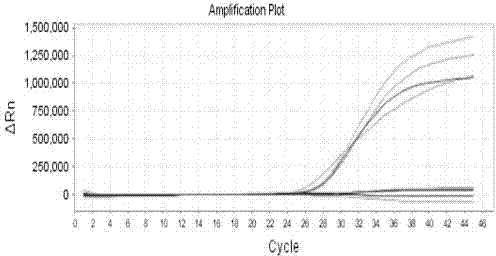
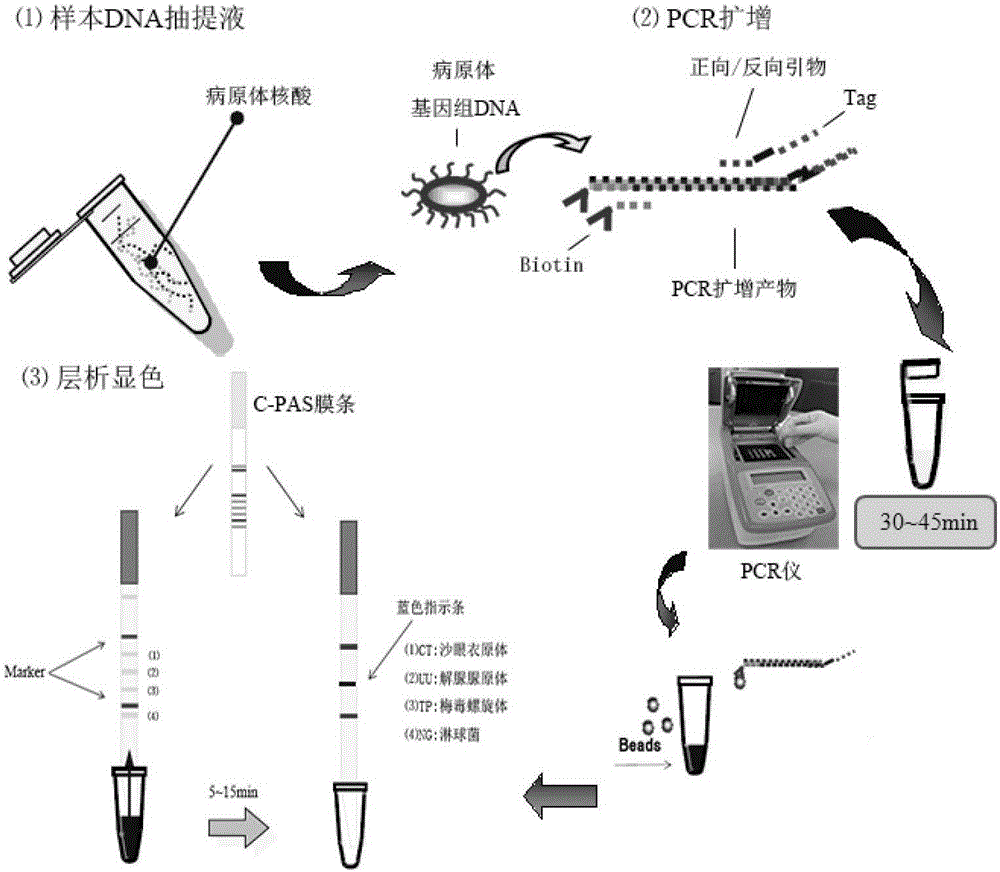
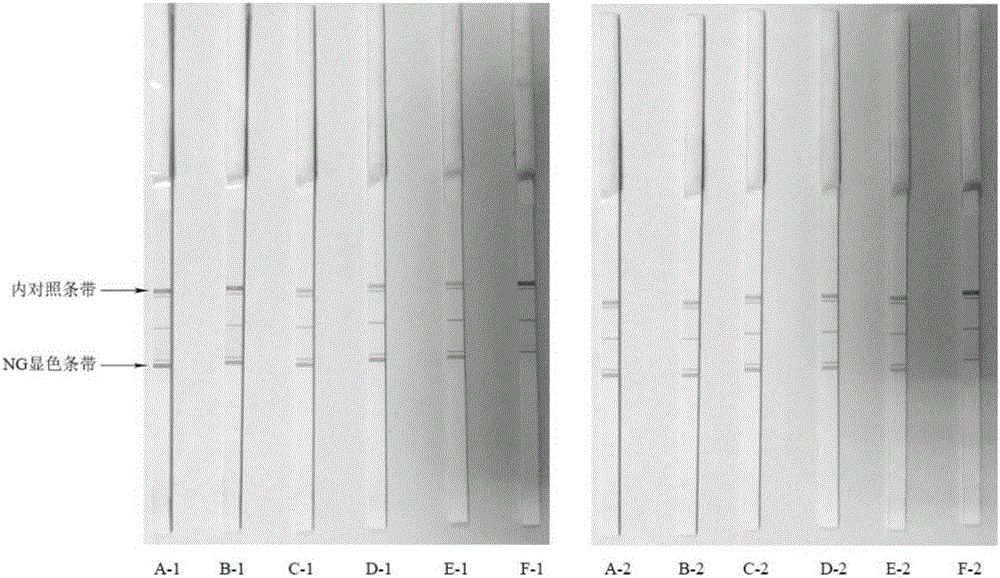
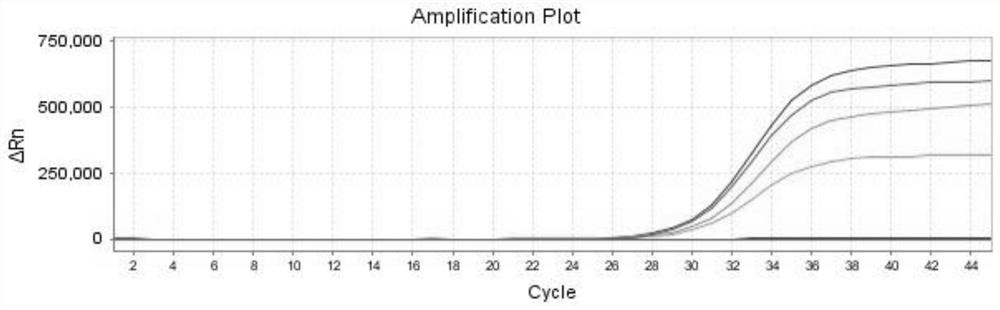
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Ureaplasma urealyticum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ureaplasma urealyticum is a bacterium belonging to the genus Ureaplasma and the family Mycoplasmataceae in the order Mycoplasmatales. This family consists of the genera Mycoplasma and Ureaplasma. Its type strain is T960.
