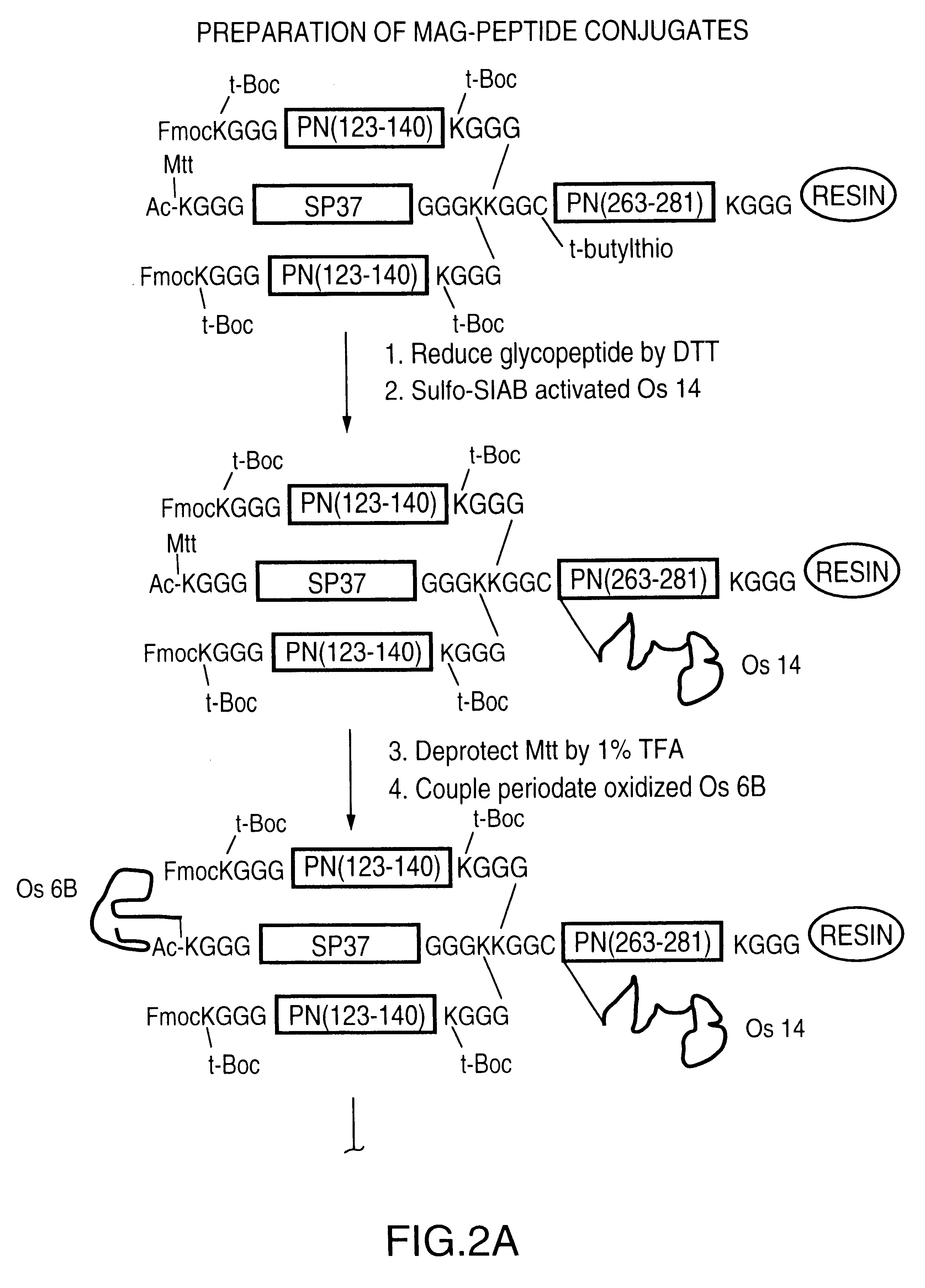
Novel multi-oligosaccharide glycoconjugate bacterial meningitis vaccines
InactiveUS20010048929A1Inhibition effectWeight increaseAntibacterial agentsPeptide/protein ingredientsSerotypeTumor antigen
Multivalent immunogenic molecules comprise a carrier molecule containing at least one functional T-cell epitope and multiple different carbohydrate fragments each linker to the carrier molecule and each containing at least one functional B-cell epitope. The carrier molecule inputs enhanced immunogenicity to the multiple carbohydrate fragments. The carbohydrate fragments may be capsular oligosaccharide fragments from Streptococcus pneumoniae, which may be serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F or 23F, or Neisseria meningitidis, which may be serotype A, B, C, W-135 or Y. Such oligosaccharide fragments may be sized from 2 to 5 kDa. Alternatively, the carbohydrate fragments may be fragments of carbohydrate-based tumor antigens, such as Globo H, LeY or STn. The multivalent molecules may be produced by random conjugation or site-directed conjugation of the carbohydrate fragments to the carrier molecule. The multivalent molecules may be employed in vaccines or in the generation of antibodies for diagnostic application.
Owner:CONNAUGHT LAB
Nucleic acid and amino acid sequences relating to Streptococcus pneumoniae for diagnostics and therapeutics
InactiveUS7098023B1Easy to adaptSugar derivativesBacteriaStreptococcus pneumoniaeNucleic acid sequencing
The invention provides isolated polypeptide and nucleic acid sequences derived from Streptococcus pneumoniae that are useful in diagnosis and therapy of pathological conditions; antibodies against the polypeptides; and methods for the production of the polypeptides. The invention also provides methods for the detection, prevention and treatment of pathological conditions resulting from bacterial infection.
Owner:SANOFI PASTEUR LTD
Injectable vaccines against multiple meningococcal serogroups
ActiveUS20070082014A1Pharmaceutical delivery mechanismDepsipeptidesStreptococcus pneumoniaeSalmonella serotype typhi
