Multiplex fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting hematogenous infectious viruses
A technology of multiple fluorescence quantification and detection kits, which is applied in the direction of recombinant DNA technology, microbial measurement/inspection, DNA/RNA fragments, etc., which can solve the problems that affect the wide application, increase the risk of pathogen transmission, detection cost and long operation time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
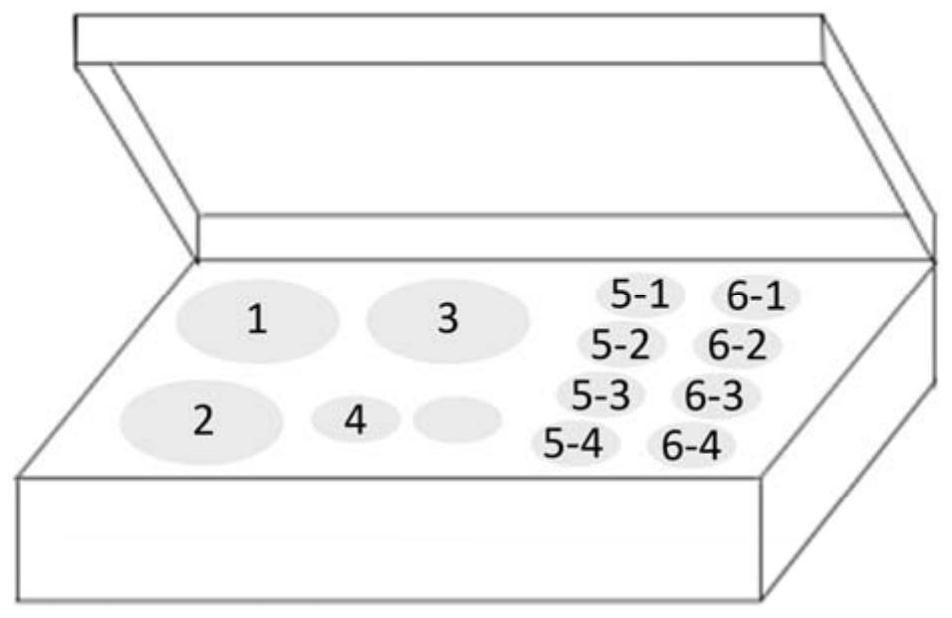
[0055] A multiple fluorescent quantitative RT-PCR detection kit for detecting blood-borne infectious viruses, the kit includes quantitative RT-PCR reaction solution, enzyme Mix solution, primer probe Mix solution, standard products (cytomegalovirus, Epstein- Barr virus, herpes simplex virus, RNase P), positive control substance (cytomegalovirus, Epstein-Barr virus, herpes simplex virus, RNaseP), negative control substance. The box body is provided with container holes, respectively placed quantitative RT-PCR reaction liquid tube, enzyme Mix liquid tube, primer probe Mix liquid tube, standard tube (cytomegalovirus, Epstein-Barr virus, herpes simplex virus, RNase P), Positive control quality control (cytomegalovirus, Epstein-Barr virus, herpes simplex virus, RNase P), negative control quality control.
[0056] see figure 1 , There are container holes in the package of this kit, which are used to place the corresponding kit components: quantitative RT-PCR reaction solution tube ...
Embodiment 2
[0058] 1 Materials and methods
[0059] 1.1 Serum samples and viral nucleic acid:
[0060] The clinical samples of cytomegalovirus, Epstein-Barr virus, and herpes simplex virus were obtained from the serum samples of patients with cytomegalovirus, Epstein-Barr virus, and herpes simplex virus nucleic acid positive in the First Affiliated Hospital of Zhejiang University School of Medicine and several other hospitals in Zhejiang Province .
[0061] In addition, positive samples of other herpes viruses such as VZV, HHV-6, HHV-7, and HHV-8 were provided by the State Key Laboratory of Diagnosis and Treatment of Infectious Diseases.
[0062] 1.2 Primers and probes
[0063]Downloaded from the NCBI gene bank multiple gene sequences covering cytomegalovirus, Epstein-Barr virus, and herpes simplex virus at home and abroad. The homology comparison was carried out using DNAman software to determine the conserved regions of the above viral genomes. Primer Express 3.0 software was used t...