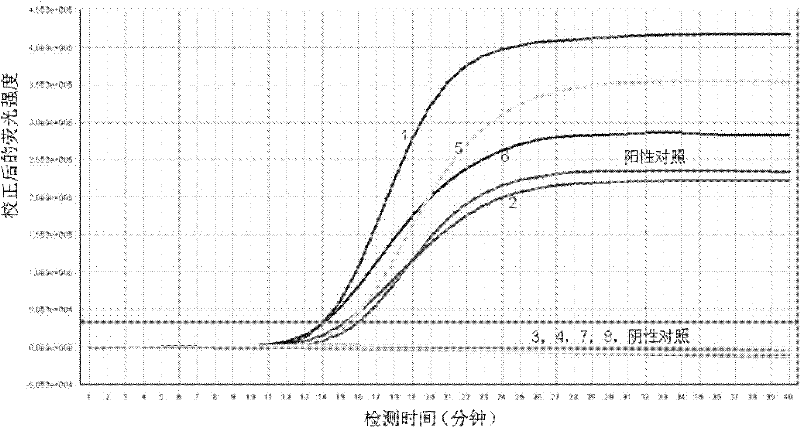
Detection kit for ureaplasma urealyticum (UU) nucleic acid by utilizing RNA constant-temperature amplification
ActiveCN102191321AHigh sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescencePositive controlAssay sensitivity
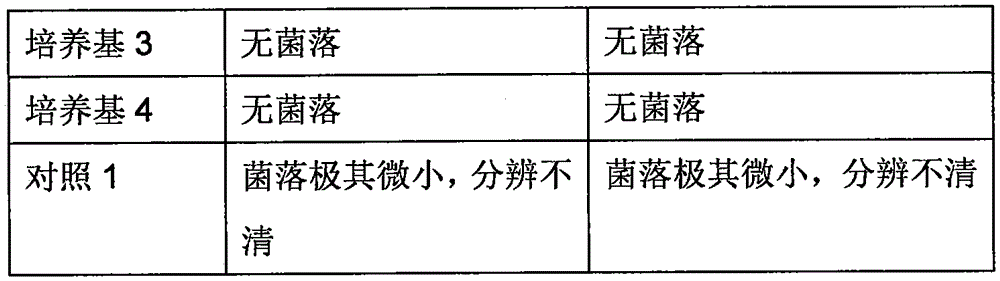
The invention relates to a detection kit for ureaplasma urealyticum (UU) nucleic acid by utilizing RNA constant-temperature amplification. The detection kit comprises a urine sample preserving solution, a nucleic acid extraction solution, a UU reaction solution, a UU detection solution, an SAT (Spermidine / Spermine N1-Acetyltransferase) enzyme solution, a UU positive control and a UU negative control. The detection kit provided by the invention has the analytical sensitivity of 50 CFU / reaction for detecting UU, and has the characteristics of high specificity and high sensitivity; and an amplification product RNA is easy to degrade in a natural environment and causes low pollution.
Owner:SHANGHAI RENDU BIOTECH
Reagent kit for rapidly and synchronously detecting Chlamydi trachomatis, Urealpasma parvum and Ureaplasma urealyticum
ActiveCN101497926AAccurate determination of starting copy numberRapid diagnosisMicrobiological testing/measurementDiseaseBiology
The invention discloses a reagent kit for quickly and synchronously detecting chlamydi trachomatis, fine urealyticum and ureaplasma urealyticum, more particular relates to a reagent kit for detecting the genes of a sexually transmitted disease pathogen. The reagent kit mainly comprises DNA nucleic acid extracting solution, a positive plasmid containing the detection sequence of three pathogens and a TaqMan PCR amplification system jointly detected by a single tube and single enzyme by adopting the one-step method, wherein the three pathogens comprise the chlamydi trachomatis, the fine urealyticum and the ureaplasma urealyticum. The invention has the advantages of good specificity, high sensitivity, accurate quantification, quick detection speed, simple use step and high repeatability, can simultaneously detect samples with high flux, and not only can carry out synchronous quantitative detection to the chlamydi trachomatis, the fine urealyticum and the ureaplasma urealyticum, but also can replace the traditional culture method and ELISA (enzyme linked immunosorbent assay) diagnostic method used always.
Owner:WUHAN ZHENFU PHARMA CO LTD
Solid culture medium for quick detection of mycoplasma, and preparation method thereof
ActiveCN102409076AImprove accuracyQuick checkMicrobiological testing/measurementMicroorganism based processesColony countingMycoplasma hominis
The invention discloses a solid culture medium for quick detection of mycoplasma, and a preparation method thereof. By using the solid culture medium disclosed by the invention, the existence of ureaplasma urealyticum and mycoplasma hominis can be accurately detected, and the content of mycoplasmas can be quantitatively concluded by colony counting, thus greatly improving the accuracy and reliability. Simultaneously, by using the culture medium disclosed by the invention for detecting mycoplasmas, the result can be read after only 18 hours, thereby realizing quick detection of mycoplasmas, and finally confirming diagnosis of urogenital tract infection caused by ureaplasma urealyticum and mycoplasma hominis.
Owner:JIANGMEN KAILIN TRADE
Immunocontraceptive synthetic peptide and immunocontraceptive chimeric peptide and application thereof
InactiveCN101906163APresence of cross-reactive antigensAntifertilityAntibody medical ingredientsHybrid peptidesAntigenSperm protein
The invention relates to immunocontraceptive synthetic peptide and immunocontraceptive chimeric peptide, wherein the amino acid sequence of the immunocontraceptive chimeric peptide is shown as SEQ ID:1 in a sequence table, the amino acid sequence of the immunocontraceptive synthetic peptide hESP103-107 is shown as SEQ ID:2 in the sequence table, and the amino acid sequence of the immunocontraceptive synthetic peptide hIzumo216-220 is shown as SEQ ID:3 in the sequence table. The invention also provides the application of the chimeric peptide, the synthetic peptide hESP103-107 or the synthetic peptide hIzumo216-220 as immunocontraceptive vaccines observed in contraception. The invention researches the chimeric peptide comprising sperm protein hESP, hIzumo and tNASP as well as the cross reacting antigens of UU (Ureaplasma Urealyticum) for the first time, and immunized mice can obtains stronger and reversible antifertility effect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Kit for detecting sexually transmitted diseases
InactiveCN102080123AHigh sensitivitySimple stepsMicrobiological testing/measurementDNA/RNA fragmentationPathogenExact results
The invention discloses a kit for detecting sexually transmitted diseases. The kit comprises 1 to 3 pairs of primers which can specifically amplify genes of pathogens such as gonococcus, chlamydia trachomatis and / or ureaplasma urealyticum respectively; and the nucleotide sequences of the primers are shown as SEQ ID NO:1-6. The kit has high specificity and high sensitivity, can be used for detecting a single pathogen and detecting various pathogens at the same time, is convenient to use, provides exact results and has an extremely good clinical application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Method for detecting three kinds of urogenital canal mycoplasma by loop-mediated isothermal amplification technology
InactiveCN107686863AStrong specificityEfficient amplificationMicrobiological testing/measurementMicroorganism based processesConserved sequenceFluorescence
The invention discloses an LAMP detection method for three kinds of urogenital canal infection mycoplasma which are common for human and a dedicated primer and a kit thereof. The method is applied toperforming isothermal amplification on ureaplasma urealyticum (UU), mycoplasma genitalium (MG) and mycoplasma hominis (MH). The LAMP primer is designed according to specific conserved sequences of theUU, the MG and the MH, and each group of the primer includes four piece of oligonucleotide (shown in a table 1, a table 2 and a table 3 in the description). When being applied to the urogenital canalmycoplasma, the LAMP primer present white precipitate when being observed by the naked eyes; in positive reaction, after SYBR GREEN is added, fluorescent green is remarkably enhanced when being observed under an ultraviolet lamp. Detection results of a real-time turbidity meter show that product turbidity can be increased along with prolonging reaction time; after being detected by gel electrophoresis, the LAMP primer is in a trapezoid stripe. The LAMP detection method disclosed by the invention provides a novel technological platform for mycoplasma detection; a sample to be measured can be applied to DNA extracted by commercialized kits or purified DNA and is also suitable for coarse extracted DNA with a boiling method as a representative; the LAMP detection method is suitable for beingpopularized and applied in grassroots units, field monitoring and bedside detection.
