Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
152 results about "N gonorrhoeae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reinfection is possible due to N. gonorrhoeae's ability to evade the immune system by varying its surface proteins. N. gonorrhoeae can cause infection of the genitals, throat, and eyes. Asymptomatic infection is common in males and females.
Nanosilver-containing antibacterial and antifungal granules and methods for preparing and using the same
InactiveUS6379712B1Improve solubilityPreventing mold build-upPowder deliveryOrganic active ingredientsEscherichia coliDisease
The present invention relates to nanosilver-containing antibacterial and antifungal granules ("NAGs"). The NAGs have longlasting inhibitory effect on a broad-spectrum of bacteria and fungi, which include, but are not limited to, Escherichia coli, Methicillin resistant Staphylococcus aureus, Chlamydia trachomatis, Providencia stuartii, Vibrio vulnificus, Pneumobacillus, Nitrate-negative bacillus, Staphylococcus aureus, Candida albicans, Bacillus cloacae, Bacillus allantoides, Morgan's bacillus (Salmonella morgani), Pseudomonas maltophila, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bacillus subtilis, Bacillus foecalis alkaligenes, Streptococcus hemolyticus B, Citrobacter, and Salmonella paratyphi C. The NAGs contain ground stalk marrow of the plant Juncus effusus L. which has