Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Gene assembly" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gene Assembly – The final step in the gene production process involves a combination of PCR heating and cooling steps with SoftLinx-directed pipetting of the appropriate reagents to create a completed gene assembly.
Anti-viral vectors
InactiveUS6783981B1Efficient exportPeptide/protein ingredientsGenetic material ingredientsNucleotideRNase P
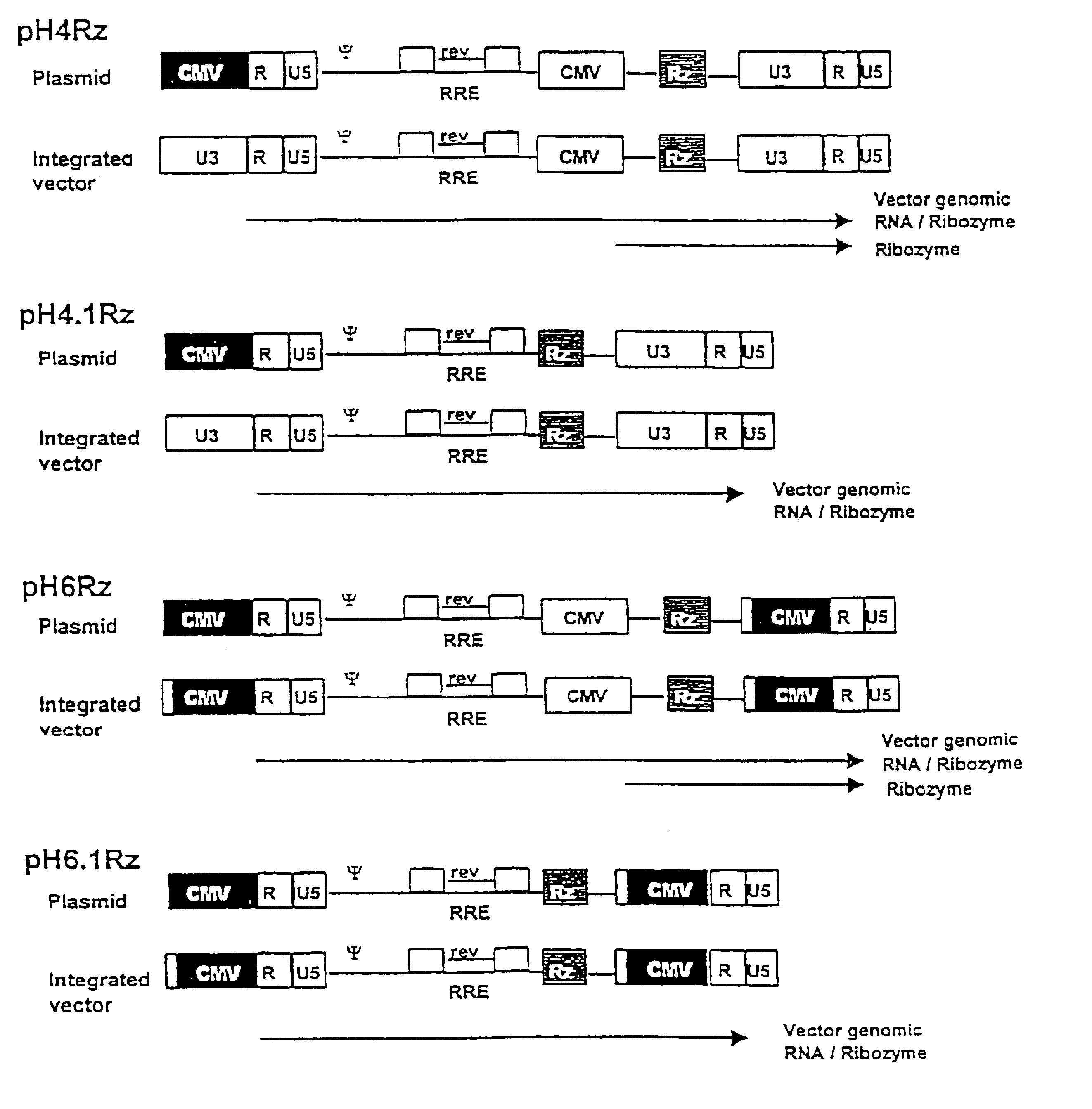
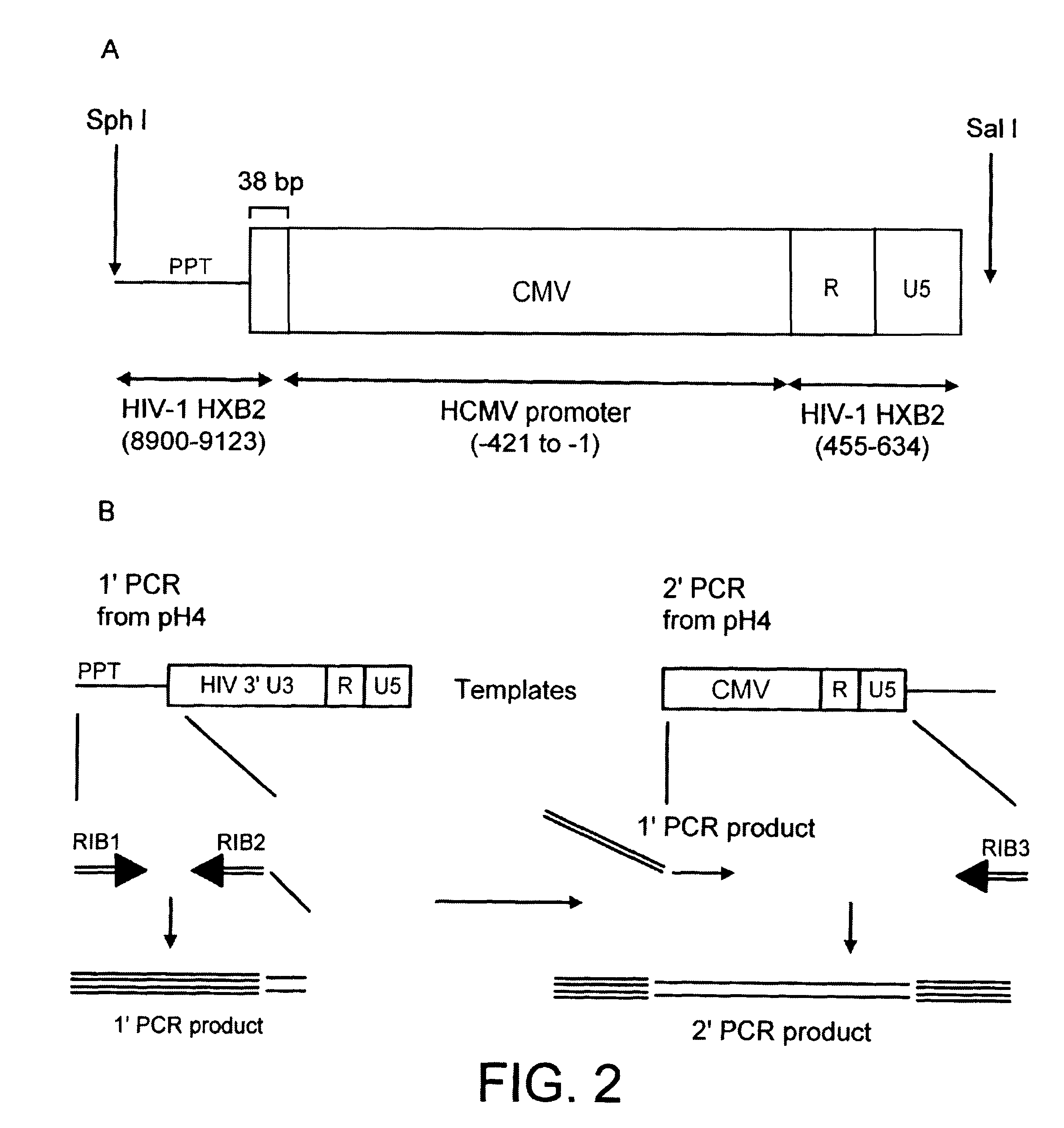
A viral vector production system is provided which system comprises: (i) a viral genome comprising at least one first nucleotide sequence encoding a gene product capable of binding to and effecting the cleavage, directly or indirectly, of a second nucleotide sequence, or transcription product thereof, encoding a viral polypeptide required for the assembly of viral particles, (ii) a third nucleotide sequence encoding said viral polypeptide required for the assembly of the viral genome into viral particles, which third nucleotide sequence has a different nucleotide sequence to the second nucleotide sequence such that said third nucleotide sequence, or transcription product thereof, is resistant to cleavage directed by said gene product; wherein at least one of the gene products is an external guide sequence capable of binding to and effecting the cleavage by RNase P of the second nucleotide sequence. The viral vector production system may be used to produce viral particles for use in treating or preventing viral infection.
Owner:OXFORD BIOMEDICA (UK) LTD
Anti-viral vectors
A viral vector production system is provided which system comprises:(i) a viral genome comprising at least one first nucleotide sequence encoding a gene product capable of binding to and effecting the cleavage, directly or indirectly, of a second nucleotide sequence, or transcription product thereof, encoding a viral polypeptide required for the assembly of viral particles;(ii) a third nucleotide sequence encoding said viral polypeptide required for the assembly of the viral genome into viral particles, which third nucleotide sequence has a different nucleotide sequence to the second nucleotide sequence such that said third nucleotide sequence, or transcription product thereof, is resistant to cleavage directed by said gene product. The viral vector production system may be used to produce viral particles for use in treating or preventing viral infection.
Owner:OXFORD BIOMEDICA (UK) LTD
Method for assembling bacteriophage gene engineering antibody library gene
InactiveCN101210241AImprove fidelityImprove connection efficiencyRecombinant DNA-technologyFermentationSingle-Chain AntibodiesHumanized antibody
The invention relates to a gene assembly method of phage genetically engineered antibody library, belonging to the field of biomedical study and clinical applications. The method aims to overcome the problems of the prior art, including poor stability, low efficiency, high mutation probability and low fidelity, and indirect application to humanized single chain antibody study. The technical proposal adopted by the invention comprises the following steps of: obtaining heavy chain variable region gene and light chain variable region gene of a humanized antibody by RT-PCR, and ligating the antibody heavy chain variable region, a linker and the antibody light chain variable region with the pre-designed restriction enzyme cutting sites on two ends of primers using PCR and restriction enzyme method at the same time.
Owner:SHAANXI CHAOYING BIOTECH
Use of non-standard bases and proximity effects for gene assembly and conversion of non-standard bases to standard bases during DNA synthesis
The present methods relate to generating nucleic acid molecules using non-natural nucleotides. In some methods, the nucleic acid molecules may be generated by hybridizing a plurality of oligonucleotides comprising one or more non-natural nucleotides and using a polymerase and / or a coupling agent to link the hybridized oligonucleotides. The methods also relate to the use of proximity effects to generate nucleic acid molecules using non-natural nucleotides. Furthermore, the methods relate to the use of at least one non-natural base in a DNA template in order to generate a replicate of the DNA template in which the non-natural base has been replaced with a natural base.