Owner:TIANJIN MEDICAL UNIV
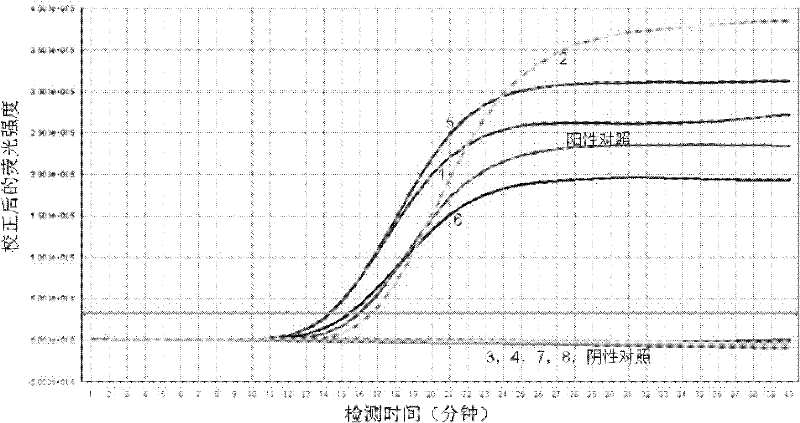
Ureaplasma urealyticum PCR (Polymerase Chain Reaction) detection kit and detection method thereof
ActiveCN102409098AEasy extractionEasy to operateMicrobiological testing/measurementFluorescence/phosphorescencePositive controlReference product
The invention relates to the field of microorganism detection, in particular to an ureaplasma urealyticum PCR (Polymerase Chain Reaction) detection kit and a detection method thereof, aims to provide the ureaplasma urealyticum PCR detection kit and the detection method thereof for detecting a specific nucleic acid sequence of ureaplasma urealyticum in a clinical sample so as to fulfill the aim ofquickly judging the existence of the ureaplasma urealyticum. In order to fulfill the aim, the technical scheme is to provide the ureaplasma urealyticum PCR detection kit, which includes DNA (Deoxyribonucleic Acid) extract, PCR reaction solution, dNTPs, tap enzyme, UNG enzyme, positive control, negative control and a quantitative reference product, wherein the PCR reaction solution includes a primer A and a fluorescent probe A; and the primer is divided into an upstream primer A and a downstream primer A. The kit has the advantages of high flexibility and specificity, stability, timeliness, convenience in operation and the like.
Owner:泰普生物科学(中国)有限公司
Preparation method for multiple mycoplasma monoclonal antibodies and multi-linked immunoassay reagent
InactiveCN102199207AAvoid painSimple and fast operationImmunoglobulins against bacteriaMaterial analysisMycoplasma hominisImmuno detection
The invention relates to a preparation method for multiple mycoplasma monoclonal antibodies and a multi-linked immunoassay reagent. The preparation method is characterized by separately immunizing mice with mycoplasma pneumoniae, mycoplasma hominis and ureaplasma urealyticum for cell fusion and cloning to obtain three monoclonal antibodies with high specificity and high potency. A mycoplasma multi-linked immunoassay reagent is prepared by mixing the three monoclonal antibodies according to a certain ratio, is used for detecting the diseases infected by the three mycoplasmas, and can quickly and accurately detect mycoplasma diseases; and sampling without phlebotomizing does not bring pain to patients, thus providing patients with timely and effective treatment. The multi-linked immunoassay reagent does not generate harmful waste gas or waste water during preparation and use processes, and also does not bring any injury to patients and operators, thereby being an environment-friendly, safe, accurate and sensitive biological preparation.
Owner:黄威权
Method for detecting ureaplasma urealyticum and tiny urealyticum
InactiveCN102952888AAccurate identificationIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesBiotin-streptavidin complexLiquid medium
The invention discloses a method for detecting ureaplasma urealyticum and tiny urealyticum, which belongs to the technical field of detection of microporous plates. The method for detecting ureaplasma urealyticum and tiny urealyticum has the characteristics that clinical samples processed by thermal denaturation after PCR (polymerase chain reaction) amplification are respectively added to micro holes of a DNA-BINDING96 hole determination plate equipped coated with Uu and Up specific probes, and positive control holes are arranged, to which Uu and Up serotype standard strains processed by thermal denaturation after PCR amplification are added; after thermostat hybridization, the plate is cleaned and is patted to be dry; streptavidin-horseradish peroxidase conjugates are added to each hole, the plate is cleaned, and the streptavidin-horseradish peroxidase conjugates are dried in air; then, chromogenic substrates A and B are added to each hole, and color development is performed in a shady place after the mixture becomes homogeneous; finally, a stop solution is added, and the result is determined according to color change. The method for detecting ureaplasma urealyticum and tiny urealyticum not only solves the problem of false positive result caused by determining a positive result according to color change of a liquid medium, but also can accurately identify Uu and Up, thereby improving the detection sensitivity. Moreover, high-flux detection is realized, and the method for detecting ureaplasma urealyticum and tiny urealyticum can be widely used for clinical screening.
Owner:TIANJIN MEDICAL UNIV
Gene chips for detecting of pathogens of sexually transmitted diseases and reagent kit for detecting
ActiveCN101407836ATimely diagnosisImprove throughputMicrobiological testing/measurementDiseaseMycoplasma hominis
The invention provides a gene chip used for detecting the common pathogens of sexually transmitted diseases and a kit used for detection, wherein, the gene chip includes a solid phase vector and an oligonucleotide probe fixed on the solid phase vector; the oligonucleotide probe mainly comprises a DNA segment or a complementary DNA segment thereof which is selected from Diplococcus gonorrhoeae, Ureaplasma urealyticum and the 16S rRNA gene of M.hominis, the outer membrane protein gene (ompA gene) of a Chlamydia trachomatis, the glycosidoprotein B gene (gB gene) of a herpes simplex virus (HSV) and the L1 gene of a papilloma virus (HPV). The gene chip and the kit can be utilized for achieving the goal of detecting the common pathogens of sexually transmitted diseases; the gene chip and the kit for detection are simple and convenient to be operated, have high flux, accuracy and repetitiveness; and the gene chip and the kit for detection can be applied to the clinic detection and epidemiology analyzing of medical and health organizations.
Owner:TIANJIN BIOCHIP TECH CO LTD
Protein chip for typing detection on helicobacter pylori infection
InactiveCN105277722AReduce dosageBeneficial to clinical treatmentBiological material analysisBiological testingSpecific iggProtein C
The invention provides a protein chip for typing detection on helicobacter pylori infection. The protein chip comprises a basement membrane and antigens which are respectively dotted on the basement membrane in the form of dot matrix, wherein the antigens include three types, i.e. CagA (cytotoxin-associated gene) antigens, VacA (Vacuolating cytotoxin A) antigens and Ure (ureaplasma urealyticum) antigens. Due to the fact that the detection antigens used by the protein chip are three single proteins, i.e. Ure, CagA and VacA, specific IgG antibodies for the three antigens in the blood serum of a patient can be detected at the same time, I-type infection or II-type infection can be judged, and clinic treatment is better facilitated; furthermore, the protein chip has the advantages of high detection sensitivity, quick, simple and convenient detection, instant readability, small using amount of antigen and the like.
