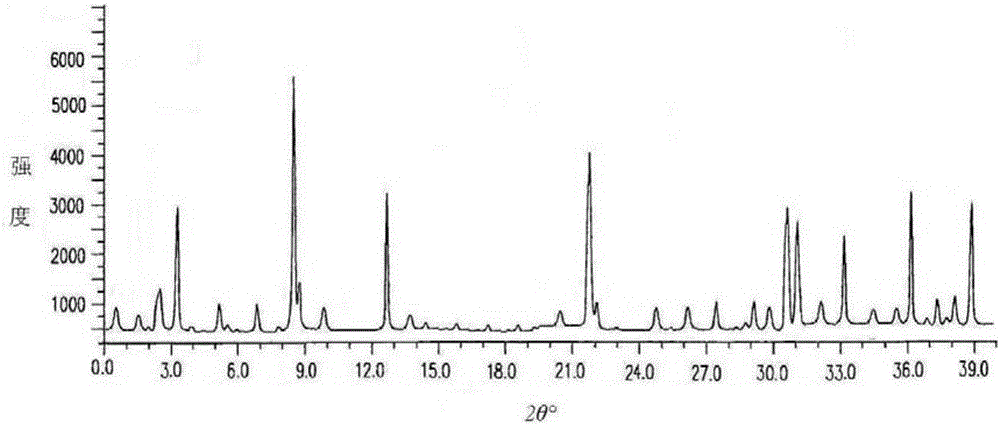
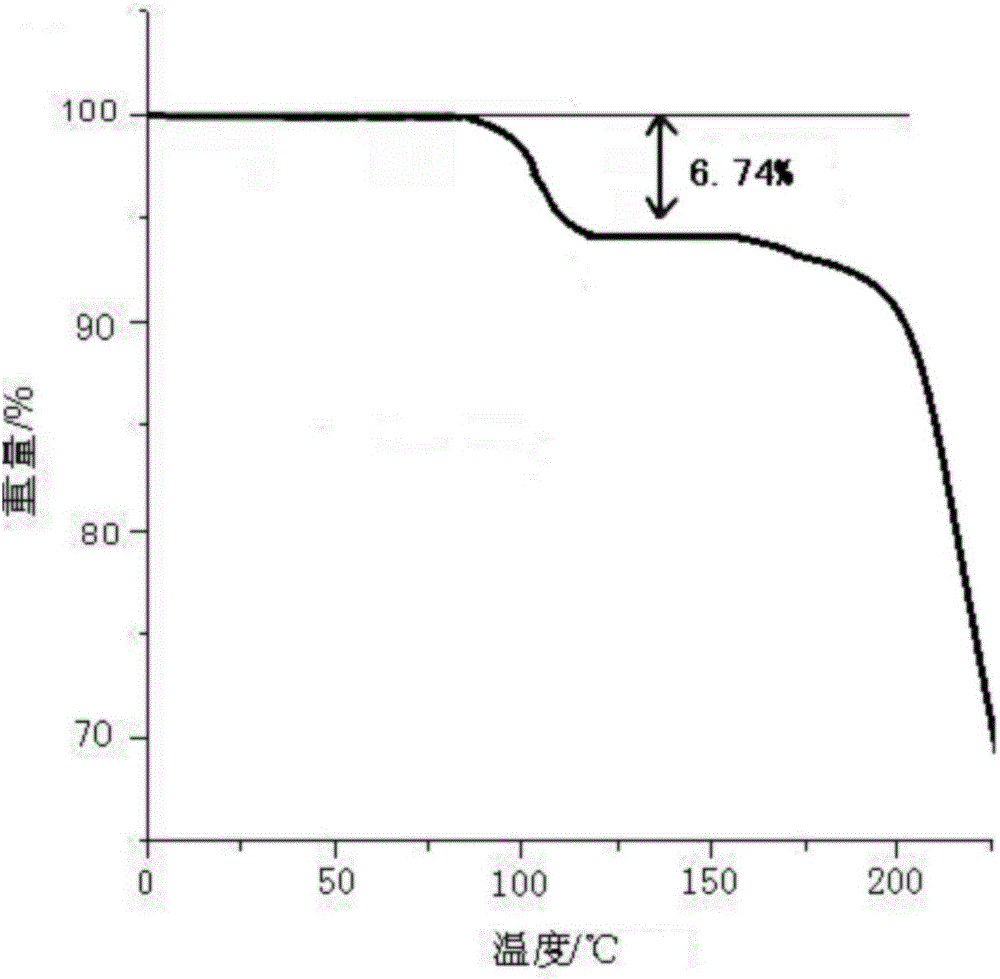
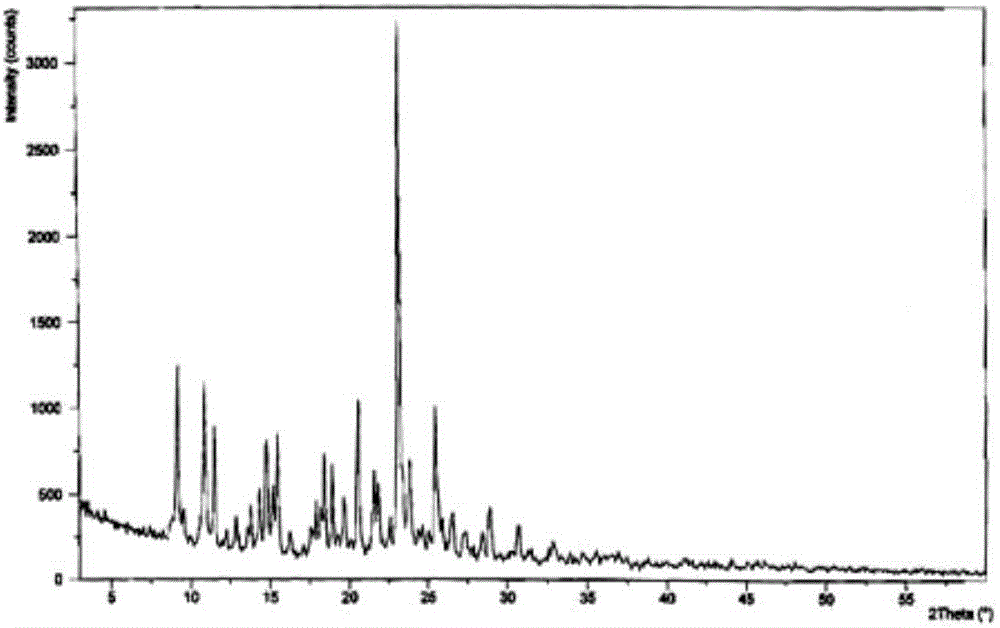
Dispersing tablet for treating diseases of respiratory systems and method for preparing dispersing tablet
A respiratory system disease, dispersion technology, applied in the direction of respiratory system disease, dispersion liquid delivery, organic chemical methods, etc., can solve the problems of poor compressibility, easy sticking, poor solubility of erdosteine, etc., to improve solubility, prevent Effects of sticking punch, improving stability and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the preparation of erdosteine hydrate
[0061] (1) Get erdosteine crude product 100g, be dissolved in the temperature that 500ml acetone and 200ml ethanol form after grinding and sieving is in the mixed solution of 30 ℃, obtains erdosteine solution;
[0062] (2) add 0.05g of activated carbon for decolorization for 25 minutes, and filter;
[0063] (3) Under the condition of a stirring rate of 23rmp, add 3 times the volume of the erdosteine solution with normal saline at a temperature of -10°C dropwise at a constant speed, and the dropwise addition is completed within 1 hour;
[0064] (4) After the dropwise addition was completed, the temperature was lowered to -10°C and the stirring was continued for 1 h at a stirring rate of 12 rpm, and white crystals were precipitated after standing for 4 h, and filtered;
[0065] (5) Washing successively with distilled water and ethyl acetate, followed by vacuum drying to obtain 98.75 g of erdosteine hydrate c...
Embodiment 2
[0073] Embodiment 2: the preparation of erdosteine hydrate
[0074] (1) Get erdosteine crude product 100g, be dissolved in the temperature that 500ml acetone and 100ml ethanol form after grinding and sieving is in the mixed solution of 25 ℃, obtains erdosteine solution;