Owner:ERAGEN BIOSIENCES INC
Gene engineering method for preparing a recombinant glutathion peroxidase
ActiveCN103224915AHigh activity of recombinant GPXAvoid yieldEnzymesFermentationInclusion bodiesPeroxidase
The invention provides a gene engineering method for preparing a recombinant glutathion peroxidase, which belongs to the field of biological technology. The method comprises the following steps: assembling a target gene to a secretion type prokaryotic expression vector, introducing a catalytic group SeCys of GPX into a substrate combination position of GPX by a gene mutant method in an auxotroph prokaryotic expression system or an auxotroph and SPP combined expression system, thereby the activity of the GPS is very high; or assembling the target gene with the SeCys insertion sequence into a secretion type mammalia cell expression vector, assembling a SeCys insertion sequence binding protein 2 into an intracellular type mammalia cell expression vector, and cotransfecting the two vectors into the same lactation cell strain, and synthesizing the GPX under the existence of sodium selenite. The invention the advantages of simple method, recombined GPX vitality, high output and good stability, thereby avoiding decreased yield and inactivation caused by renaturation of an inclusion body, and solving the problem that the natural GPX source is limited and the property is not stable.
Owner:JILIN UNIV
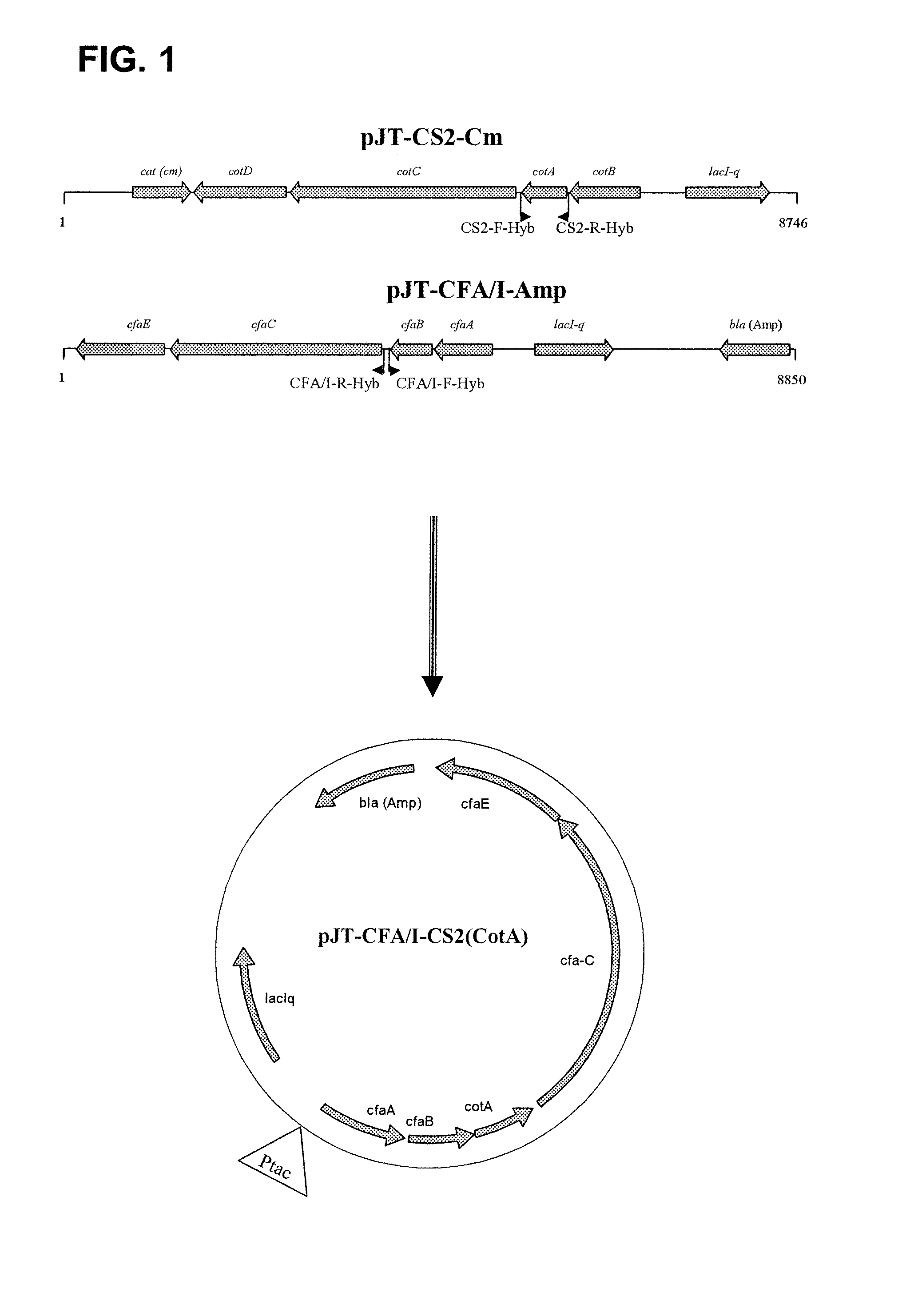
Hybrid operon for expression of colonization factor (CF) antigens of enterotoxigenic escherichia coli
InactiveUS20100136059A1Reduce in quantityAvoid restrictionsAntibacterial agentsBacteriaAntigenVaccine Production
A recombinant operon comprising a gene assembly wherein there are at least two structural genes coding for at least two major subunits of colonization factor antigens (CFs) associated with enterotoxigenic Escherichia coli bacteria (ETEC), is disclosed. Further disclosed is a host cell, such as an Escherichia coli cell, genetically engineered to comprise such a recombinant operon, wherein said operon is located on an episomal element, such as a plasmid, or integrated in the chromosome of said host cell. Also disclosed is a method of producing a host cell capable of expressing from said operon at least two major subunits of colonization factor antigens (CFs) associated with enterotoxigenic Escherichia coli bacteria (ETEC). In addition, a vaccine composition against diarrhea comprising at least one such host cell together with pharmaceutically acceptable excipients, buffers, and / or diluents is disclosed. Finally is disclosed the use of said operon in the production of such a vaccine.
Owner:GOTOVAX AB
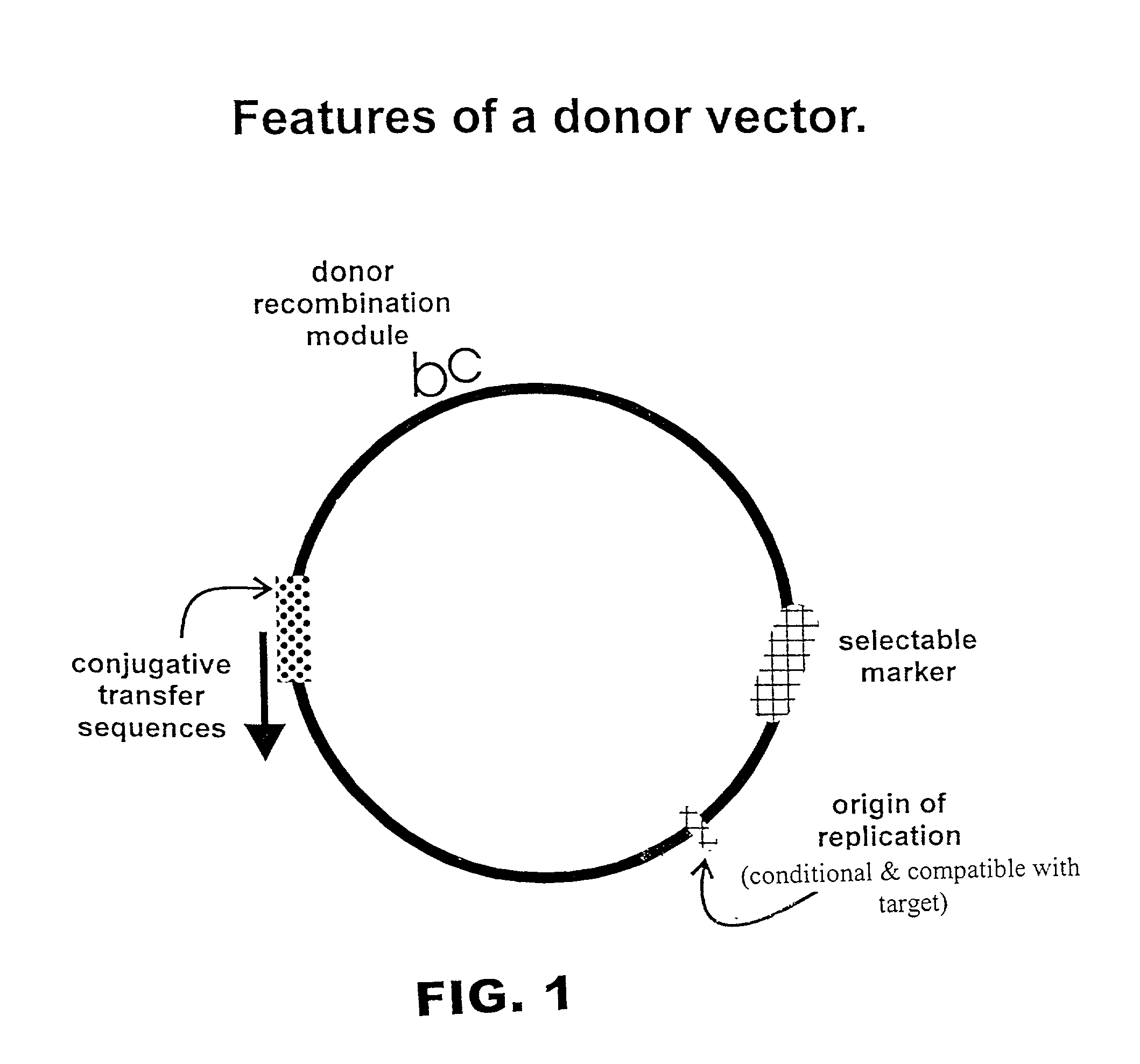
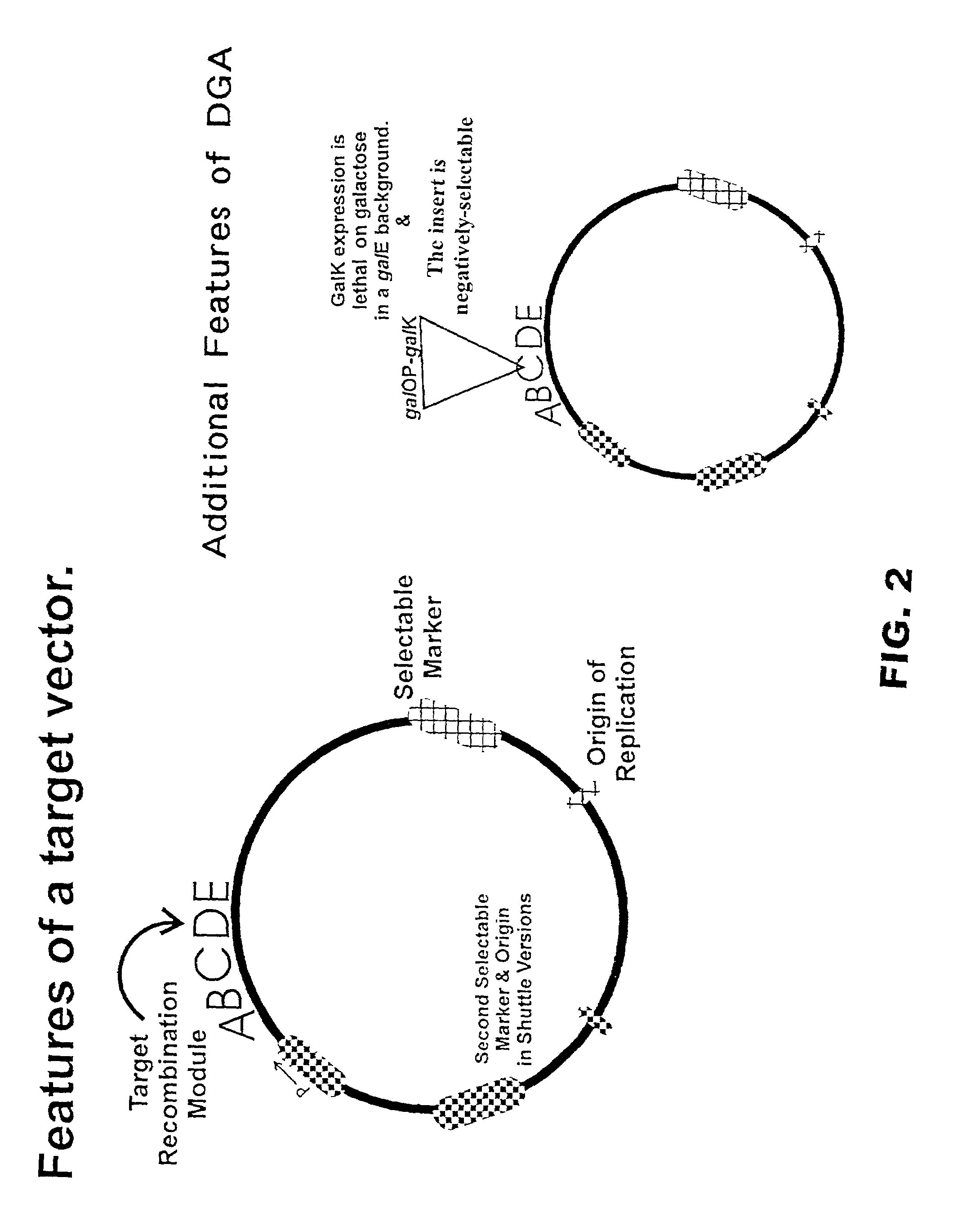
Methods and compositions for directed gene assembly
InactiveUS20020102734A1Reduce the burden onBacteriaMicrobiological testing/measurementGene assemblyDirected evolution
The present invention is directed to methods and compositions for use of homologous recombination for directed evolution, gene reassembly, and directed mutagenesis. One aspect of the present invention relates to methods for use of bacterial conjugative transfer and homologous recombination for directed evolution, gene reassembly, and directed mutagenesis. Another aspect of the present invention relates to compositions for use in or produced by the methods of the present invention, including libraries, archives and databases.