Owner:TAIZHOU SYNO GENE DIGITAL TECH CO LTD
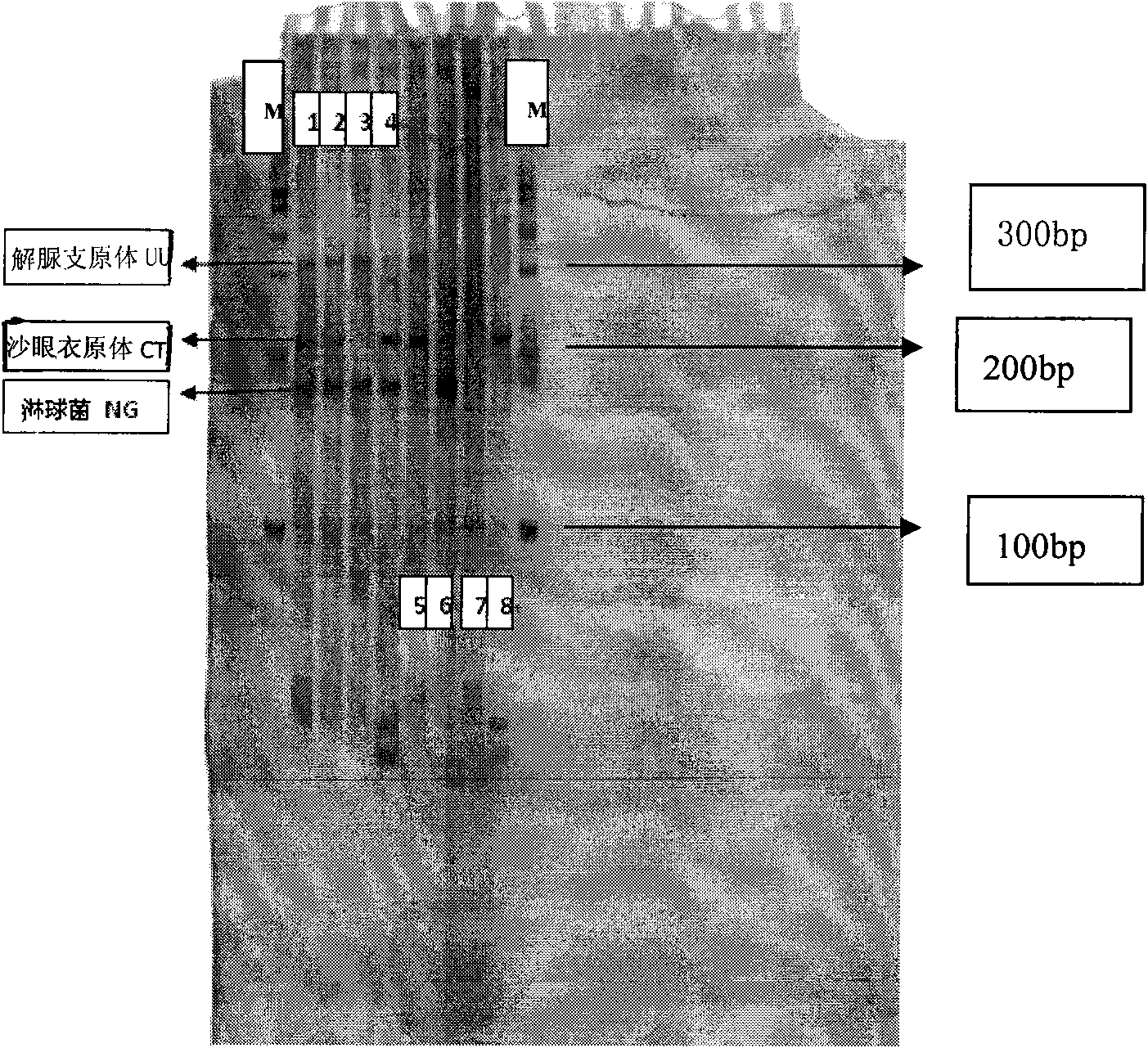
Neisseria gonorrhoeae, Chlamydia trachomatis and Ureaplasma urealyticum detection kits
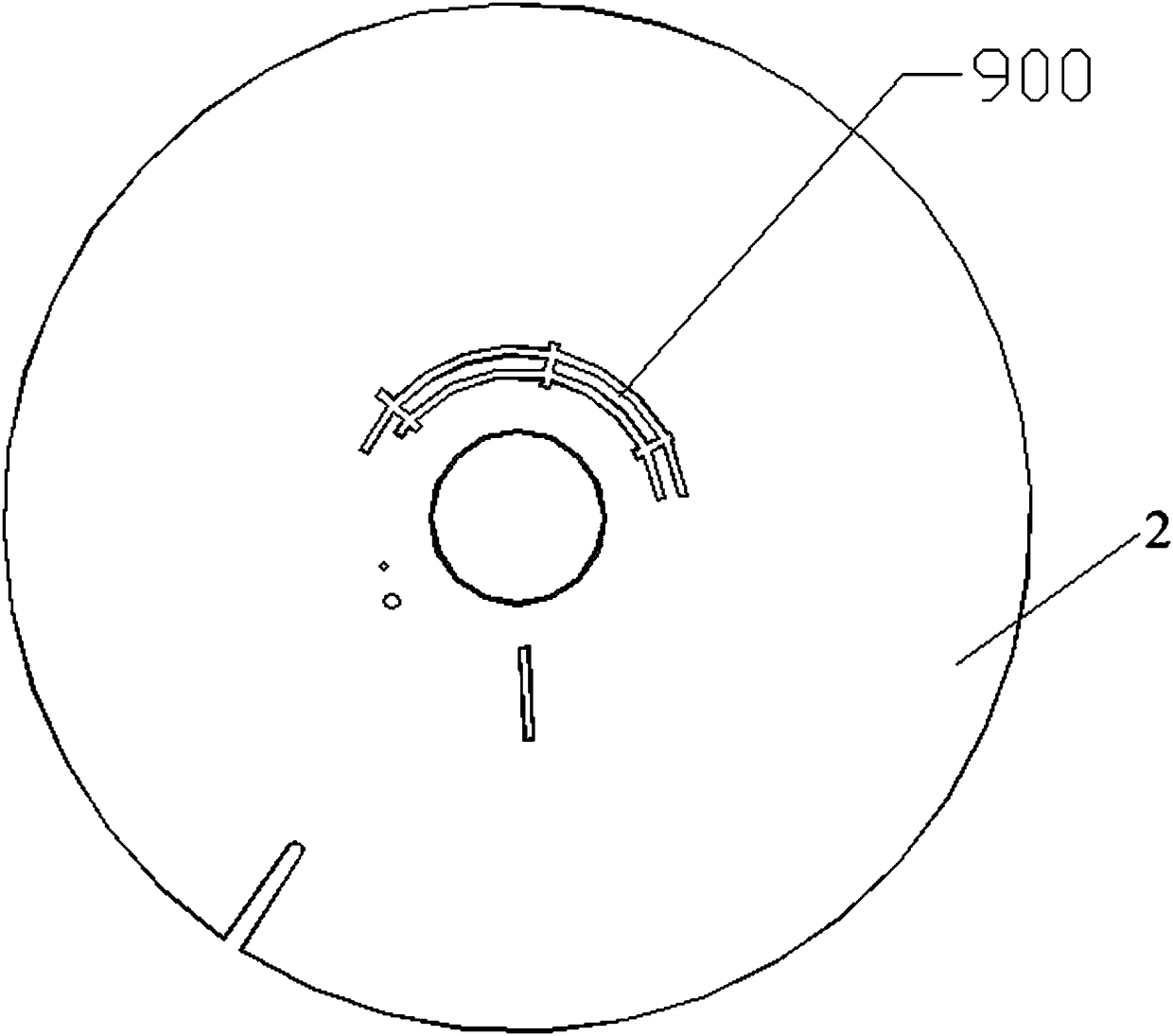
ActiveCN102277429AWill not affect the results of the hybridizationGood synchronizationMicrobiological testing/measurementBiotinA-DNA
The invention discloses a gonococcus, chlamydia trachomatis and ureaplasma urealyticum detection kit. The detection kit comprises a gene chip (1) and various primers (2), wherein the gene chip carries (i) nucleotide probes of different pathogens, (ii) a biotin point-labeled DNA (deoxyribonucleic acid) sequence and (iii) a DNA sequence carrying beta-globulin encoding part; the nucleotide probes are a sequence of SEQ ID Nos:1-3 or a sequence complementary to SEQ ID Nos:1-3; the biotin point-labeled DNA sequence is SEQ ID No.4; the DNA sequence carrying beta-globulin encoding part serving as an internal control point is SEQ ID No.5; and various primers are used for amplifying the DNA sequences in a clinical sample, and have DNA sequences of SEQ ID Nos.6-11. The kit can quickly and accuratelydetect infections of gonococcus, chlamydia trachomatis and ureaplasma urealyticum, and has magnificent meaning to pathogen detection.
