Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
344 results about "Cysteine l" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
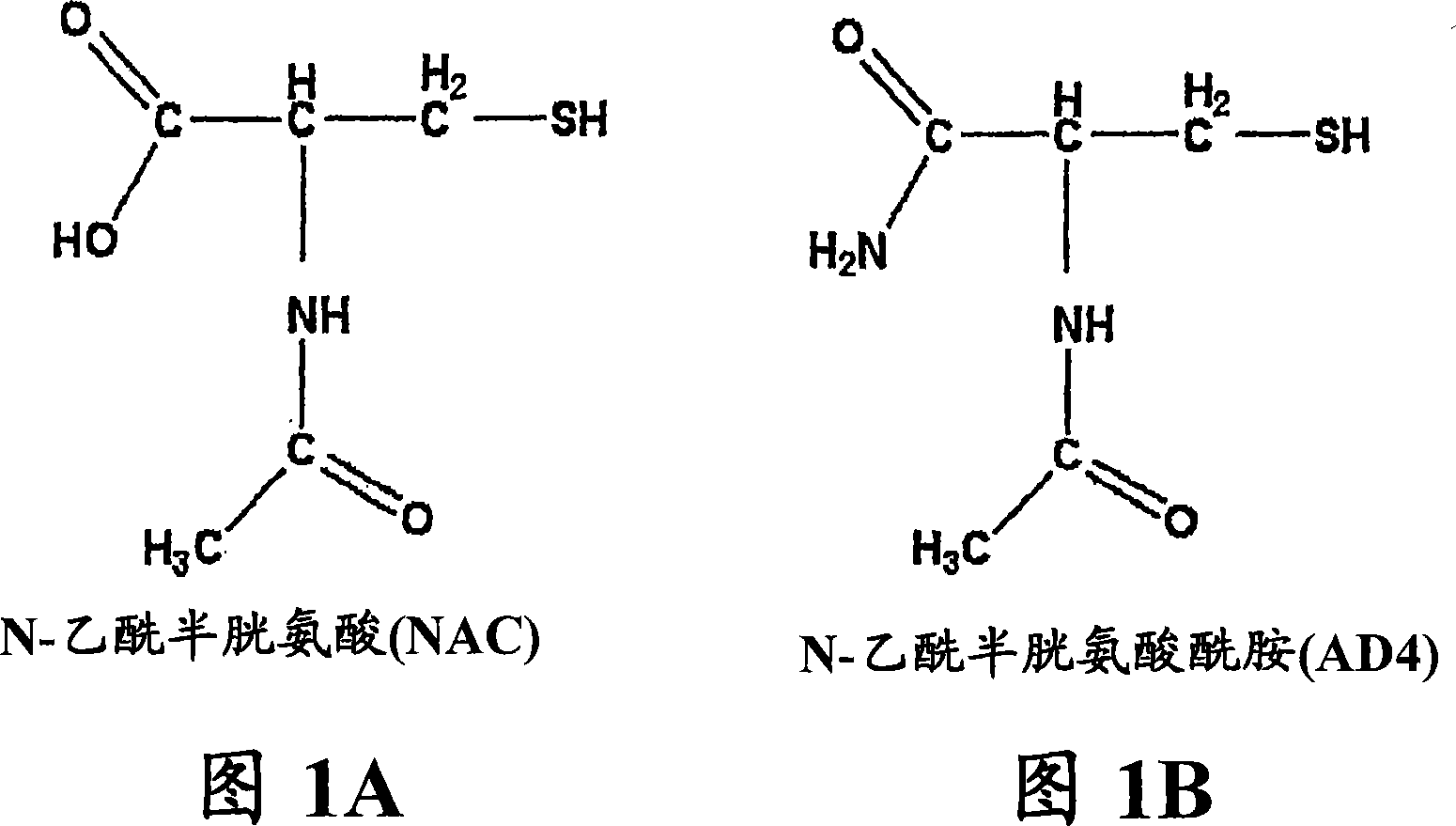
Cysteine is an amino acid, which functions as a building block of proteins. When used as a supplement, cysteine is generally in the form of N-acetyl-L-cysteine, or NAC. Your body converts NAC into cysteine and then into an antioxidant called glutathione.
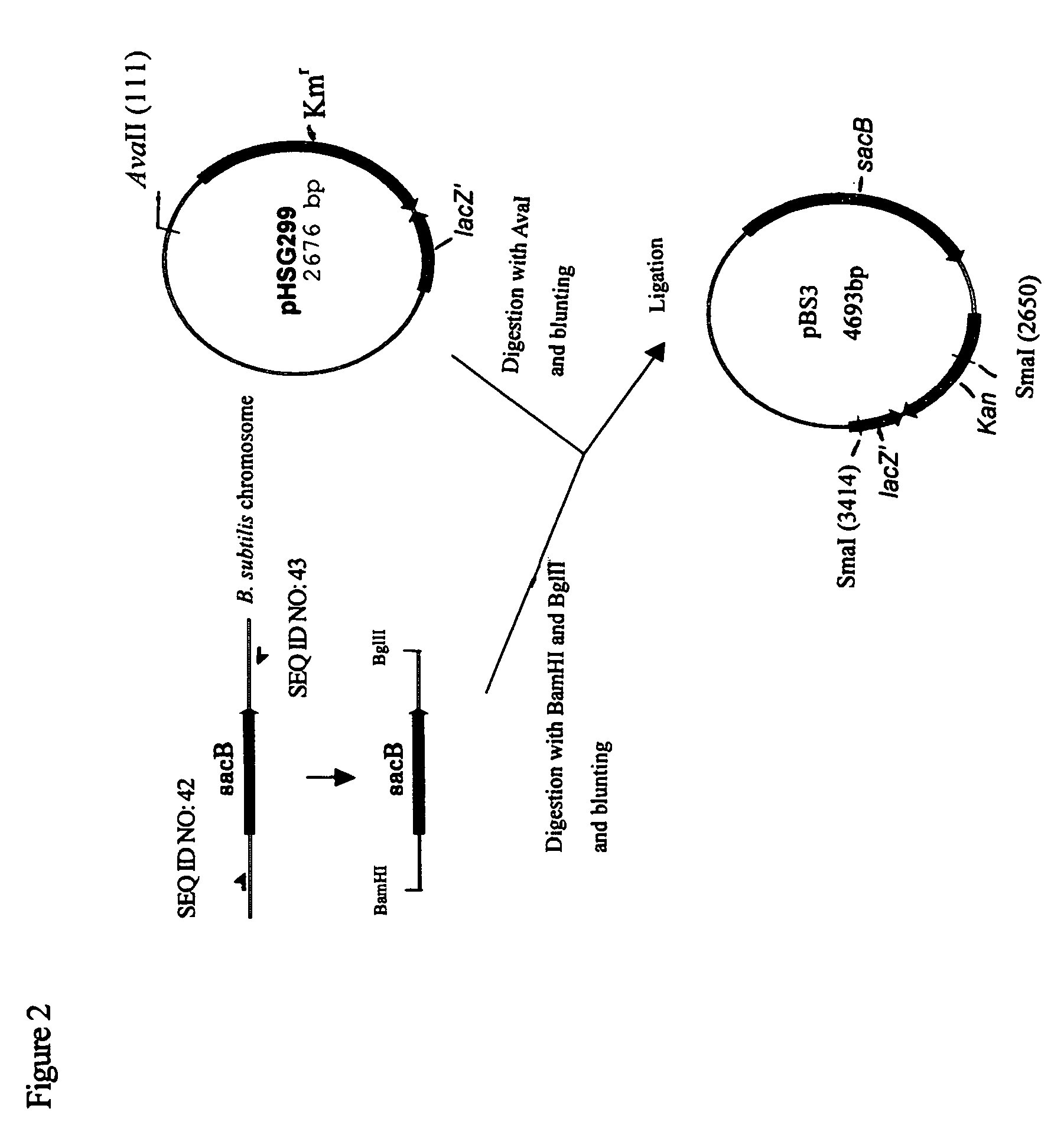
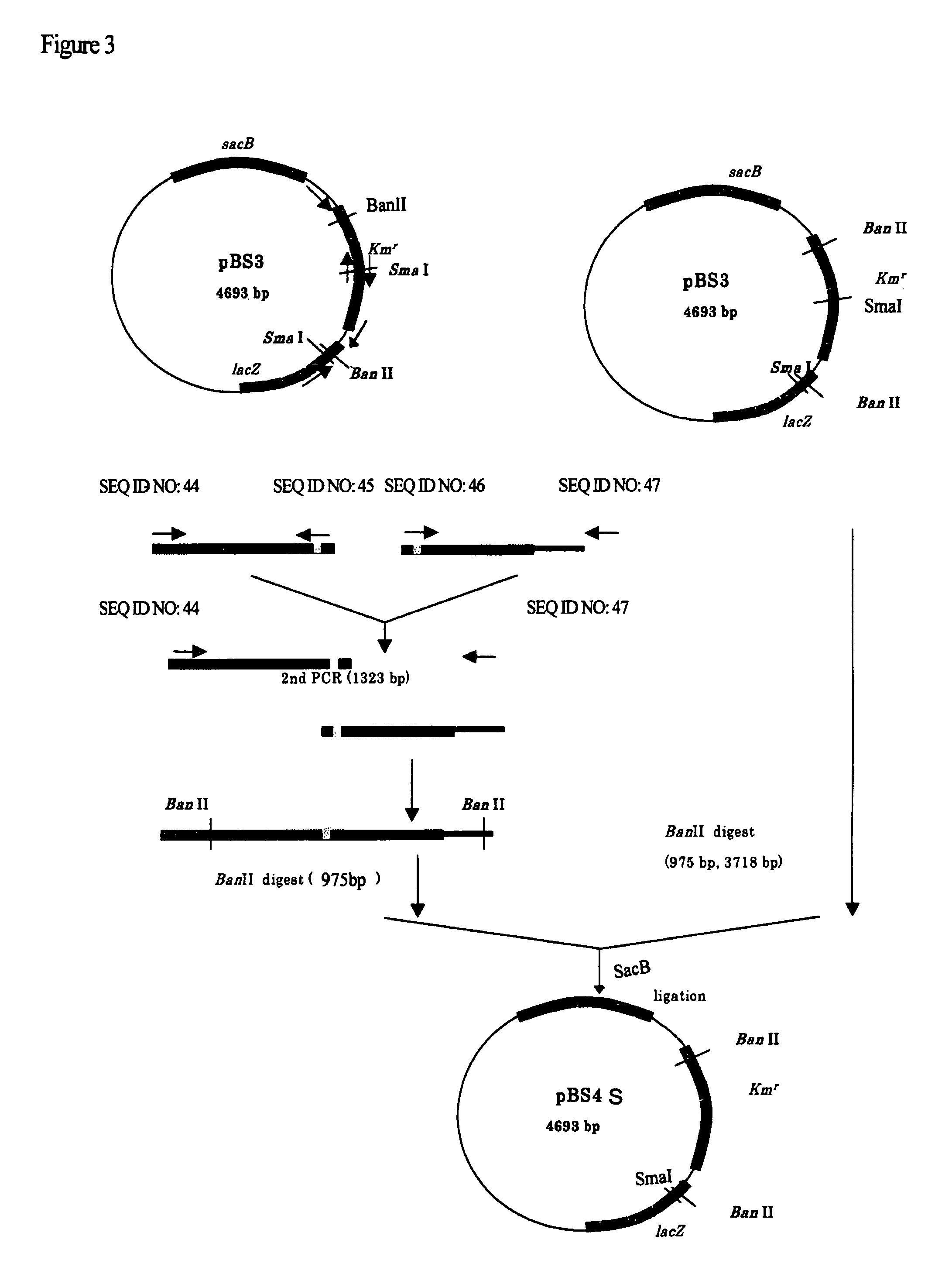
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Dietary Supplement Cognitive Support System
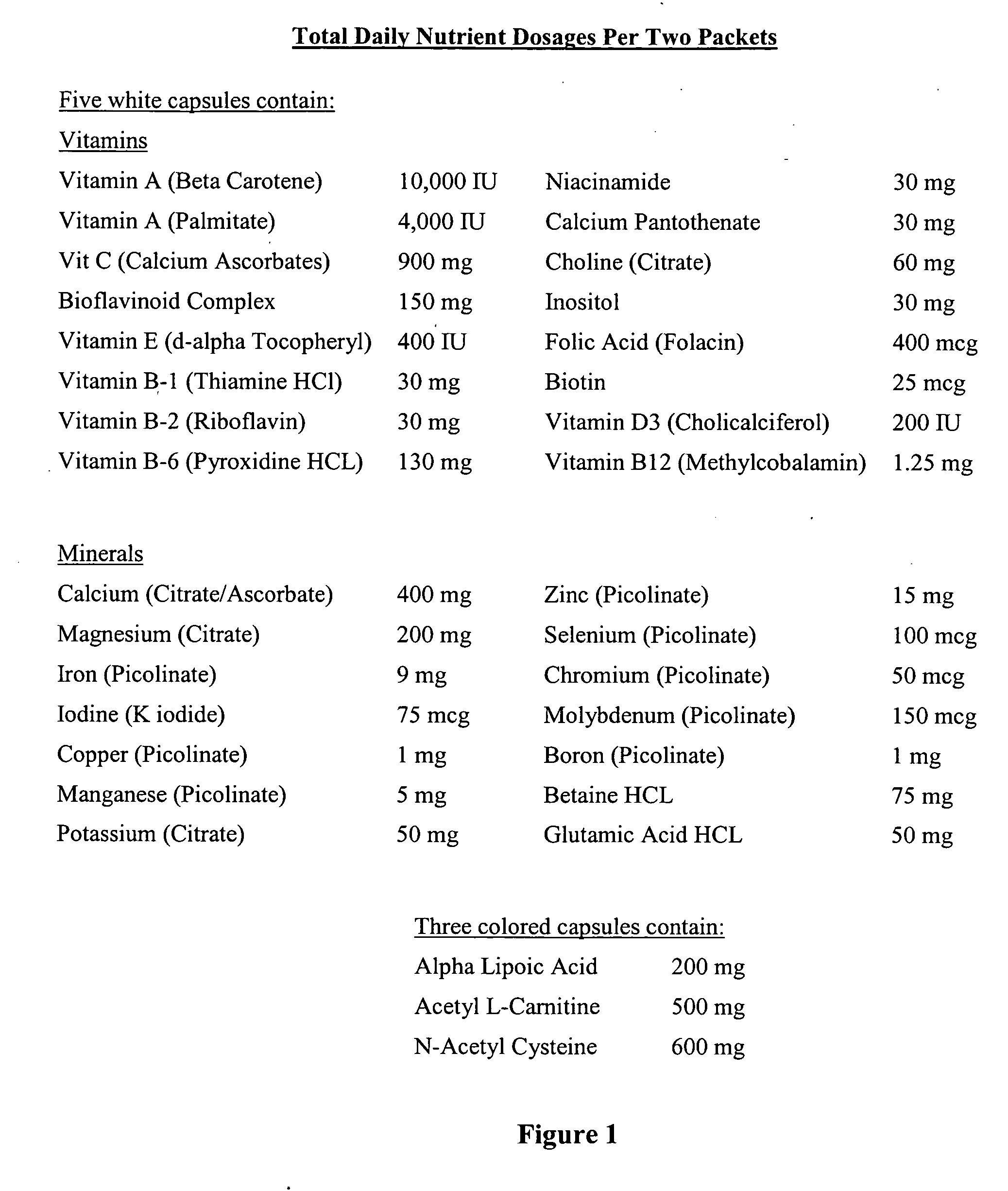
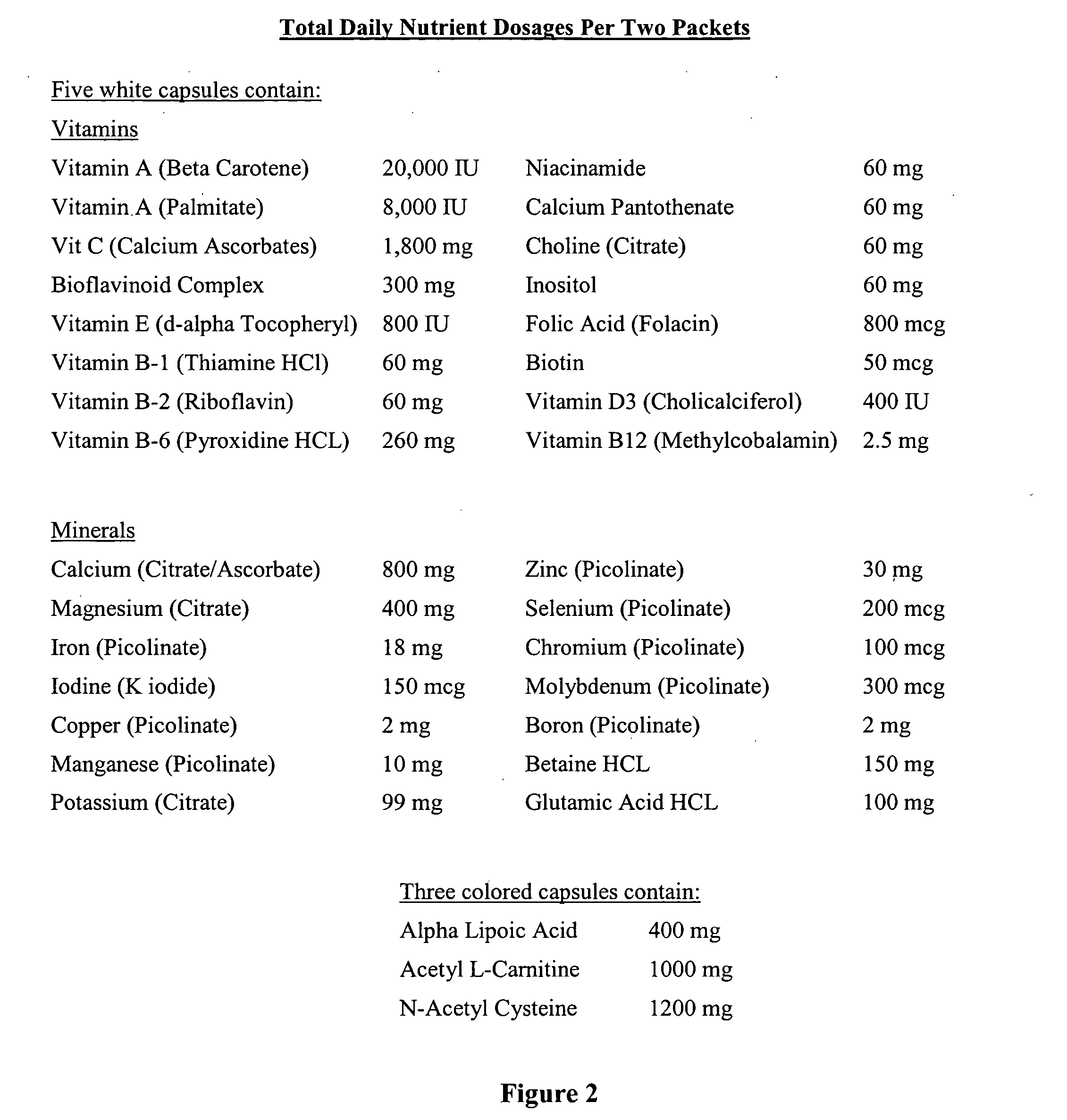
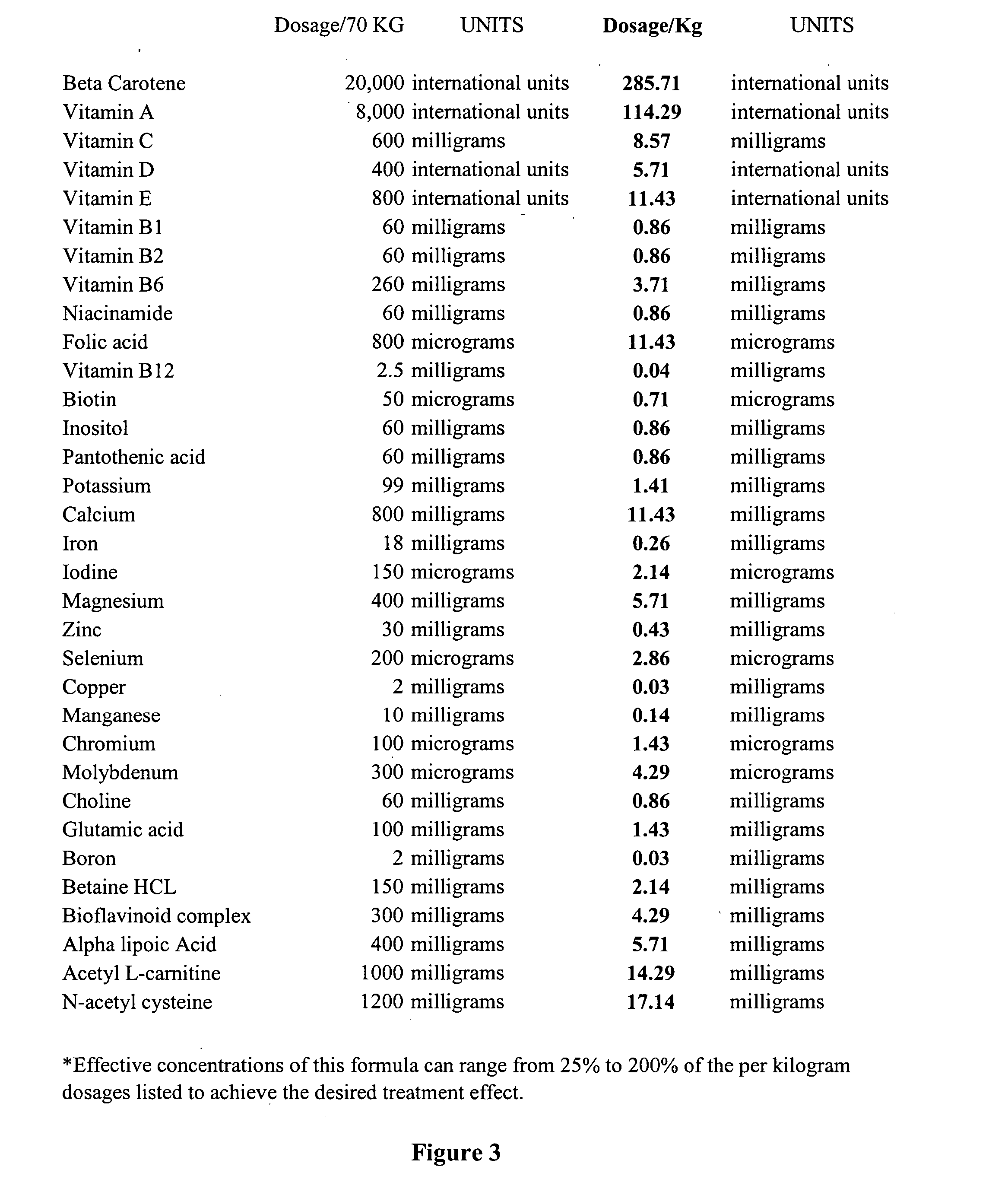
The present invention relates to a nutritional supplement composition, comprising a therapeutically effective amounts of Vitamin C, Vitamin D3, Thiamin, Riboflavin, Niacin, Vitamin B6, Folic acid, Vitamin B12, Pantothenic acid, Calcium, Magnesium, Zinc, Chromium, Sugar, Protein, Acetyl-L-Carnitine, Dimethylaminoethanol complex, Phosphatidylserine complex, L-Glutamine, N-Acetyl-L-Tyrosine, L-Phenylalanine, Taurine, Methionine, Valine, Isoleucine, 5 Hydroxytryptophan, L-Taurine, N-Acetyl-Tyrosine, N-Acetyl-L-Cysteine, Alpha Lipoic Acid, Alpha Glycerylphosphoricholine complex, Bacopa Monnieri extract, Gingko Biloba extract, Passion flower, Lemon Balm, Gotu Kola, Ashwagandha, Choline Bitartrate complex, Panax Ginseng extract, Turmeric, Organic freeze dried fruit juice blends (concord grape, red raspberry, pineapple, cranberry, acai, pomegranate, acerola cherry, bilberry, lingonberry, black currant, aronia, sour cherry, black raspberry), Organic freeze dried greens blends (barley grass, broccoli, beet, carrot, alfalfa, oat), and Protein digestive enzyme blends (Protease 4.5, peptidase, bromelain, protease 6.0, protease 3.0, L planatrum, B bifidum) in a mixture to provide optimal cognitive function.