Owner:ATHENA BIOTECH INC
Recombinant CL7-CVN protein, and preparation method and applications thereof
ActiveCN110894242AIncreased expression of solubleSoluble expression achievedBacteriaPeptide/protein ingredientsEscherichia coliGene assembly
The invention discloses a recombinant CL7-CVN protein, and a preparation method and applications thereof. The preparation method includes cloning a coding sequence on an expression vector to obtain arecombinant expression vector; and converting the recombinant expression vector into host cells to perform expression and purification so as to obtain the recombinant CL7-CVN protein. Through a synthesized CVN gene and gene assembly method, the fusion expression vector can be built, and the highly soluble expression of the vector in escherichia coli can be realized; and according to the heat resistance of the recombinant CL7-CVN protein and through heat treatment and 3C protease digestion, the recombinant CL7-CVN protein and CVN protein can be rapidly obtained through one-step affinity chromatography, and purity can reach 99%. The provided preparation method can make the recombinant protein expression significantly enhanced, make activity more stable and make a purification method simpler;and both the prepared recombinant CL7-CVN protein and CVN protein have antiviral activity.
Owner:HUBEI UNIV
Novel method for gene assembly using improved CRISPR-Cpf1
ActiveCN109825520ASticky end longSuitable for high-throughput cloningStable introduction of DNAVector-based foreign material introductionTularemiaNucleotide
The invention discloses a novel method for gene assembly using improved CRISPR-Cpf1. The method includes utilizing a DNA gene editing enzyme FnCpf1 from a Francisella tularemia noticed U112 strain (gene sequence number: MF193599) for gene recombinant cloning. The seed sequence of crRNA (an RNA sequence generated by transcription of regular clustering spaced CRISPR) from 23 nucleotides reported inthe literature to 18 without affecting the FnCpf1 cleavage efficiency. At the same time, oligo(dN)6 (oligonucleotide hexamer) is used as the viscous end of the cleavage target site, the problem of terminal heterogeneity after FnCpf1 digestion is solved, the assembly efficiency of exogenous DNA fragments is remarkably improved, and the DNA fragment assembly efficiency reaches about 85%. The gene editing enzyme FnCpf1 can be replaced with other nucleic acid editing enzymes Cpf1 such as AsCpf1 and LbCpf1.
Owner:HUBEI UNIV
Assembling method for de novo sequencing data based on optics map platform Irys
ActiveCN106021978AImprove gene assembly effectSpeed up the calculation processProteomicsGenomicsContigAccessory genome
The invention relates to an assembling method for de novo sequencing data based on an optics map platform Irys. The method comprises: using the optics map platform Irys to obtain a gene assembly file; getting a scaffold file: fai file of NGS; preprocessing data: through setting a threshold value, filtering a comparison result whose confidence level is low, combining cmap files, sorting, calculating N50; counting assembling effects: counting comparison results of BioNano and NGS, including the contig of the BioNano, and the length, number, and total quantity of scaffold of NGS; according to the contig of the BioNano and network topological relations among the scaffold of the NGS, analyzing length of the assembled new contig and length of scaffold in a classified manner. The method can assist genome assembly, and obviously improves gene assembly effects of species.
Owner:GENERGY BIO TECH SHANGHAI CO LTD
Construction method of multigene carrier and its application
InactiveCN1263860CEfficient assemblyOvercoming technical hurdlesVector-based foreign material introductionDNA preparationMultigene expressionGene assembly
A multi-gene carrier system is composed of a receptor carrier and at least two donor carriers, and is prepared by altermative gene assemblings with the said carriers. It can be used for conversion of multiple genes to obtain different genetically engineered products and multi-gene expression characteristics.
Owner:SOUTH CHINA AGRI UNIV
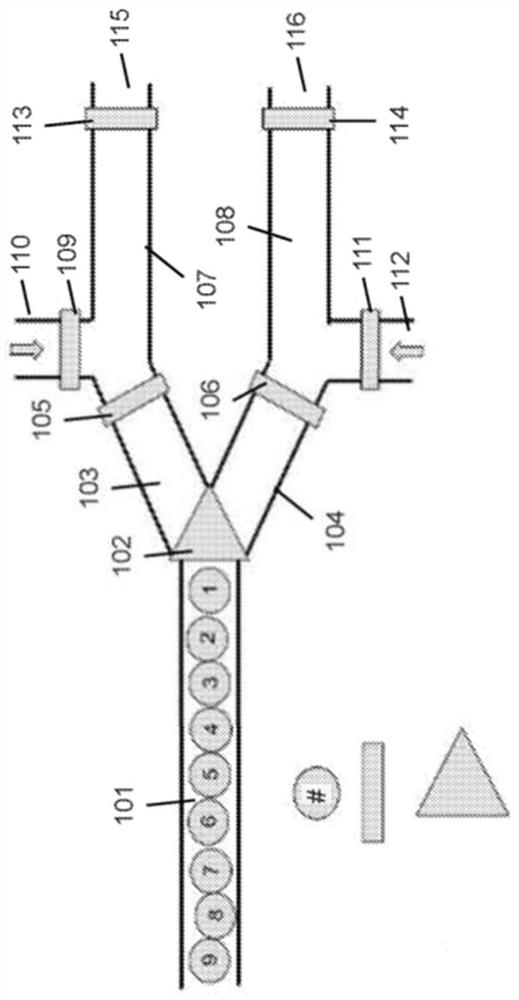
Methods of using microfluidic positional encoding devices
PendingUS20220126298A1Sequential/parallel process reactionsHeating or cooling apparatusComputer hardwareChemical reaction
The invention relates to methods and compositions useful for routing and tracking multiple mobile units within a microfluidic device. Mobile units may be routed through a plurality of chemical environments, and the mobile units may be tracked to determine the path and / or environments that the mobile units have routed through. Mobile units may be routed in accordance with a predetermined algorithm. Mobile units may be routed through microfluidic devices in ordered flow. Mobile units routed through the microfluidic device can be used to perform various chemical reactions uniquely associated to the units, including without limitation peptide synthesis, enzymatic gene synthesis and gene assembly.