been dispersed with nanosilver particles. The nanosilver particles are about 1-100 nm in diameter. Each of the nanosilver particles contain a metallic silver core which is surrounded by silver oxide. The present invention also provides a process for making the NAGs. The NAGs can be used in a variety of healthcare and industrial products. Examples of the healthcare products include, but are not limited to, ointments or lotions to treat skin trauma, soaking solutions or cleansing solutions for dental or women hygiene, medications for treating gastrointestinal bacteria infections, sexual related diseases, and eye diseases. Examples of industrial products include, but are not limited to, food preservatives, water disinfectants, paper disinfectants, construction filling materials (to prevent mold formation).
Owner:LEGEND WIN FINANCE
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS6822076B2Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsTetanusBasophilia
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-lIe-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Gonococcal proteins and nucleic acids
InactiveUS7504111B2Stable maintenanceAntibacterial agentsOrganic active ingredientsAntigenSalmonella serotype typhi
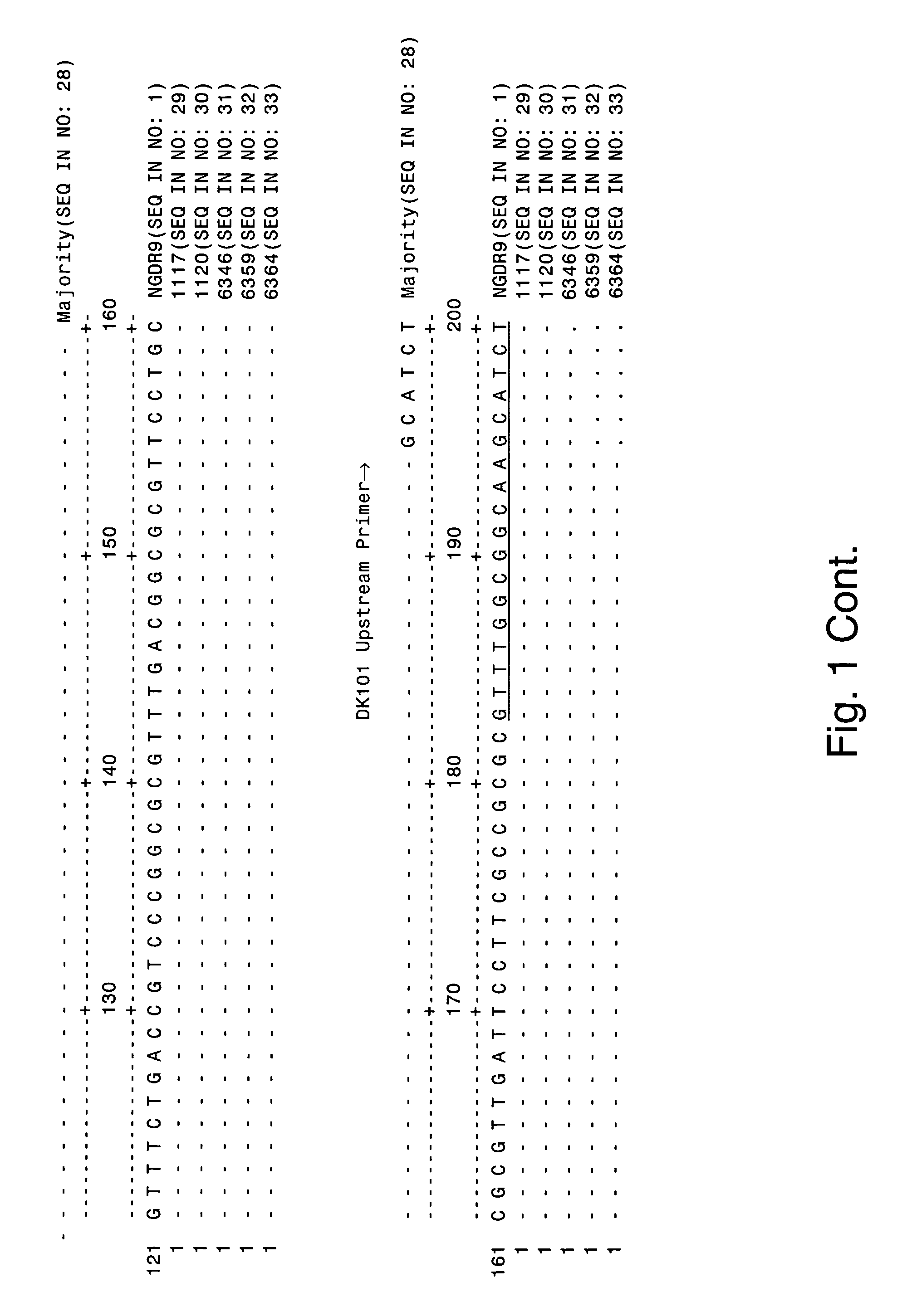
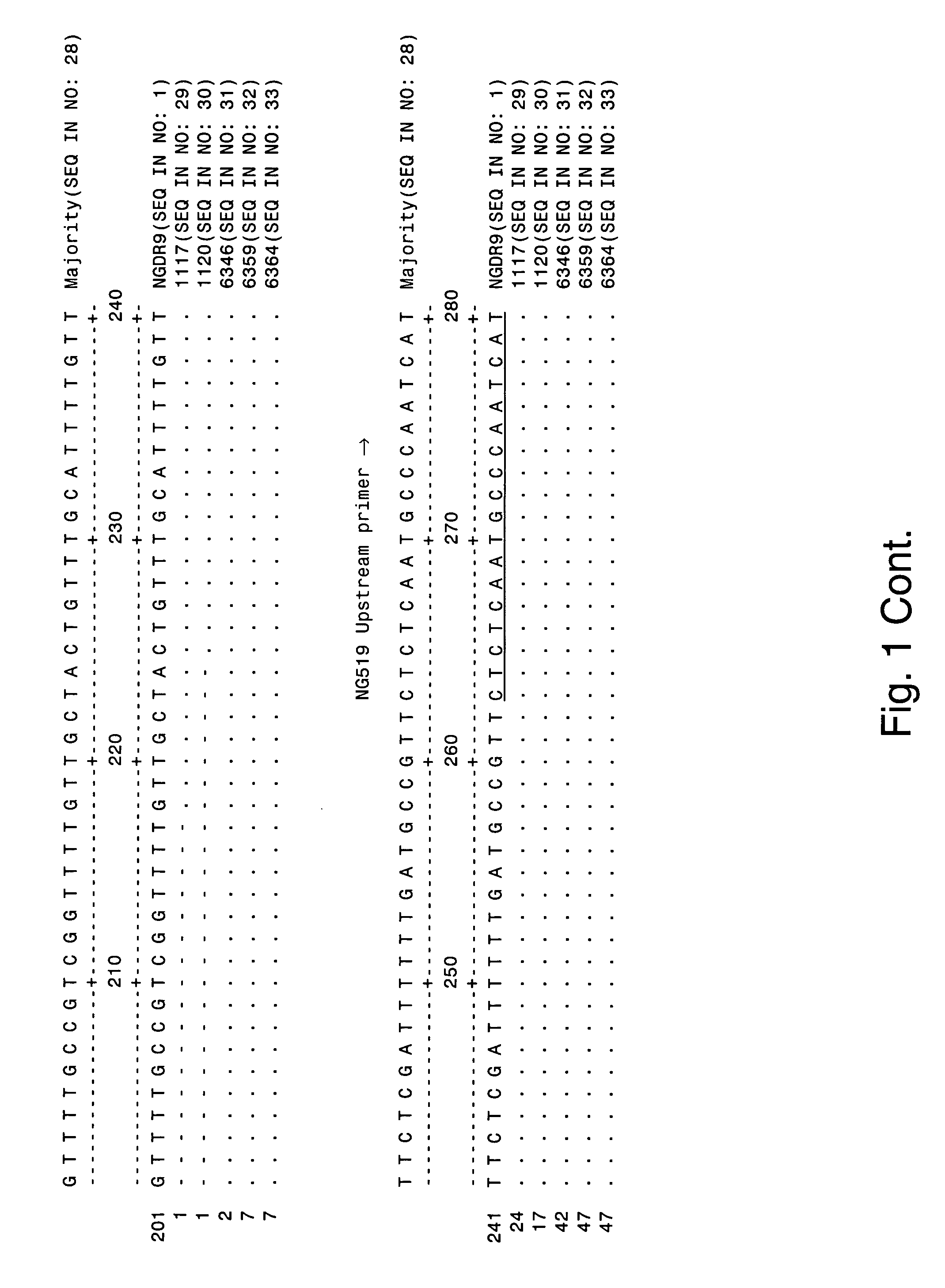
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Gonococcal proteins and nucleic acids