An injectable immunogenic composition comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of Neisseria meningitidis, wherein said capsular saccharides are conjugated to carrier protein(s) and / or are oligosaccharides, and wherein (i) the composition comprises <50 mug meningococcal saccharide per dose, and / or (ii) the composition further comprises an antigen from one or more of: (a) serogroup B N. meningitidis; (b) Haemophilus influenzae type B; and / or (c) Streptococcus pneumoniae. Saccharide antigens in the compositions are generally conjugated to a carrier.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Nucleic acid and amino acid sequences relating to Streptococcus pneumoniae for diagnostics and therapeutics
InactiveUS7074914B1Easy to adaptAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeNucleic acid sequencing
Owner:SANOFI PASTEUR LTD
15-valent pneumococcal polysaccharide-protein conjugate vaccine composition
ActiveUS20110195086A1Antibacterial agentsBacteria material medical ingredientsConjugate vaccineDisease
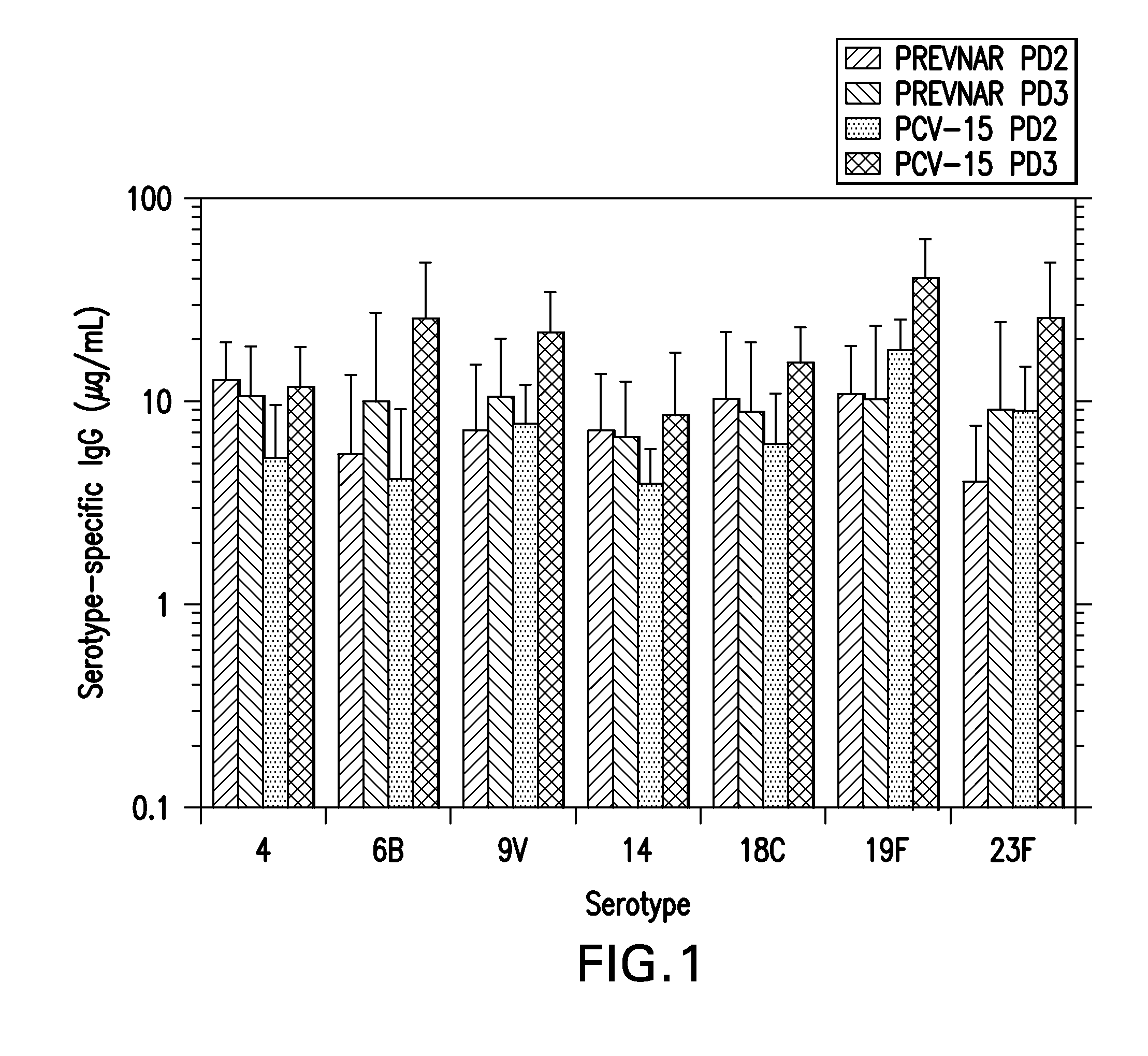
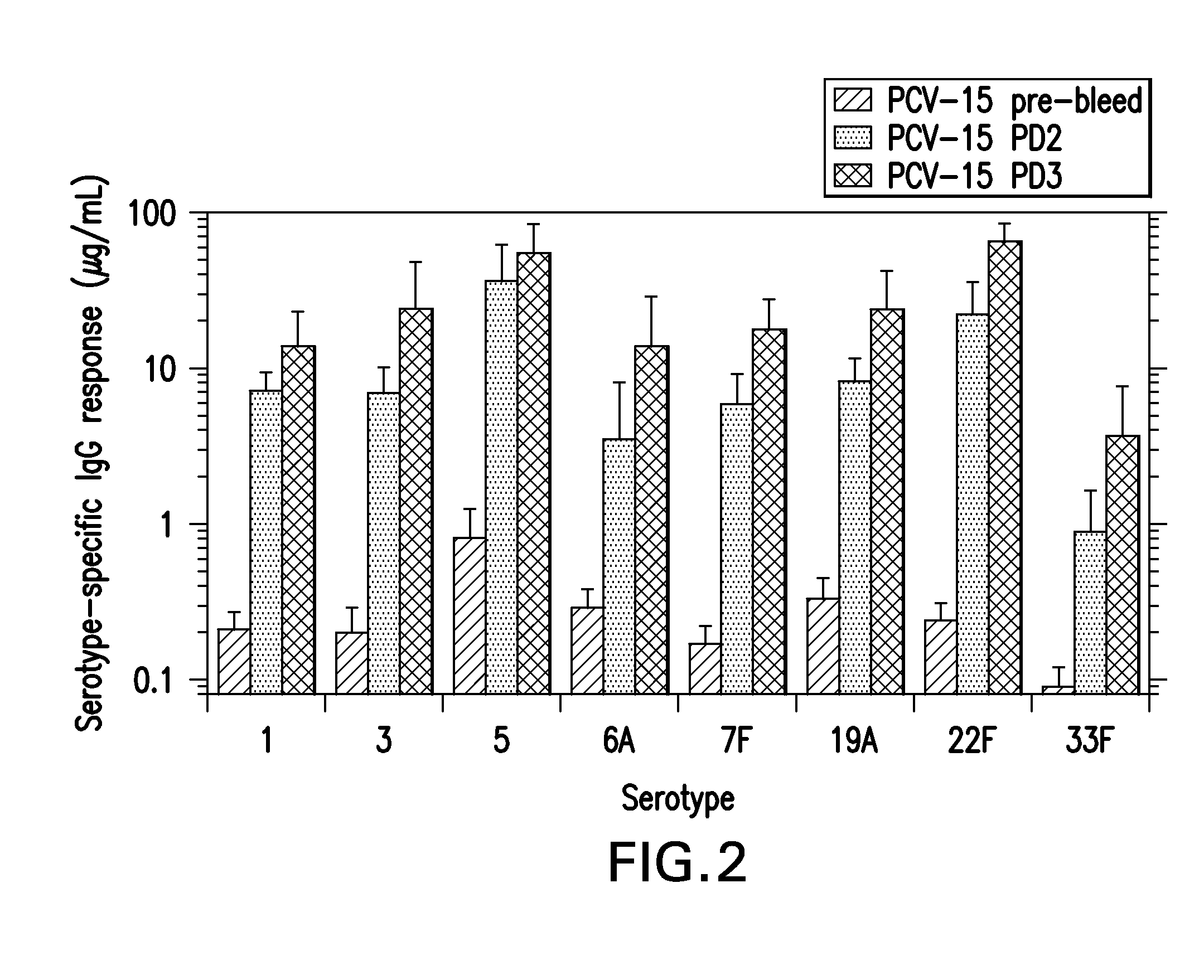
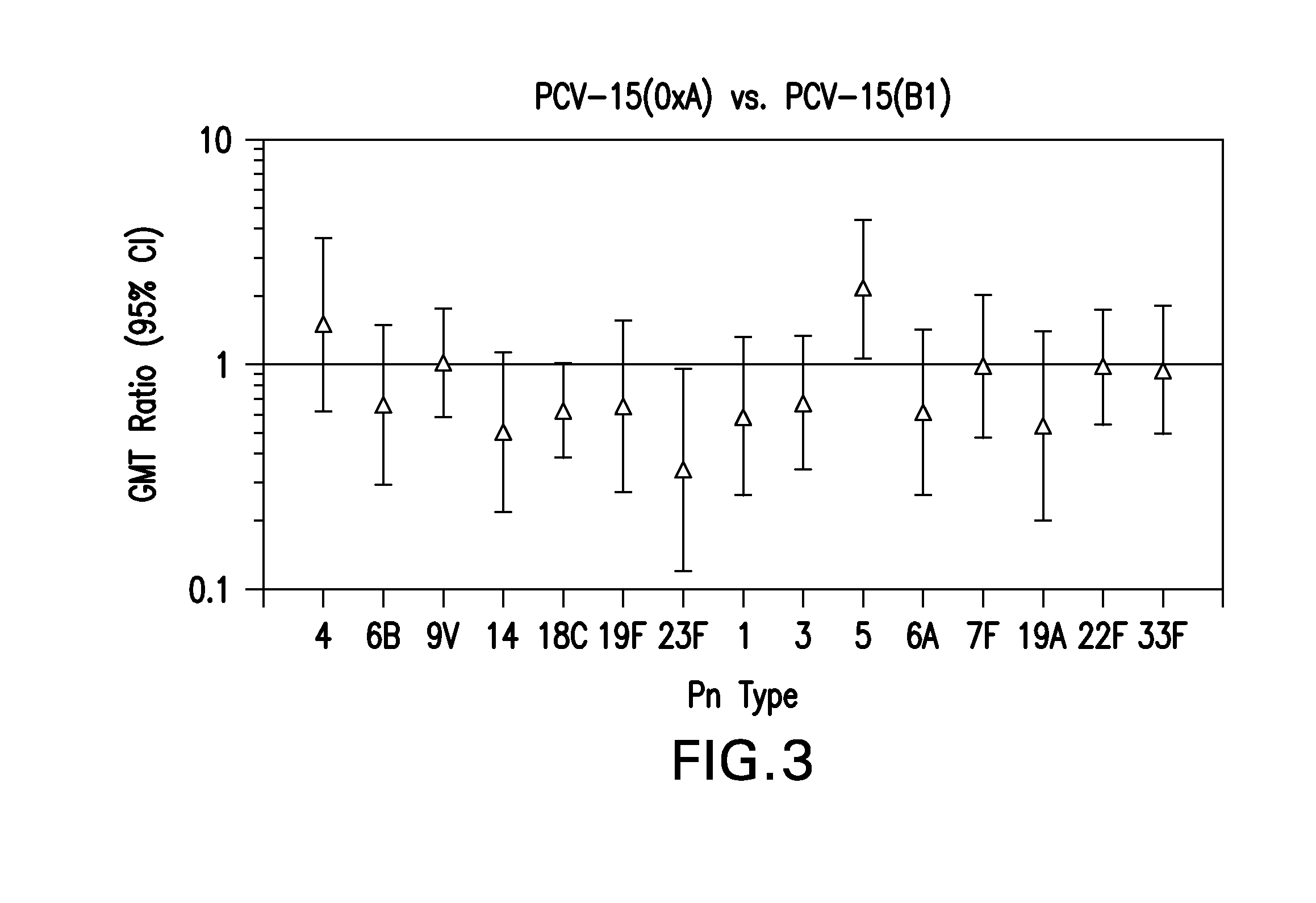
The present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197. The immunogenic composition, preferably formulated as a vaccine on an aluminum-based adjuvant, provides broad coverage against pneumococcal disease, particularly in infants and young children.
Owner:MERCK SHARP & DOHME LLC
Nucleic acid and amino acid sequences relating to Streptococcus pneumoniae for diagnostics and therapeutics
InactiveUS7081530B1Easy to adaptBacteriaSugar derivativesStreptococcus pneumoniaeNucleic acid sequencing
The invention provides isolated polypeptide and nucleic acid sequences derived from Streptococcus pneumoniae that are useful in diagnosis and therapy of pathological conditions; antibodies against the polypeptides; and methods for the production of the polypeptides. The invention also provides methods for the detection, prevention and treatment of pathological conditions resulting from bacterial infection.