Owner:GUANGDONG HYBRIBIO BIOTECH CO LTD
Fluorescence quantitative detection method of ureaplasma urealyticum and microureaplasma
ActiveCN103045721ANo cross amplificationStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
The invention relates to a fluorescence quantitative detection method of ureaplasma urealyticum and microureaplasma. The method comprises the following steps of: processing a sample and extracting DNA (Deoxyribonucleic Acid), and specifically comprises the following steps of: using oligonucleotide sequence complemented with ureaplasma urealyticum and microureaplasma topoisomerase IV DNA as a forward primer, a reverse primer and a fluorescently-labeled probe in PCR reaction to respectively add in two different PCR amplification tubes, simultaneously amplifying DNA target sequences of ureaplasma urealyticum and microureaplasma in the above two amplification tubes respectively by a same amplification condition; judging whether the sample to be detected containing ureaplasma urealyticum and microureaplasma or not through a standard curve according to change of different fluorescence signals in a detection reaction system, and determining the corresponding content of ureaplasma urealyticum and microureaplasma. As for identification and quantitative analysis of reaplasma urealyticum and microureaplasma, the method provided by the invention has characteristics of speediness, sensitivity, high specificity and low cost.
Owner:上海国兴医疗器械有限公司
Pathogen nucleic acid-drug resistant gene detection kit and application thereof
ActiveCN104513854AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesMycoplasma hominisChlamydiae
The invention relates to the technical field of medical biology, and especially relates to a pathogen nucleic acid-drug resistant gene detection kit and an application technology thereof. The kit designed by the invention includes the following two kinds of compositions: a PCR reaction buffer solution, an enzyme mixed solution, and a chlamydia trachomatis / mycoplasma urealytium / mycoplasma hominis / drug resistant gene multiple reaction solution; or a PCR reaction buffer solution, an enzyme mixed solution, a chlamydia trachomatis / mycoplasma urealytium / mycoplasma hominis multiple reaction solution, and a drug resistant gene reaction liquid. The kit adopts a multi-fluorescence quantitative PCR technology, can simultaneously detect multiple target genes in a same PCR reaction tube, and provides a powerful technical support for simultaneous detection of pathogens and drug resistant genes. A method has the characteristics of less required amount on samples, low cost, simple and convenient operation, high sensitivity and good specificity, and has extremely great social and economic significances.
Owner:常州百代生物科技股份有限公司
PCR (Polymerase Chain Reaction) kit for detecting ureaplasma urealyticum biogroup
InactiveCN103361405AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceUreaplasma urealyticumVirology
The invention relates to a PCR (Polymerase Chain Reaction) detection kit and particularly relates to a kit used for detecting ureaplasma urealyticum biogroup and a detecting method. The invention further provides two groups of primers SEQIDNo.1-4. Compared with the common PCR technology, the invention has the advantages that the detection specificity and the detection sensitivity are greatly improved, the pollution opportunity is reduced, and the automation degree is improved as well. The kit used for detecting ureaplasma urealyticum (UU) biogroup not only achieves rapid diagnosis (within 3 hours), but also performs quantitative analysis on the UU biogroup.
Owner:SHANGHAI PULMONARY HOSPITAL
Urogenital tract infection pathogen multi-detection primer group and detection device comprising same
ActiveCN108531623ASimplify the experimental operation processHigh detection throughputBioreactor/fermenter combinationsBiological substance pretreatmentsSingle sampleMycoplasma hominis
Owner:无锡科智达科技有限公司
Rapid genotyping analysis and kits thereof
ActiveCN106906306AMicrobiological testing/measurementOrgan movement/changes detectionDiseaseMycobacterium tuberculosis
The present invention provides methods, primers, probes, and kits for identifying gene mutation or material genotyping. In one embodiment, the materials are disease-causing agents. In another embodiment, the present invention is applied to detecting multidrug-resistant mycobacterium tuberculosis, hepatitis B virus, beta-globulin mutation, thrombosis-related mutations; or multiple sexually transmitted diseases causing agents, including but not limited to, trichomonas vaginalis, chlamydia, neisseria gonorrhoeae, mycoplasma, mycoplasma hominis, ureaplasma urealyticum, Mycelia, Treponema pallidum, Herpes simplex virus type 1 and type 2 and human papilloma virus type 6 and type 11.
Owner:DIAGCOR LIFE SCI LTD
Rapid culture method and culture medium of ureaplasma urealyticum
InactiveCN105331671AImprove accuracyShorten detection timeMicrobiological testing/measurementMicrobiologyUreaplasma urealyticum
The invention discloses a rapid culture method and a culture medium of ureaplasma urealyticum. Compared with a culture medium in the prior art, the culture medium disclosed by the invention has the characteristic that multiple components are added and have effects of rapidly improving the culture efficiency and effectively identifying the ureaplasma urealyticum. The rapid culture method and the culture medium have the advantages of short culture time and easiness in identification.