Owner:ELEGEN CORP
Error correcting method of test sequence, corresponding system and gene assembly equipment
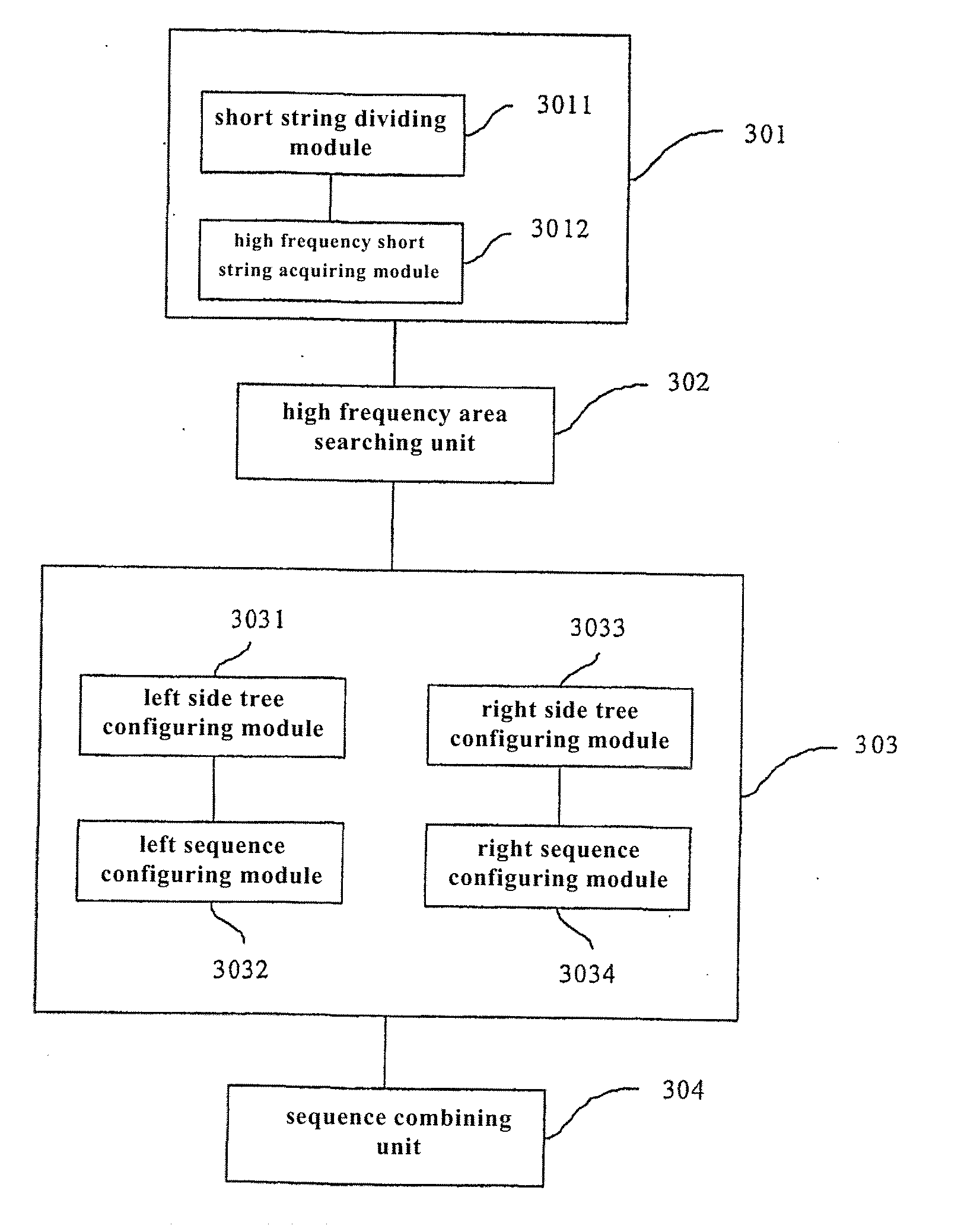
ActiveUS20110295784A1Increase profitReduce memory usageMicrobiological testing/measurementGenetic modelsShort stringGene assembly
The present invention provides an error correcting method of test sequence, which involves receiving test sequences, configuring high frequency short string list based on a preset high frequency threshold value, traversing each received test sequence, searching an area with the largest number of continuous high frequency short strings on each test sequence in combination with high frequency short string list, configuring whole left sequence and / or right sequence of high frequency short strings at left side and / or right side of searched area according to corresponding received test sequence and high frequency short string list, and constituting corresponding test sequence according to configured left and / or right sequence and searched area. The present invention also provides corresponding error correcting system of test sequence and gene assembly equipment.
Owner:BGI TECH SOLUTIONS
Methods and compositions for directed gene assembly
InactiveUS7087415B2Reduce the burden onBacteriaMicrobiological testing/measurementGene assemblyBacterial conjugation
The present invention is directed to methods and compositions for use of homologous recombination for directed evolution, gene reassembly, and directed mutagenesis. One aspect of the present invention relates to methods for use of bacterial conjugative transfer and homologous recombination for directed evolution, gene reassembly, and directed mutagenesis. Another aspect of the present invention relates to compositions for use in or produced by the methods of the present invention, including libraries, archives and databases.
Owner:ATHENA BIOTECH INC
Method for improving caffeic acid content and/or biological self-luminous intensity of plant
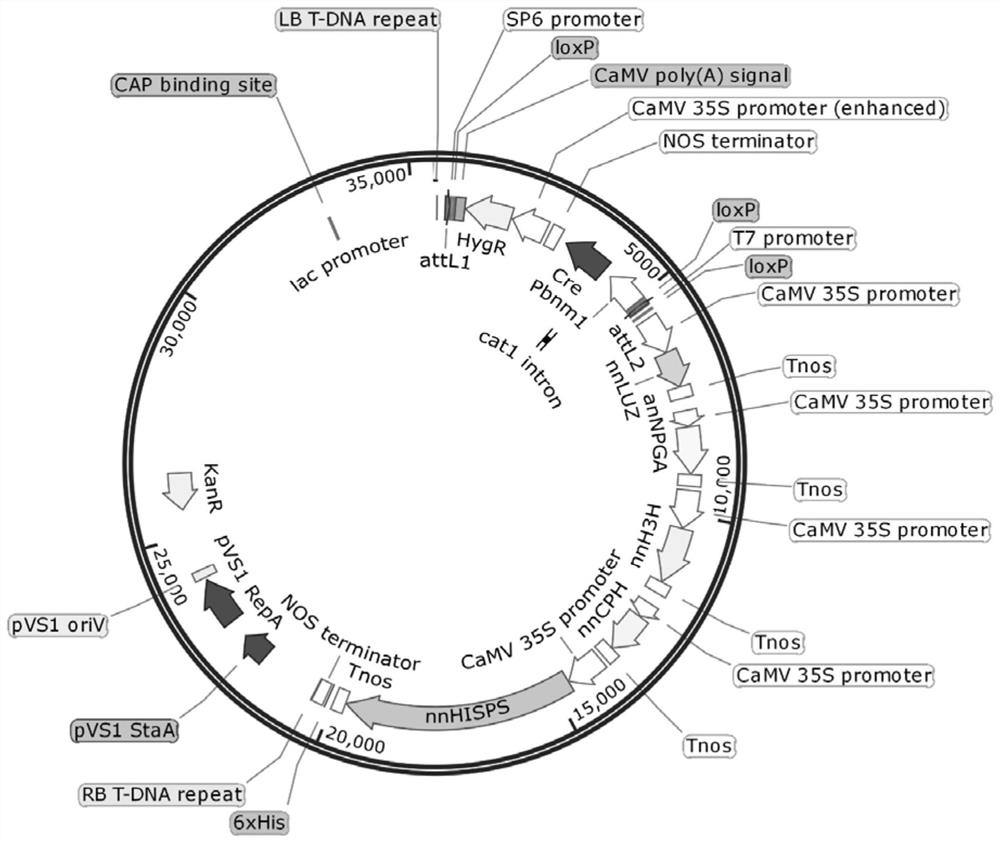
ActiveCN114591974AIncrease contentIncrease self-illumination intensityCarbon-nitrogen lyasesTransferasesBiotechnologyReceptor
The invention discloses a method for improving caffeic acid content and / or biological self-luminous intensity of plants, and belongs to the technical field of biology. The method comprises the following steps: (1) sequentially integrating an NtCM1 gene, an NtPAT gene, an NtADT2 gene, an NtPAL1 gene and an NtC4H gene into a receptor vector by utilizing a multi-gene assembly technology, and constructing to obtain a multi-gene vector; and (2) introducing the target gene segment into a receptor plant by using a transgenic technology to obtain a transgenic plant with enhanced caffeic acid content and / or biological self-luminous intensity. The gene participates in synthesis of caffeic acid in the plant for the first time, and co-expression of the gene and a self-luminous Hisps gene, a CPH gene, an H3H gene, an NPGA gene and a Luz gene is carried out, so that the biological self-luminous intensity of the plant can be remarkably improved. The invention provides a feasible technical scheme for breeding and production of high caffeic acid plants and sustainable luminous plants.
Owner:ZHEJIANG UNIV HANGZHOU GLOBAL SCI & TECH INNOVATION CENT
Antiviral vectors
A viral vector production system is provided which system comprises: (i) a viral genome comprising at least one first nucleotide sequence encoding a gene product capable of binding to and effecting the cleavage, directly or indirectly, of a second nucleotide sequence, or transcription product thereof, encoding a viral polypeptide required for the assembly of viral particles; (ii) a third nucleotide sequence encoding said viral polypeptide required for the assembly of the viral genome into viral particles, which third nucleotide sequence has a different nucleotide sequence to the second nucleotide sequence such that said third nucleotide sequence, or transcription product thereof, is resistant to cleavage directed by said gene product. The viral vector production system may be used to produce viral particles for use in treating or preventing viral infection.
Owner:OXFORD BIOMEDICA (UK) LTD
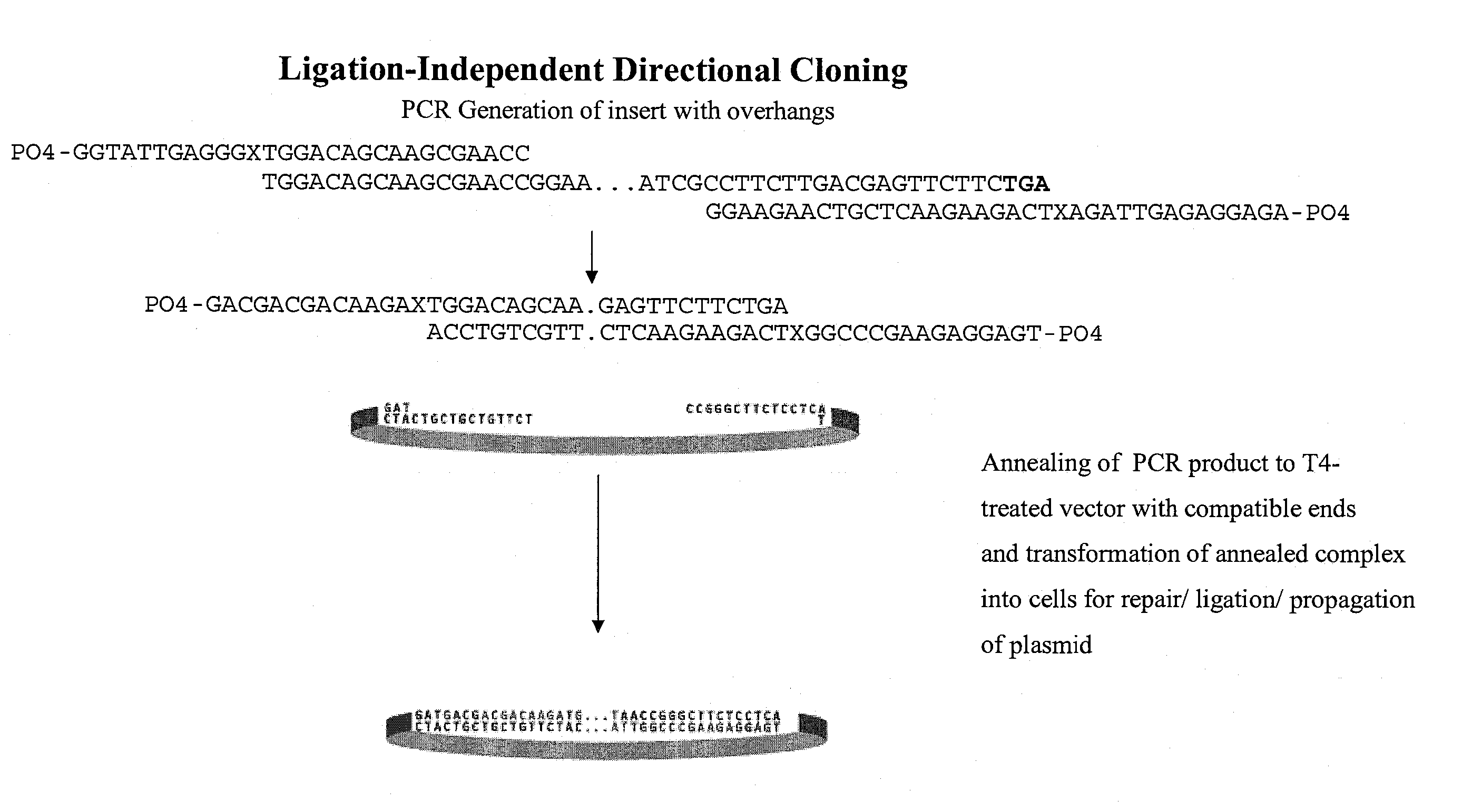
Novel systems, methods and compositions for the direct synthesis of sticky ended polynucleotides
PendingUS20220162687A1Eliminate needMicrobiological testing/measurementLigasesOligonucleotide primersDNA construct
The current inventive technology includes systems, methods, and compositions for directly synthesizing sticky ended DNA fragments for subsequent gene assembly. In a preferred embodiment, the inventive technology includes strategies for the direct synthesis of sticky ended DNA with 5′ overhangs that have any desired length and base composition, using typical PCR protocols with no additional manipulation. In another embodiment, the inventive technology includes the direct synthesis of sticky ended DNA using chemically modified oligonucleotide primers in a polymerase chain reaction (PCR). In certain embodiments, the inventive technology allows for the generation of larger DNA constructs formed by the sticky-ended assemblies generally described herein compared to traditional synthesis and ligation applications.
