Pharmaceutical composition containing ambroxol and erdosteine or acetylcysteine and application thereof
A technology of acetylcysteine and composition, which is applied in the directions of drug combination, medical preparation containing active ingredients, drug delivery, etc., can solve the problem that the water solubility of ambroxol hydrochloride is not ideal, and the limitation of preparation application and clinical application, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
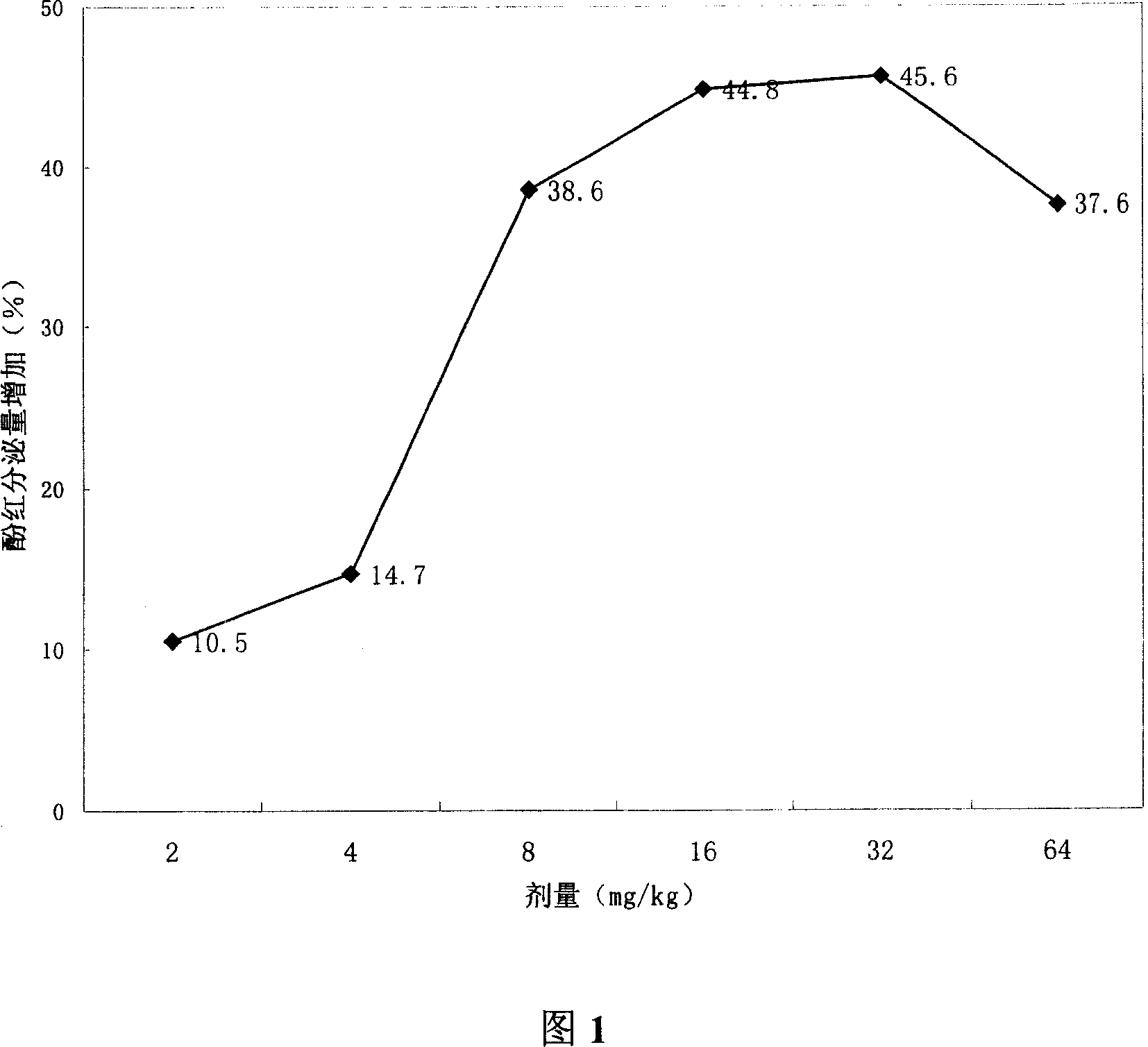
[0041] Experimental Example 1 Effects on Excretion of Phenol Red in the Respiratory Tract of Mice
[0042] 1), test method
[0043] 360 healthy male mice were randomly divided into 12 groups, respectively negative control group, erdosteine group, compound erdosteine ambroxol (equal quality) 6 dosage groups, ambroxol hydrochloride low, medium, High-dose group and potassium iodide group. The test sample was orally administered once a day for 3 consecutive days, with an administration volume of 0.4ml / 20g, and the negative control group was orally administered normal saline of the same volume. Fasting 1 day before the last administration, intraperitoneally inject 2.5% phenol red solution 500mg / kg, 0.4ml / 20g 0.5 hours after the last administration. After another 0.5 hour, overdose of anesthesia was given to kill the animal, the trachea was separated, an injection needle was inserted, rinsed with 2ml of normal saline, and 0.1ml of 1M NaOH was added to the rinse solution for co...
experiment example 2
[0054] Effect of Experimental Example 2 on Rabbit Sputum Secretion
[0055] 1) Experimental method
[0056] 80 rabbits were randomly divided into 8 groups, which were respectively compound erdosteine ambroxol (mass ratio 2: 1) 1, 2, 4 and 8 mg / kg four dosage groups and ambroxol hydrochloride 2, 4 and 8 mg / kg. 8mg / kg three dose groups, negative control group and erdosteine 4mg / kg group. Animals were intraperitoneally anesthetized with urethane, intubated with a tracheal tube, and connected to a device that constantly provided preheated (37° C.) and constant humidity (80%) breathing air. A fixed graduated cylinder is connected to the lower end of the cross-shaped endotracheal tube to collect secretions. To prevent condensation, all animals breathed freely and provided the required amount of breathing air in a cotton-padded enclosure. After 2 hours of stable observation, the experimental drug was delivered to the stomach through the esophagus. Immediately after administra...
experiment example 3
[0066] Effect of Experimental Example 3 on Movement of Dog Trachea Cilia in Vitro
[0067] 1), test method
[0068] Grass dog about 10kg, male and female, separate the trachea quickly after anesthesia, and put O 2 Tyrode's solution, save for later use. During the experiment, the trachea was cut to a length of 0.5-1 cm. Cut it in the middle, divide it into two pieces longitudinally, take a single piece and put it obliquely on cotton moistened with Tyrode solution at 37°C (throat end facing upward). Put the hair (0.2mm) at the lower end of the trachea, observe with a low-power microscope, and record the time required for the hair to run 2mm, observe 3 times, and put the tracheal piece into Tyrode's solution at 37°C for rinsing after each observation . Then put it into the nutrient solution containing the medicinal solution for 5 minutes, and observe 3 times in the same way. Calculate the difference in hair running time before and after administration, and compare it with th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com