Owner:FANTZ DAVID R
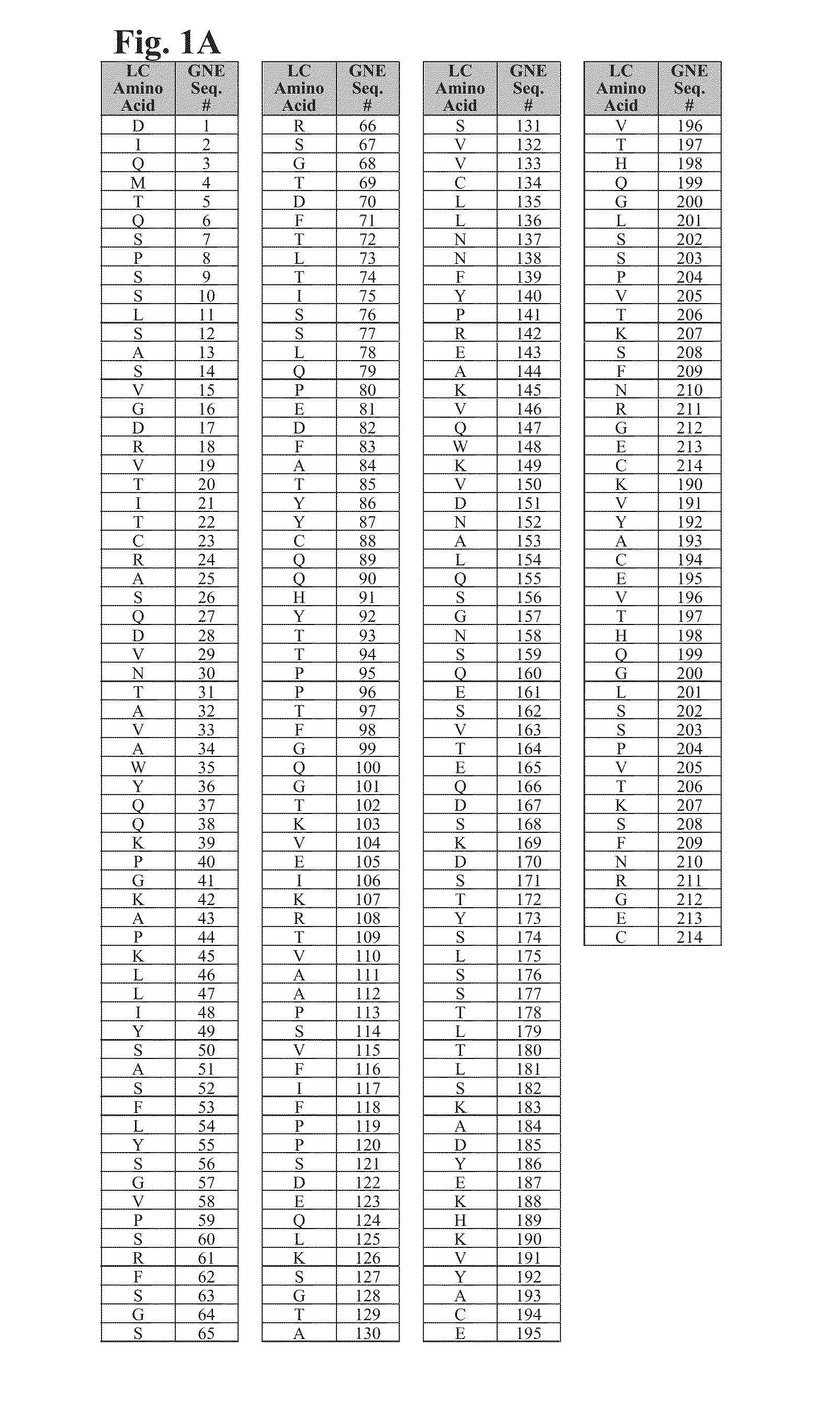
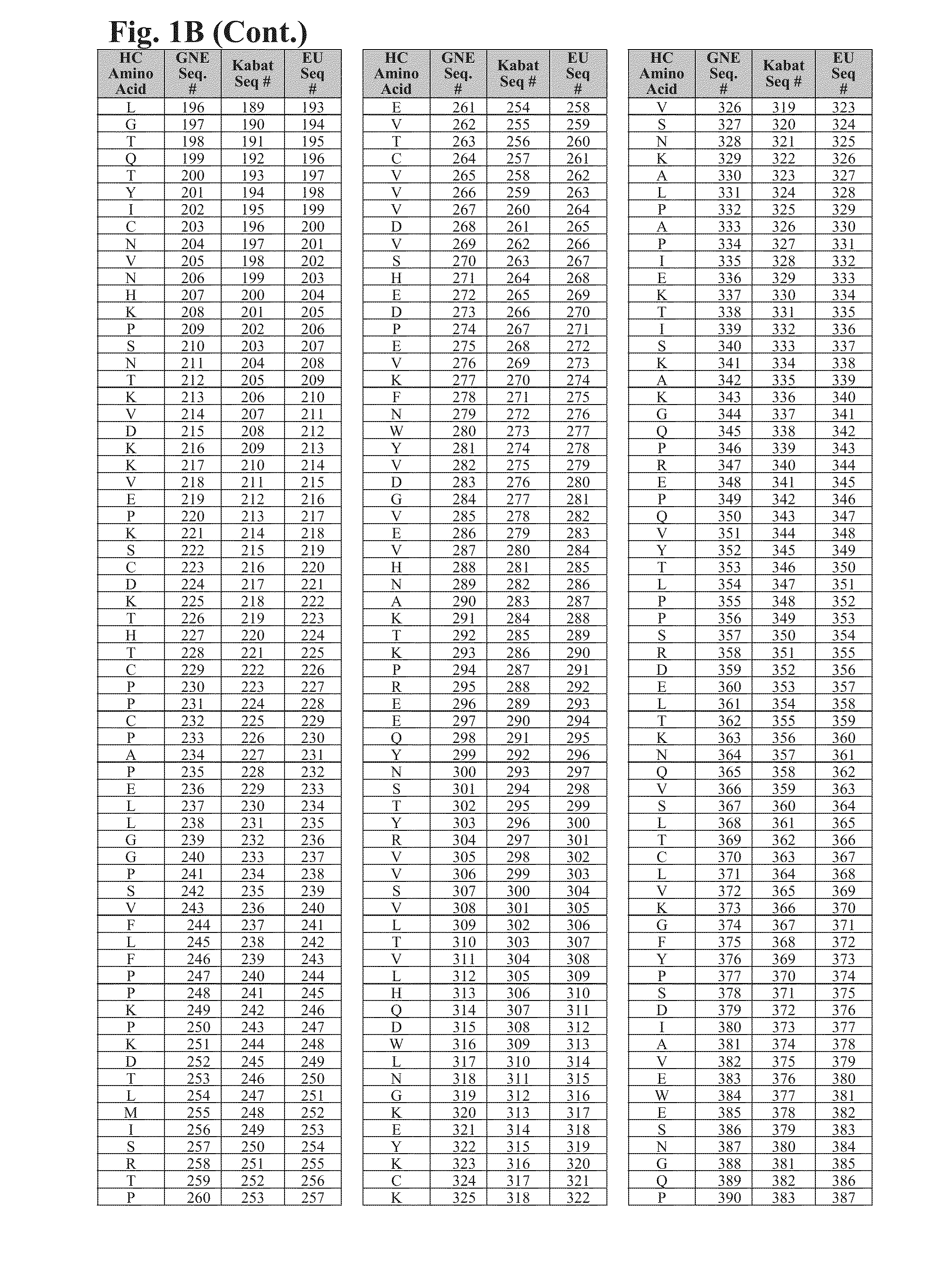
Cysteine engineered antibodies and conjugates
ActiveUS20160130358A1Immunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersAntiendomysial antibodiesHeavy chain
Cysteine engineered antibodies comprising a free cysteine amino acid in the heavy chain or light chain are prepared by mutagenizing a nucleic acid sequence of a parent antibody and replacing one or more amino acid residues by cysteine to encode the cysteine engineered antibody; expressing the cysteine engineered antibody; and isolating the cysteine engineered antibody.
