Liquid preparation of recombinant anti-PD-L1 whole-human monoclonal antibody
A monoclonal antibody, liquid preparation technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc., can solve the problem of difficulty in obtaining liquid preparations, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
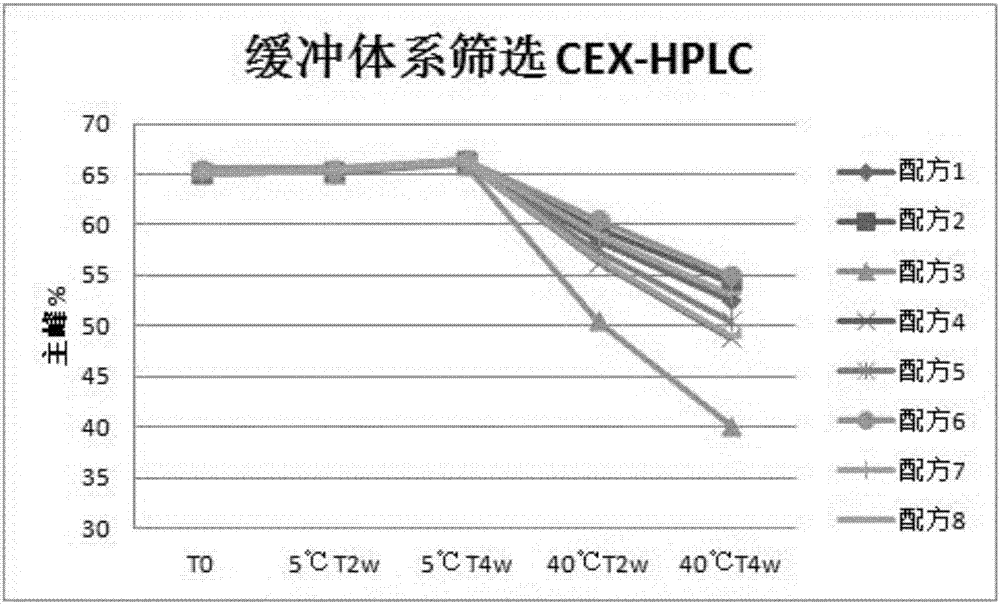
[0047] The screening of embodiment 1 buffer system kind
[0048] The formula of the recombinant anti-PD-L1 fully human monoclonal antibody liquid preparation of this embodiment is as follows:
[0049] Formula 1: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM acetic acid / sodium acetate buffer system, pH value is 4.5;
[0050] Formula 2: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM acetic acid / sodium acetate buffer system, pH value is 5.0;
[0051] Formula 3: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM citric acid / sodium citrate buffer system, pH 5.0;
[0052] Formula 4: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM citric acid / sodium citrate buffer system, pH value is 5.5;
[0053] Formula 5: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM citric acid / sodium citrate buffer system, pH value is 6.0;
[0054] Formula 6: 30mg / ml recombinant anti-PD-L1 fully hum...
Embodiment 2
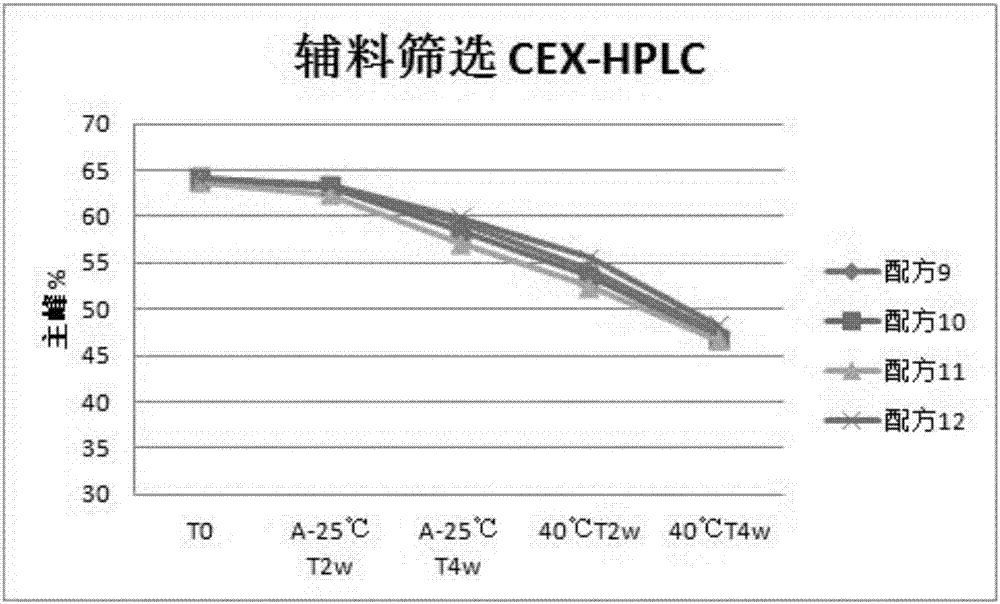
[0063] Embodiment 2 auxiliary material screening test
[0064] The formula of the recombinant anti-PD-L1 fully human monoclonal antibody liquid preparation of this embodiment is as follows:
[0065] Formula 9: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM histidine-histidine hydrochloride buffer + 150mM trehalose + 55mM sodium chloride + 0.01wt% polysorbate 80;