Owner:SANOFI PASTEUR LTD
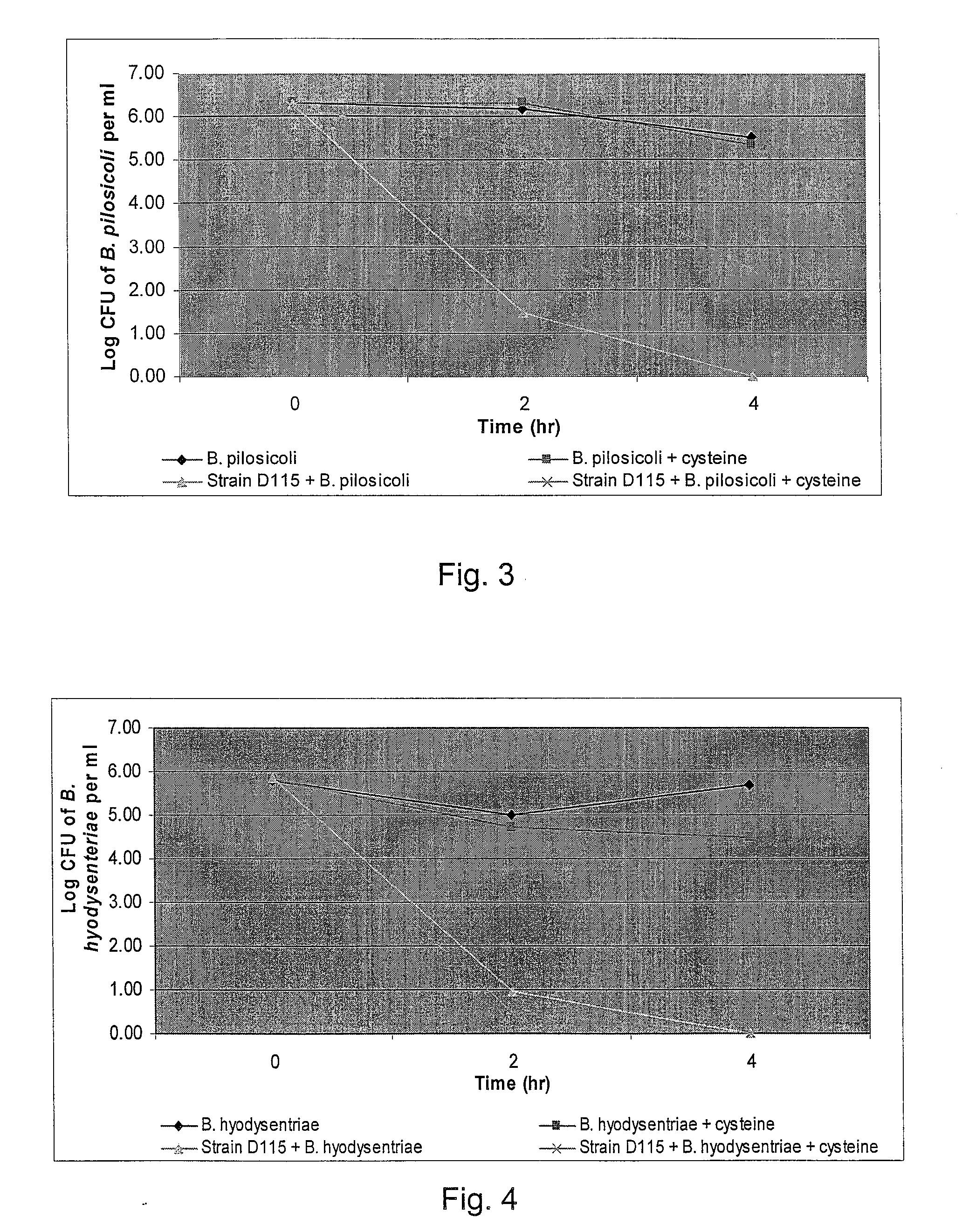
Broad-Spectrum Antibacterial and Antifungal Activity of Lactobacillus Johnsonii D115
The present invention demonstrated the potential use of Lactobacillus johnsonii D115 as a probiotic, as a prophylactic agent or as a surface treatment of materials against human and animal pathogens such as Brachyspira pilosicoli, Brachyspira hyodysenteriae, Shigella sonnei, Vibrio cholera, Vibrio parahaemolyticus, Campylobacter jejuni, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, Clostridium perfringens, Yersinia enterocolitica, Escherichia coli, Klebbsiella pneumoniae, Staphylococcus aureus, Salmonella spp., Bacillus cereus, Aspergillus niger and Fusarium chlamydosporum. The proteineous antimicrobial compound was partially characterized and found to be heat tolerant up to 121° C. for 15 min, and acid tolerant up to pH1 for 30 min at 40° C. The compound is also stable to enzymatic digestion, being able to retain more than 60% antimicrobial activity when treated with pepsin and trypsin.
Owner:KEMIN IND INC
Antimicrobial compounds from Bacillus subtilis for use against animal and human pathogens
ActiveUS7247299B2Enhanced inhibitory effectRetain viabilityAntibacterial agentsBiocideBacillus perfringensClostridium difficile (bacteria)
Antimicrobial compounds from Bacillus subtilis for use against animal and human pathogens. A novel strain of Bacillus subtilis was isolated from the gastrointestinal tract of poultry and was found to produce a factor or factors that have excellent inhibitory effects on Clostridium perfringens, Clostridium difficile, Campylobacter jejuni, Campylobacter coli, and Streptococcus pneumoniae. The factor(s) retain full viability and antimicrobial activity after heat treatment. The invention provides a method of treatment of pathogenic microorganisms including C. perfringens.
Owner:KEMIN IND INC
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20150202309A1Excellent characteristicsHigh yieldAntibacterial agentsMedical devicesStreptococcus pneumoniaeVaccination
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
Nucleic acid and amino acid sequences relating to streptococcus pneumoniae for diagnostics and therapeutics
InactiveUS20050136404A1Easy to adaptAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeNucleic acid sequencing
The invention provides isolated polypeptide and nucleic acid sequences derived from Streptococcus pneumonia that are useful in diagnosis and therapy of pathological conditions; antibodies against the polypeptides; and methods for the production of the polypeptides. The invention also provides methods for the detection, prevention and treatment of pathological conditions resulting from bacterial infection.
Owner:SANOFI PASTEUR LTD
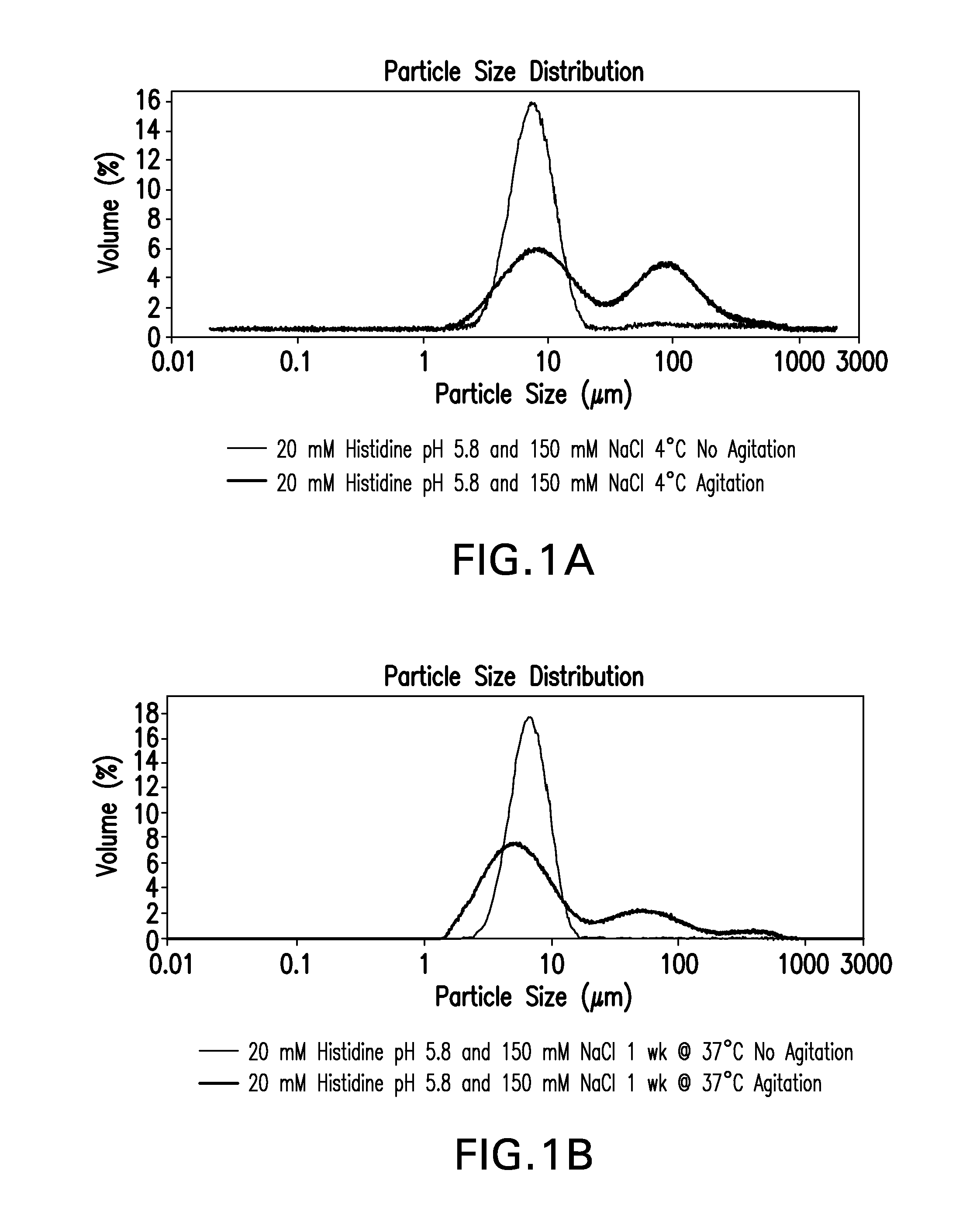
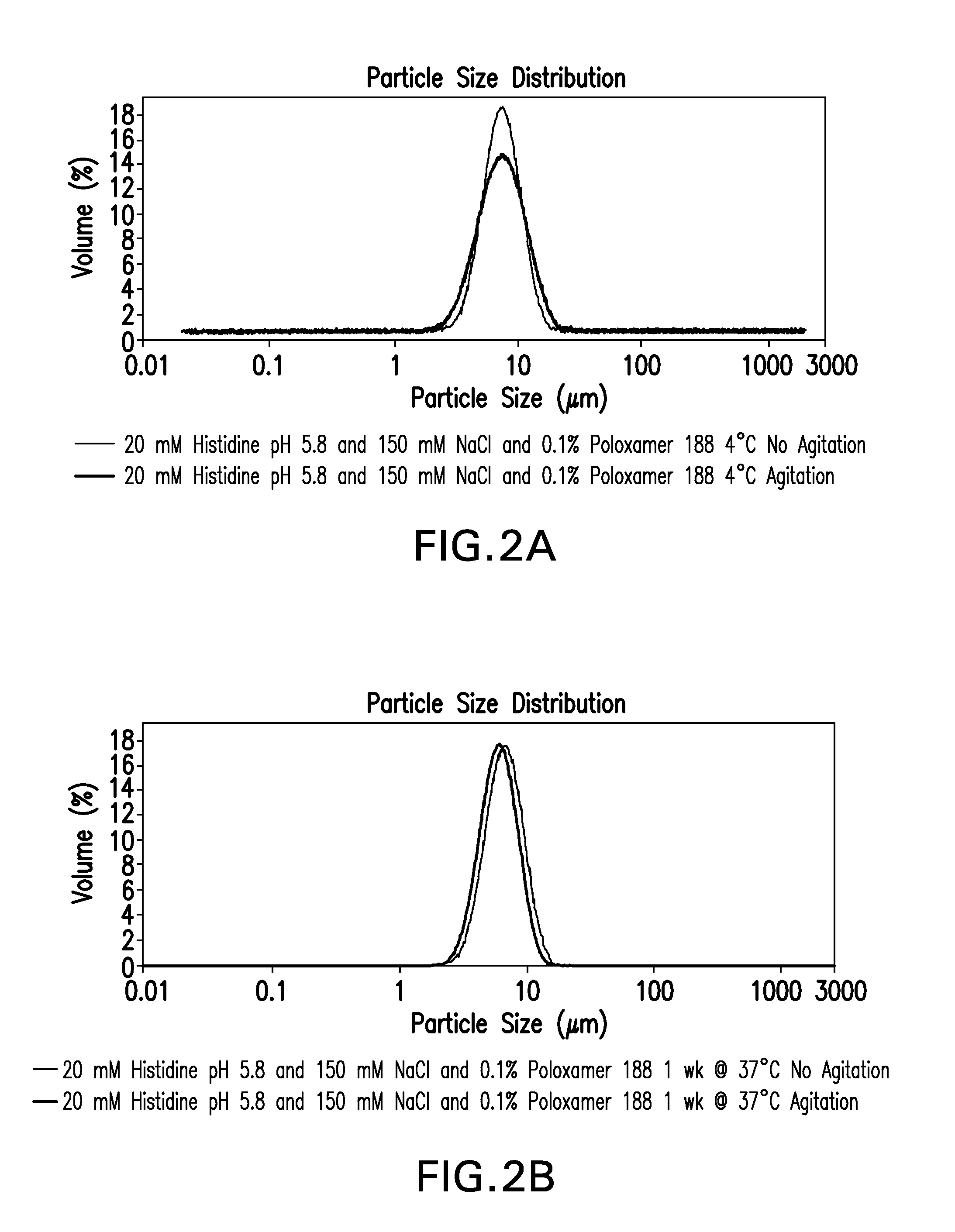
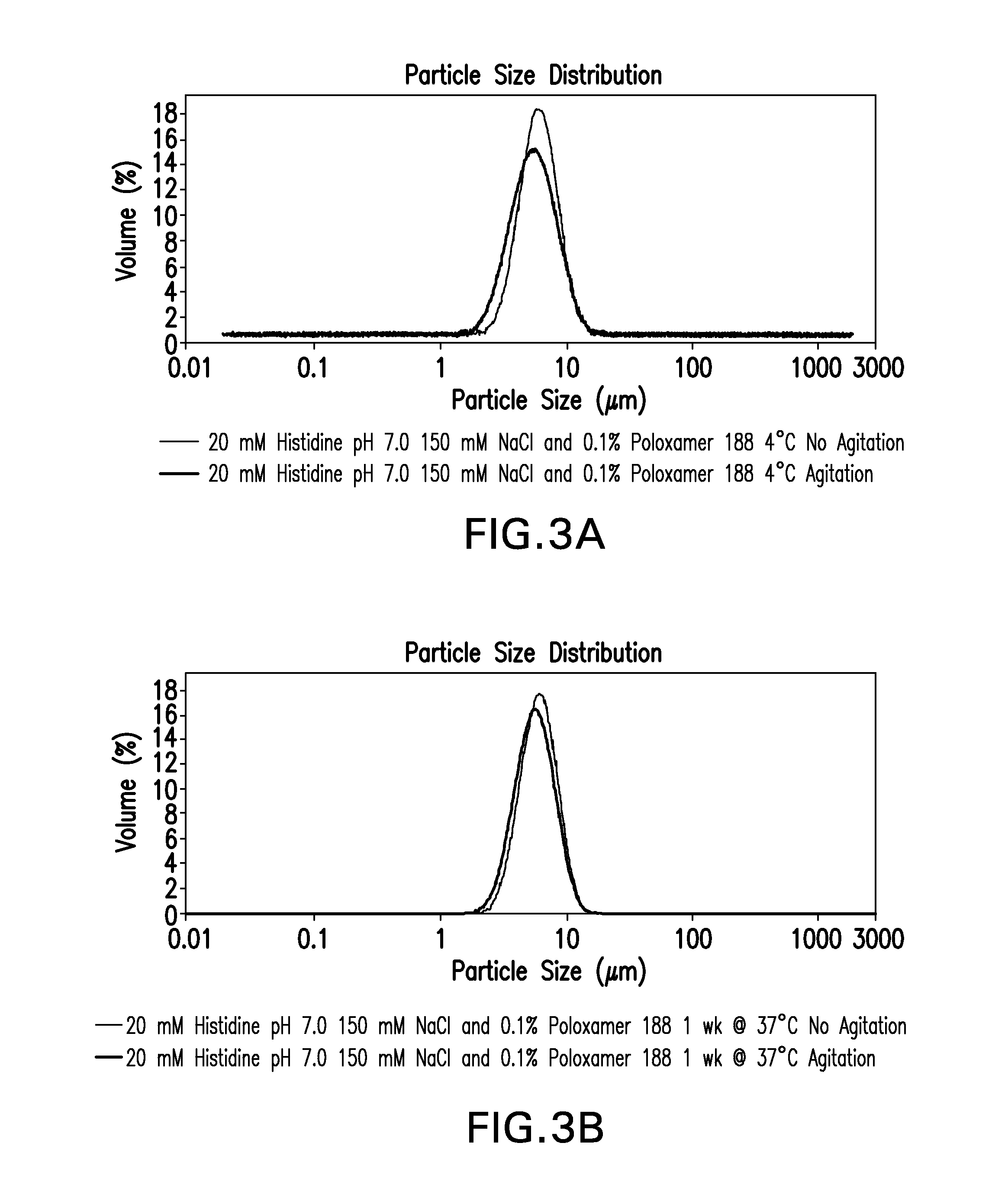
Novel formulations which mitigate agitation-induced aggregation of immunogenic compositions
