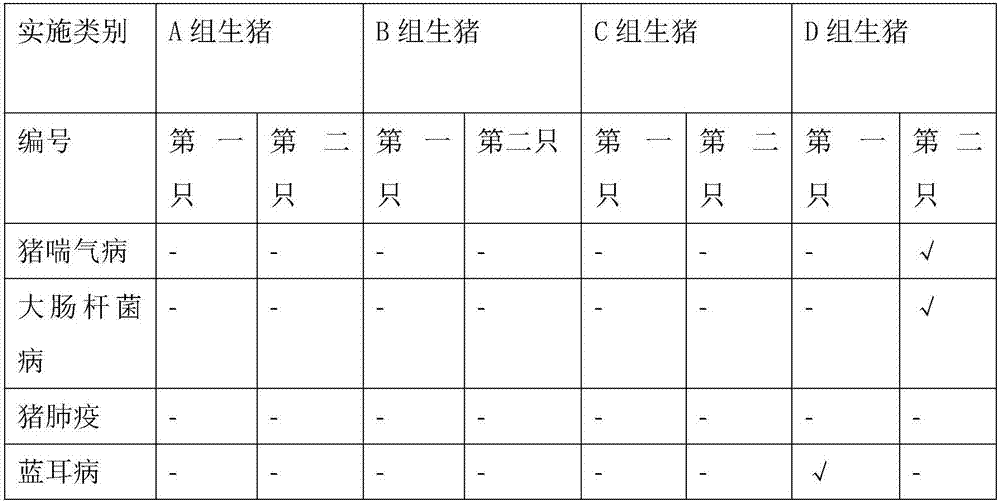
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "Mycoplasmal pneumonia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151AStrengthen cellsEnhance humoral immune stimulationAntibacterial agentsBacterial antigen ingredientsCholesterolVaccine antigen
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Mycoplasma Hyopneumoniae Vaccine
ActiveUS20130266601A1Antibacterial agentsSsRNA viruses positive-senseImmunoglobulin IgEMycoplasma hyopneumoniae
This invention provides an immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M.hyo) whole cell preparation, wherein the soluble portion of the M.hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Feed for preventing and treating mycoplasmal pneumonia of swine and traditional Chinese medicine composition as well as preparation method
ActiveCN103947887AImprove the body's immunityLow priceAntibacterial agentsInorganic active ingredientsClinical efficacySide effect
The invention discloses feed for preventing and treating mycoplasmal pneumonia of swine and a traditional Chinese medicine composition as well as a preparation method. The feed comprises a feed carrier, a feed adhesive and the traditional Chinese medicine composition, wherein the traditional Chinese medicine composition comprises meadowrue root and rhizome, common andrographis herb, cordate houttuynia, wild buckwheat rhizome, calyx seu fructus physalis, agilawood, perillaseed, fingered citron, bulbus fritillariae cirrhosae, snakegourd fruit, rhizoma anemarrhenae, bamboo juice, tabasheer, platycodon grandiflorum, concha meretricis seu cyclinae, bryozoatum, aster, birthwort, folium eriobotryae, herba ardisiae japonicae, folium rhododendri daurici, radix astragali, liquorice, herb of little platanthera, pueraria montana merr and all-grass of longsepaled crotalaria. The feed has the beneficial effects that the feed overcomes various defects of traditional Chinese medicines and western medicines in the prior art, has the effects of clearing heat and eliminating phlegm, relieving cough and asthma, strengthening body resistance and eliminating evil on treatment of mycoplasmal pneumonia of swine and has a remarkable clinical effect, is high in curative ratio and has no toxic or side effects and no drug residue, no drug resistance is generated by using the feed, and the mycoplasmal pneumonia of swine is difficult to relapse by using the feed; in addition, the feed is capable of improving the immunity of organisms of ill pigs and is low in cost, and meat of pigs fed by using the feed has a certain health effect on bodies of customers eating the meat.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Method for detecting pneumonia causative bacteria using nucleic acid chromatography
InactiveUS20130023443A1Quick and accurate identificationNucleotide librariesMicrobiological testing/measurementBacteroidesStaphylococcus aureus