Owner:F HOFFMANN LA ROCHE & CO AG
Composition of amino acid
ActiveCN101049500AImprove metabolic disordersNutritional support therapy works wellOrganic active ingredientsDipeptide ingredientsNutrition supportTryptophan
An amino acid composition with high nutrition supporting effect is composed of acetylcysteine, acetyltyrosine and tryptophan in weight ratio of (0.81-2): (0.81-2): (0.9-1.6).
Owner:BEIJING SHIQIAO BIOPHAM
Method for the preparation of a high-temperature stable oxygen-carrier-containing pharmaceutical composition and the use thereof
ActiveUS7989593B1Increase oxygenationHigh sensitivityPeptide/protein ingredientsMammal material medical ingredientsArteriolar VasoconstrictionWhite blood cell
A high temperature-stable and highly purified cross-linked (optionally ≧70% β-β linked) tetrameric hemoglobin with high efficiency of oxygen delivery suitable for use in mammals without causing renal injury and vasoconstriction is provided. The dimeric form of hemoglobin is degenerated and purification processes are performed on red blood cells from whole blood. Controlled hypotonic lysis in an instant cytolysis apparatus prevents lysis of white blood cells. Nucleic acids from white blood cells and phospholipids impurities are not detected. Blocking of reactive sulfhydryl groups by a sulfhydryl reagent is performed in an oxygenated environment. Flowthrough column chromatography removes different plasma protein impurities. N-acetyl cysteine is added to the cross-linked tetrameric hemoglobin to maintain a low level of met-hemoglobin. The stabilized hemoglobin is preserved in an infusion bag with aluminum overwrap to prevent formation of inactive met-hemoglobin from oxygen intrusion. The product finds use in tissue oxygenation and cancer treatment.
Owner:BILLION KING INT
Nutrient compositions and methods for enhanced effectiveness of the immune system
InactiveUS20050142124A1Reduce the burden onTimelyOrganic active ingredientsAntimycoticsDiseaseBeta-Carotene
The invention provides a nutrient composition for augmenting immune strength or physiological detoxification. The nutrient composition consists of an optimal combination of an effective amount of at least one vitamin antioxidant, at least one mineral antioxidant and a highly saturable amount of at least three high potency antioxidants. The at least one vitamin antioxidant can be vitamin C, bioflavonoid complex, vitamin E, vitamin B6 or beta-carotene and the at least one mineral antioxidant can be zinc or selenium. The at least three high potency antioxidants can be alpha lipoic acid, acetyl L-carnitine, N-acetyl-cysteine, co-enzyme Q10 or glutathione. Also provided is a nutrient composition for augmenting immune strength or physiological detoxification that consists of an optimal combination of an effective amount of at least three vitamin antioxidants, at least two mineral antioxidants and a highly saturable amount of at least three high potency antioxidants. Further provided is a method of stimulating immune system function or a method of augmenting a therapeutic treatment of a disease. The method consists of administering to an individual a nutrient composition of the invention one or more times a day over a period of about 5-7 weeks, the immune system function being stimulated to result in an increase of CD4+ cells of at least about 15% compared to pre-administration levels. A method of stimulating a physiological detoxification function of an individual or a method of augmenting a therapeutic treatment of a disease is also provided. The method consists of administering to an individual a nutrient composition of the invention one or more times a day over a period of about 5-7 weeks, the physiological detoxification function being stimulated to result in a decrease of one or more free radical markers by about 20% compared to pre-administration levels.
Owner:INTEGRATIVE HEALTH CONSULTING INC
Kit for Treatment of Cancer
InactiveUS20080287541A1Effective treatmentBiocideOrganic active ingredientsRate limiting enzymeAdduct
The present invention relates to a kit for the treatment of cancer comprising (a) a container for containing a first compound (i) or a precursor thereof, said first compound or precursor being a compound that oxidizes glutathione (GSH); (b) a container for containing a second compound (ii) or a precursor thereof, said second compound or precursor being a compound that forms an adduct or conjugate with GSH; (c) a container for containing a third compound (iii) or a precursor thereof, said third compound or precursor being a compound that inhibits the rate-limiting enzyme of GSH biosynthesis, gamma-glutamylcysteine synthetase (GCS); and (d) a container for containing a fourth compound (iv) or a precursor thereof, said fourth compound or precursor being a compound that inhibits the enzyme responsible for the conversion of GSSG to GSH, glutathione reductase (GR).
Owner:REDOXIA ISRAEL
Mutant serine acetyltransferase
O-acetylserine, L-cysteine and sulphurous compounds derived therefrom may be produced using a bacterium belonging to the genus Escherichia which harbors a mutant feedback-resistant serine acetyltransferases in which the amino acid sequence corresponding to positions from 89 to 96 in a wild-type serine acetyltransferase is replaced with any one of the amino acid sequences shown in SEQ ID NOS: 4 to 9, and feedback inhibition by L-cysteine in the bacterium is desensitized.
Owner:AJINOMOTO CO INC
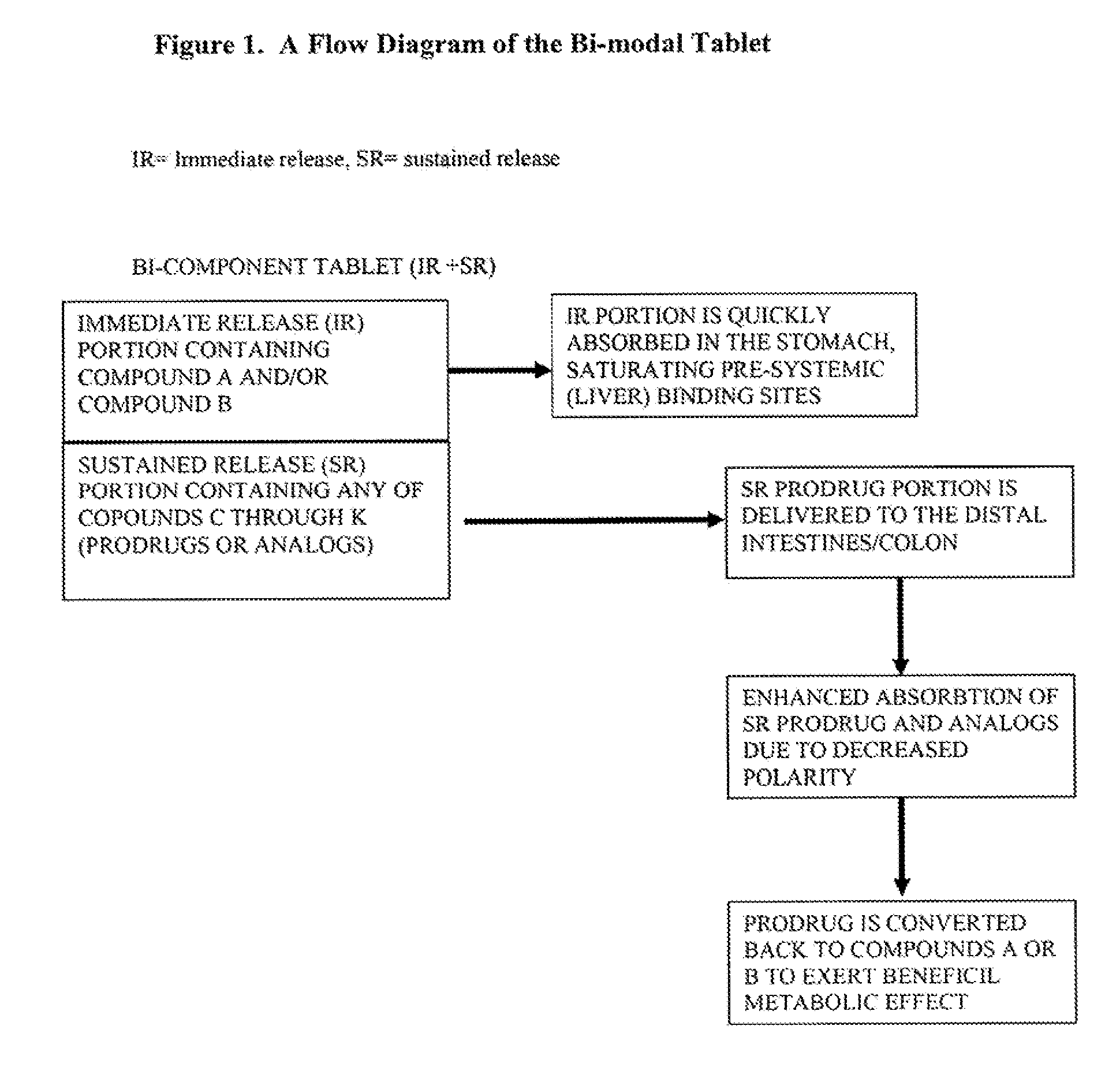
Controlled release of N-acetylcysteine (NAC) for reduction of systemic and/or vascular inflammation
The present invention provides a controlled-release composition which provides a therapeutically effective plasma concentration of N-acetylcysteine over prolonged period of time. The present invention also includes the use of the controlled-release composition, either alone or in combination with at least one additional active agent, for reduction of vascular inflammation marker and treatment of diseases, conditions, and / or symptoms associated with systemic and / or vascular inflammation in a patient. Furthermore, the present invention provides a process of making granules comprising N-acetylcysteine, or a salt, solvate, prodrug, and / or analog thereof.