Owner:UNIV OF COLORADO THE REGENTS OF
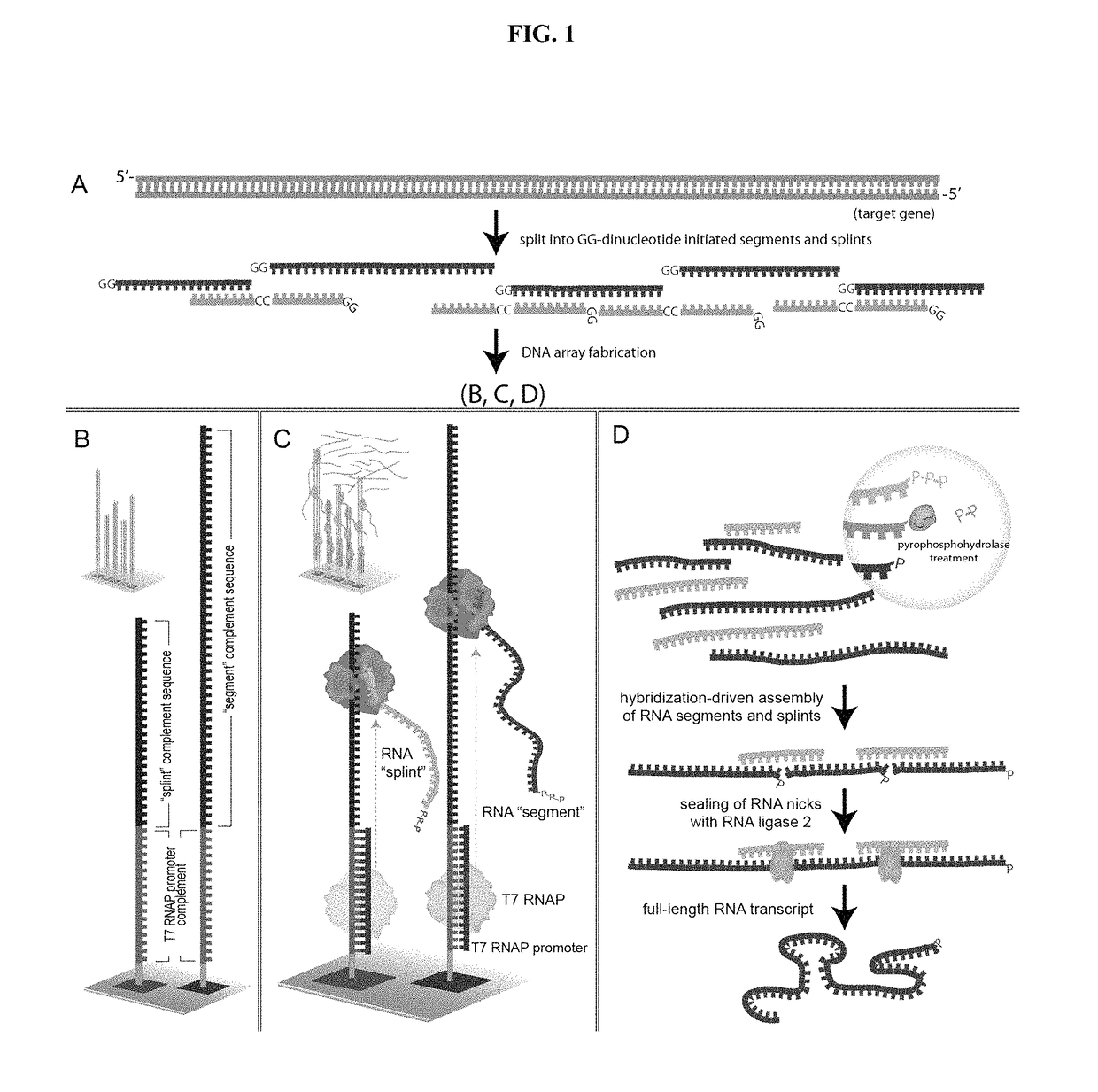
RNA-mediated gene assembly from DNA oligonucleotides
ActiveUS9873900B2Easy to assembleLow costFermentationVector-based foreign material introductionADAMTS ProteinsGene assembly
The present invention is directed to methods and materials for RNA-mediated gene assembly from oligonucleotide sequences. In some embodiments, the oligonucleotides used for gene assembly are provided in an array format. An RNA polymerase promoter is appended to surface-bound oligonucleotides and a plurality of RNA copies of each oligonucleotide are then produced with an RNA polymerase. These RNA molecules self-assemble into a desired full-length RNA transcript by hybridization and ligation. The resulting RNA transcript may then be converted into double strand DNA useful in a variety of applications including protein expression.
Owner:WISCONSIN ALUMNI RES FOUND
Method and system for gene transcript assembly and quantification based on information theory
ActiveCN107944226BImprove accuracyImprove Sequencing AccuracySequence analysisHybridisationGene PositionInformation transmission
The invention provides an informatics-based gene transcript assembly and quantification method and system, wherein the method comprises: aligning a sequencing read with a reference genome, and predicting initial start and end positions of a gene and transcript according to the alignment results for the sequencing read and reference genome; after prediction, establishing a directed graph to simulate possible transcripts to obtain a candidate transcript set; subjecting the candidate transcript set to transcript prediction and kurtosis estimation according the mode of maxium information transmission capacity. The method and system of the invention have the advantages that dependence on external gene positional marking is avoided, gene assembly accuracy is significantly improved, and sequencing precision is improved.
Owner:TSINGHUA UNIV
RNA-mediated gene assembly from DNA oligonucleotides
ActiveUS20130225446A1Easy to assembleLow costFermentationVector-based foreign material introductionProtein insertionGene assembly
The present invention is directed to methods and materials for RNA-mediated gene assembly from oligonucleotide sequences. In some embodiments, the oligonucleotides used for gene assembly are provided in an array format. An RNA polymerase promoter is appended to surface-bound oligonucleotides and a plurality of RNA copies of each oligonucleotide are then produced with an RNA polymerase. These RNA molecules self-assemble into a desired full-length RNA transcript by hybridization and ligation. The resulting RNA transcript may then be converted into double strand DNA useful in a variety of applications including protein expression.
Owner:WISCONSIN ALUMNI RES FOUND
Application of oilseed rape BnC3H gene in improving biological self-luminous intensity of plants
ActiveCN114015703AIncrease the intensity of biological self-luminescenceOxidoreductasesFermentationBiotechnologyTransgenic technology
The invention discloses an application of an oilseed rape BnC3H gene in improving the biological self-luminous intensity of plants, and belongs to the technical field of biology. The CDS sequence of the BnC3H gene is as shown in SEQ ID NO.1. The application comprises the steps: (1) sequentially integrating a Hisps gene, a CPH gene, a H3H gene, a NPGA gene, a Luz gene and a BnC3H gene into a receptor vector by utilizing a multi-gene assembly technology, and constructing to obtain a multi-gene vector; and (2) introducing a target gene segment into a receptor plant by using a transgenic technology to obtain a transgenic plant with enhanced biological self-luminous intensity. The invention discloses co-expression of the BnC3H gene and the Hiss gene, the CPH gene, the H3H gene, the NPGA gene and the Luz gene participating in plant self-luminescence for the first time, and the biological self-luminescence intensity of the plant can be remarkably improved. The invention provides a feasible technical scheme for breeding and production of sustainable luminous plants.