Provided are a method and a kit for accurately and rapidly detecting ten types of targeting pneumonia bacteria: Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Klebsiella pneumoniae, Pseudomonas aeruginosa, Moraxella catarrhalis, methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus aureus. A set of primer pairs directed to their respective target regions contained in the DnaJ gene, etc., of the ten types of pneumonia causative bacteria is designed for the ten bacterial strains and used to amplify gene products. A set of bacterial strain-specific probe pairs is further designed for the ten bacterial strains such that the probe pairs hybridize with the amplification products via sequences in the respective target regions differing from the sequences hybridized by the set of primer pairs. A first probe-bound labeled high molecular carrier in which plural types of first probes for the pneumonia bacteria are bound to a labeled high molecular carrier and a solid-phase second probe-carrying developing support are used as the set of probe pairs to perform nucleic acid chromatography.
Owner:YAMAGUCHI TECH LICENSING ORG
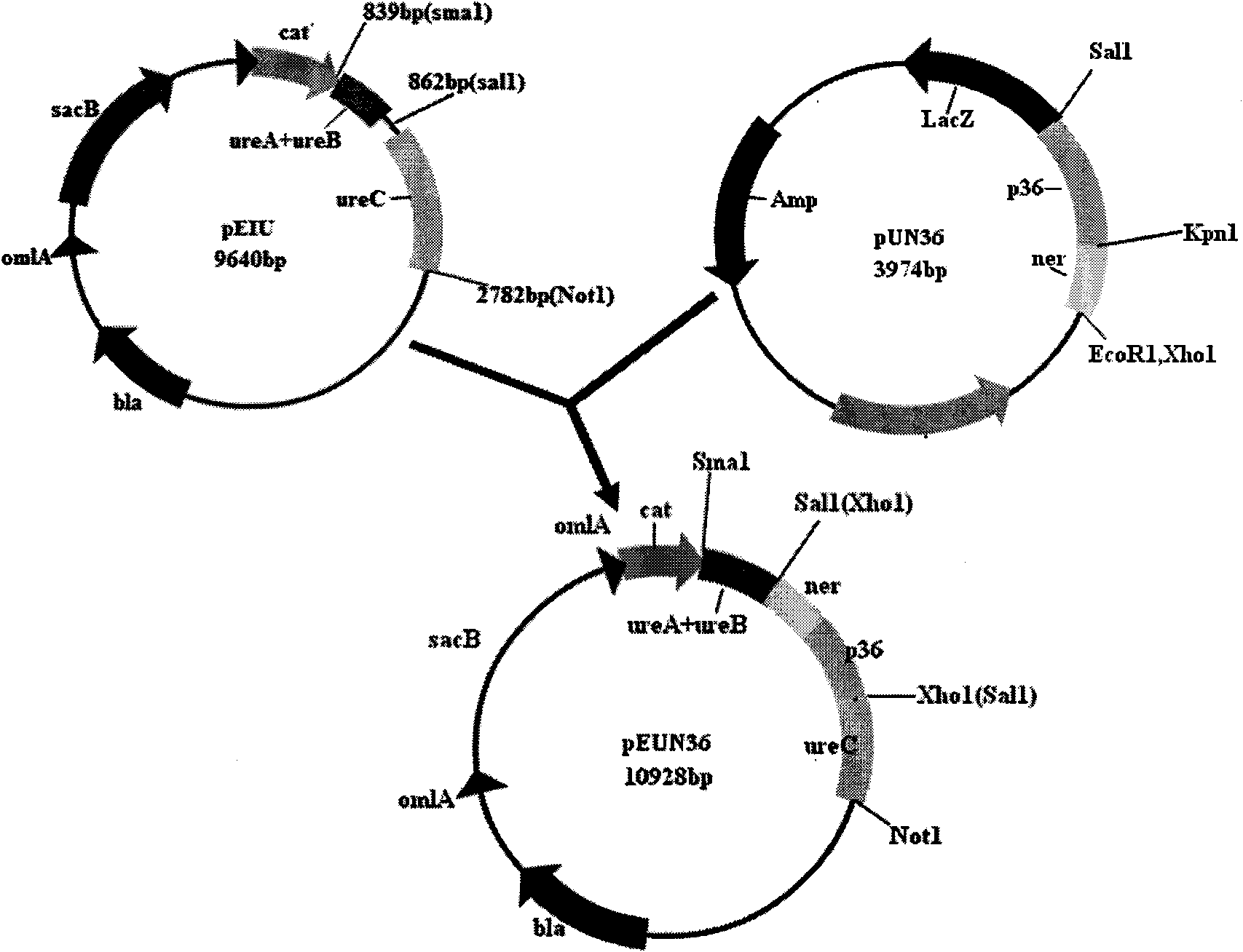
Porcine mycoplasmal pneumonia and porcine infectious actinobacillus pleuropneumoniae serum 1 type gene engineering strain vaccine and application thereof
InactiveCN101603024AEasy to buildStimulate systemic immunityAntibacterial agentsBacterial antigen ingredientsBacteroidesNucleotide
The invention belongs to the technical field of domestic animal infectious disease, relating to the technical field of bacterial gene engineering, in particular to a construction, vaccine preparation and application of a reconstruction porcine actinobacillus pleuropneumoniae serum 1 type and porcine mycoplasmal pneumonia double-gene engineering strain. The invention is characterized in that a strain of reconstruction porcine actinobacillus pleuropneumoniae serum 1 type low virulent strain SLW05, the reconstruction strain is preserved in China Center for Type Culture Collection, the preservation number is CCTCC NO: M209068, porcine mycoplasmal pneumonia antigenicity P36 gene segment is inserted into the chromosome of porcine actinobacillus pleuropneumoniae serum 1 type low virulent strain SLW03, and the nucleotide sequence of the P36 gene segment is shown in sequence table SEQ ID NO: 1. the invention also discloses a bivalent gene engineering vaccine prepared by using the reconstruction strain and an application thereof.
Owner:HUAZHONG AGRI UNIV
Method for preparing tiamulin fumarate effervescent granules for livestock and poultry
InactiveCN101897679AImprove bioavailabilityPreparation Technology ScienceAntibacterial agentsPharmaceutical delivery mechanismBiotechnologySodium bicarbonate
The invention relates to a method for preparing tiamulin fumarate effervescent granules for livestock and poultry, which comprises the following steps of: taking raw and auxiliary materials in part by weight for later use; mixing the weighed and crushed tiamulin fumarate, citric acid, polyethylene glycol 6000 in an amount which is one second of the total amount, dextrin and sucrose uniformly in a groove-shaped mixer, adding purified water into the mixture to prepare a soft material, granulating and drying the soft material, and settling the granules; weighing crushed sodium bicarbonate, mixing the reset