Preparation method and application of novel oil-free adjuvant
An adjuvant, mineral oil technology, applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of unclear immune mechanism, aseptic abscess and inflammatory reaction, and high viscosity of vaccines, etc. Achieve the effect of improving the quality of vaccine products, improving the effect of vaccine immunity, and reducing the probability of immunization failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 The preparation method of adjuvant (605 oil-free adjuvant) of the present invention
[0016] Adjuvant group 1: disodium hydrogen phosphate 3.5g, potassium dihydrogen phosphate 0.6g, sodium chloride 7g, trehalose 7g, astragalus polysaccharide 7g, sodium glutamate 3g, gelatin 13g, polyethylene glycol 30007g, dextran 3g, 1000ml of water for injection, after ultrasonic treatment, sterilized by autoclaving at 121°C for 20min and then ready for use.
[0017] Adjuvant group 2: Weigh disodium hydrogen phosphate 5g, potassium dihydrogen phosphate 0.2g, sodium chloride 10g, trehalose 2g, astragalus polysaccharide 10g, sodium glutamate 1g, gelatin 20g, polyethylene glycol 30003g, dextran Sugar 5g, 1000ml water for injection, ultrasonic treatment, 121 ℃, autoclave for 20min, and then set aside.
[0018] Adjuvant group 3: disodium hydrogen phosphate 2g, potassium dihydrogen phosphate 1g, sodium chloride 3g, trehalose 10g, astragalus polysaccharide 2g, sodium glutamate ...
Embodiment 2
[0021] The research that embodiment 2 adjuvants are used for bird flu inactivated vaccine
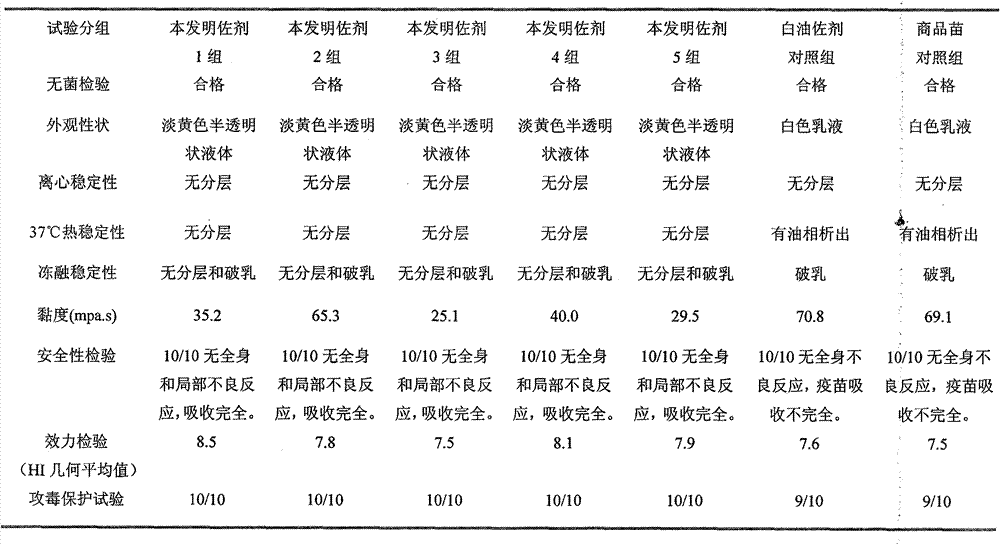
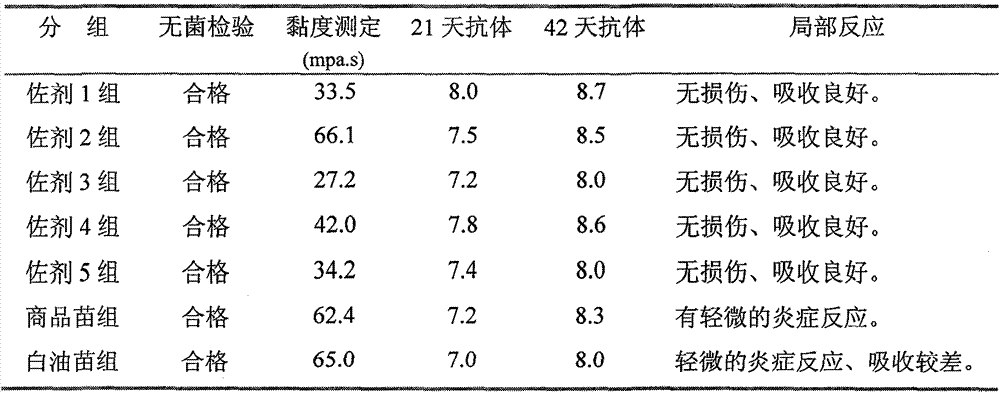
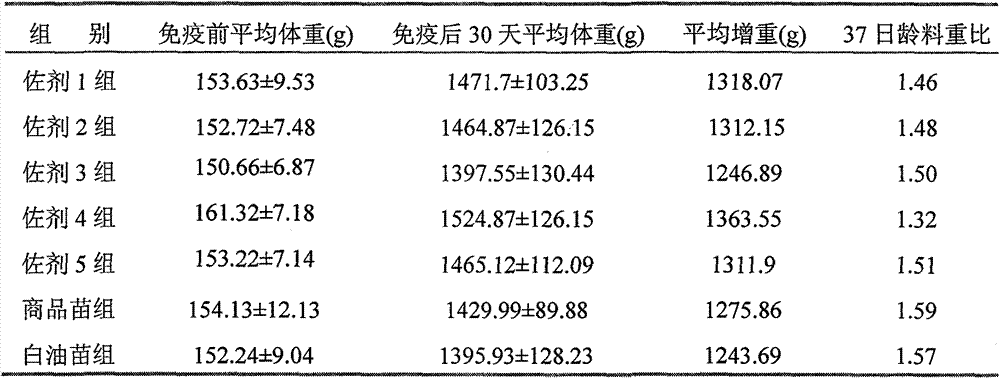
[0022] Mix the qualified avian influenza virus inactivation solution with the adjuvant of the present invention in a ratio of 4:6, and stir at 500-1000 rpm for 30 minutes. Sterility test, physical properties, stability, viscosity, safety and efficacy tests were carried out according to the methods stipulated in the three parts of the current "Veterinary Pharmacopoeia of the People's Republic of China". The results show that compared with the inactivated vaccine made with the traditional oil adjuvant, the adjuvant of the present invention has significant advantages in terms of stability, viscosity, safety and vaccine preparation process. The results are shown in Table 1.
[0023] Table 1 Test results of each batch of Newcastle disease inactivated vaccine
[0024]
Embodiment 3
[0025] Example 3 The adjuvant used in the immune effect test of Newcastle disease inactivated vaccine immunized SPF chickens.
[0026] 1. Materials and methods
[0027] 1) Antigen: Newcastle disease La Sota strain antigen (toxicity is 10 9.3 ELD 50 / ml)
[0028] 2) Test animals: SPF chickens aged 1 to 2 months
[0029] 3) Antigen inactivation of ND La Sota strain: add 10% formaldehyde solution to the antigen, and shake as it is added to make it fully mixed. The final concentration of formaldehyde is 0.2%, inactivated at 37°C for 16 hours (the inactivation temperature is calculated when the temperature in the bottle reaches 37°C), shaken 3-4 times during the inactivation period, and stored at 4-8°C after inactivation .
[0030] 4) Test grouping: the test was divided into white oil control group, commercial seedling control group, adjuvant group 1, group 2, group 3, group 4 and group 5 of the present invention. The adjuvant and antigen were prepared according to the ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com