Owner:ZHEJIANG UNIV HANGZHOU GLOBAL SCI & TECH INNOVATION CENT
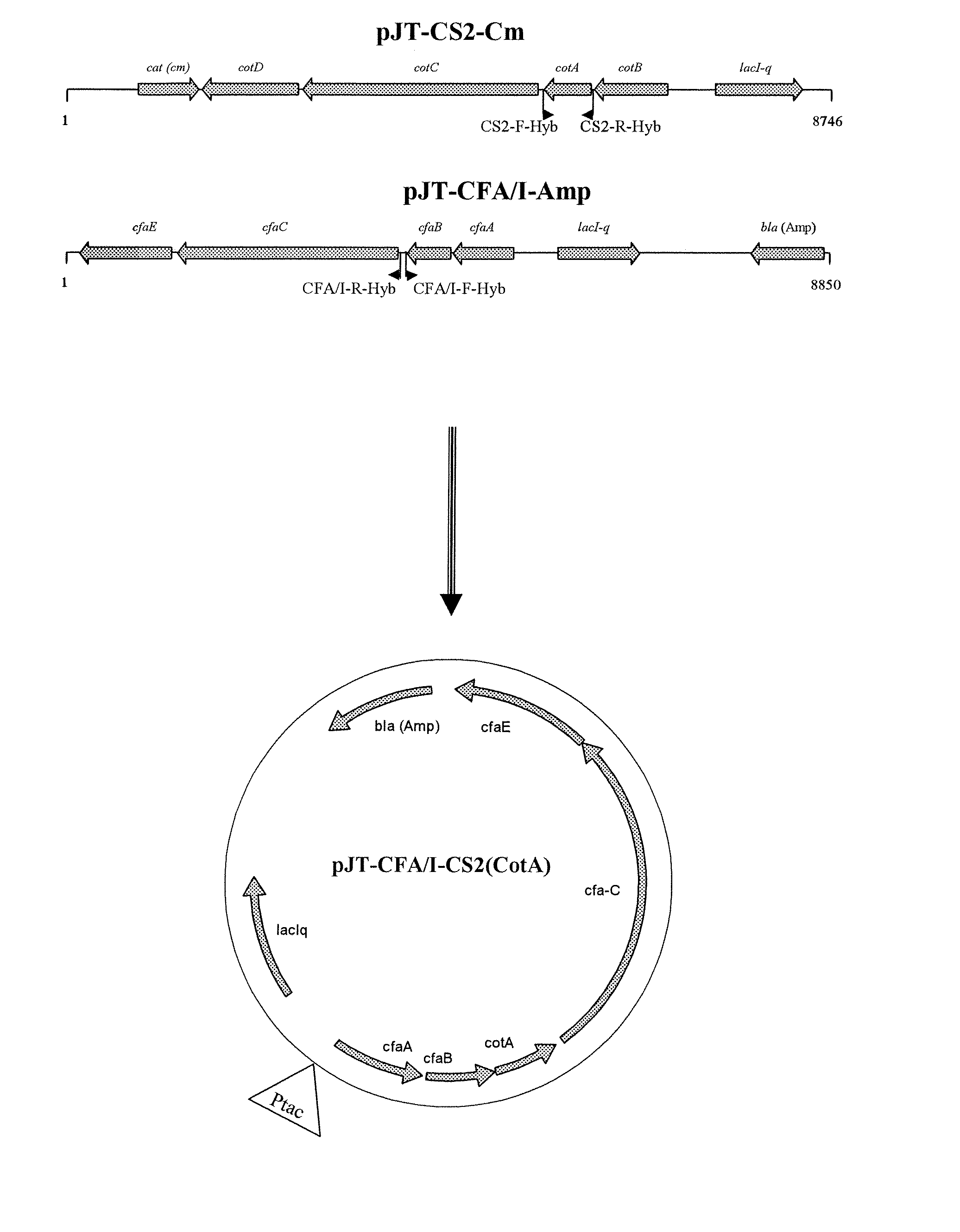
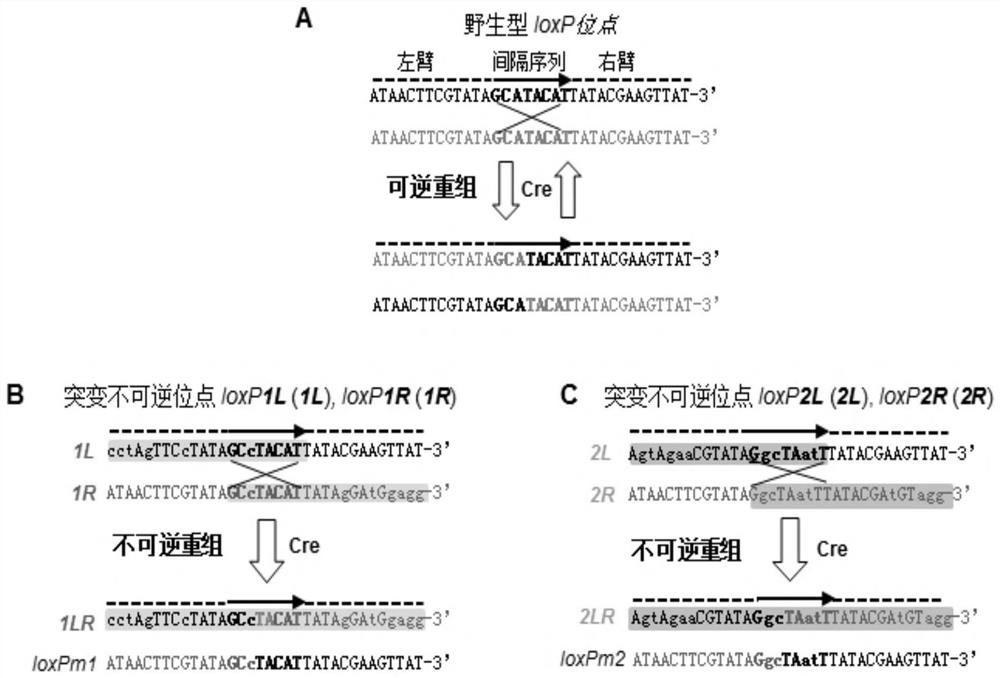
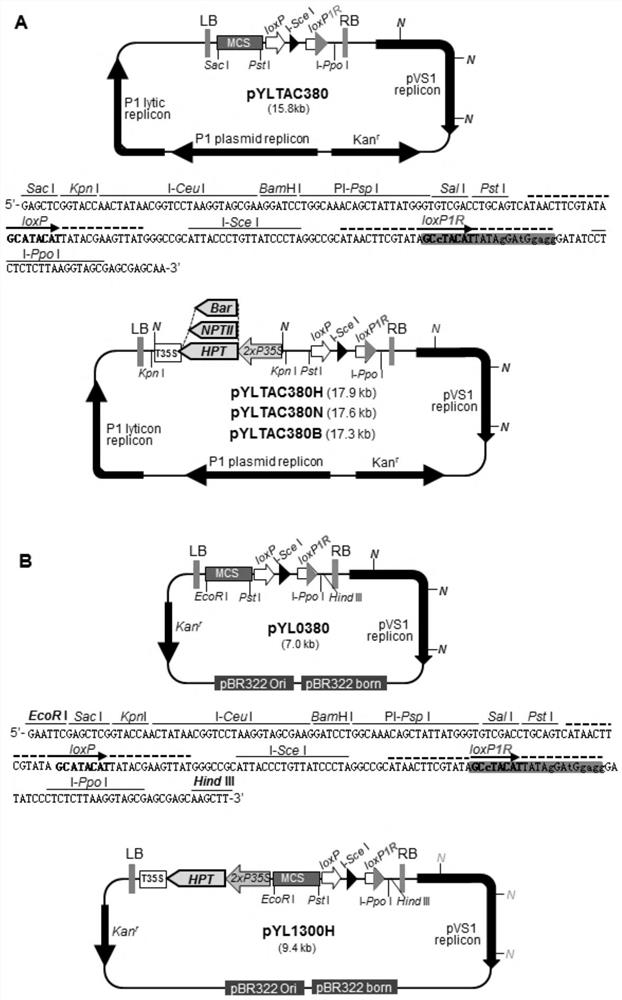
A kind of multi-gene assembly vector system and its multi-gene assembly method
ActiveCN107177616BQuick assemblyEasy to buildNucleic acid vectorVector-based foreign material introductionBiotechnologyVector system
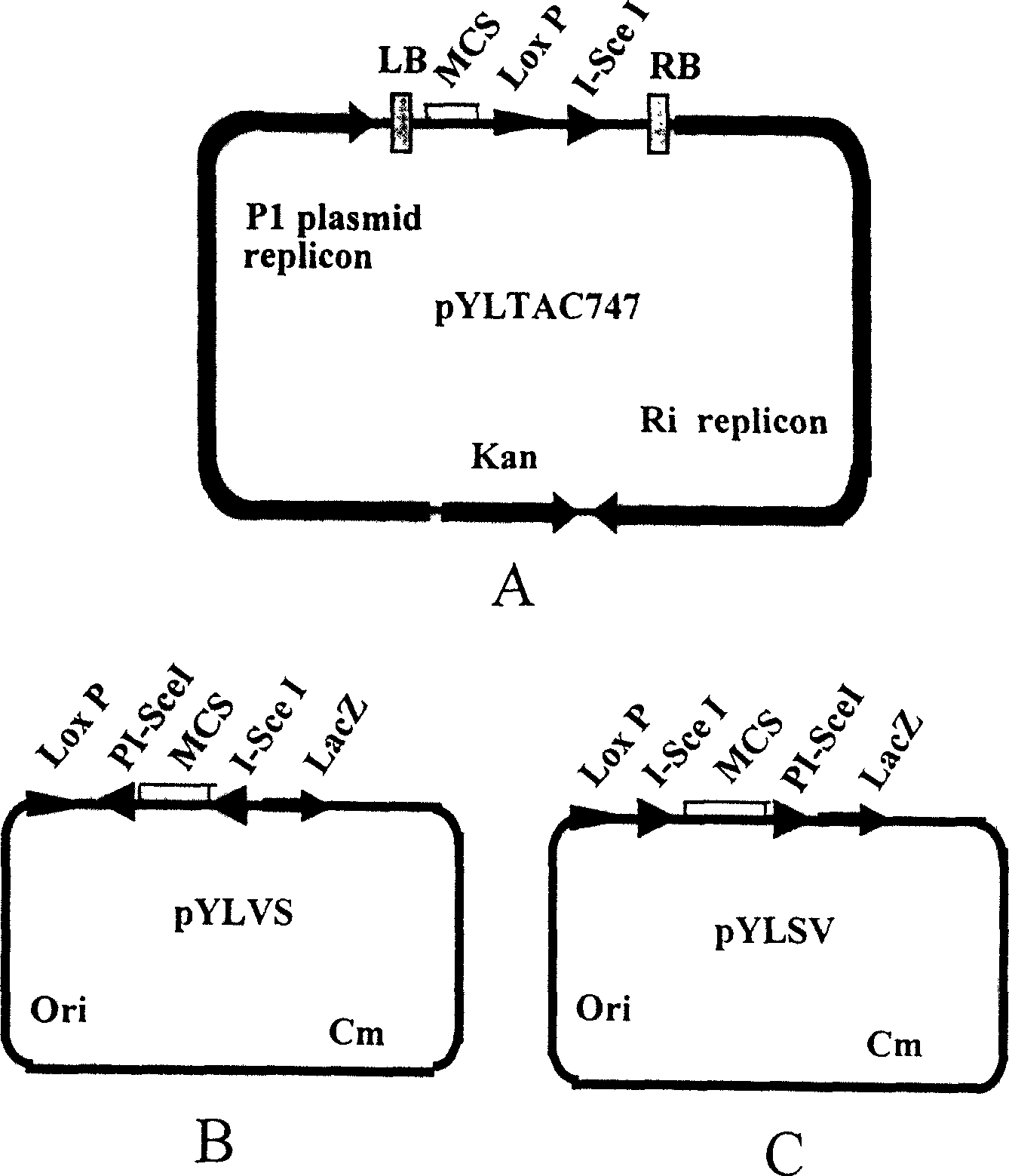
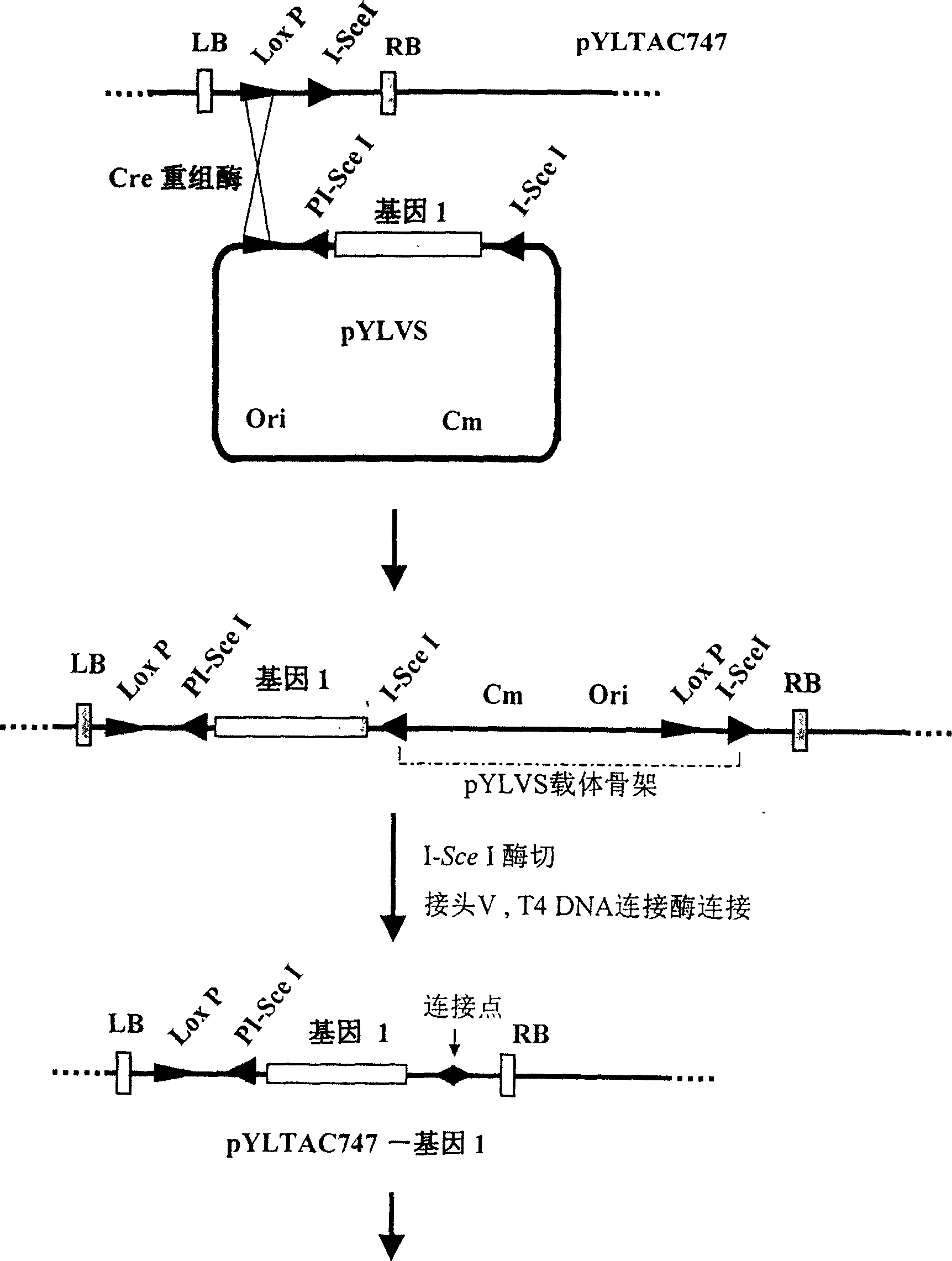
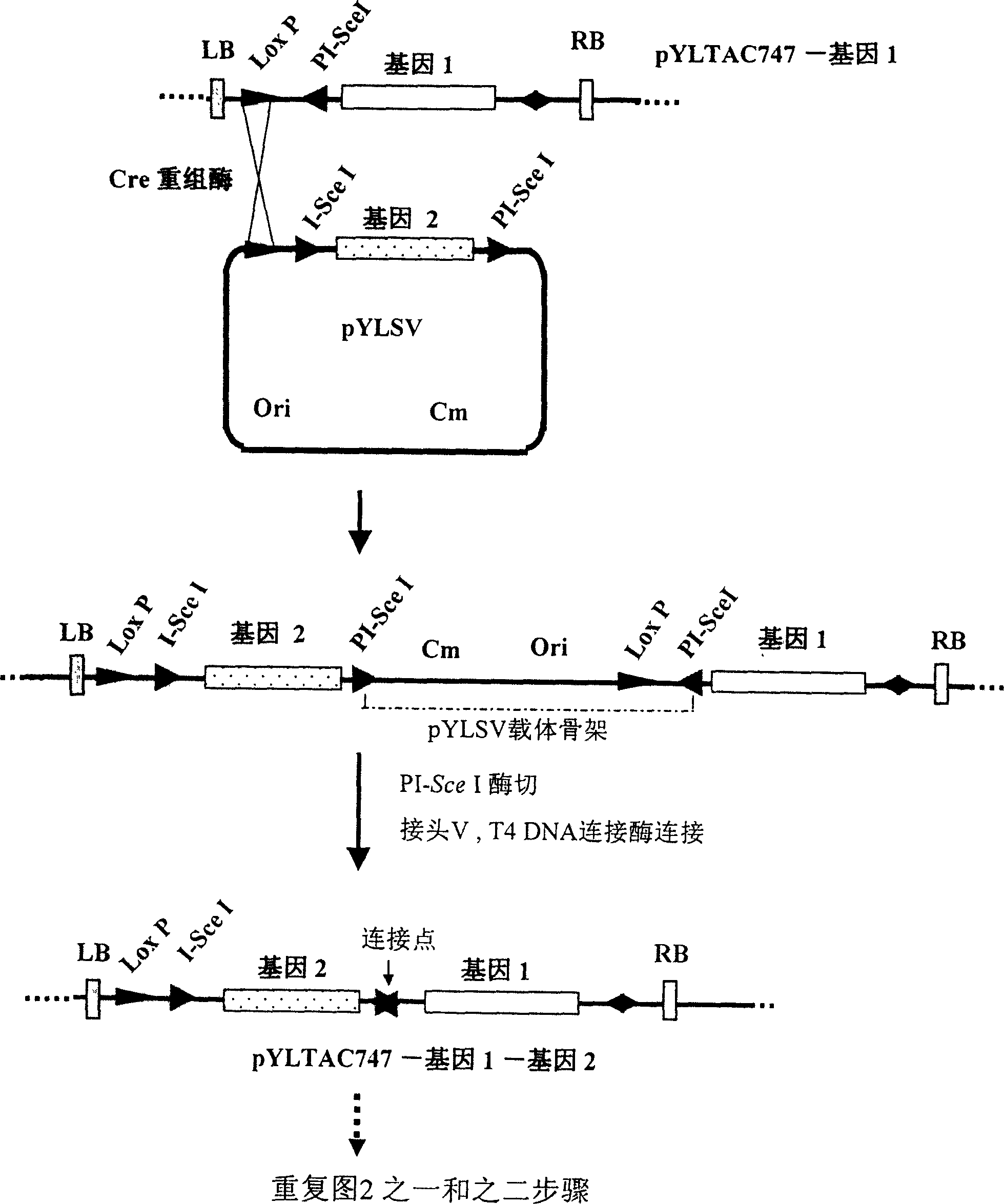
The invention discloses a multigene assembly carrier system and a multigene assembly method thereof. The system includes a type 1 acceptor vector, a type 2 donor vector, and an optional donor plasmid capable of self-deleting selection markers. The system utilizes the Cre / loxP recombination system and two sets of irreversible recombination sites of mutant loxP. Under the action of Cre enzyme, the recombination of wild-type loxP sites is used to realize the integration of the donor vector into the acceptor vector, and then a set of mutant loxP The irreversible recombination of loxP automatically deletes the backbone of the donor vector, leaving only the target gene on the acceptor carrier; repeating the assembly of the two types of donor vectors and acceptor vectors alternately can realize the rapid assembly of multiple genes. The present invention is the most simple and efficient multigene carrier system at present. Therefore, the invention provides an operable technical platform for the multi-gene genetic engineering operation of important and complex biosynthetic pathways and important agronomic traits, and has important application value.
Owner:SOUTH CHINA AGRI UNIV
Informatics-based gene transcript assembly and quantification method and system
ActiveCN107944226AImprove assembly accuracyImprove accuracyHybridisationSpecial data processing applicationsGene PositionDirected graph
The invention provides an informatics-based gene transcript assembly and quantification method and system, wherein the method comprises: aligning a sequencing read with a reference genome, and predicting initial start and end positions of a gene and transcript according to the alignment results for the sequencing read and reference genome; after