auxiliary materials in the groove-shaped mixer uniformly, adding purified water into the mixture to prepare a soft material, granulating and drying the soft material, and settling the granules; and mixing the two kinds of dried granules uniformly in a three-dimensional mixer, and packing the mixed granules. The prepared tiamulin fumarate effervescent granules have the advantages of good mouthfeel and stable quality after oral administration of sick livestock and poultry, and are an ideal preparation for treating mycoplasma pneumonia, actinomycetic pleuropneumonia and treponema dysentery at present.
Owner:SRICK TIANJIN BIO TECH
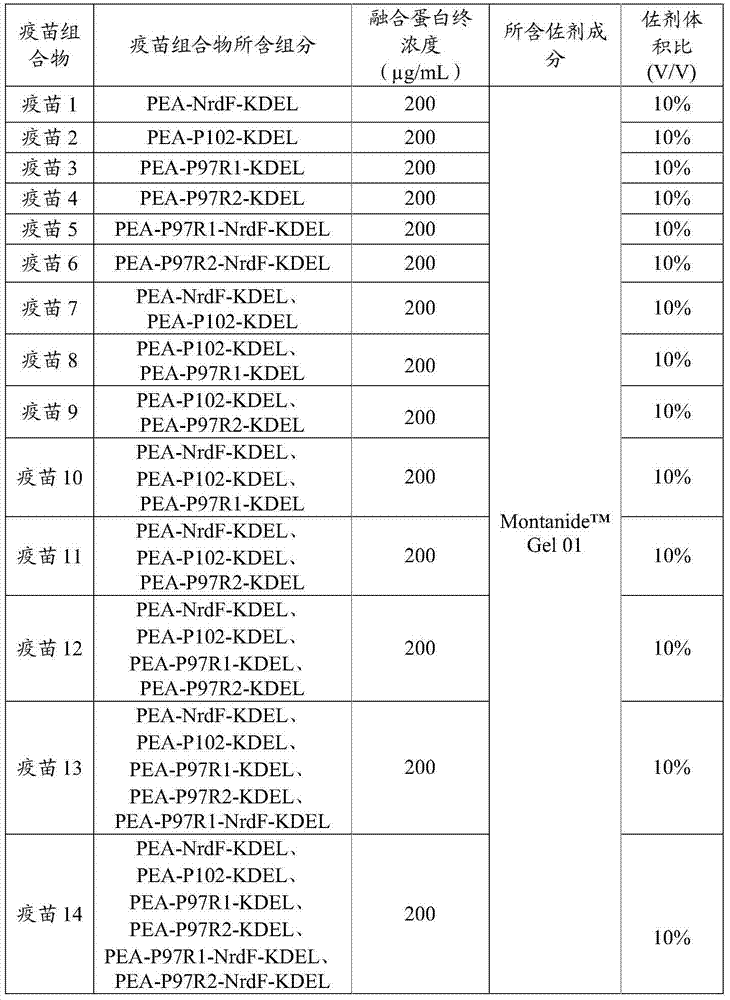
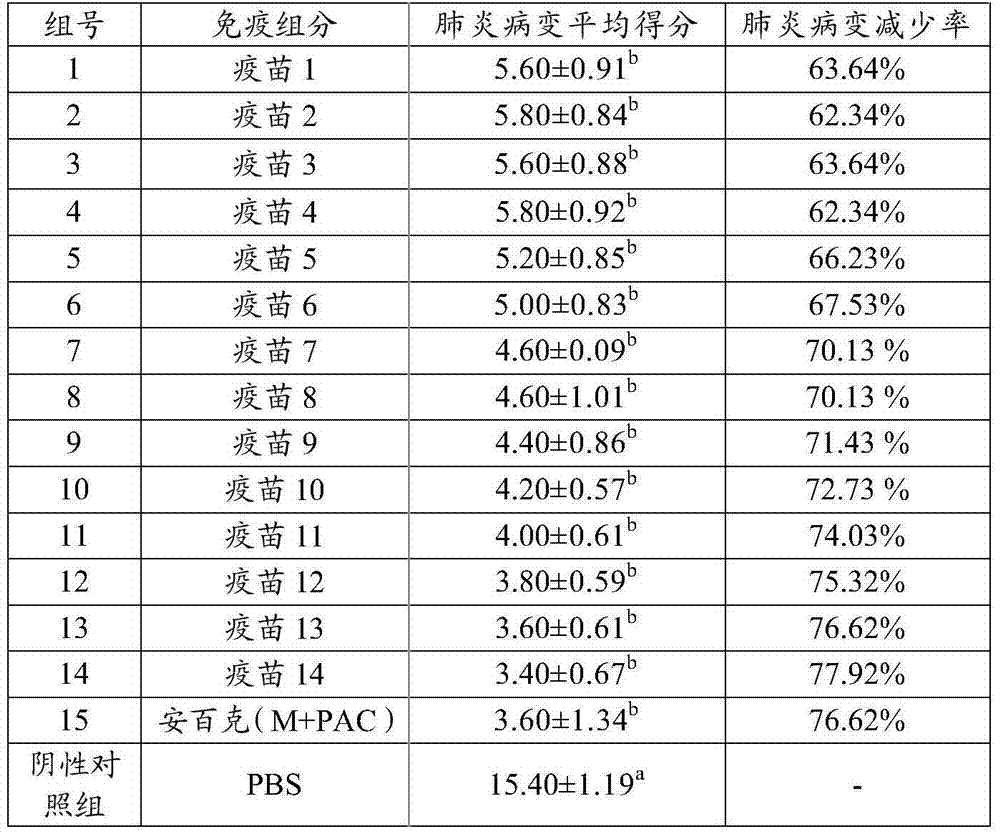
Preparation method and application of fusion protein and vaccine composition containing same
ActiveCN104277115AShort timeEase of mass productionAntibacterial agentsBacterial antigen ingredientsPseudomonas aeruginosa exotoxin AMycoplasmal pneumonia
The invention provides a fusion protein, a fusion protein-containing vaccine composition for mycoplasmal pneumonia of swine (MPS) and an application of the vaccine composition. The fusion protein comprises pseudomonas exotoxin A structural domains I and II, an antigenic protein for MPS and a carboxyl terminal part, wherein the antigenic protein for MPS comprises any one of an NrdF protein, a P102 protein, a P97R1 protein, a P97R2 protein, a P97R1-NrdF protein and a P97R2-NrdF protein. The invention also discloses the vaccine composition containing immune dosage of fusion protein and the application of the vaccine composition to preparation of drugs for preventing and / or treating MPS. The vaccine composition has the advantages that recombinant expression can be carried out on the components of the vaccine composition in quantity by means of genetic engineering, thus not only consuming short time but also facilitating large-scale production; the vaccine composition can generate humoral immunity and cellular immunity and can gain better immune effects.
Owner:PU LIKE BIO ENG
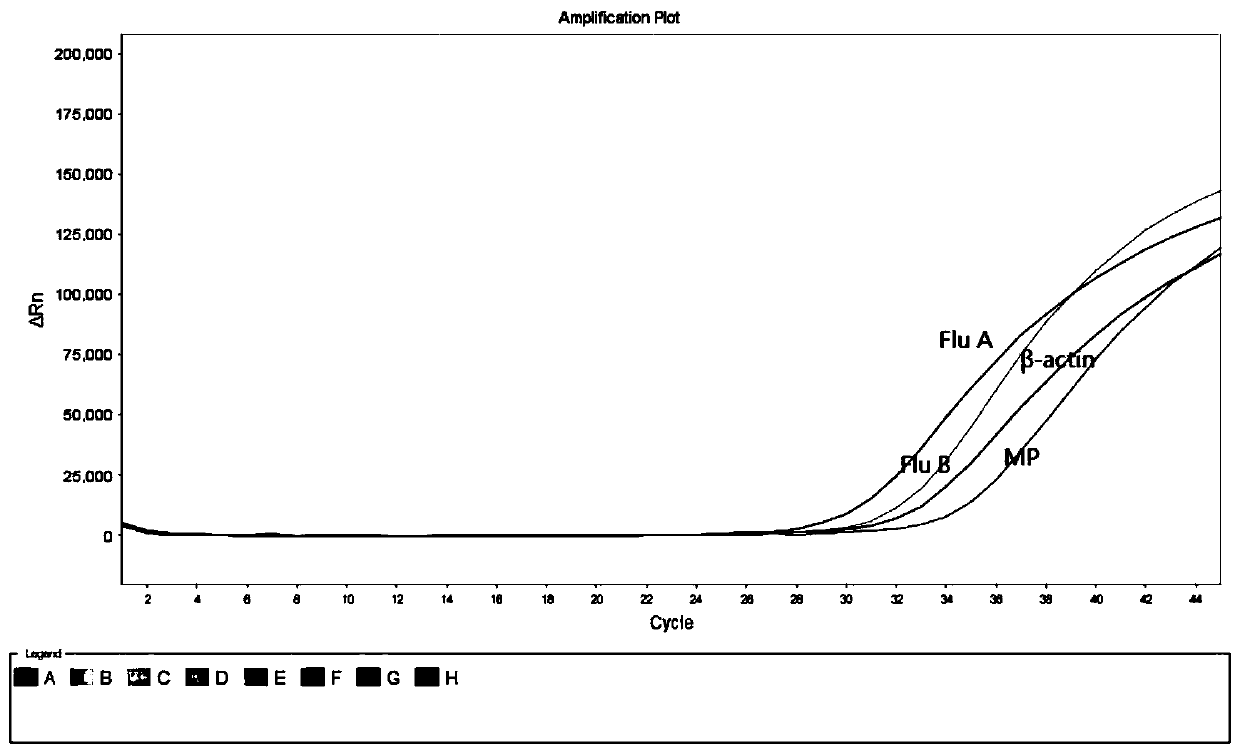
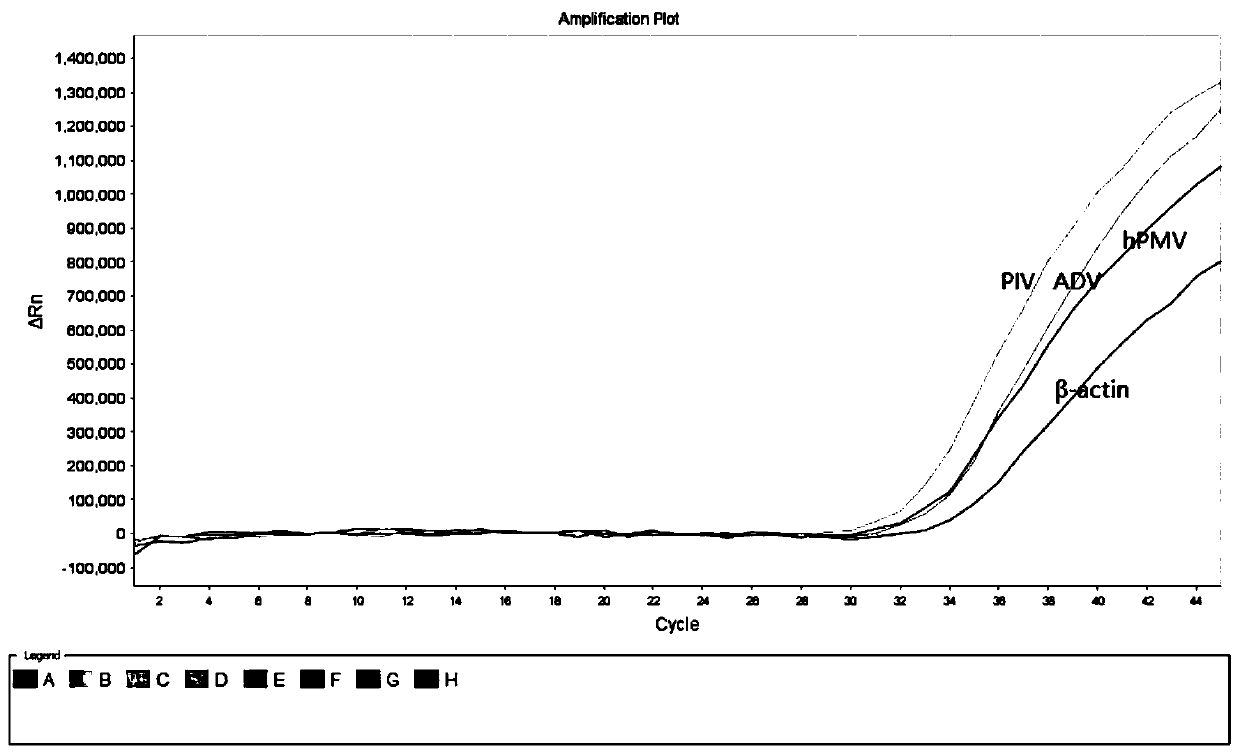
Collaborative multiplex PCR detection method for nucleic acids of nine respiratory tract pathogens
ActiveCN111074001ANo cross contaminationNo distractionMicrobiological testing/measurementMicroorganism based processesMultiplexMycoplasmal pneumonia
The invention discloses a collaborative quadruple real-time fluorescence PCR detection method for simultaneously detecting target nucleic acids of nine pathogens by using three PCR reaction tubes. Themethod is used for detecting influenza A virus (FluA), influenza B virus (FluB), parainfluenza virus (PIV), respiratory syncytial virus (RSV), mycoplasma pneumoniae (MP), chlamydia pneumoniae (Cpn),adenovirus (ADV), human rhinovirus (HRV) and human metapneumovirus (hMPV) in samples. The method is rapid to operate, can be completed by one-step cooperation and has high sensitivity. In addition, the invention also relates to a reagent involved in the PCR method, a detection kit used for the method, preparation and application of the detection kit and the like.