InactiveUS20130273098A1Stabilize immunogenic compositionInhibit aggregationAntibacterial agentsBiocideStreptococcus pneumoniaeCarrier protein
The present invention provides novel formulations which mitigate agitation-induced aggregation of immunogenic compositions particularly those having polysaccharide-protein conjugates. Specifically, the novel formulations comprise a poloxamer within a molecular weight range of 1100 to 17,400 which provides significant advantages over previously used surfactants including polysorbate 80. In one embodiment, the present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates and a poloxamer. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197.
Owner:MERCK SHARP & DOHME CORP
Streptococcal heat shock proteins of the Hsp60 family
Methods and compositions comprising isolated nucleic acid molecules specific to Streptococcus pneumoniae and Streptococcus pyogenes, as well as vector constructs and isolated polypeptides specific to Streptococcus pneumoniae and Streptococcus pyogenes are provided. Such compositions and methods are useful for the diagnosis of Streptococcal infection and for generating an immune response to Streptococcal bacteria.
Owner:NVENTA BIOPHARMACEUTICALS CORP
Pneumococcal surface proteins and uses thereof
InactiveUS6500613B1Improving immunogenicityLow production costAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeCoccidia
The present invention relates to pneumococcal genes, portions thereof, expression products therefrom and uses of such genes, portions and products; especially to genes of Streptococcus pneumoniae, e.g., the gene encoding pneumococcal surface protein A (PspA), i.e., the pspA gene, the gene encoding pneumococcal surface protein A-like proteins, such as pspA-like genes, e.g., the gene encoding pneumococcal surface protein C (PspC), i.e., the pspC gene, portions of such genes, expression products therefrom, and the uses of such genes, portions thereof and expression products therefrom.
Owner:ALABAMA AT BIRMINGHAM UNIVERISTY OF
Kit for quickly detecting 15 pneumonia pathogenic bacteria
ActiveCN107338315AMicrobiological testing/measurementMicroorganism based processesBacteroidesStaphylococcus aureus
The invention discloses a kit for quickly detecting 15 pneumonia pathogenic bacteria. The kit can detect streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, baumanii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae which cover clinically common pneumonia pathogenic bacteria difficult to culture. 16S rDNA and specific gene sequences corresponding to the pneumonia pathogenic bacteria are detected by combining gene chips with multiple asymmetric PCR reactions, and the categories of the bacteria in a to-be-detected sample are identified in genus and species. The kit makes up for the defect that current clinical detection of pneumonia pathogenic bacteria is not in time or comprehensive and a novel detection means for early diagnosis and early treatment of patients suffering from pneumonia is provided.