Owner:TIARA PHARMA
Unifying mechanism and methods to prevent cancer and neurodegenerative diseases
InactiveUS20050164911A1Grow moreInhibition formationBiocideNervous disorderCancer preventionMetabolite
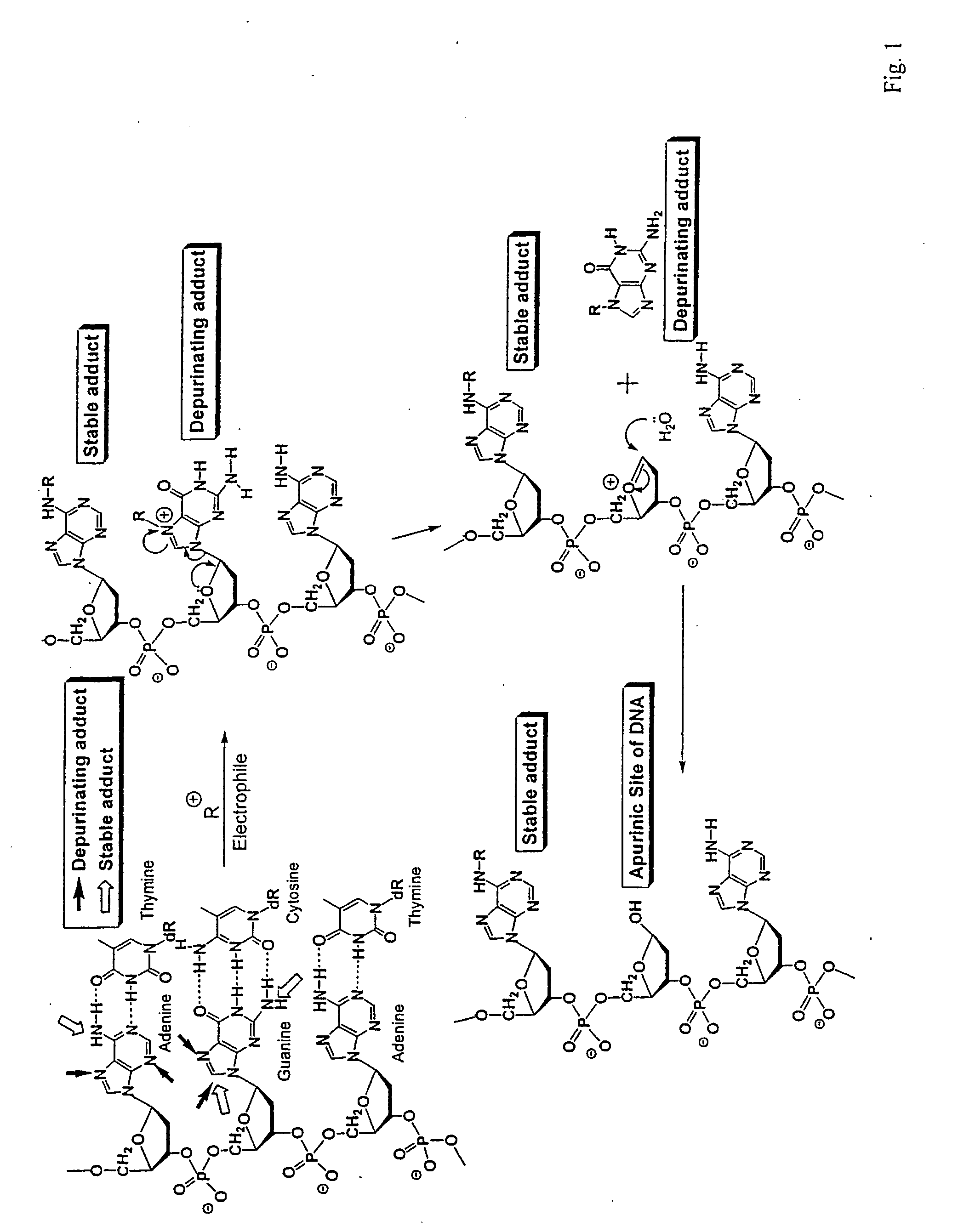
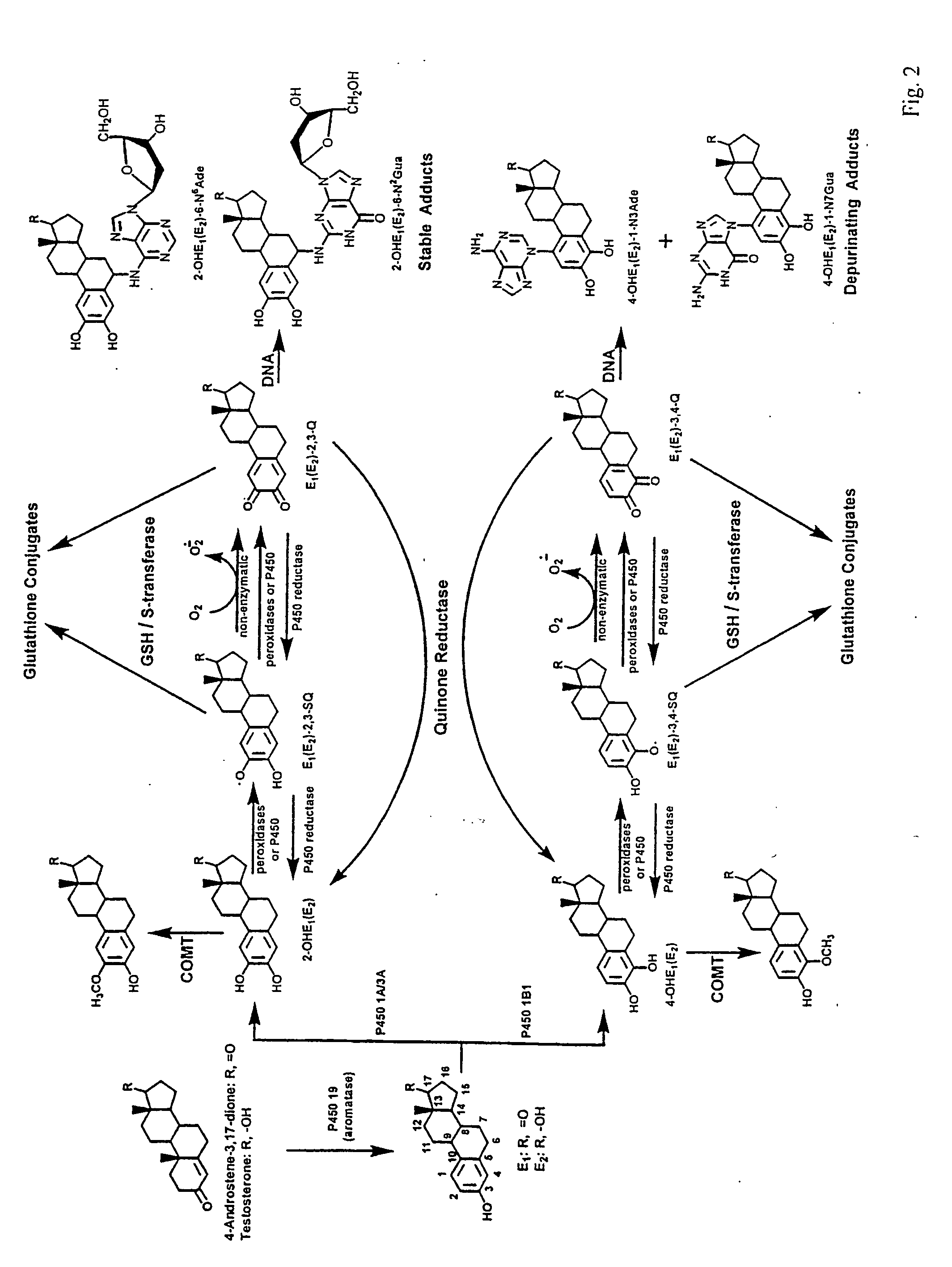
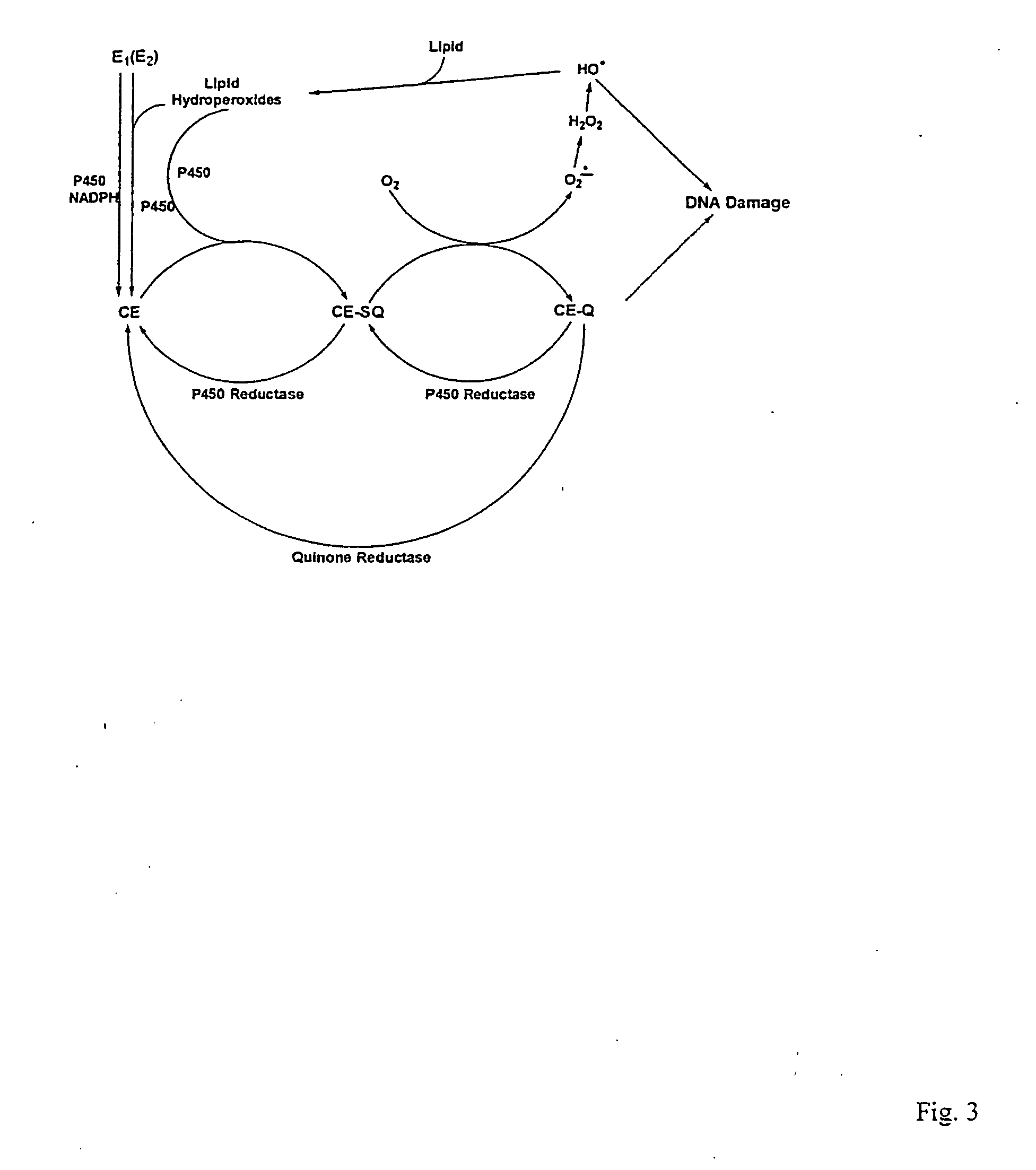
The present invention relates to methods for preventing the development of cancer or neurodegenerative diseases by administering N-Acetylcysteine (NAC), melatonin, or a combination thereof. The present invention also relates to methods for diagnosing cancer and / or neurdegenerative disease by detecting or determining the amount of dopamine metabolites, 4-CE, 2-CE, methylation of CE or CE-Q conjugates.