Owner:刘慧
Fast detection method of ureaplasma urealyticum based on RPA
InactiveCN111534624AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesBiochemistryUreaplasma urealyticum
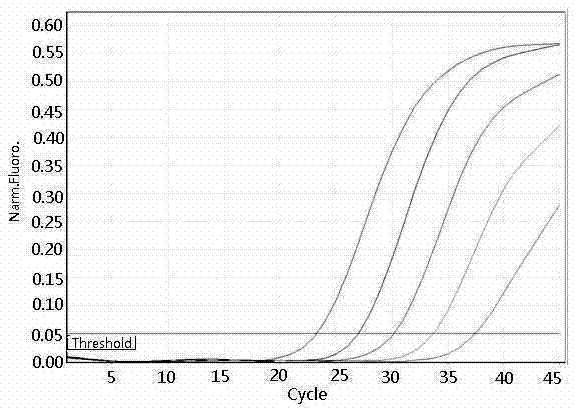
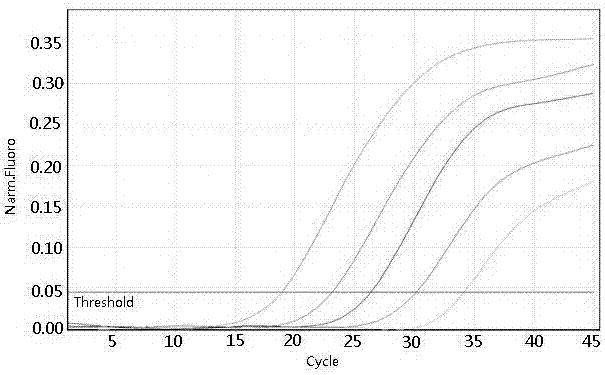
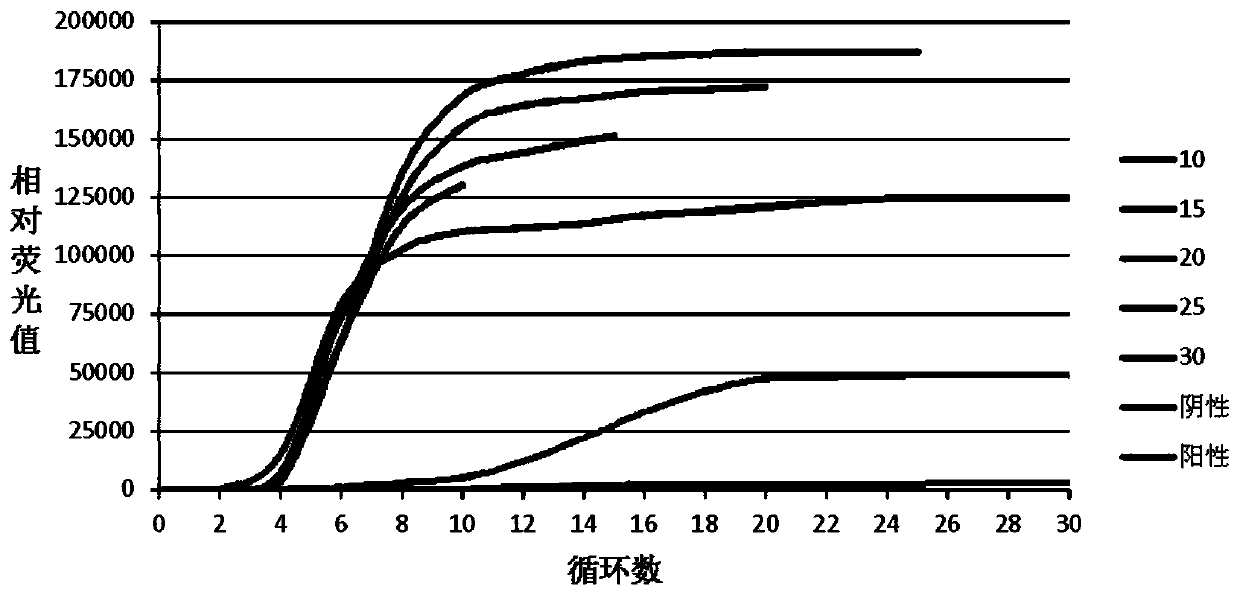
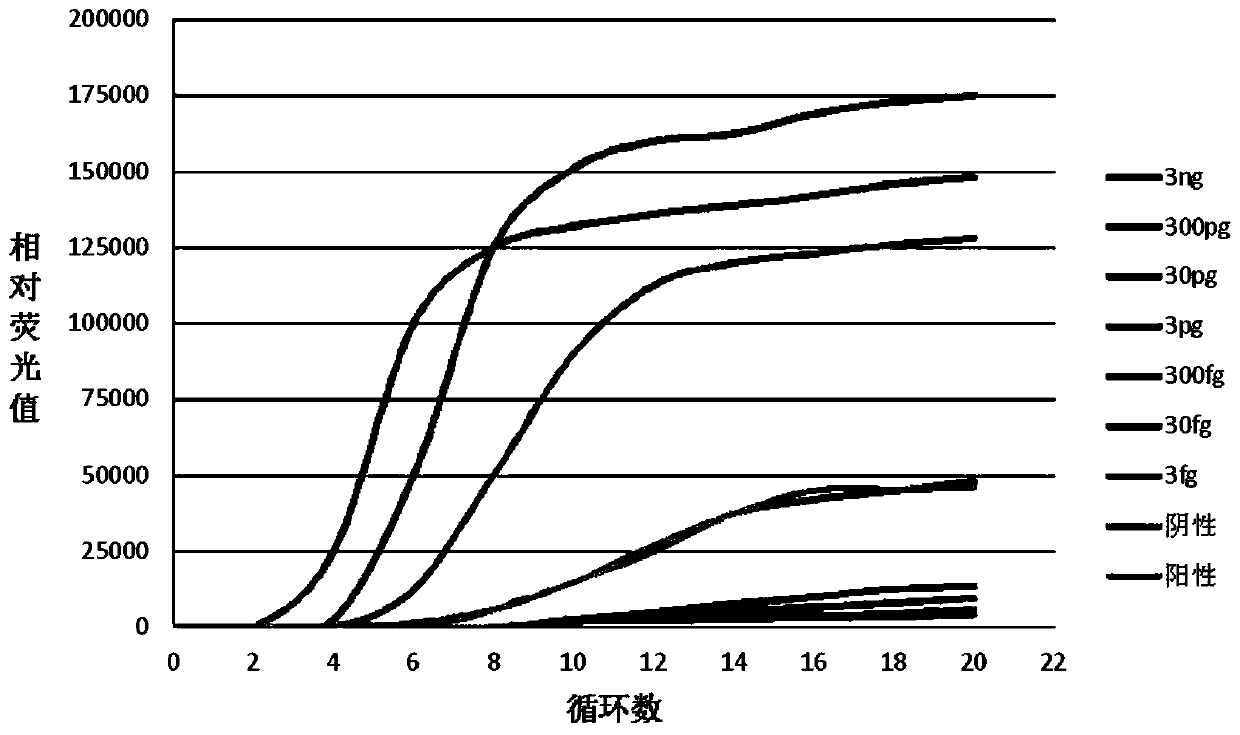
The invention concretely discloses a fast detection method of ureaplasma urealyticum based on RPA, and belongs to the technical field of ureaplasma urealyticum detection. The method comprises the following step of material preparation: Step 1, strains, and Step 2, reagents. The fast detection method of the ureaplasma urealyticum based on RPA has the advantages that the RPA technology is used for the fast detection of the ureaplasma urealyticum; meanwhile, high sensitivity and good specificity are realized; and the fast detection on the ureaplasma urealyticum is completed by a built RPA amplification method. Compared with a reference pathogen, only the ureaplasma urealyticum shows a typical amplification curve; during the detection of the ureaplasma urealyticum based on RPA, the lowest detection limit is 3 pg; and clinical case sample evaluation detection effects prove that the RPA method has the feasibility, and the clinical detection requirements can be met. A built ureaplasma urealyticum real-time fluorescent RPA method has the advantages of high specificity, high sensitivity, simplicity, convenience, high speed and the like, can be used for fast detection of the ureaplasma urealyticum, and provides feasibility and good study basis for the further studies.
Owner:台州市中心医院
Triple-joint-detection kit for ureaplasma urealyticum, chlamydia trachomatis and neisseria gonorrhoeae
PendingCN110373485AReduce workloadMicrobiological testing/measurementMicroorganism based processesCombined testChlamydia trachomatis
The invention provides a triple-joint-detection kit for ureaplasma urealyticum, chlamydia trachomatis and neisseria gonorrhoeae. Specifically, different primers and probes for ureaplasma urealyticum,chlamydia trachomatis and neisseria gonorrhoeae are screened for combined tests, a multiple detection system with excellent specificity and sensitivity for ureaplasma urealyticum, chlamydia trachomatis and neisseria gonorrhoeae is obtained, three kinds of pathogens can be detected by a single tube and tested through a single experiment, and the detection workload is greatly reduced.