Owner:苏州华益美生物科技有限公司
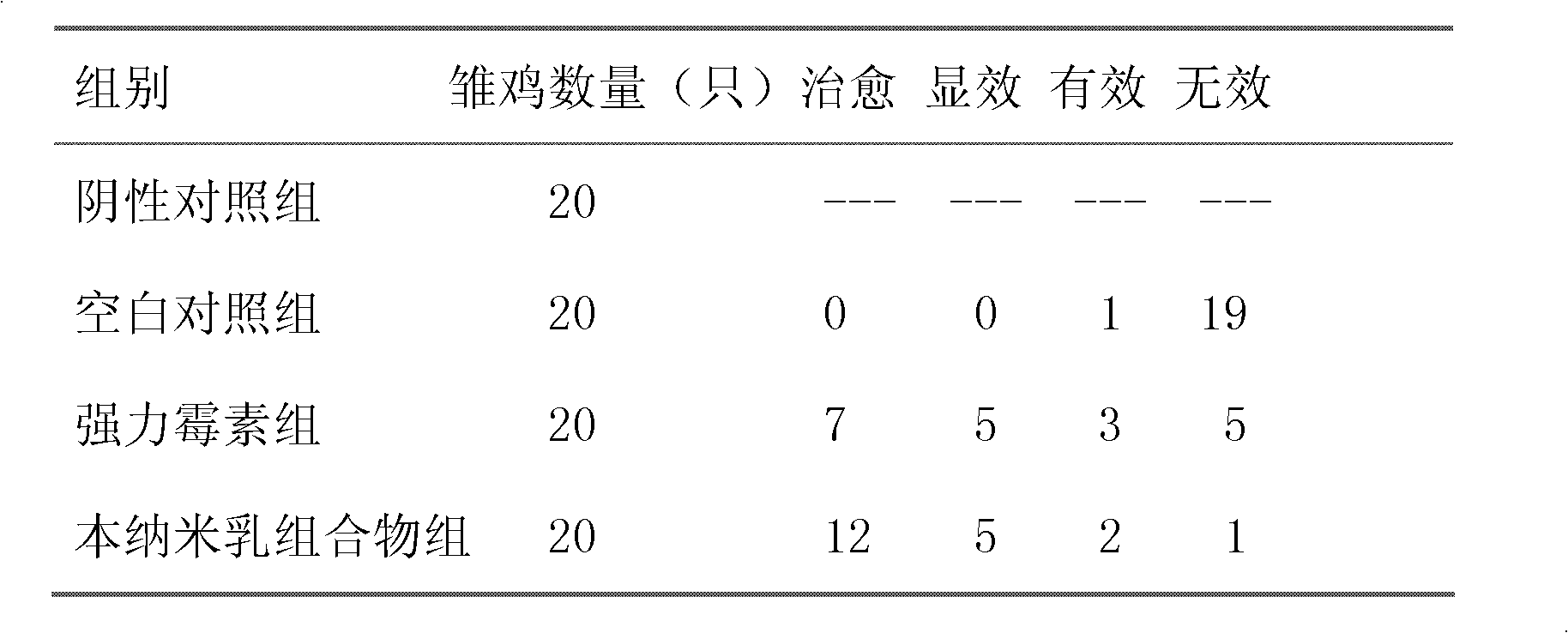
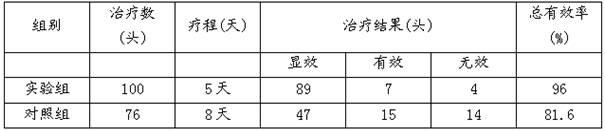
Nanoemulsion composition for treating mycoplasma pneumonia of livestock and preparation method of nanoemulsion composition
InactiveCN102600304AGood water solubilityWill not break milkAntibacterial agentsSulfur/selenium/tellurium active ingredientsBiotechnologyDisease
The invention discloses a nanoemulsion composition for treating mycoplasma pneumonia of livestock, which consists of raw materials including, by mass percent, from 0.1 to 8.0% of amyris oil, from 0.1 to 5.0% of vitex oil, from 0.1 to 3.0% of perilla oil, from 0.1 to 2.0% of bupleurum oil, from 0.01 to 0.1% of allicin, from 0.1 to 4.0% of tiamulin fumarate, from 19.0% to 31.0% of surfactant and the balance distilled water. The sum of the mass ratios of the ingredients is 100%. The nanoemulsion composition has effects of dilating the trachea of the livestock, suppressing cough reflex, increasing immunity of the organism, resisting inflammation and killing mycoplasma pneumoniae or other mixed infected causative agent, can be used for preventing and curing respiratory diseases including chronic respiratory diseases of the livestock, mycoplasma pneumoniae of swine, porcine contagious pleuropneumonia and the like, is fine in stability, is high in intestinal absorption utilization rate after being orally taken, can avoid liver first pass effect, and is remarkable in curative effect.