Owner:PREVENTION
N-Acetylcysteine Compositions and Methods for Treating Acute Exacerbations of Inflammatory Lung Disease
The present invention relates to N-acetylcysteine compositions and methods for treating inflammation and redox imbalance in acute exacerbations of inflammatory lung disease.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Culture media for hepatocyte culture and liver organ preparation
The invention provides a proliferation culture medium and differentiation culture medium for hepatocyte culture and liver organ preparation. The proliferation culture medium and the differentiation medium both take a culture medium for growth of mammalian cells as a basic culture medium, and an agent for supplementing L-glutamine, a pH value modifier for maintaining the pH values of the culture media stable, a primary cell culture antibiotic, a serum substitute, N-acetylcysteine, arbitrary nicotinamide and any one or more of a BMP inhibitor, a Wnt agonist, a growth factor, a Rock signaling pathway inhibitor, a P38 signal path inhibitor, a Notch signal path inhibitor, dexamethasone, BMP7 and a cAMP activator are added into the culture media. By using the culture media for culturing liver cells, functional liver organs can be obtained.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Specific culture medium for lung tumor organ and stentless 3D culturing method
ActiveCN110592022AStrong cell stemnessRetain heterogeneityCulture processCell culture active agentsY-27632HEPES
The invention discloses a specific culture medium for a lung tumor organ and a stentless 3D culturing method. The specific culture medium is prepared from the following components: FBS, double antibody, N-2, Noggin, B-27, EGF, FGF-10, Y-27632, A 83-01, SB202190, N-acetylcysteine, HEPES, Glutamax, IGF-1, hydrocortisone and Advanced DMEM / F12. The culturing method comprises the following steps: adding a tumor cell into a low serum culture medium, re-suspending the tumor cell, inoculating the tumor cell into a culture vessel, adding the specific culture medium into the culture vessel, changing thespecific culture medium once a day, and performing culturing until an organoid is formed. According to the culture medium and culturing method, a tumor organoid can quickly generate, can be stably cultured for a long time, is regular in spheroid form and has uniform and controllable size, and the heterogeneity of a tumor tissue of a patient can be well maintained in vitro.
Owner:浙江弘瑞医疗科技有限公司
Ratiometric fluorescent probe and preparation thereof, and application of ratiometric fluorescent probe in detection of glutathione
InactiveCN107603593AThe synthesis method is simpleShort timeMaterial nanotechnologyNanoopticsSilica nanoparticlesMicrosphere
The invention discloses a ratiometric fluorescent probe and a preparation thereof, and an application of the ratiometric fluorescent probe in detection of glutathione. The ratiometric fluorescent probe is prepared through the following steps: with green quantum dots as cores, carrying out embedding in silica nanoparticles, then subjecting the surface of the silica nanoparticles to amination modification, and covalently coupling red quantum dots onto the amination modified surface of the silica nanoparticles so as to obtain the ratiometric fluorescent probe, wherein the green quantum dots are cadmium telluride quantum dots modified by N-acetyl-L-cysteine. According to the invention, a St ber method is utilized to prepare quantum dot embedded silica nanoparticles, so the synthetic method issimple and has less time consumption. The probe provided by the invention has low detection limit and can realize specific detection during detection of GSH; meanwhile, the probe has advantages like simple preparation and good stability, reduces the detection limit of glutathione to 45 nM, and provides convenience for rapid visual detection.
Owner:ZHEJIANG UNIV OF TECH
Compositions and methods for treating traumatic brain injury
ActiveUS20140170211A1Symptoms improvedPromote healingBiocideKetone active ingredientsL-GlutaminTaurine
The disclosure provides compositions treating traumatic brain injuries such as concussions. In one embodiment, the composition comprises phosphatidylserine, phosphatidylcholine, quercetin, astaxanthin, R-alpha lipoic acid, N-acetyl cysteine, taurine, L-glutamine, carnitine, D-ribose, creatine, epigallocatechin gallate, melatonin, ginkgo leaf extract, curcumin and L-glycine. The disclosure also provides methods for treating traumatic brain injuries such as concussions by administering an effective amount of the compositions described within.
Owner:HAVN LIFE SCI INC
N-acetylcysteine amide (NAC amide) for the treatment of diseases and conditions associated with oxidative stress
Compositions containing N-acetylcysteine amide (NAC amide) and derivatives thereof, and their use in the treatment and therapy of diseases, conditions, disorders and conditions in humans and non-human mammals. Administration of pharmaceutically or physiologically acceptable compositions of NAC amide or derivatives thereof alone or in combination with other suitable drugs to reduce, prevent or antagonize oxidative stress and the formation and excess of free radical oxidants in cells and tissues Produce, and provide a new source of glutathione.
Owner:格伦·A·戈尔茨坦
Cysteine Prodrugs
Novel cysteine prodrugs and their use in the treatment of diseases and / or conditions, including but not limited to diseases and / or conditions of the Central Nervous System (CNS), including but not limited to schizophrenia, drug craving, drug addiction, bipolar disorder, anxiety, depression, Parkinson's disease, Alzheimer's disease, cognitive dysfunction, multiple sclerosis, Amyotrophic lateral sclerosis (ALS), ischemic stroke, HIV dementia, and Huntington's disease.
Owner:PROMENTIS PHARMA
Utilization of lipopeptides or lipoproteins in wound treatment and infection prophylaxis
The invention concerns a pharmaceutical preparation for the treatment of wounds in animals or humans containing or consisting of a lipopeptide or lipoprotein which carries at the N-terminals a dihydroxypropyl-cysteine group with two, optionally long-chain, fatty acids bonded via ester bonds.
Owner:MUHLRADT PETER +1
L-cysteine producing microorganism and a method for producing l-cysteine
ActiveUS20100093045A1Reducing and eliminating activityHigh activityBacteriaTransferasesMicroorganismMicrobiology
L-cysteine is produced by culturing an Escherichia bacterium having L-cysteine producing ability and containing a gene encoding an O-acetylserine sulphydrylase B or MalY regulatory protein that is modified so that cysteine desulfhydrase activity is reduced or eliminated. The bacterium is cultured in a medium to produce and cause accumulation of L-cysteine in the medium, and collecting L-cysteine from the medium.
Owner:AJINOMOTO CO INC
Supplement composition and method of use in enhancement of methylation process
InactiveUS20070021376A1Increase heightIncrease of methylationBiocideSulfur/selenium/tellurium active ingredientsS-Adenosyl-l-methionineMethylsulfonylmethane
A supplement composition for enhancement of methylation process is provided, which contains vitamin B6 (as pyridoxine HCl), folic acid, vitamin B12 (as cyanocobalamin), betaine HCl, and methylsulfonylmethane; and also contains S-adenosylmethionine. The supplement composition further includes silymarin (from milk thistle seed extract), N-acetyl L-cysteine, and cruciferious blend which includes broccoli (brassica oleracea var. talica), kale (brassica oleracea var. acephala), and radish (raphanus sativus). Further provided is a method of using the supplement composition for enhancement of methylation process.
Owner:SURACELL
Stable and safe edaravone injecta
ActiveCN102144964AImprove stabilityQuality improvementOrganic active ingredientsInorganic non-active ingredientsEdaravone InjectionPhosphate
The invention belongs to the field of pharmaceutical preparations, in particular to an edaravone injecta and a preparation process thereof. The edaravone injecta contains edaravone and pharmaceutically acceptable auxiliary materials and is characterized in that the injecta contains an antioxidant and a pH regulator, wherein the antioxidant contains phosphate, L-cysteine hydrochloride and sodium bisulfite. A whole-process nitrogen-filing and oxide-emitting method and a post-sterilization rapid cooling method are adopted in the preparing and filling process of the edaravone injecta to improve the stability of the edaravone injecta, remarkably reduce the content of a related substance, i.e., dimmer and provide the stable and safe edaravone injecta for clinical administration.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Factor IX polypeptide mutant, its uses and a method for its production
Disclosed are a modified FIX (factor IX) polypeptide comprising a leucine, cysteine, aspartic acid, glutamic acid, histidine, lysine, asparagine, glutamine or tyrosine in position 338; pharmaceutical preparations containing said modified FIX polypeptide; a nucleotide sequence coding for the modified FIX polypeptide; and a method for producing the modified FIX polypeptide.