prediction, establishing a directed graph to simulate possible transcripts to obtain a candidate transcript set; subjecting the candidate transcript set to transcript prediction and kurtosis estimation according the mode of maxium information transmission capacity. The method and system of the invention have the advantages that dependence on external gene positional marking is avoided, gene assembly accuracy is significantly improved, and sequencing precision is improved.
Owner:TSINGHUA UNIV
Methods of using microfluidic positional encoding devices
PendingCN113811391ASequential/parallel process reactionsHeating or cooling apparatusComputer hardwareChemical reaction
Embodiments relate to methods and compositions useful for routing and tracking multiple mobile units within a microfluidic device. Mobile units may be routed through a plurality of chemical environments, and the mobile units may be tracked to determine the path and / or environments that the mobile units have routed through. Mobile units may be routed in accordance with a predetermined algorithm. Mobile units may be routed through microfluidic devices in ordered flow. Mobile units routed through the microfluidic device can be used to perform various chemical reactions uniquely associated to the units, including without limitation peptide synthesis, enzymatic gene synthesis and gene assembly.
Owner:艾勒根公司
Methods of using microfluidic positional encoding devices
PendingUS20220090183A1Sequential/parallel process reactionsHeating or cooling apparatusComputer hardwareChemical reaction
Embodiments relate to methods and compositions useful for routing and tracking multiple mobile units within a microfluidic device. Mobile units may be routed through a plurality of chemical environments, and the mobile units may be tracked to determine the path and / or environments that the mobile units have routed through. Mobile units may be routed in accordance with a predetermined algorithm. Mobile units may be routed through microfluidic devices in ordered flow. Mobile units routed through the microfluidic device can be used to perform various chemical reactions uniquely associated to the units, including without limitation peptide synthesis, enzymatic gene synthesis and gene assembly.