Owner:NORTHWEST A & F UNIV
Feed for treating mycoplasmal pneumonia of swine and preparation method
ActiveCN102640884AEffective treatmentTreatment without residueAnimal feeding stuffRespiratory disorderSalvia miltiorrhizaSide effect
The invention provides a feed for treating mycoplasmal pneumonia of swine, wherein the feed comprises traditional Chinese medicine as a feed additive and a feed carrier; the traditional Chinese medicine comprises cassia twig, ginger, anemarrhena, rhizoma phragmitis, cortex dictamni, forsythia, blackberry lily, toad, salvia miltiorrhiza, radix cyathulae, pinellia ternata, inula japonica thunb, ginkgo, codonopsis, licorice, loquat leaf and wild buckwheat. Relative to 10 parts by weight of feed carrier, the additive amount of the traditional Chinese medicine can be 4-8 parts by weight. The feed containing the traditional Chinese medicine composition for treating mycoplasmal pneumonia of swine has the advantages of high efficiency, low toxic and side effects, no residue, no generation of drug resistance, no environment pollution, short treatment course and no repetition of illness state, and organism resistance can be increased, and the like.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Lincomycin hydrochloride-spectinomycin sulfate injection and preparation method thereof
InactiveCN102793713AGood treatment effectHigh antibacterial activityAntibacterial agentsOrganic active ingredientsDiseaseSide effect
Owner:上海公谊药业有限公司
Preparation method, formula and use method of bovine mycoplasma pneumonia inactivated vaccine
InactiveCN104857509AStrong targetingReduce Lesion IndexAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention provides a preparation method, a formula and a use method of a bovine mycoplasma pneumonia inactivated vaccine. The preparation method of the bovine mycoplasma pneumonia inactivated vaccine comprises the following steps: 1, selecting diseased cattle which naturally infect mycoplasma pneumonia and is dying, taking diseased lungs, lymph glands and spleens to homogenize and removing precipitates and grease through filtering and centrifugation to obtain an antigen solution; 2, adding formaldehyde into the antigen solution to inactivate for 72 hours, adding 1,000 units of penicillin and 1,000 units of streptomycin into the antigen solution per millimeter and uniformly mixing to obtain an inactivated antigen solution; 3, mixing the inactivated antigen solution with an aluminum hydroxide adjuvant according to the proportion of 4:1 to obtain the inactivated vaccine. The vaccine prepared by the preparation method is safe in application, strong in pertinence and indeed in immune protection effect on mycoplasma causing bovine pleuropneumonia by infection, the lung lesion score of the infected cattle can be obviously reduced, the material weight ratio is improved, and the good effects of prevention and disease control can be achieved.
Owner:福清市默克兽医院
Primer group for multiple RPA detection of respiratory tract pathogens, detection reagent and kit
ActiveCN111154916AMicrobiological testing/measurementMicroorganism based processesStaphylococcus aureusRespiratory syncytial virus (RSV)
The invention provides a primer group for multiple RPA detection of respiratory tract pathogens, a detection reagent and a kit. The high-specificity primer group for multiple RPA detection is successfully obtained through RPA primer design improvement of at least 5 respiratory tract pathogens of human cytomegalovirus, adenovirus, parainfluenza virus 1, parainfluenza virus 2, parainfluenza virus 3,respiratory syncytial virus, staphylococcus aureus, streptococcus pneumoniae, mycoplasma pneumoniae, chlamydia pneumoniae, klebsiella pneumoniae, acinetobacter baumannii, pseudomonas aeruginosa, stenotrophomonas maltophilia and legionella pneumophila, the detection reagent is prepared with the adoption of the primer group, and the kit is prepared, so that RPA detection of up to 15 respiratory tract pathogens can be realized according to speculation, time required for the whole detection procedure can be shortened, and steps are simplified.
Owner:FUJIAN PROVINCIAL HOSPITAL +2
Combined strain for preparing mycoplasma ovipneumoniae vaccine, mycoplasma ovipneumoniae trivalent inactivated vaccine and preparation method of inactivated vaccine
ActiveCN111690554AHigh immune protection rateImprove protectionAntibacterial agentsBacterial antigen ingredientsBiotechnologyStructural protein
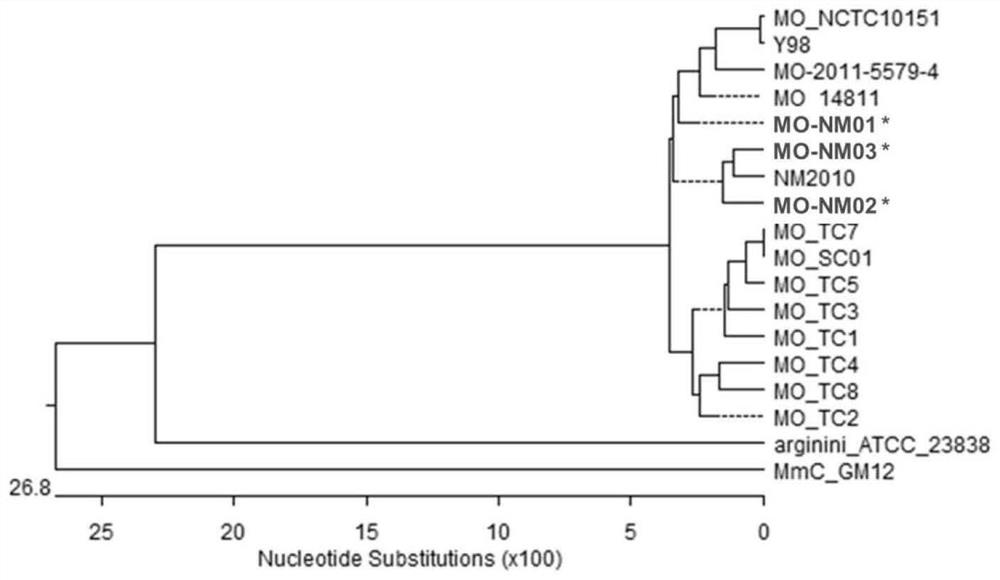
The invention provides a combined strain for preparing a mycoplasma ovipneumoniae vaccine, a mycoplasma ovipneumoniae trivalent inactivated vaccine and a preparation method of the inactivated vaccine,and belongs to the technical field of veterinary biological products. The combined strain comprises mycoplasma ovipneumoniae strains MO_NM01, MO_NM02 and MO_NM03, the preservation number of the mycoplasma ovipneumoniae strain MO_NM01 is CGMCC No.19698, the preservation number of the mycoplasma ovipneumoniae strain MO_NM02 is CGMCC No.19699, and the preservation number of the mycoplasma ovipneumoniae strain MO_NM02 is CGMCC No.19700. Housekeeping genes, structural proteins and polyclonal antibodies of the strains in the combined strain are different, the trivalent inactivated vaccine preparedfrom the combined strain can play a good role in protecting different strains, and has high immune protection rate.
Owner:INNER MONGOLIA AUTONOMOUS REGION ACAD OF AGRI & ANIMAL HUSBANDRY SCI
Medicine composition for treating mycoplasmal pneumonia and preparation method thereof
InactiveCN104984292AQuick effectImprove efficiencyAntibacterial agentsHeavy metal active ingredientsHouttuyniaTherapeutic effect
The invention discloses a medicine composition for treating mycoplasmal pneumonia and a preparation method thereof. The medicine composition comprises the raw materials of lobelia, herbs of threeflower bluebeard, fructus aurantii, great burdock achene, stemona tubers, balloon flowers, meadowrue roots and rhizomes, heartleaf houttuynia herbs, liquorice, Japanese ardisia, Japanese metaplexis, Indian Kalimeris herbs, ginger, thorny elaeagnus fruits, magnetite, Russian boschniakia herbs, bamboo juice, plantain seeds, white mustard seeds, narrow-leaf lepisorus, tabasheer, perilla fruits, agilawood, white mulberry root-bark, raspberries, immature fruits of medicine terminalia, pepperweed seeds, sharpleaf galangal fruits, pennycress, woody hawkbeard roots, wallich eriophyton and lemmaphyllum subrostratum. The medicine composition has the advantages that the effects of clearing away heat through releasing the lungs, regulating the lungs, relieving asthma and reducing phlegm are achieved, meanwhile, the effects of tonifying the kidneys, removing blood stasis, promoting urination and replenishing vital essence to cultivate qi are achieved, the medicine composition takes effect rapidly, is high in effective rate and free of toxic and side effects and has a definite treatment effect, the treatment course is short, and the price is low.