Owner:GENERAL HOSPITAL OF PLA +1
Multi oligosaccharide glycoconjugate bacterial meningitis vaccines
InactiveUS6656472B1Inhibition effectWeight increaseAntibacterial agentsAntipyreticSerotypeTumor antigen
Owner:AVENTIS PASTEUR LTD
Vaccine
InactiveUS20040081662A1Avoid toxicityEnhance immune responseAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeMedicine
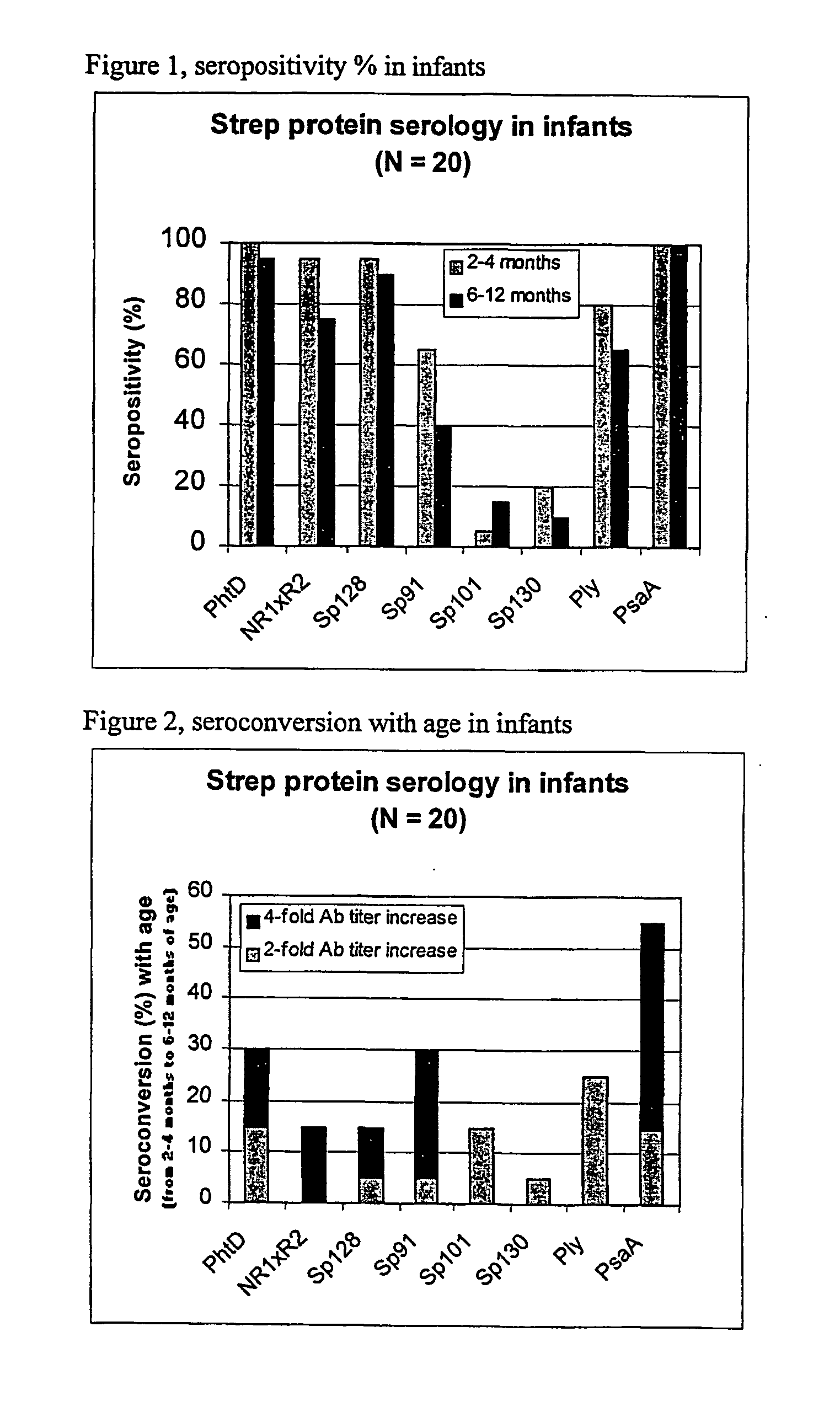
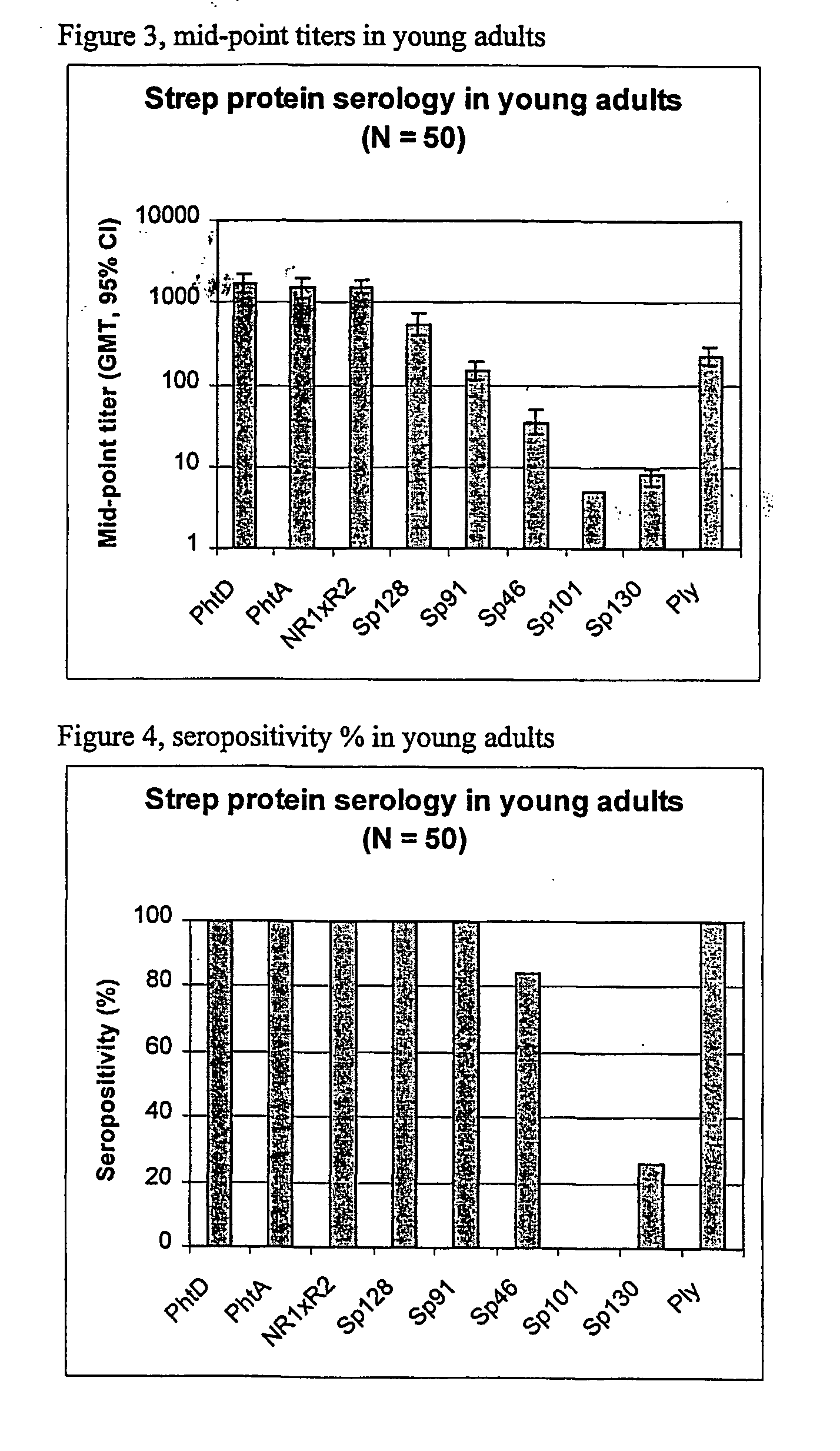
The present invention relates to a combination of 2 or more S pneumoniae proteins, their manufacture and use in medicine as a vaccine. Such combinations are particularly useful for the protection of infants and elderly against streptococcal infection.
