Sample dilution composition for detecting sugar antigen 72-4, detection reagent and kit containing detection reagent
A composition and carbohydrate antigen technology, applied in the field of medical testing, can solve the problems of unfavorable improvement of laboratory pipeline, cumbersome calibration operation, narrow linear range, etc., to eliminate the difference between old and new samples, low HOOK risk, and linear range. wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation and use of the kit for the detection of carbohydrate antigen 72-4 1. Preparation of the detection kit for carbohydrate antigen 72-4
[0066] The kit of the invention consists of four components: enzyme conjugate, sample diluent, magnetic bead suspension and calibrator.
[0067] (1) Conventional equipment and reagents
[0068] 1. Enzyme conjugate (packed in a 12mL magnetic bead bottle);
[0069] 2. Sample diluent (packed in a 12mL magnetic bead bottle);
[0070] 3. Magnetic bead suspension (packed in a 4mL magnetic bead bottle);
[0071] 4. Reagent rack (for immobilizing the three components of enzyme conjugate, sample diluent, and magnetic bead suspension);
[0072] 5. Calibrator (packed in 4mL HDPE bottle);
[0073] 6. Calibration card;
[0074] (2) Preparation of enzyme conjugates
[0075] Preparation of enzyme conjugate dilution: use one of the buffer solutions Tris-NaCl, Bis-tris, PBS, MOPS, MES or Taps with a concentration of 0.01-0.1M, ...
Embodiment 2
[0109] Embodiment 2 kit sensitivity assessment
[0110] LoB, LoD, and LOQ were established according to the performance confirmation (establishment) method of the CLSI standard "EP17-A: Evaluation of Detection Limits of Clinical Laboratories". There is no standard substance in this project, and it cannot be traced to SI units. LOQ is not applicable, and the functional sensitivity (FS) is assessed.
[0111] The establishment method is as follows:
[0112] LoB, prepare 5 clinical samples with a value close to 0, repeat 3 times for each sample, do a total of 4 days, and get 60 data; LoD, prepare 5 serial clinical samples with a concentration range of 1 to 4 times LOB, each sample Repeat 3 times, do a total of 4 days, and get 60 data; FS: Using the data in the LoD experiment, 5 concentration samples are measured 3 times a day, a total of 4 days, each sample gets 12 results, and the calculation of each sample Mean, SD and CV%, the concentration to the nearest 20% is the functiona...
Embodiment 3
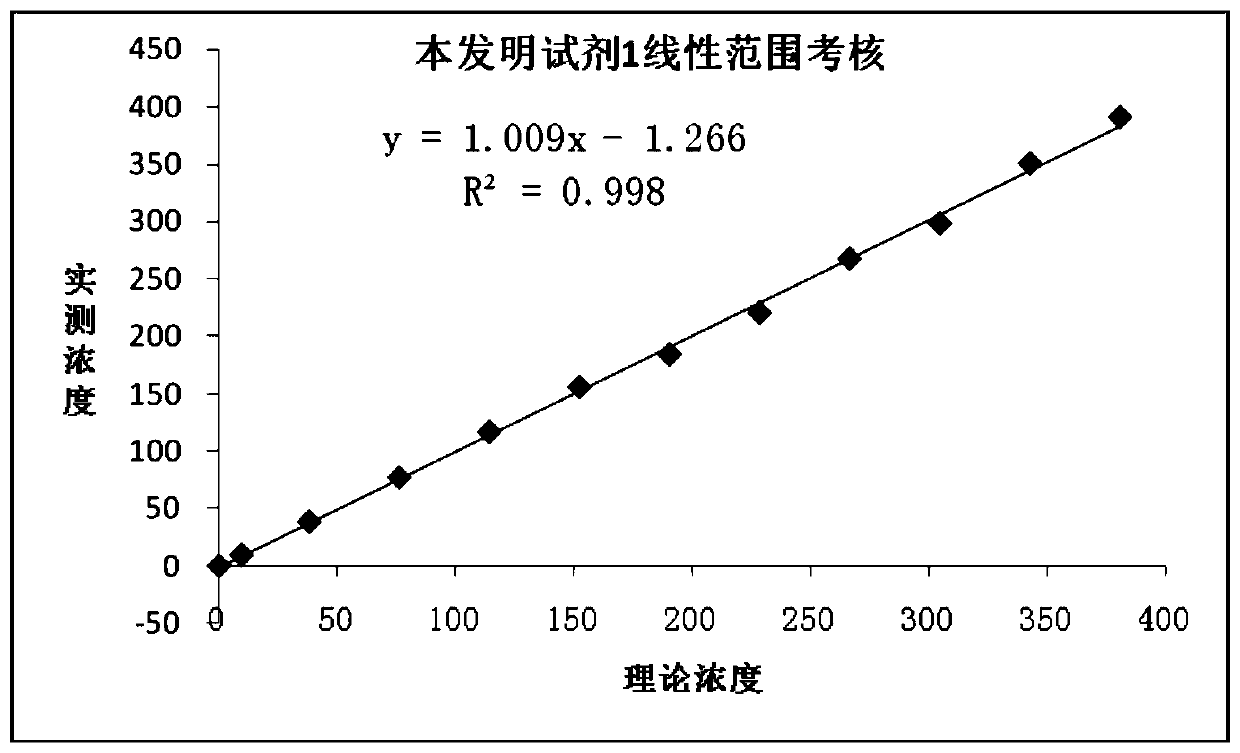
[0120] Embodiment 3 test kit linear range examination
[0121] According to the method of CLSI standard "EP6-A: Linearity Evaluation of Quantitative Detection Methods", the linear range of this kit was established.
[0122] The establishment method is as follows:
[0123] Take 1 high-value sample H (>300U / mL) and 1 low-value sample L (0.99.
[0124] The result is as attached figure 1 , figure 2 As shown, it can be seen that the linear range of the kit of the present invention can reach 0.3-300 U / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com