Owner:吴强
Dual fluorescence quantitation method for MO (mycoplasma ovipneumonia) and Mmc (Mycoplasma mycoides subsp.capri)
InactiveCN108823324AEasy to operateStrong specificityMicrobiological testing/measurementMicroorganism based processesSpecific detectionPcr method
The invention provides a dual fluorescence quantitation PCR (polymerase chain reaction) method for MO (mycoplasma ovipneumonia) and Mmc (Mycoplasma mycoides subsp.capri). A pair of specific detectionprimers Mo-F and Mo-R and a fluorescent probe Mo-P for MO as well as a pair specific detection primers Mmc-F and Mmc-R and a fluorescent probe Mmc-P for Mmc are designed respectively, and concentrations of the specific primers and corresponding fluorescent probes for the dual fluorescence quantitation PCR method for MO and Mmc are determined. Two kinds of mycoplasmas of MO and Mmc can be detectedand identified simultaneously with the dual fluorescence quantitation PCR method; the method has the advantages of rapidness, accuracy, high specificity, good repeatability and the like, can save cost, and is suitable for providing effective means for rapid detection and epidemiological investigation of caprine mycoplasmal pneumonia caused by the two kinds of mycoplasmas.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Epidemic diseases prevention feed for live pigs and preparation method thereof
The invention relates to the field of breeding of live pigs, and specifically discloses epidemic disease prevention feed for live pigs and a preparation method thereof. The feed disclosed by the invention can be added to the feed for live pigs, the live pigs can quickly intake the components on the feed, the resistibility of the live pigs to diseases can be effectively enhanced before the live pigs suffer from the diseases, and the risk that the live pigs suffer from colibacillosis, swine plague, mycoplasmal pneumonia of swine, porcine reproductive and respiratory syndrome and other diseases can be greatly reduced The scheme can improve the resistibility of the live pigs to epidemic diseases.
Owner:贵州三人行食品开发有限公司
Traditional Chinese medicine composition used for preventing and treating mycoplasmal pneumonia of swine as well as preparation method and application thereof
ActiveCN103623313AShort use timeImprove efficiencyAntibacterial agentsAnimal feeding stuffSide effectMycoplasmal pneumonia
The invention discloses a traditional Chinese medicine composition used for preventing and treating mycoplasmal pneumonia of swine. The traditional Chinese medicine composition comprises the following components in parts by weight as main active components: 20-30 parts of herba houttuyniae, 15-25 parts of membranous milkvetch roots, 15-25 parts of radix stemonae, 15-20 parts of platycodon grandiflorum, 15-20 parts of garlic, 10-20 parts of rhizoma anemarrhenae, 10-15 parts of ephedra, 10-15 parts of roots of redroot gromwell, and 5-10 parts of radix glycyrrhizae. The invention also discloses a preparation method and application of the traditional Chinese medicine composition. Tests show that the traditional Chinese medicine composition can inhibit in-vitro growth of mycoplasma hyopneumoniae, can well control the mycoplasma hyopneumoniae for infection and pathogenicity of mice, and is clinically used for preventing and treating infection and pathogenicity of the mycoplasma hyopneumoniae, has a good pharmaceutical effect and no toxic or side effects, and is convenient to take.
Owner:HUNAN TAIFENG ANIMAL PHARMA
Compound genticin-micronomicin injection, preparing method and use thereof
InactiveCN101015561AImprove immunityRelative weight gainOrganic active ingredientsPharmaceutical delivery mechanismMicronomicinCurative effect
The invention discloses an injection of compound micironomicin sulfate, its preparation method and application. the injection comprises (by weight part) micironomicin sulfate injection 1-20, chlorpheniramine maleate 0.1-1, and dexamethasone sodium phosphate 0.01-1. the preparation method comprises heating 50% water for injection to 40-50deg.C, dissolving each components, regulating pH to 3.5-6.5 with hydrochloric acid or sodium hydroxide, and adding water for injection to 1000. The invention has the advantages of rational formulation, reduced drugfastness, and shortened withholding period. It can enhance therapeutic effect, improve swinish immunity, increase swinish body weight; and can be used for treating mycoplasmal pneumonia of pig.
Owner:TIANJIN SHENGJI GRP CO LTD
Attenuated live vaccine against mycoplasmal pneumonia of swine (MPS) and use thereof
ActiveUS20160346372A1Prevention and control of diseaseEffectively activate the immune systemAntibacterial agentsBacterial antigen ingredientsAdjuvantLung tissue
Disclosed are an attenuated live vaccine against mycoplasmal pneumonia of swine (MPS) and use thereof. In the present invention, pathological lung tissues of swine having typical Mycoplasma hyopneumoniae (Mhp) infection and no obvious other pathogenic infections are screened, and subcultured 100 generations in lungs of newborn rabbits; then, Mhp strains are isolated and serially subcultured in a medium; and the Mhp strain AN306 is obtained by screening a plurality of strains, which is deposited with an accession number: CCTCC NO. M2014176. Also disclosed is a live vaccine formulation against MPS prepared on the basis of the attenuated strain and comprising live attenuated strain, a pharmaceutically acceptable carrier or excipient, and optionally an adjuvant and immunogens of other pathogens.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Compound florfenicol powder for animals and preparation thereof
InactiveCN101297795AAvoid damageReduce stress responsePowder deliveryPharmaceutical non-active ingredientsMycoplasmal pneumoniaWeight gain
The invention discloses a veterinary compound florfenicol powder and a preparation method thereof, which aims at providing the veterinary compound florfenicol powder that has rapid onset of action for the treatment for porcine infectious pleuropneumonia and mycoplasmal pneumonia and can treat both manifestation and root causes of the diseases, alleviate the stress reactions when having the attacks of diseases of the pigs and improve the weight gain rate, and the preparation method that has simple process and easy realization. The powder is composed of the components with the following weight percentages: 5 to 20 percent florfenicol, 0.5 to 5 percent doxophylline and the rest of starch. The powder of the invention is a compound preparation which combines anti-bacterial drugs and the drugs for relieving cough and asthma, the florfenicol and the doxophylline are combined for usage, thus having rapid onset of action for the treatment for infectious pleuropneumonia and mycoplasmal pneumonia, effectivity and high efficiency; furthermore, the power can ease the stress reactions of the affected animals, significantly ease the clinical symptoms caused by the infectious pleuropneumonia and the mycoplasmal pneumonia, treat both manifestation and root causes of diseases, reduce the suffering of the affected pigs and increase the weight of the pigs.
Owner:天津市海纳德动物药业有限公司
Tiamulin fumarate effervescent granules for treating pneumonia and dysentery of livestock and poultry
InactiveCN101889988AImprove bioavailabilityPreparation Technology ScienceAntibacterial agentsPharmaceutical delivery mechanismSodium bicarbonateSucrose
The invention relates to tiamulin fumarate effervescent granules for treating pneumonia and dysentery of livestock and poultry, which comprise the following raw materials and auxiliary materials in part by weight: 80 to 120 parts of tiamulin fumarate, 100 to 140 parts of citric acid, 150 to 170 parts of sodium bicarbonate, 20 to 40 parts of polyethylene glycol 6000, 190 to 230 parts of dextrin and 360 to 400 parts of sucrose. A method for preparing the tiamulin fumarate effervescent granules comprises the following steps of: grinding the raw materials and the auxiliary materials, respectively mixing the ground raw materials and the ground auxiliary materials, drying the mixture, pelletizing the dried mixture and sufficiently mixing the products. The tiamulin fumarate effervescent granules prepared by the method are used for diseased livestock and poultry in an oral administration mode, have the advantages of good taste and stable quality and are an ideal preparation for treating mycoplasma pneumonia, actinomycotic pleuropneumonia and treponema dysentery of the livestock and poultry currently.