Owner:DAAN GENE CO LTD
Method for shortening mycoplasma clinical detection time under A-PEG-B array
InactiveCN101851661AExtension of timeMicrobiological testing/measurementControl releasePolyethylene glycol
The invention discloses a method for shortening mycoplasma clinical detection time under an A-PEG-B array, which belongs to the technical field of biology. The method comprises the following steps of: dissolving polyethylene glycol (PEG) having a repeated structure of --(OH2CH2O)n-- into an in-vitro diagnostic kit for culturing, identifying, quantifying and medicinally sensitizing ureaplasmaurealyticum (Uu) and mycoplasma hominis (Mh) to promote clinical recombination with the A-PEG-B array. In the method, in recombination reaction at the right end of the A-PEG-B array, the functions of the PEG in the complementation and controlled-release of all active substances are highlighted; in the recombination reaction at the left end of the A-PEG-B array, the osmotic pressure function of the PEG is highlighted; at the right end of the A-PEG-B array, the antibiotic capacity and biological activity of a culture medium are simultaneously improved; and at the left end of the A-PEG-B array, the detected Uu and Mh are repaired to different extents and the activity of the detected Uu and Mh on the culture medium is improved. Compared with the prior art, the method has the advantages of obtaining detection results 12 to 24 earlier and remarkably shortening time for curing sicknesses to save patients.
Owner:其昌达生物高科技(上海)有限公司
Primer set for triple detection of Neisseria gonorrhoeae, Chlamydia trachomatis and Ureaplasma urealyticum, product and application
InactiveCN111471781AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesUreaplasma urealyticumChlamydiae
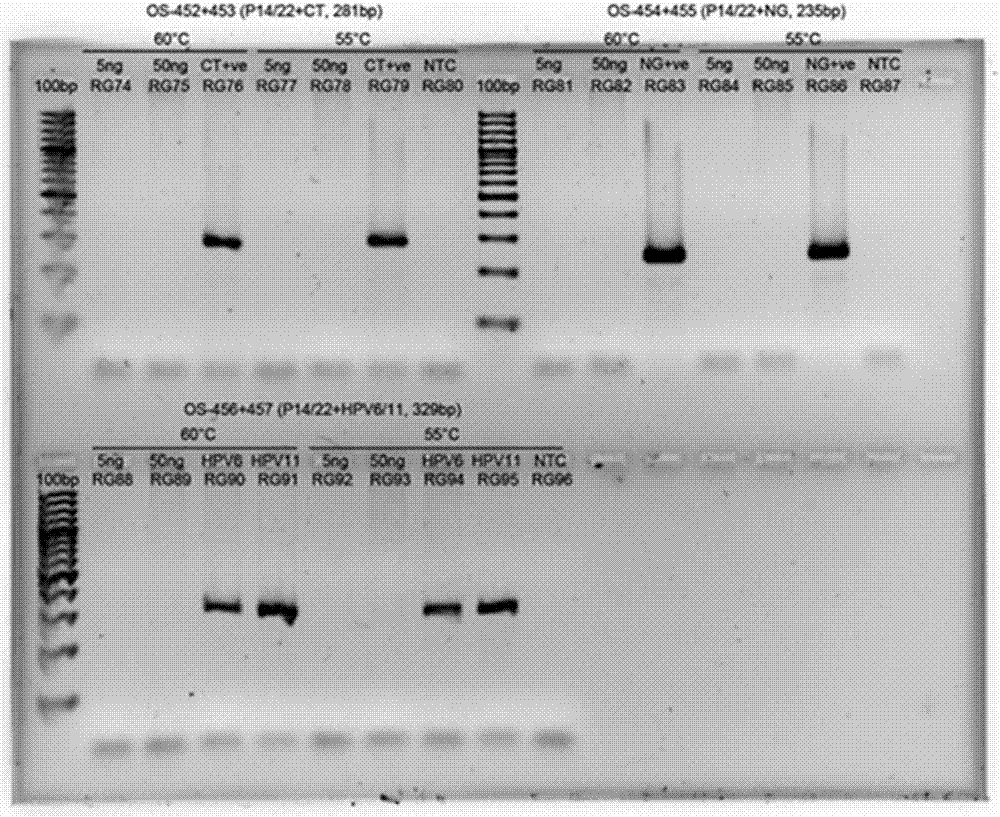
The invention provides a primer set for the triple detection of Neisseria gonorrhoeae, Chlamydia trachomatis and Ureaplasma urealyticum, a product and an application, and relates to the technical field of molecular biology. The primer set provided by the invention includes a primer pair for detecting Neisseria gonorrhoeae, a primer pair for detecting Chlamydia trachomatis, and a primer pair for detecting Ureaplasma urealyticum, the simultaneous detection of three pathogens of Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT), and Ureaplasma urealyticum (UU) in one detection system is realized, and the primer set provided by the invention also has the advantages such as high sensitivity, good specificity, strong reproducibility, and rapid and intuitive detection results.
Owner:HANGZHOU BIOER TECH CO LTD
Gonococcus, treponema pallidum, chlamydia trachomatis and ureaplasma urealyticum hybrid membrane strip and detection kit
ActiveCN106701983ARealize detectionQuick checkMicrobiological testing/measurementPathogenic microorganismTreponema palidum
The invention discloses a gonococcus, treponema pallidum, chlamydia trachomatis and ureaplasma urealyticum hybrid membrane strip and a detection kit. The kit comprises the hybrid membrane strip; a nitrocellulose membrane of the hybrid membrane strip is immobilized with at least one of the following probes: oligonucleotide sequences for gonococcus, treponema pallidum, chlamydia trachomatis and ureaplasma urealyticum and completely complementary with SEQ ID NO.13, SEQ ID NO.14, SEQ ID NO.15 and SEQ ID NO.16; and amplification primers. By selecting special detection fragments, the kit can detect single microorganism, also can simultaneously detect the presence of 4 kinds of pathogenic microorganisms in a same-tube PCR reaction, and provides simple, fast and cheap selection for screening.
Owner:广州市宝创生物技术有限公司
Ureaplasma urealyticum/mycoplasma hominis combined rapid culture and drug sensitivity detection kit
InactiveCN104988206AResolve detectionResolve accuracyMicrobiological testing/measurementMicroorganism based processesPenicillinArginine
Owner:姜洪波 +1
Primer set and kit for detecting Ureaplasma urealyticum through loop-mediated isothermal amplification method
InactiveCN109112221AImprove clinical detection rateImprove featuresMicrobiological testing/measurementMicroorganism based processesPositive controlLoop-mediated isothermal amplification
The present invention discloses a primer set and a kit for detecting Ureaplasma urealyticum through a loop-mediated isothermal amplification (LAMP) method. According to the present invention, the primer set is designed for six different regions on the 16S ribosomal RNA gene sequence of Ureaplasma urealyticum, and comprises a pair of specific internal primers, a pair of specific outer primers and aloop primer; the kit comprises 10 reaction tubes containing 23 [mu]L of a reaction solution, 1 positive control tube containing 25 [mu]L of Uu DNA, and 1 negative control tube containing 100 [mu]L ofsterile ultrapure water; the primer set and the kit can quickly and sensitively detect Ureaplasma urealyticum, have characteristics of high specificity and easy operation, and are suitable for rapiddiagnosis and screening in hospitals at all levels.