Owner:UNIQURE BIOPHARMA BV
Ambroxol cysteine analogs and their preparation process and use thereof
The invention relates to an ambroxol cysteine analogs and their preparation , the medicinal composition containing the compound, and the use in preparing apophlegmatic medicaments. The method for preparation comprises reacting ambroxol and sulfo-aminolactic acid analogue in solvent, the crystallizing, wherein n=1, 2, A is defined in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Oral formulations
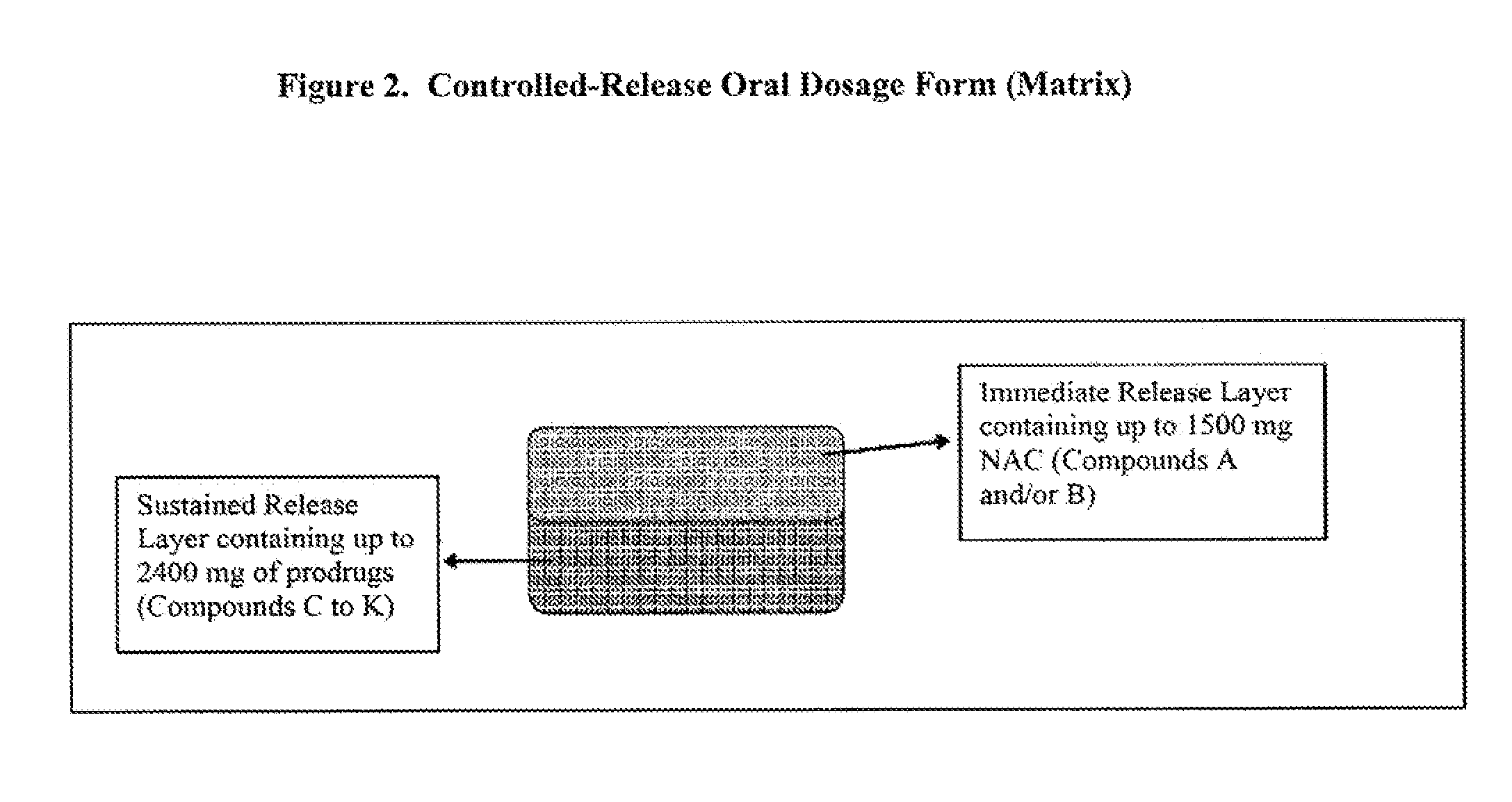
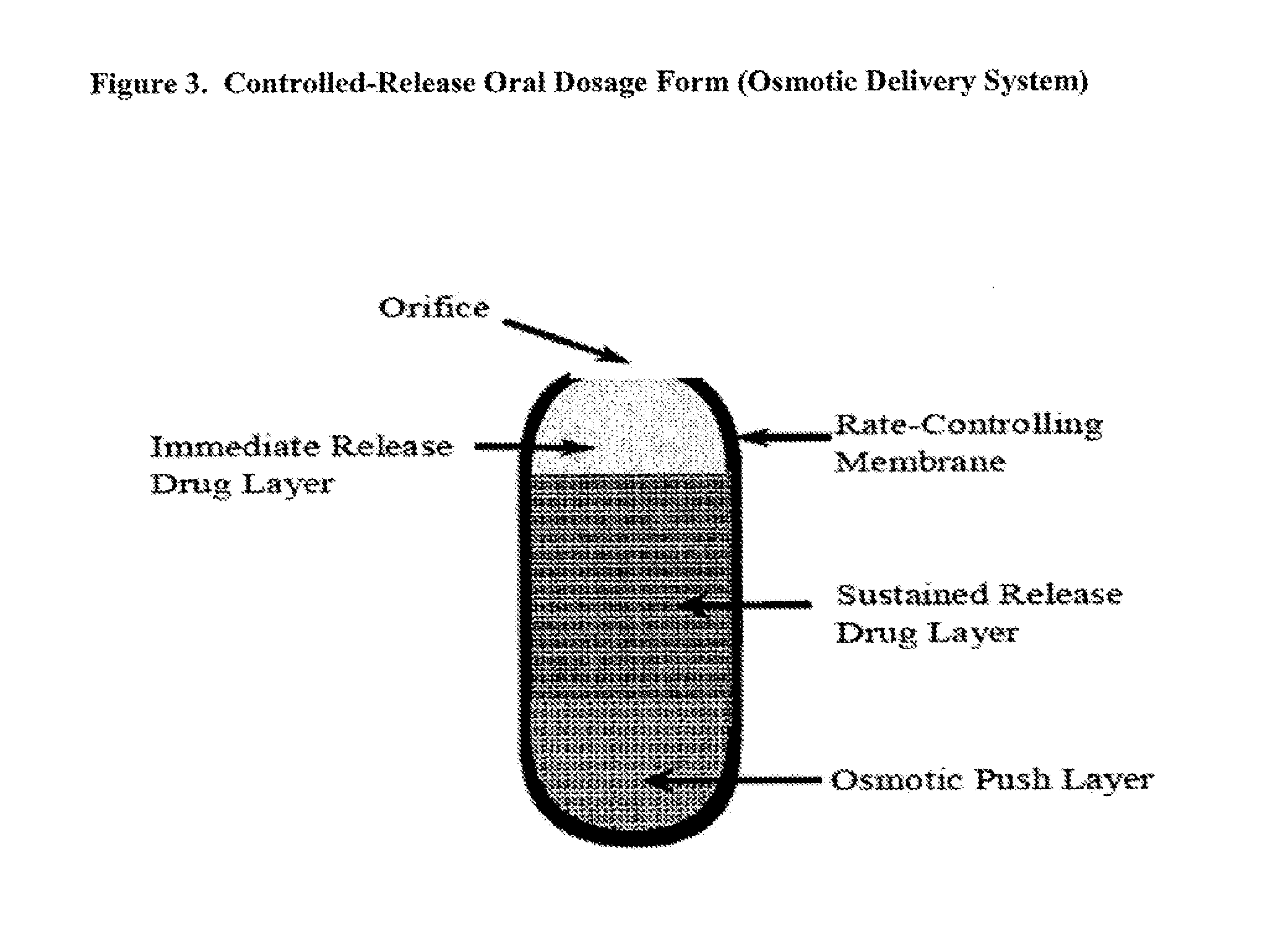
InactiveUS20120093939A1Powder deliverySalicyclic acid active ingredientsLower Gastrointestinal TractImmediate release
Disclosed are pharmaceutical compositions comprising immediate release and sustained release formulations of 5-aminosalicylic acid, or a pharmaceutically acceptable salt or ester thereof, and / or N-acetylcysteine, or a pharmaceutically acceptable salt or ester thereof, for release in the lower gastrointestinal tract.
Owner:ALTHEUS THERAPEUTICS
Flavor development material and preparation thereof
The invention discloses flavoring. The main components of the flavoring and the weight portions are: 5 to 25 portions of water, 5 to 30 portions of meat zymolyte, 5 to 15 portions of soy sauce, 5 to 15 portions of hydrolyzed vegetable protein, 0.5 to 2 portions of glucose, 0.8 to 6 portions of L-cysteine hydrochloride, 0.6 to 3.8 portions of IMP, 0.6 to 5 portions of revertose, 3 to 28 portions of sugar, 15 to 30 portions of salt, 10 to 25 portions of monosodium glutamate, 2 to 15 portions of oil, 10 to 20 portions of starch and 0.2 to 1.5 portions of edible essence. The invention also discloses a preparation method of the flavoring, consisting of the processes of dissolution, adjusting pH, reaction, blending, drying and shattering. The main process parameters are as follows: the pH is 3.3 to 10.5, the reaction temperature is 90 DEG C to 135 DEG C and the reaction time is 0.5 to 5 hours. The flavoring of the invention can satisfy the demands of smell and flavor and is convenient to use, and the processing cost is low. Thereby the flavoring can be used in instant noodles, recreation puffed food, compound condiment and meat products.
Owner:南京汇肽生物科技有限公司
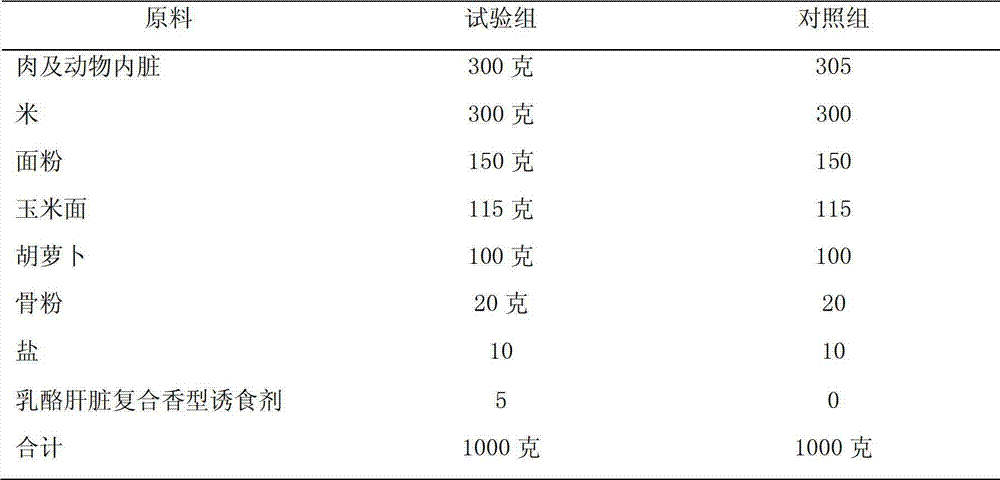
Composite cheese liver flavor attractant and preparation method thereof
The invention discloses a composite cheese liver flavor attractant and a preparation method of the composite cheese liver flavor attractant. The composite cheese liver flavor attractant comprises the following raw materials in parts by weight: 1-15 parts of natural cream zymolyte, 30-60 parts of fresh pork liver zymolyte, 0.5-3.5 parts of yeast extract, 0.5-4 parts of xylose, 0-2 parts of cysteine hydrochloride, 0-2 parts of vitamin B1, 0-3.5 parts of glutamic acid, 0-30 parts of dextrin and 15-30 parts of water. The composite cheese liver flavor attractant and the preparation method of the composite cheese liver flavor attractant reasonably utilize waste resources, such as animal livers, as alternative products of meat flavor essence or primary liver products in the pet feed and the special animal feed. The composite cheese liver flavor attractant and the preparation method of the composite cheese liver flavor attractant can ensure the intensity of the liver flavor and the meat flavor of the finished product, efficiently reduce the direct usage of the liver, and ensure the body health of pets and carnivores. The cheese flavor and the liver flavor are properly coordinated. The composite cheese liver flavor attractant has the advantages that the composite cheese liver flavor attractant is rich, heavy, natural, lasting and soft in flavor, and favorable in the animal attracting effect, and the composite cheese liver flavor attractant has favorable high temperature resistance. The composite cheese liver flavor attractant meets national edible standards, and the composite cheese liver flavor attractant is safe and reliable when being used as an attractant.
Owner:成都大帝汉克生物科技有限公司
Pegylated mutated clostridium botulinum toxin
InactiveUS20090118193A1Improve stabilityProlong the action timeSenses disorderNervous disorderHeavy chainOrganic chemistry
The invention relates to a modified botulinum toxin comprising a natural heavy chain and a modified light chain, characterized in that the modification of the light chain resides in that it comprises (i) an extension of the chain on its N-terminus which has the structure —(C)n-(tag)m-(X)l— in the direction from the N- to the C-terminal end, whereinC represents a cysteine residue,tag represents any tag andX represents the residue of any naturally occurring amino acid,n represents an integer from 1 to 50,m represents 0 or 1, andl represents 0 or an integer from 1 to 50,and in that (ii) at least one of the cysteine residues in the extension of the chain is coupled to at least one chain of PEG.