Owner:SRICK TIANJIN BIO TECH
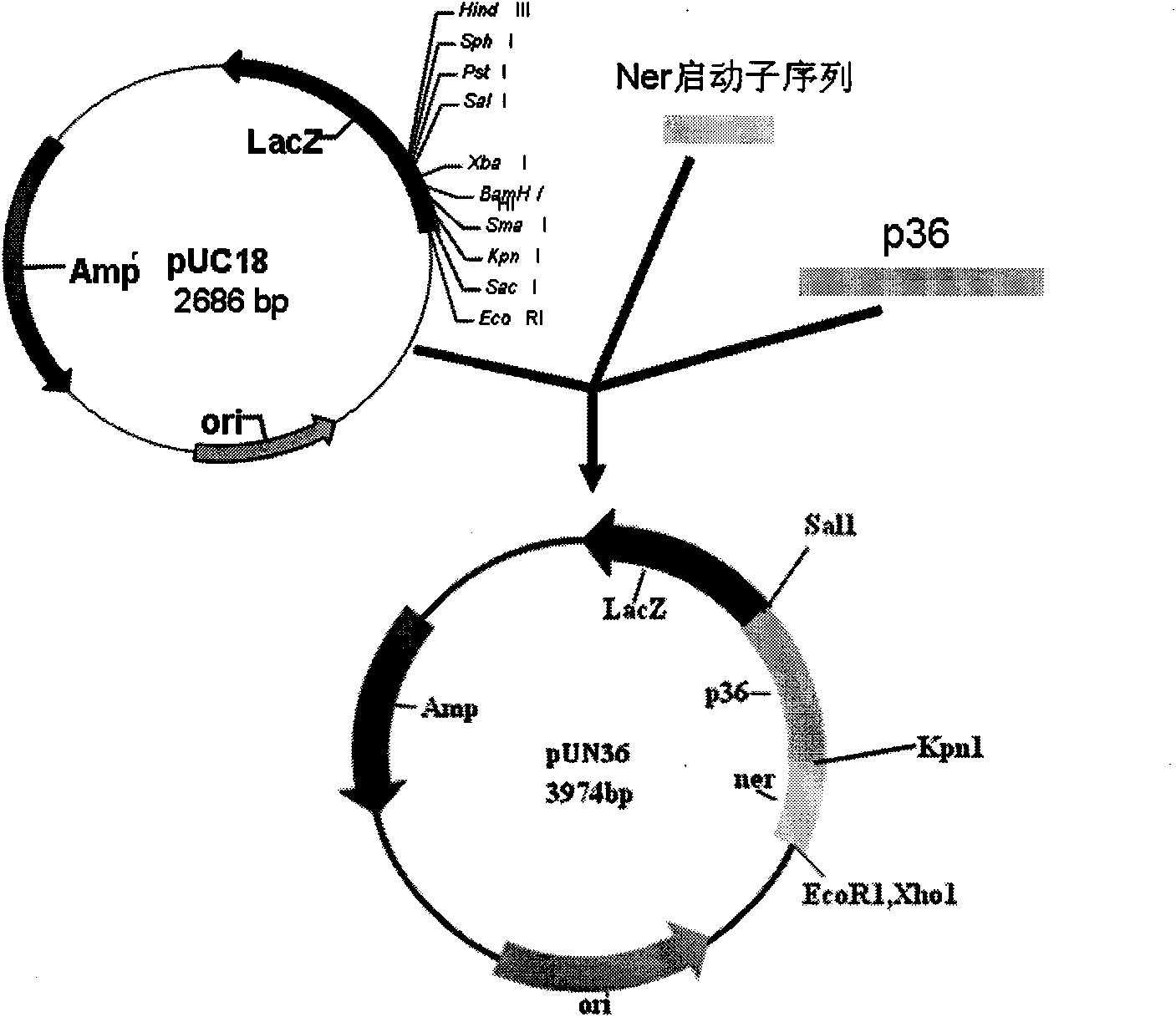
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151BStrengthen cellsImprove the effectiveness of anti-virus protectionAntibacterial agentsBacterial antigen ingredientsCholesterolLevamisole
The special diluent for swine mycoplasma pneumonia vaccine of the present invention mainly contains recombinant P97R1 protein immunostimulatory complex (ISCOM-P97R1), and may also contain other aqueous adjuvant components, including levamisole, or / and astragalus polysaccharide, or / and carboxy bohm. Each ml solution of the diluent contains 0.1-1 mg of QuilA, 0.02-0.2 mg of cholesterol, 0.02-0.2 mg of phosphatidylcholine, 0.4-4 mg of P97R1 antigen, and may further contain 2-20 mg of levamisole , or / and astragalus polysaccharide 20-100 mg, or / and carbomer 3-10 mg, and the rest is phosphate buffer. This diluent is specially used for dissolving or diluting Mycoplasma pneumoniae vaccine, including live vaccine and inactivated vaccine. It has the dual effects of supplementing the protective spectrum of vaccine antigens and enhancing the immune stimulating ability of vaccine cells and humoral, which can significantly improve the protective efficacy of vaccines against viruses. At the same time, due to the use of the water solvent system, it also has the advantages of simple operation, good needle penetration, and no obvious toxic and side effects.