Owner:JINAN CENTER HOSPITAL
Kit for rapidly detecting urogenital tract pathogens
InactiveCN111647672ARealize synchronous detectionQuick checkMicrobiological testing/measurementMicroorganism based processesUreaplasma urealyticumChlamydiae
The invention provides a kit for rapidly detecting urogenital tract pathogens. The kit is characterized by comprising PCR reaction liquid, Taq / UDG enzyme mixture liquid, positive control and negativecontrol; the PCR reaction liquid comprises 10*PCR buffer, a primer and a probe of chlamydia trachomatis CT, a primer and a probe of gonococcus NG, a primer and a probe of ureaplasma urealyticum UU, aprimer and a probe of human beta-globin HBB, 25 mM MgCl<2>, dNTPs (dATP, dGTP, dCTP, dTTP, dUTP mixtures), and sterile distilled water. The kit in the invention has the advantages that: sample nucleicacid does not need to be extracted; detection is carried out after direct treatment; operation is simple, convenient and rapid; a single tube can detect multiple pathogens at the same time; the kit is economic and high-efficiency; high-flux sample detection can be carried out; the detection efficiency is increased; and the kit is good in specificity, high in pollution and interference resistance,good in result repeatability, and relatively precise.
Owner:安徽同科生物科技有限公司
Fluorescence quantitative PCR (polymerase chain reaction) detection method for 14 serotypes of Uu (ureaplasma urealyticum)
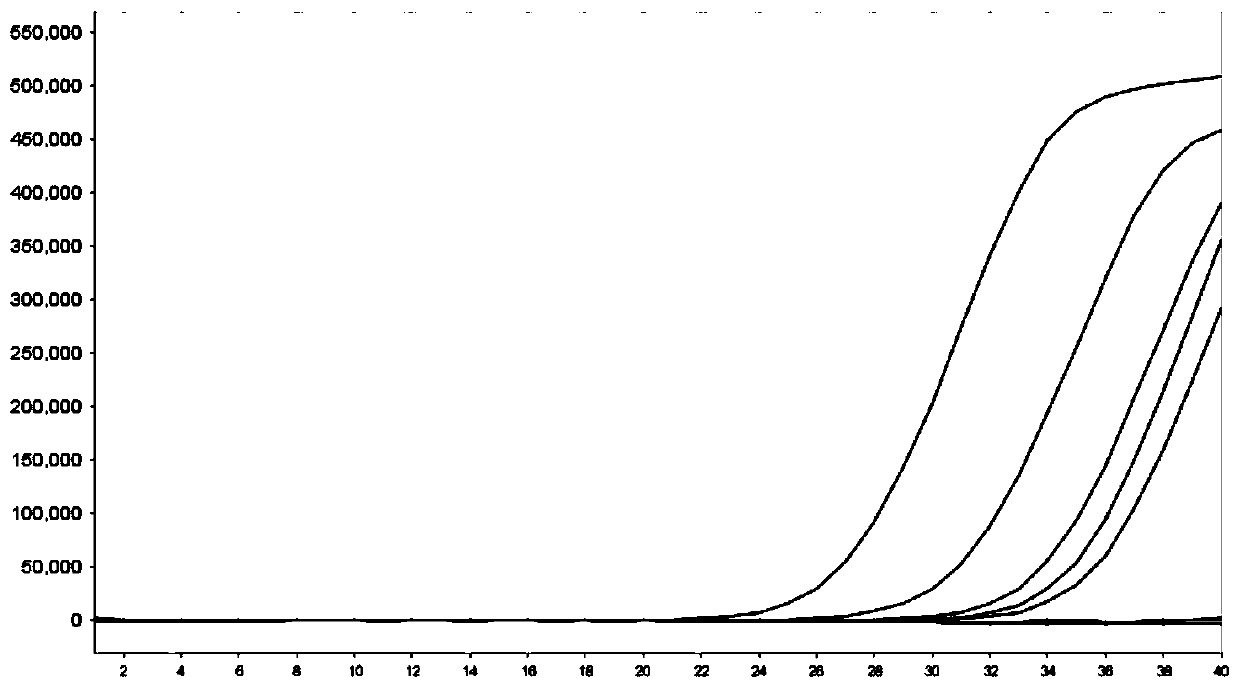
The invention discloses a fluorescence quantitative PCR (polymerase chain reaction) detection method for 14 serotypes of Uu (ureaplasma urealyticum). According to the method, the 14 serotypes adopt 20 microliterreaction systems, thefinal concentration of primers and probes is 0.2 mu mol / L (mu M), the final concentration of magnesium ions is 2 mmol / L (mM) for a dye method and 3 mM for a probe method, 10 microliters of qPCRMasterMix is added for the dye method, and 10 microliters of quantitativePCRSuperMix-UDG is added for the probe method. According to the method, the 14 serotypes of the Uu are typed with a molecular biological method for the first time, defects that a traditional typing method is low in sensitivity and poor in repeatability are overcome, a methodology basis is laid for further study on thepathogenicity of the Uu at a serotype level in the future, and the method can be widely applied clinically.
Owner:ZHEJIANG UNIV
Yolk bioprotein foam filler and preparation method thereof
InactiveCN105749277AAvoid infectionMaintain a healthy physiological environmentAntibacterial agentsEgg immunoglobulinsEscherichia coliBacteroides
The invention discloses a yolk bioprotein foam filler which is mainly composed of 8-10% of multi-link yolk protein, 0.1-0.3% of preservative, 40-50% of matrix and the balance of a foamer.The multi-link yolk protein is yolk bioprotein capable of being combined with various pathogens, and the pathogens include, but not limited to ureaplasma urealyticum, mycoplasma hominis, staphylococcus, streptococcus, Escherichia coli, Gardnerella vaginalis, gonococcus and Tritirachium album.The yolk bioprotein foam filler is mainly composed of yolk bioprotein aiming at inhibiting pathogenic bacteria, can be combined with corresponding pathogens, can effectively inhibit and remove pathogens, is effective on most bacteria, mycete and mycoplasma, does not have impact on probiotics, can maintain health and physiological environment of a reproductive system, does not enter blood, is higher in safety during local use, is free of irritation and side effect on human body, dependency, drug resistance and hypersensitivity and can be used for various clinical therapeutic products for vaginitis.
Owner:西安昱子生物科技有限公司
Mycoplasma quick detection reagent for non-gonococcal urethritis
InactiveCN101566621AFull of nutritionEasy to prepareMicrobiological testing/measurementBiological testingFiltrationNutrition
The invention relates to a mycoplasma quick detection reagent for non-gonococcal urethritis, in particular to a quick detection test paper for macoplasma hominics and mycoplasma hominis and a preparation method thereof. The quick detection reagent can detect out the positive result of the mycoplasma in a genital tract, thereby having the function of the clinical diagnosis guidance and the clinical treatment and the significant meaning of preventing the abuse of antibacterial drugs. The mycoplasma quick detection reagent for the non-gonococcal urethritis is rich in nutrition and easy to prepare, achieves satisfied clinical application effect, solves the difficulty of bedside inoculation, is less polluted, has simple detection method, short time, strong selectivity, sensitive color change, high detection rate and good stability and can obtain a liquid for filtration enrichment and achieve the effects of permanently observing the growth condition of a colony after a sample is inoculated for 24 hours.
Owner:曲奕
Quintuple fluorescent PCR (polymerase chain reaction) quick and hypersensitive detection kit and application thereof
ActiveCN102888464BGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceMycoplasma hominisFluorescent pcr
The invention relates to a real-time PCR (polymerase chain reaction) method for quintuply detecting target nucleic acid in a nucleic acid extracting solution in a single PCR reaction vessel, which is used for detecting Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in a sample.
Owner:苏州华益美生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com