Owner:MERZ PHARMA GMBH & CO KGAA
Conjugate of Folate and Antibody Preparation Method and Use Thereof
InactiveUS20130259882A1Function increaseInducing effectAntipyreticDigestive systemCysteamineAutoimmune disease
Anti-tumor conjugates, which consists of foliate or analogues thereof, linkers, an antibodies such as immunoglobulin G. The linker comprises glutathione, cysteamine or cysteine residue, and further comprises N-hydroxysuccinimide. The Conjugates target folate-receptor-positive tumor cells. Also provided are preparation methods and anti-tumor and anti-autoimmune disease uses of the conjugates.
Owner:ZHEJIANG JIANFENG HANSHENG BIOSCI
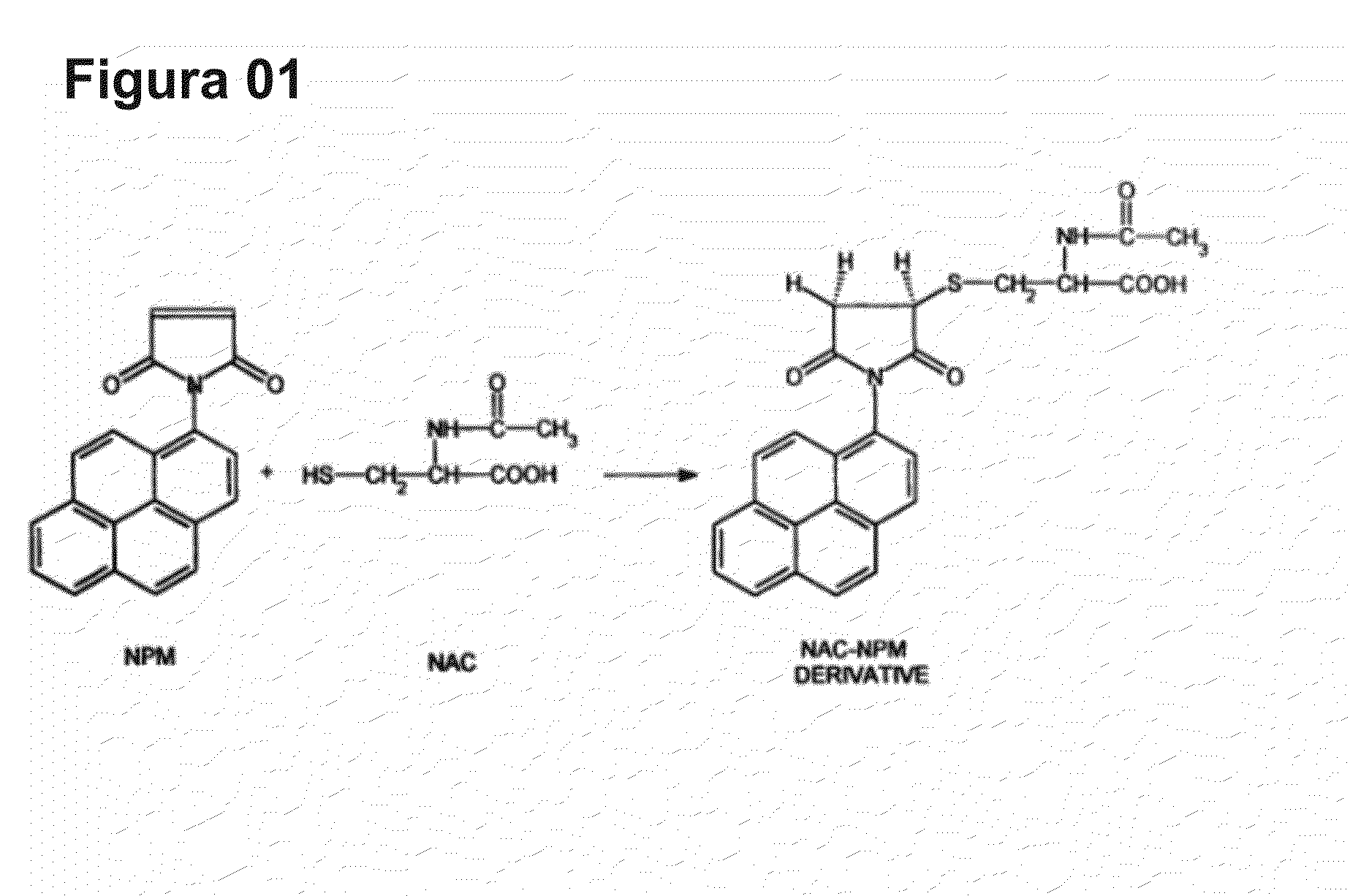
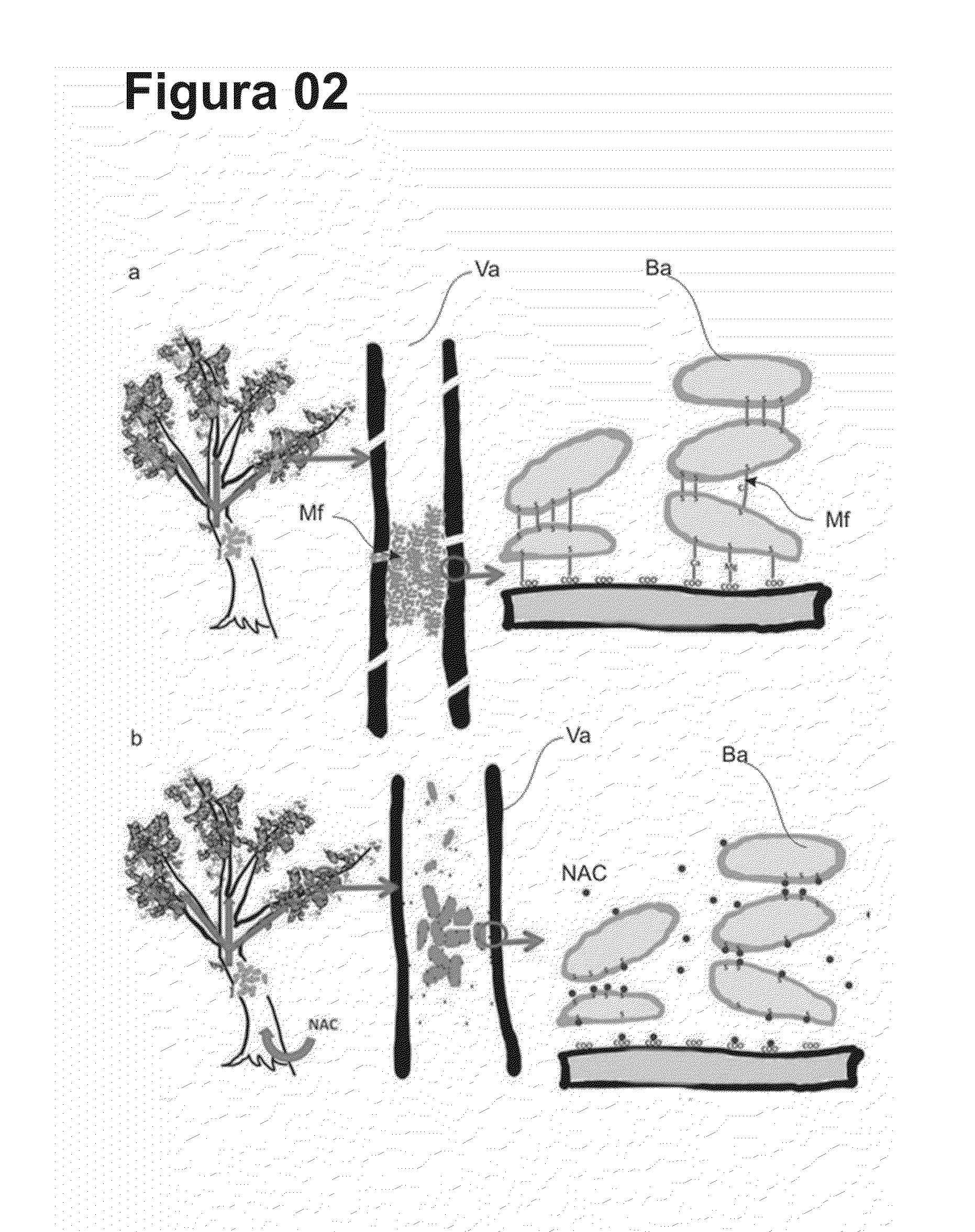
Cysteine amino-acid compound (or its analogues) used in the disruption of microbial biofilms when treating or preventing diseases caused by phytopathogenic bacteria known to attack plants of agricultural interest
Cysteine amino-acid compound (or its analogues) used in the disruption of microbial biofilms by treatment of prevention of diseases generated by phytopathogenic bacteria attacking plants of agricultural interest represented by an innovative solution within the agriculture sector, where said compound can be used in the pharmacological form, as a drug associated with fertilizer for the combat of bacterial diseases which form microbial biofilms, such as citrus variegated chlorosis (CVC), citric canker’, huanlongbing (HLB) disease or ‘greening’, amongst other, which inventive concept, as such, never before completed, resides in the benefit deriving from the cysteine amino-acid, and all of its analogues, in the inhibitory action and progressive disruption of the microbial biofilm thus liberating the nutritive flux and hydration of the root to the upper part of the plant and the subsequent regression of the disease symptoms, with the added advantage that the cysteine amino-acid compound is non-toxic, guaranteeing healthy production of foods by plants of agricultural interest that are totally healthy, without toxic residues in their composition, as well as when said compound is applied there is risk to the environment due to the rapid absorption, notably within the area in which it is applied, where such predicates of disease combat, with the exception of toxic collateral effects still guarantee that the final crop and harvest will have a higher productivity per hectare.
Owner:INST AGRONOMICO DE CAMPINAS
Health-care product and preparation method thereof
InactiveCN106036823APrevent Vascular DiseaseIncreased methyl transport capacityFood ingredient functionsOxidation resistantMagnesium
The invention discloses a health-care product and a preparation method thereof. The health-care product is prepared from the following raw materials of folic acid, betaine, choline, vitamin B1, vitamin B2, niacin, pantothenic acid, vitamin B6, vitamin B12, biotin, vitamin C, soluble zinc, soluble selenium, soluble magnesium, soluble calcium and soluble iron. The health-care product disclosed by the invention has the beneficial effects that the choline and the betaine provide methyl, the folic acid transfers the methyl, and the methyl is used for methylating DNA and protein; the vitamins B2 and Vc contribute to conversion of the folic acid into 5-methyltetrahydrofolic acid, so that the methyl transfer capacity is improved; B12 is used as a coenzyme to be used for promoting transformation of homocysteine, so that occurrence of vascular diseases is prevented; and selenium, zinc, magnesium and calcium can improve the activity of resultant-glutathione. The Vc and selenium glutathione form an oxidation resistant network, so that the self antioxidant ability of bodies is improved as a whole. The health-care product disclosed by the invention has the efficacies of promoting normal methylating level, reducing the homocysteine and improving the self antioxidant ability of the bodies.
Owner:沈阳翰臣益医疗科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com