Owner:JIANGSU ACAD OF AGRI SCI
Construction method of sheep mycoplasmal pneumonia bivalent nucleic acid vaccine containing adjuvant gene
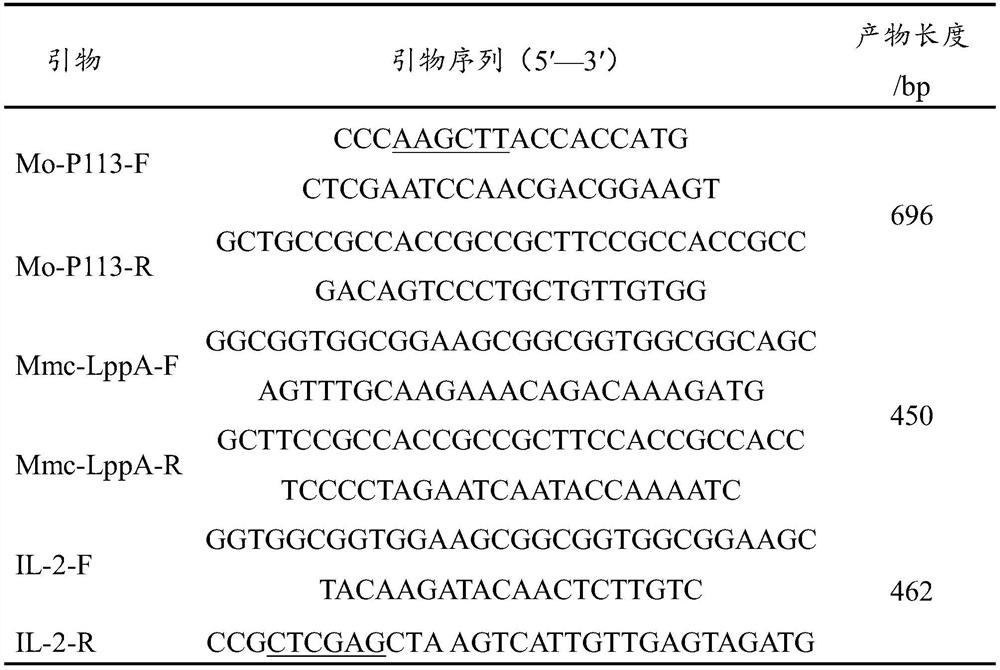
PendingCN113444743AEffective against weak immunity and other problemsSolve many problems faced by R&DAntibacterial agentsAntibody mimetics/scaffoldsMycoplasmal pneumoniaTGE VACCINE
The invention discloses a construction method of a sheep mycoplasmal pneumonia bivalent nucleic acid vaccine containing an adjuvant gene. The construction method comprises the following specific steps of: step 1, extraction of a Mo / Mmc genome: S1, recovery and culture of a Mo / Mmc Guizhou strain, and S2, extraction of the DNA of the Mo / Mmc Guizhou strain; step 2, amplifying a P113-LppA-IL-2 fusion gene: S1, amplification of a mucosal immunologic adjuvant gene and mycoplasma ovipneumoniae and Mycoplasma mycoides subsp. Capri antigen gene, S2, the amplification of the P113-LppA fusion gene, and S3, the amplification of the P113-LppA-IL-2 fusion gene; and step 3, preparing the sheep mycoplasmal pneumonia bivalent nucleic acid vaccine containing the mucosal immunologic adjuvant gene. According to the construction method disclosed by the invention, the nucleic acid vaccine is developed by selecting two different antigen genes and mucosal immunologic adjuvant genes, so that the problems of a weak immune effect and the like of the sheep mycoplasmal pneumonia DNA vaccine can be effectively solved, and many problems confronted by current vaccine research and development are solved.
Owner:GUIZHOU UNIV
Selective separation medium for caprine mycoplasmal pneumonia subspecies and preparation method of selective separation medium
PendingCN106399203AImprove separation rateFast growthBacteriaMicroorganism based processesHydrolysateMycoplasmal pneumonia
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Medicinal composition for treating mycoplasmal pneumonia and application thereof
ActiveCN103655965AShorten cooling timeShorten cough disappear timeAntibacterial agentsRespiratory disorderBelamcanda chinensisHouttuynia
The invention relates to a medicinal composition for treating mycoplasmal pneumonia and application thereof. The medicinal composition is prepared from the following Chinese herbs in part by weight: 26-40 parts of cordate houttuynia, 26-40 parts of blackberry lily, 18-30 parts of Chinese arborvitae twig, 15-25 parts of scutellaria baicalensis, 18-30 parts of duckweed, 18-30 parts of dried orange peel, 8-16 parts of ephedra and 8-16 parts of moutan bark. The medicinal composition is matched with erythrocin for treating the pediatric mycoplasmal pneumonia, and can effectively shorten the defervescence time, the cough disappearing time and the lung rale disappearing time of a patient and reduce pains of an ill child.
Owner:NANTONG FINC PHARMA CHEM
Chinese herbal medicine feed for pigs and preparation method of feed
InactiveCN107173542AGood curative effectHigh in ironFood processingAnimal feeding stuffAnimal scienceRapeseed
The invention discloses Chinese herbal medicine feed for pigs and a preparation method of the feed. The feed is prepared from raw materials as follows: corn starch, wheat bran, soybean meal, rapeseed meal, radix astragali, fructus lycii, radix angelicae sinensis, poria cocos, fructus psoraleae, rhizoma atractylodis, silymarin, flastem milkvetch seeds, rhizoma cimicifugae, pomegranate rind, rape pollen, piper laetispicum, xylo-oligosaccharide, arabinose and galactose. On the basis of the corn starch, the wheat bran, the soybean meal and the rapeseed meal, a Chinese herbal medicine formula and saccharides are selected, a specific Chinese herbal medicine extraction method is adopted, and the prepared feed can treat PRRS (porcine reproductive and respiratory syndrome), mycoplasmal pneumonia of pigs and other diseases and can increase the content of protein and other nutrients in pork and the content of iron in pig blood.
Owner:江西派尼生物科技有限公司 +1
Reagent kit for culturing and differentiating pathogenic mycoplasma and preparing method thereof
InactiveCN101109746AMicrobiological testing/measurementBiological testingMycoplasma hominisMycoplasmal pneumonia
The invention pertains to the field of medical inspection, and relates to a reagent box and preparing method for the reagent box for culturing and distinguishing pathogenic mycoplasma, in particular to a method for culturing and distinguishing Ureaplasma urealyticum, mycoplasma hominis, and mycoplasmal pneumonia. The invention prepares basic culture medium based on the common features of clinically common pathogenic mycoplasma, which is applicable for direct culturing of mycoplasma hominis; prepares distinguishing reagent for mycoplasma hominis and mycoplasmal pneumonia based on the different culturing features of mycoplasma hominis and mycoplasmal pneumonia. After adding the distinguishing reagent for mycoplasma hominis or mycoplasmal pneumonia in the basic culture reagent, the culture medium is only applicable for the growth of mycoplasma hominis or mycoplasmal pneumonia, and culture and distinguish various pathogenic mycoplasma for human being. The invention further prepares the reagent box, which obviously makes convenience for the clinic detection.
Owner:SHANGHAI EASTDIAGNO MEDICAL TECH
Acetylisovaleryltylosin tartrate formulation preparation method and prepared acetylisovaleryltylosin tartrate formulation thereof, and pharmaceutical composition
ActiveCN105030801AReduced bioavailabilityReduce releaseAntibacterial agentsTetracycline active ingredientsGum arabicPolylactic acid
The present invention provides an acetylisovaleryltylosin tartrate formulation preparation method, which comprises: (1) adding a wall material to water, and dissolving to prepare a binder solution, wherein the wall material comprises polylactic acid and arabic gum according to a ratio of 1:1; (2) adding an acetylisovaleryltylosin tartrate core material to the binder solution, and uniformly mixing to prepare a mixture; and (3) carrying out spray drying of the mixture prepared in the step (2) to prepare the acetylisovaleryltylosin tartrate formulation. According to the present invention, the acetylisovaleryltylosin tartrate formulation prepared through the method is stable and lastly act, and the cure rate is extremely high when the composition comprising the acetylisovaleryltylosin tartrate formulation and doxycycline is used to treat swine mycoplasma pneumonia.
Owner:LUOYANG HUIDE BIO ENG CO LTD
Vaccine strain for mycoplasmal pneumonia of swine
InactiveCN104513804AShort training timeHigh bacterial contentAntibacterial agentsBacteriaMicroorganismVeterinary Drugs
The invention discloses a vaccine strain for mycoplasmal pneumonia of swine. The vaccine strain for mycoplasmal pneumonia of the swine, the strain number of which is CJ, is named as mycoplasma hyopneumoniae by classification, and preserved in the China General Microbiological Culture Collection Center of China Committee for Culture Collection of Microorganisms (CCCCM) with the preservation number of CGMCC No.9909 on November 6th of 2014. The mycoplasma hyopneumoniae CJ strain grows rapidly and is high in content of bacteria during the preparation of an improved friis liquid medium; moreover, the mycoplasma hyopneumoniae has good immunogenicity and can be prepared into a prophylactic veterinary biological product or applied to veterinary drugs.
Owner:TECON BIOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com