Embodiment 3
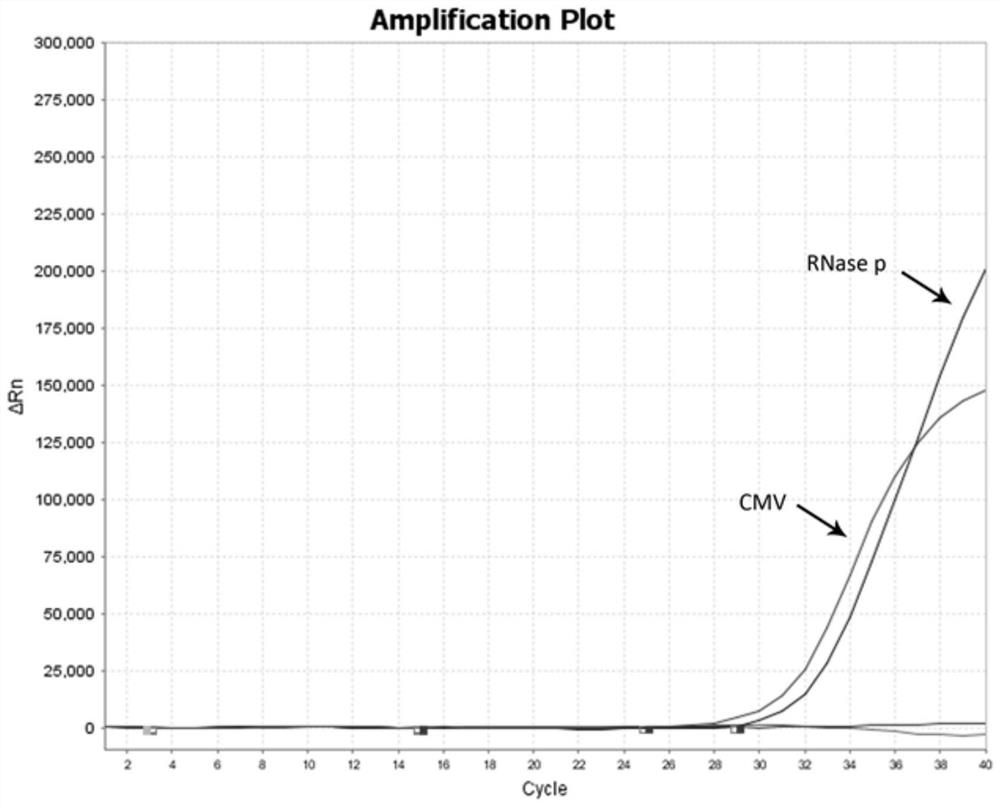
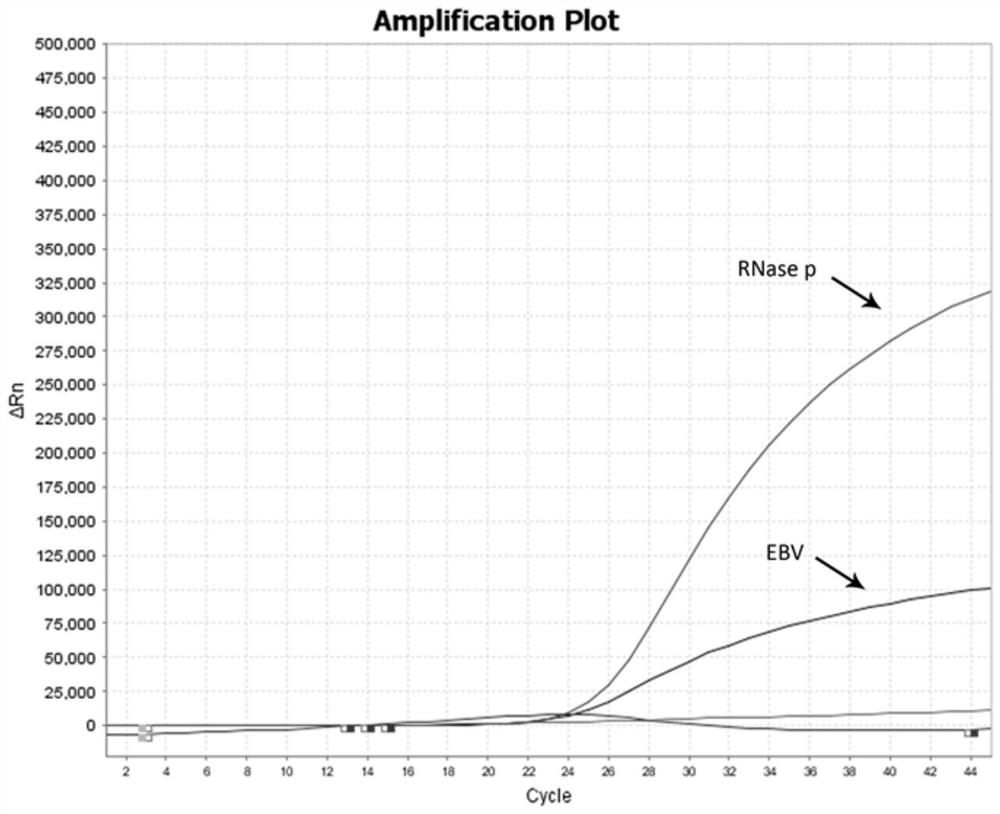
[0098] The clinical samples collected by this kit mainly come from a total of 200 preoperative four test specimens between May 2021 and July 2021. The collected specimens were detected by multiple real-time fluorescence quantitative RT-PCR in this method, and the results were as follows: a total of 200 specimens were tested for four items before surgery, 2 positive specimens for cytomegalovirus, 2 positive specimens for EB virus, There were 0 positive herpes simplex virus samples, and all the test results of the internal reference RNase P gene were positive. The positive result of this kit is consistent with the positive result of the commercial kit (Zhijiang Biology) by 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com