[0066] Formula 10: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM histidine-histidine hydrochloride buffer + 150mM sucrose + 55mM sodium chloride + 0.03wt% polysorbate 80;
[0067] Formula 11: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM histidine-histidine hydrochloride buffer + 150mM glycine + 55mM sodium chloride + 0.05wt% polysorbate 80;
[0068] Formula 12: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM histidine-histidine hydrochloride buffer + 150mM mannitol + 55mM sodium chloride + 0.05wt% polysorbate 80;
[0069] ...
Embodiment 3
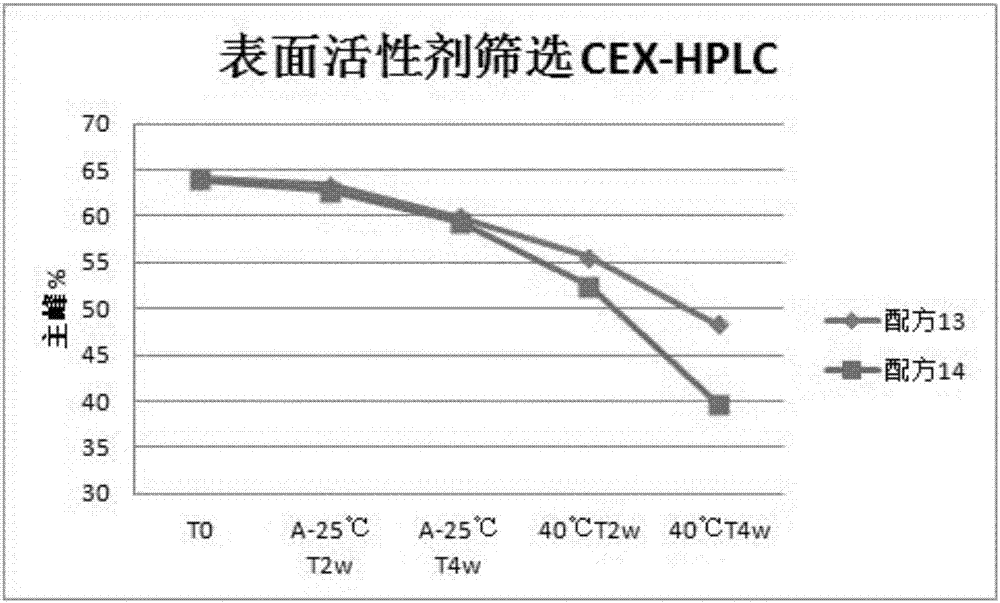
[0075] The screening test of embodiment 3 surfactants
[0076] Formula 13: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM histidine-histidine hydrochloride buffer + 150mM mannitol + 55mM sodium chloride + 0.01wt% polysorbate 80;
[0077] Formula 14: 30mg / ml recombinant anti-PD-L1 fully human monoclonal antibody + 20mM citrate buffer + 150mM mannitol + 55mM sodium chloride + 0.05wt% polysorbate 80;
[0078] The preparation method of the recombinant anti-PD-L1 fully human monoclonal antibody liquid preparation of the above formulations is the same as that in Example 1.
[0079]The samples of the above formulations 13 and 14 were sampled and divided into two groups, which were respectively placed under the conditions of 40°C 75% RH and 25°C 100rpm shaking conditions for investigation. The samples were taken at 2 weeks and 4 weeks for purity testing, and cation exchange chromatography CEX -HPLC detects, and the investigation results are shown in Table 3:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com