Triple-joint-detection kit for ureaplasma urealyticum, chlamydia trachomatis and neisseria gonorrhoeae
A technology of Chlamydia trachomatis and Ureaplasma urealyticum, applied in biochemical equipment and methods, measurement/testing of microorganisms, microorganisms, etc., can solve problems such as unclear pathogenic mechanism of Uu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1, detection limit test of Ureaplasma urealyticum, Chlamydia trachomatis and Neisseria gonorrhoeae triple detection kit
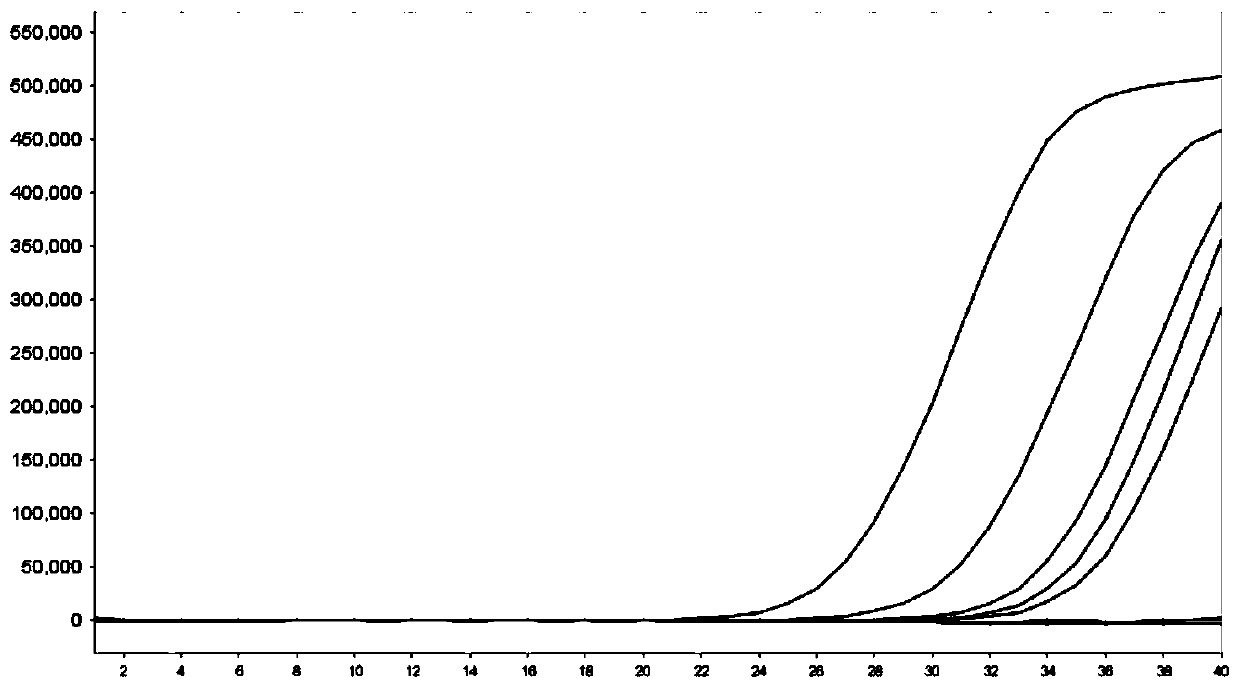
[0110] Dilute the inactivated cultures of Ureaplasma urealyticum, Chlamydia trachomatis, and Neisseria gonorrhoeae to a concentration of 10 5 copies / ml, serially diluted to 10 4 、10 3 , 500, 250, 100copies / ml, each concentration sample was added to the final concentration of 10 4 Copies / ml of the plasmid bacteria containing the amplified fragment of the internal standard was used as the sample to be tested, and the sensitivity of the 3-fold detection reagent was tested.
[0111] Test results such as figure 1 , 2 , 3, the results show that the multiple detection system of the present invention can reach 250copies / ml for the detection sensitivity of Ureaplasma urealyticum, the detection sensitivity for Chlamydia trachomatis can reach 250copies / ml, and the detection sensitivity for Neisseria gonorrhoeae can reach 250copies / ml ml.
Embodiment 2
[0112] Example 2, Specificity Test of Triple Detection Kit for Ureaplasma urealyticum, Chlamydia trachomatis and Neisseria gonorrhoeae
[0113] With normal saline, human papillomavirus, herpes simplex virus type Ⅰ, herpes simplex virus type Ⅱ, Neisseria meningitidis, Brevibacterium haemophilus, Escherichia coli, Epstein-Barr virus, and Staphylococcus aureus as specific reference substances, the test solution Specificity of a triple test kit for Ureaplasma ureaplasma, Chlamydia trachomatis and Neisseria gonorrhoeae.
[0114] Test results such as Figure 5 and Figure 6 As shown, the test results show that the multiplex detection system of the present invention has good specificity, and there is no non-specific amplification reaction for non-target pathogens and negative control (normal saline). Test-specific reference products (normal saline, human papillomavirus, herpes simplex virus type Ⅰ, herpes simplex virus type Ⅱ, Neisseria meningitidis, Brevibacterium haemophilus, Esc...
Embodiment 3
[0115] Example 3, precision test of triple detection kit for Ureaplasma urealyticum, Chlamydia trachomatis and Neisseria gonorrhoeae
[0116] Dilute the inactivated cultures of Ureaplasma urealyticum, Chlamydia trachomatis, and Neisseria gonorrhoeae to 10 5 and 10 3 Copies / ml was used as a precision reference product, each repeated 10 times, and the coefficient of variation of each concentration precision reference product was calculated.
[0117] The test results are as Figure 7 , Figure 8 ,and Figure 9 As shown, the test results show that the multiple detection system of the present invention has better precision, and there is no significant difference in different test batches for samples with different concentrations, which can fully meet the requirements of clinical precision. After testing, the coefficients of variation of the high concentration and low concentration precision reference products of Ureaplasma urealyticum were 1.16% and 1.54% respectively; the vari...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com