Owner:ELEGEN CORP
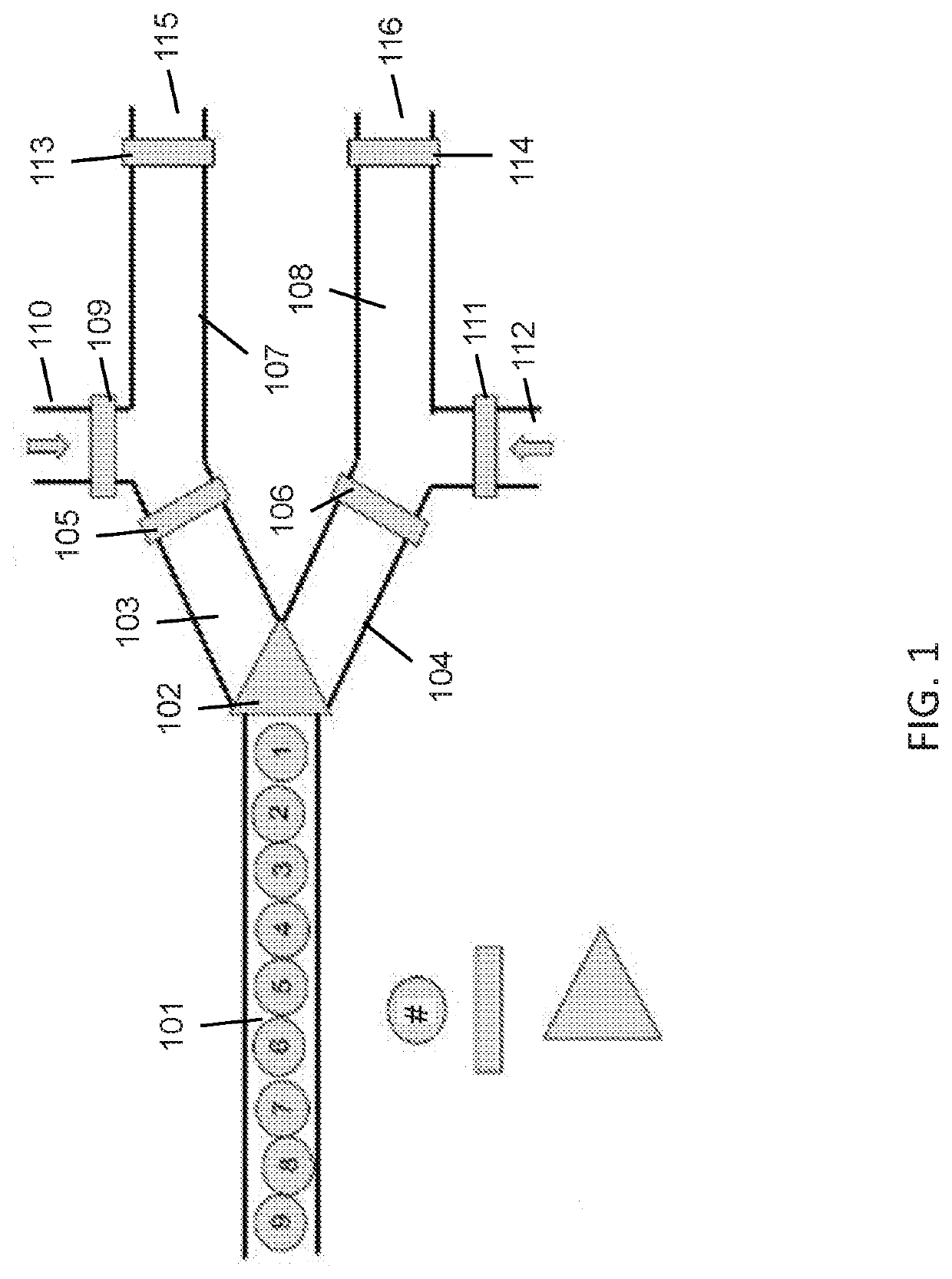
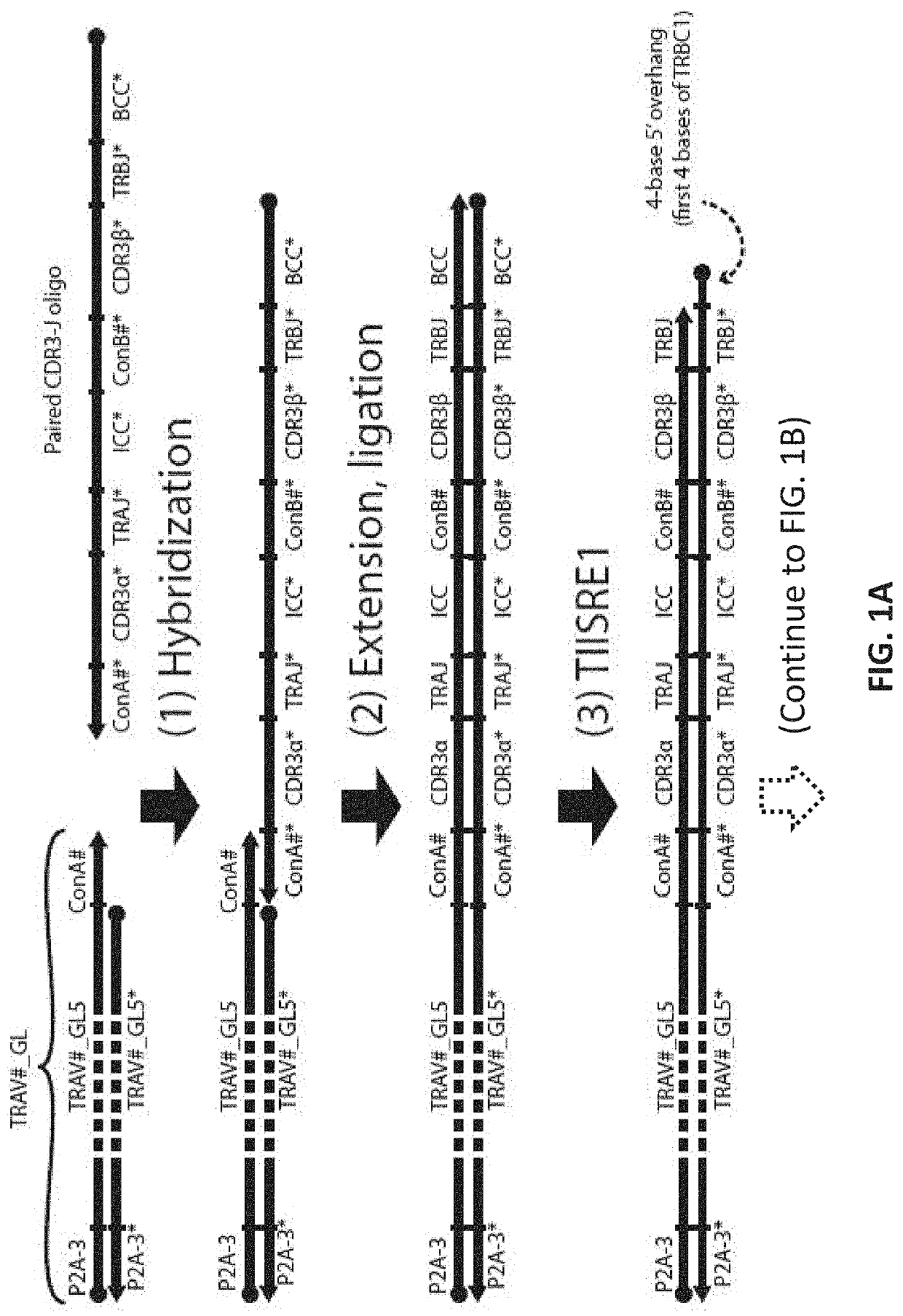
Compositions and methods for t-cell receptor gene assembly
Provided herein are compositions and methods for assembling nucleic acid sequences encoding T-cell receptors.
Owner:ROOTPATH GENOMICS INC
Recombinant cl7-cvn protein and its preparation method and application
ActiveCN110894242BIncreased expression of solubleSoluble expression achievedBacteriaPeptide/protein ingredientsEscherichia coliGene assembly
The invention discloses a recombinant CL7-CVN protein, its preparation method and application. The preparation method of the recombinant CL7-CVN protein comprises: cloning its coding sequence into an expression vector to obtain a recombinant expression vector; transforming the recombinant expression vector into a host cell for expression and purification to obtain a recombinant CL7-CVN protein. The present invention constructs a fusion expression vector through a synthetic CVN gene and a gene assembly method to realize its highly soluble expression in Escherichia coli; according to the heat resistance of the recombinant CL7-CVN protein, through heat treatment and 3C protease digestion, it can be passed One-step affinity chromatography quickly obtains recombinant CL7‑CVN protein and CVN protein with a purity of up to 99%. The preparation method provided by the invention can significantly increase the expression level of the recombinant protein, make the activity more stable, and the purification method is simpler; both the recombinant CL7‑CVN protein and the CVN protein prepared by the invention have antiviral activity.
Owner:HUBEI UNIV
Construction method of stem cell bank for preventing and treating novel coronavirus pneumonia
InactiveCN112626624AGood treatment effectSolve the problem of ultra-low temperature permanent storageVirus peptidesTransferasesViral antibodyIndividualized treatment
A construction method of a stem cell bank for preventing and treating novel coronavirus pneumonia comprises the following steps of: collecting residual amniotic fluid cells, newborn umbilical cord blood, umbilical cord and placenta tissues after prenatal diagnosis, carrying out stem cell induction, immortalized gene transfection, novel coronavirus targeted interference gene assembly, novel coronavirus susceptibility gene knockout or knock-in and novel coronavirus antibody generation gene assembly to prepare immortalized stem cells, DNA recombinant stem cells, RNA interference stem cells, gene silencing stem cells and various stem cell vector vaccines for preventing and treating novel coronavirus pneumonia, collecting the prepared stem cell products in -196 DEG C liquid nitrogen in a centralized mode according to donor names, ABO blood types or HLA types, and establishing a renewable special stem cell bank. When the novel coronavirus is epidemic, various types of stem cells or vaccines thereof can be extracted from the stem cell bank to be cultured and amplified, and individualized treatment is performed by the patient's own or homologous stem cells, so that side effects such as immunological rejection and the like of traditional stem cell or vaccine prevention and treatment are reduced, and the problem of lack of COVID-19 special-effect medicines and stem cells is solved.
Owner:翁炳焕
Error correcting method of test sequence, corresponding system and gene assembly equipment
ActiveUS8751165B2Increase profitReduce memory usageMicrobiological testing/measurementBiostatisticsShort stringGene assembly
The present invention provides an error correcting method of test sequence, which involves receiving test sequences, configuring high frequency short string list based on a preset high frequency threshold value, traversing each received test sequence, searching an area with the largest number of continuous high frequency short strings on each test sequence in combination with high frequency short string list, configuring whole left sequence and / or right sequence of high frequency short strings at left side and / or right side of searched area according to corresponding received test sequence and high frequency short string list, and constituting corresponding test sequence according to configured left and / or right sequence and searched area. The present invention also provides corresponding error correcting system of test sequence and gene assembly equipment.
Owner:BGI TECH SOLUTIONS
Gene engineering method for preparing a recombinant glutathion peroxidase
ActiveCN103224915BHigh activity of recombinant GPXAvoid yieldEnzymesFermentationBiotechnologyTwo-vector
The invention provides a gene engineering method for preparing a recombinant glutathion peroxidase, which belongs to the field of biological technology. The method comprises the following steps: assembling a target gene to a secretion type prokaryotic expression vector, introducing a catalytic group SeCys of GPX into a substrate combination position of GPX by a gene mutant method in an auxotroph prokaryotic expression system or an auxotroph and SPP combined expression system, thereby the activity of the GPS is very high; or assembling the target gene with the SeCys insertion sequence into a secretion type mammalia cell expression vector, assembling a SeCys insertion sequence binding protein 2 into an intracellular type mammalia cell expression vector, and cotransfecting the two vectors into the same lactation cell strain, and synthesizing the GPX under the existence of sodium selenite. The invention the advantages of simple method, recombined GPX vitality, high output and good stability, thereby avoiding decreased yield and inactivation caused by renaturation of an inclusion body, and solving the problem that the natural GPX source is limited and the property is not stable.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com