[0075] (2) Add 0.1 g of activated carbon for decolorization for 20 minutes, and filter;
[0076] (3) Under the condition of a stirring rate of 20rmp, add 4 times the volume of erdosteine solution with physiological saline at a temperature of 5°C dropwise at a constant speed, and the dropwise addition is completed within 1.5 hours;
[0077] (4) After the dropwise addition was completed, the temperature was lowered to 5° C. and the stirring was continued for 2 h at a stirring rate of 10 rpm, and white crystals were precipitated after standing for 5 h, and filtered;
[0078] (5) Washing with distilled water and ethyl acetate successively, followed by vacuum drying to obtain 98.52 g of erdosteine hydra...
Embodiment 3
[0084] Embodiment 3: the preparation of erdosteine hydrate
[0085] (1) Get erdosteine crude product 100g, be dissolved in the temperature that 500ml acetone and 300ml ethanol form after grinding and sieving is in the mixed solution of 35 ℃, obtains erdosteine solution;
[0086] (2) add 0.02g of activated carbon for decolorization for 30 minutes, and filter;
[0087] (3) Under the condition of a stirring rate of 25rmp, add 2 times the volume of erdosteine solution with normal saline at a temperature of -15°C dropwise at a constant speed, and the dropwise addition is completed within 0.5h;
[0088] (4) After the dropwise addition, cool down to -15°C and continue to stir for 0.5h at a stirring rate of 15rmp, let stand for 3h to precipitate white crystals, and filter;
[0089] (5) Washing successively with distilled water and ethyl acetate, followed by vacuum drying to obtain 98.67 g of erdosteine hydrate crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com