Owner:SMITHKLINE BECKMAN CORP +1
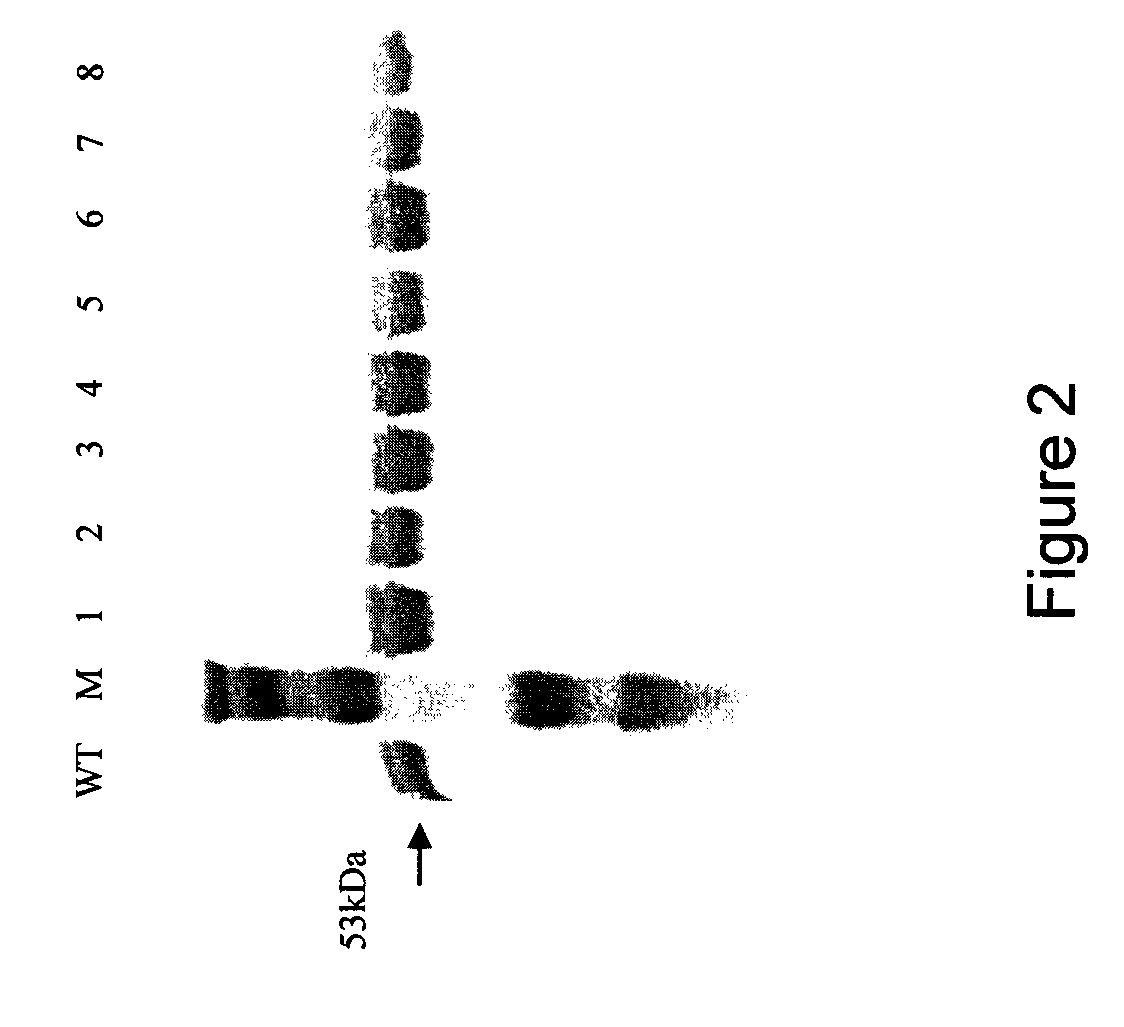
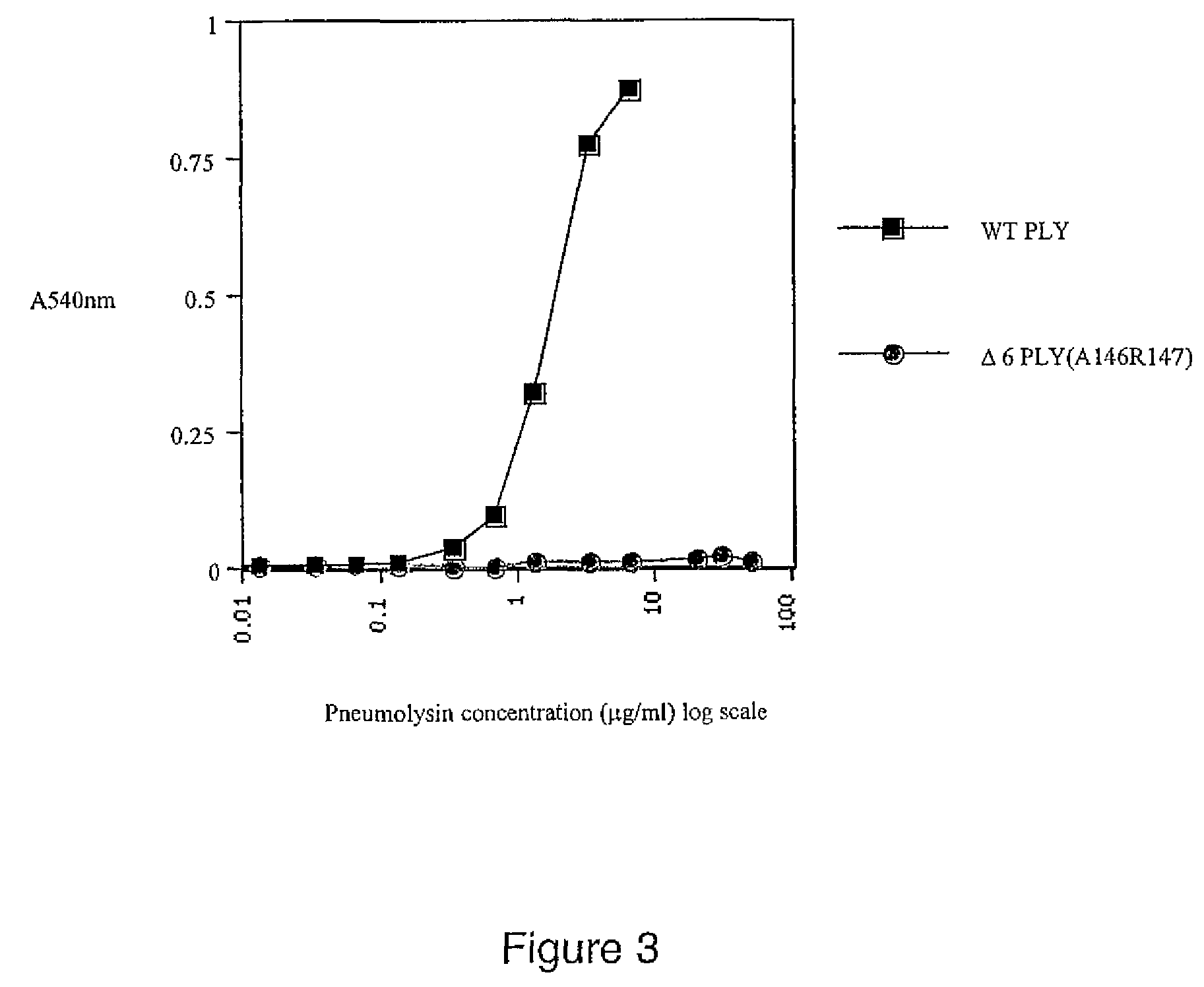
Mutant pneumolysin proteins
InactiveUS7820789B2Low toxicityReduced oligomerisation activityAntibacterial agentsPeptide/protein ingredientsSynechococcusPyrococcus
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
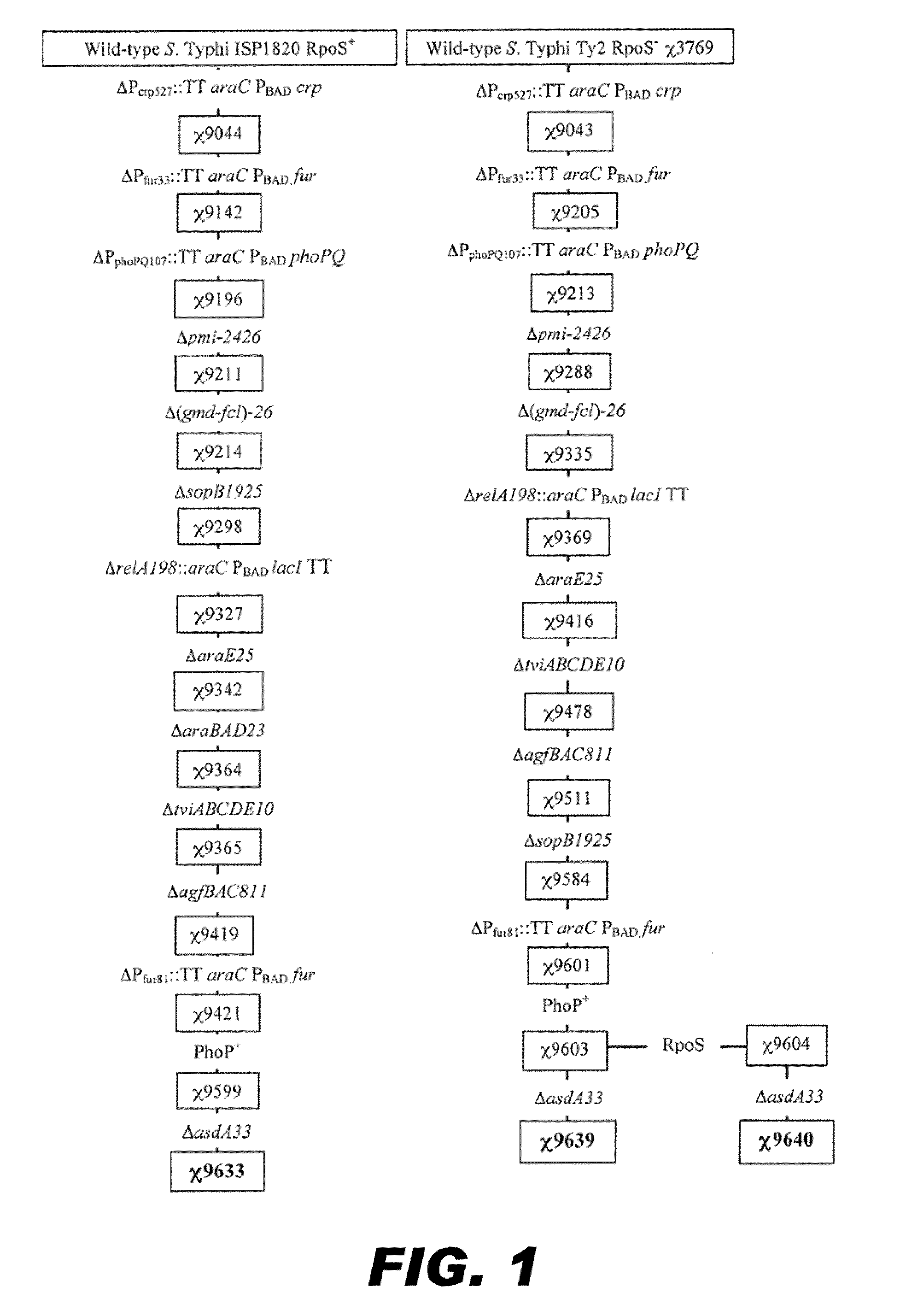
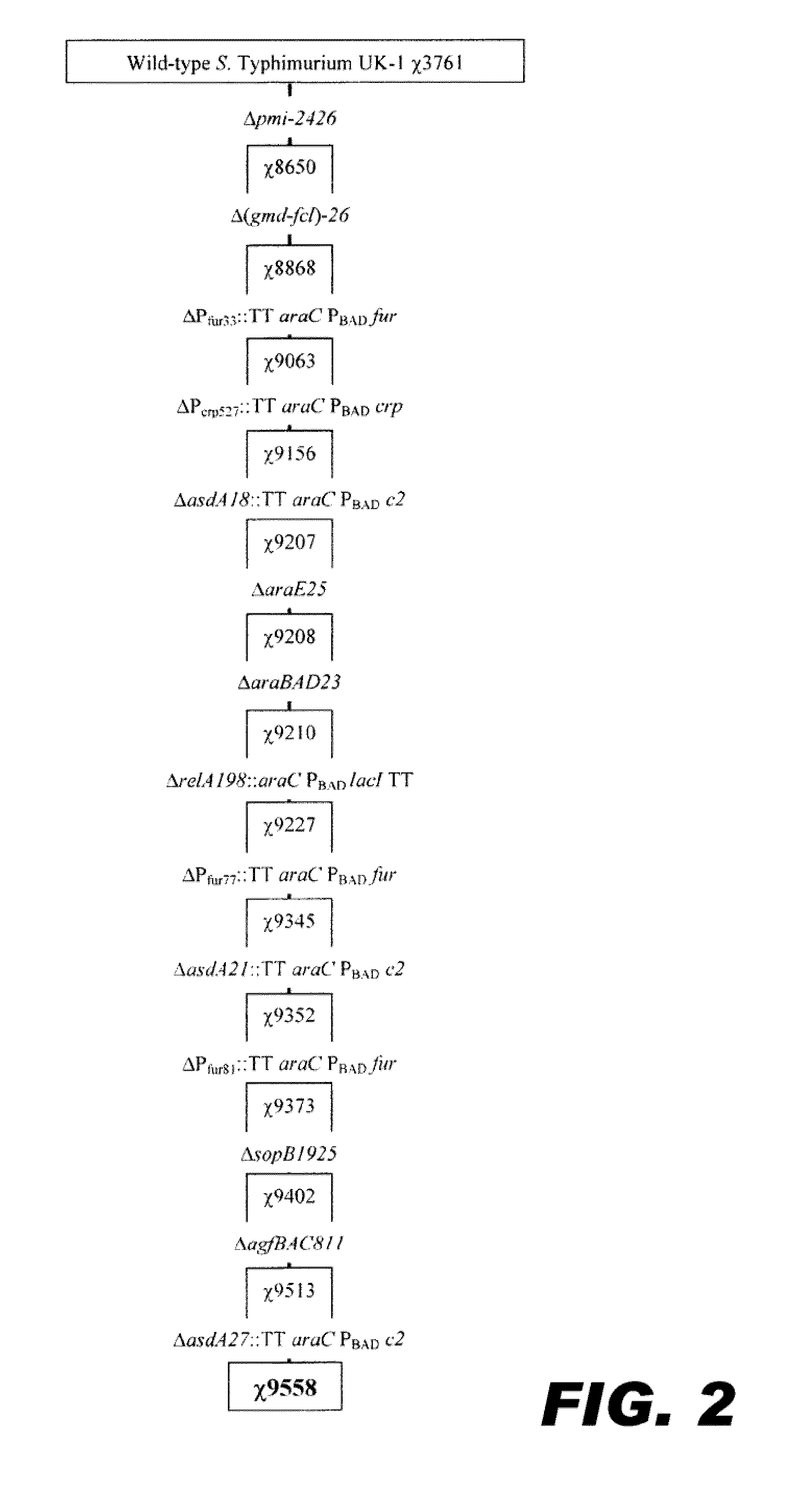
Recombinant bacterium capable of eliciting an immune response against streptococcus pneumoniae
ActiveUS20110287052A1Reduces fluid secretionAntibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
The invention encompasses a recombinant bacterium capable of eliciting an immune response against Streptococcus pneumoniae, a vaccine comprising the bacterium, and methods of using the bacterium.
Owner:ARIZONA STATE UNIVERSITY
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20180099039A1Excellent characteristicsHigh yieldPowder deliveryBacterial antigen ingredientsStreptococcus pneumoniaeVaccination
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
Vaccine against Streptococcus pneumoniae
InactiveUS20060051361A1Good synergyAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeAdjuvant
The present invention relates to the field of bacterial polysaccharide antigen vaccines. In particular, the present invention relates to vaccines comprising a pneumococcal polysaccharide antigen, typically a pneumococcal polysaccharide conjugate antigen from Streptococcus pneumoniae selected from the group consisting of PhtA, PhtD, PhtB, PhtE, SpsA, LytB, LytC, LytA, Sp125, Sp101, Sp128, Sp130 and Sp133, and optionally a Th1-inducing adjuvant.
Owner:LAFERRIERE CRAIG A J +1
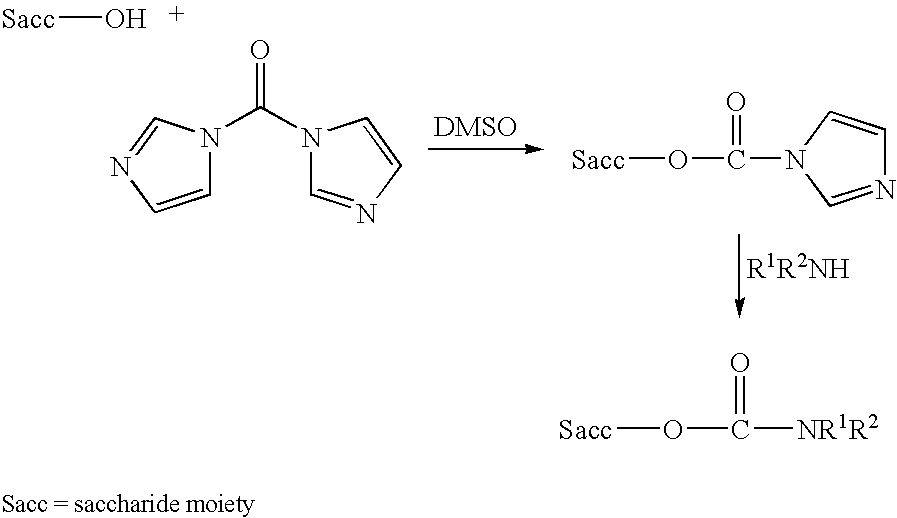
Conjugation process of bacterial polysaccharides to carrier proteins
ActiveUS8753645B2Antibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
Process for conjugation of bacterial saccharides including Streptococcus pneumoniae and Haemophilus influenzae saccharides by reductive amination are provided herein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Secreted Streptococcus Pneumoniae Proteins
Novel proteins from Streptococcus pneumoniae are described, together with nucleic acid sequences encoding them. Their use in vaccines and in screening methods is also described.
Owner:SANOFI PASTEUR LTD
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
Polypeptides And Immunogenic Conjugates Capable of Inducing Antibodies Against Pathogens, and Uses Thereof
InactiveUS20080171053A1Efficient responseAntibacterial agentsBacterial antigen ingredientsKexinDNA construct
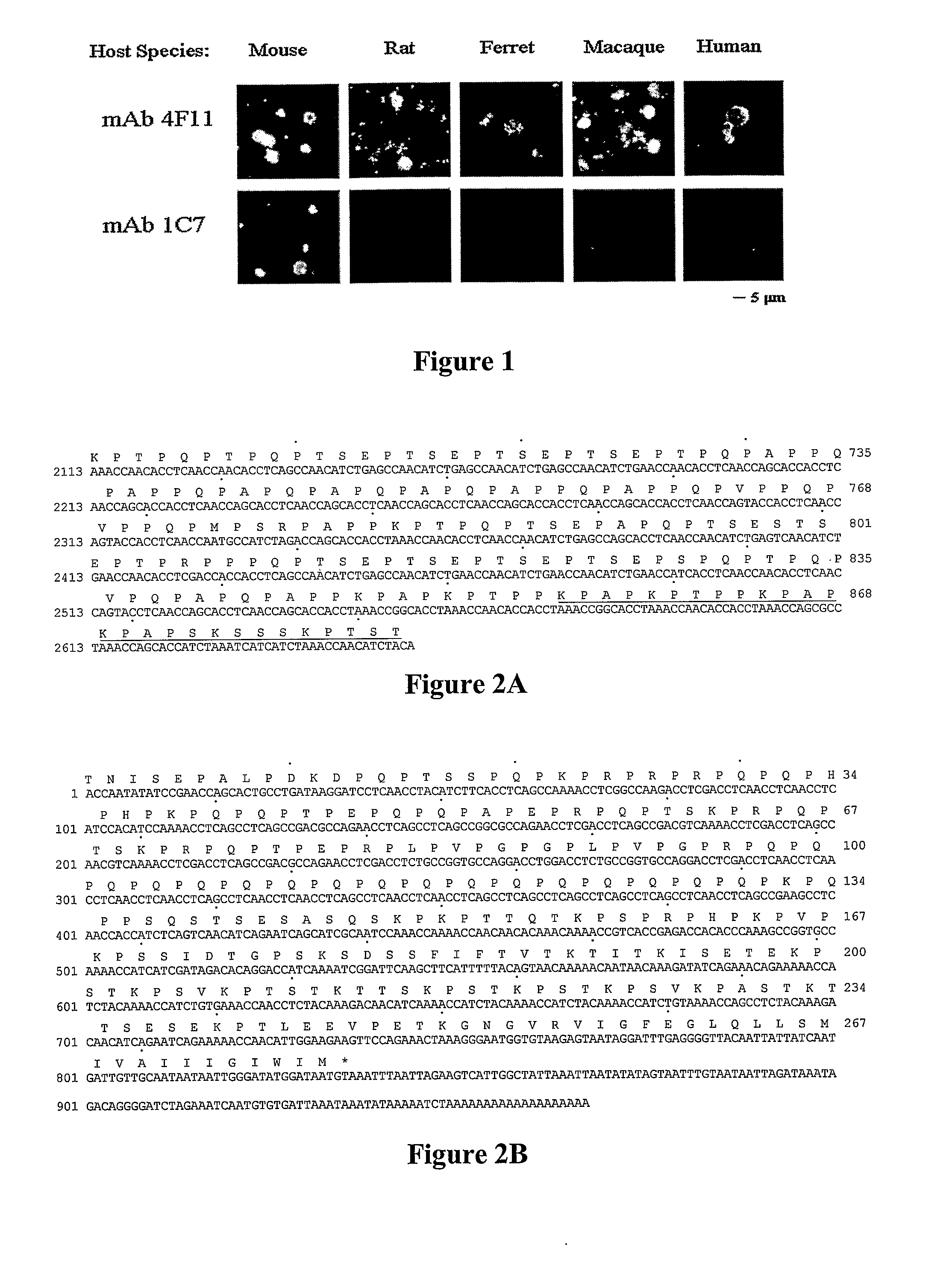
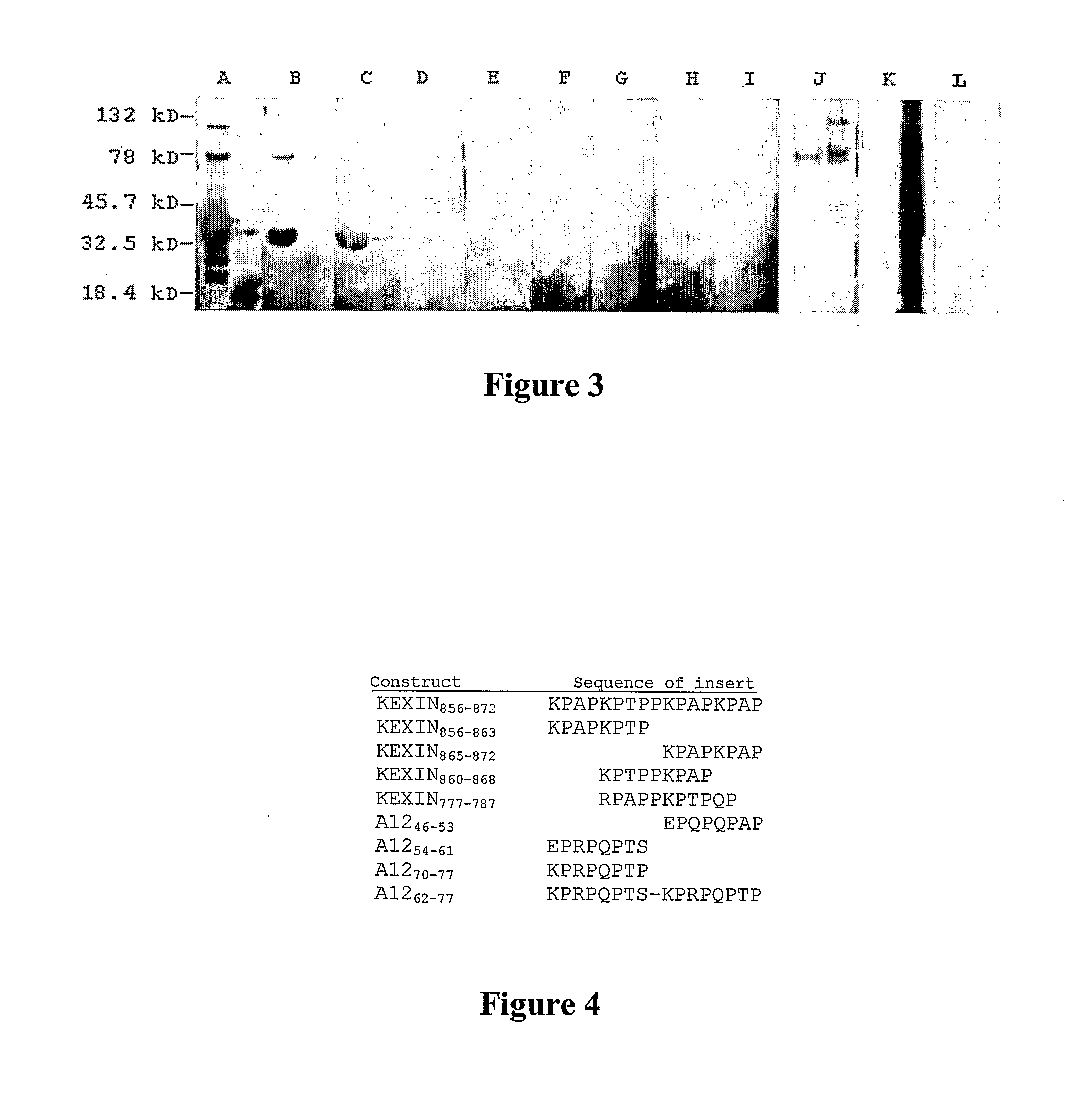
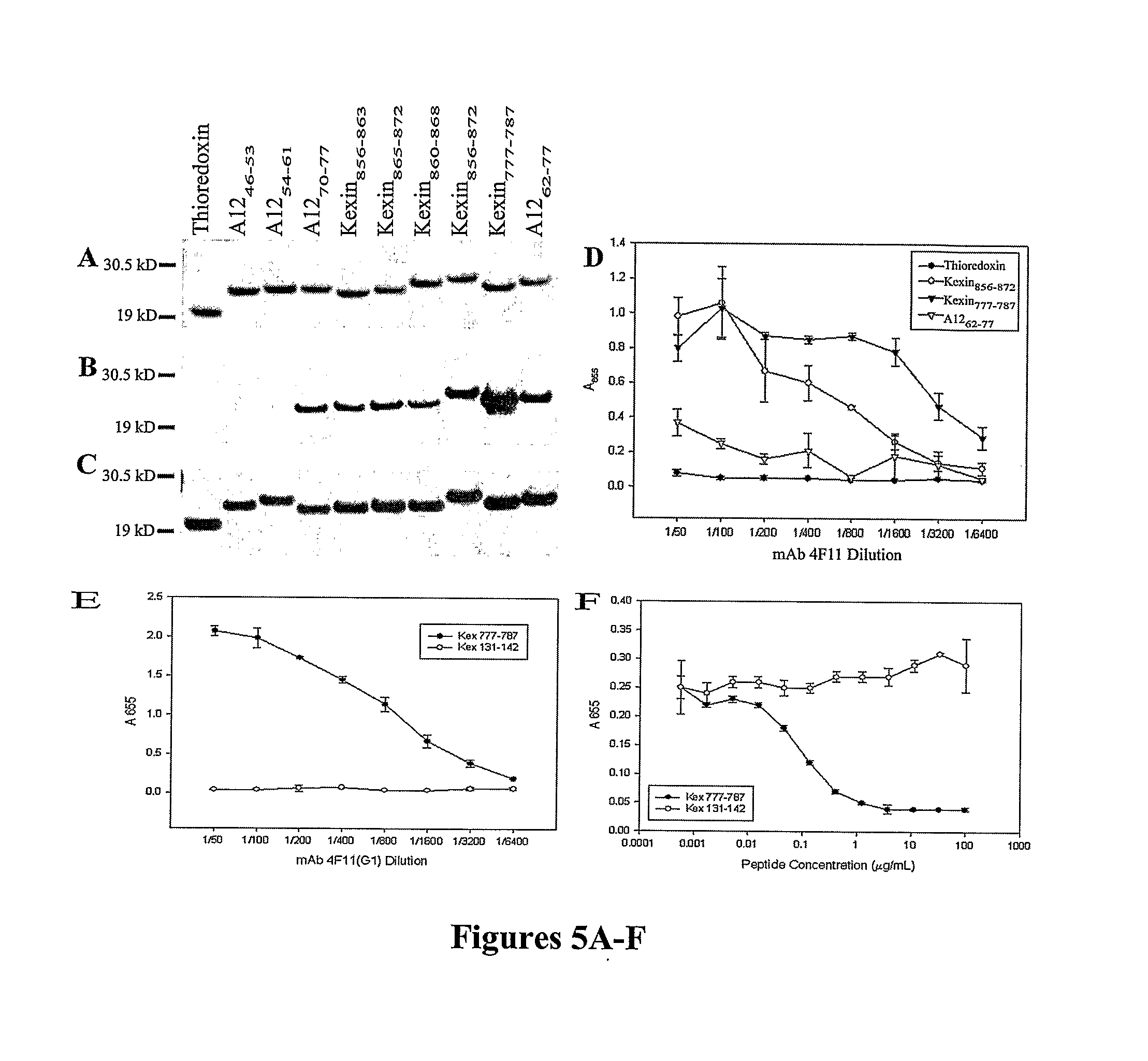
A number of immunologically active agents are described, including an isolated protein or polypeptide that includes the amino acid sequence of SEQ ID NO: 1, immunogenic conjugates containing either the protein or polypeptide, a full-length Pneumocystis kexin, or a full length Streptococcus pneumoniae pneumococcal surface protein A (PspA), antibodies recognizing the protein or polypeptide or the immunogenic conjugates (particularly the epitope of SEQ ID NO: 1), and nucleic acid molecules that encode the protein or polypeptide, as well as DNA constructs, expression vectors, and host cells that contain the nucleic acid molecules. Disclosed uses of the antibodies, immunogenic conjugates, and DNA constructs include inducing passive or active immunity to treat or prevent pathogen infections, particularly by a Pneumocystis organism, in a patient.
Owner:UNIVERSITY OF ROCHESTER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com