InactiveUS20050260581A1Easy to insertEfficient HarvestingAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiVaccine Immunogenicity
The invention provides proteins from gonococcus (Neisseria gonorrhoeae), including amino acid sequences, the corresponding nucleotide sequences, expression data, and serological data. The proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. They are also useful for distinguishing between gonococcus and meningococcus and, in particular, between gonococcus and serogroup B meningococcus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
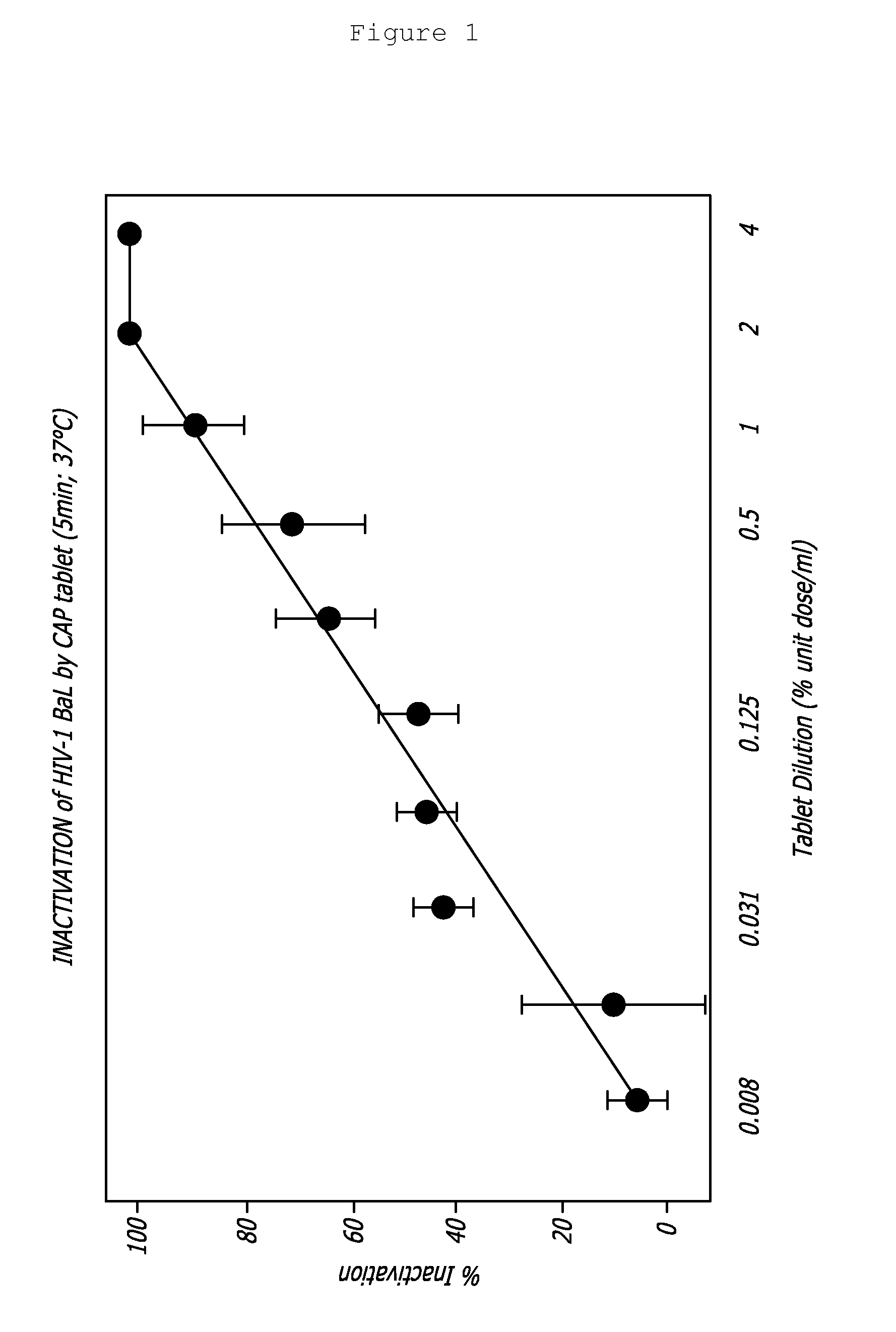
Rapidly dispersible vaginal tablet that provides a bioadhesive gel
InactiveUS20110159091A1Safe and relatively inexpensive methodAvoid spreadingAntibacterial agentsBiocideBacterial vaginosisSpiroplasma
A tablet for insertion into a vagina including 0.01 to 500 mg of a vaginal medication, such as a microbicide, such as cellulose acetate 1,2-benzenedicarboxylate (CAP); 100 to 500 mg of mannitol powder; 50 to 300 mg of inert microcrystalline cellulose; 10 to 80 mg of hydroxypropyl methylcellulose; 50 to 250 mg of glycerol and optionally 2 to 4 mg of at least one preservative which protects against microbicidal contamination and discourages the growth of yeast in the vagina. The tablet which includes CAP as the vaginal medication is vaginally administered before coitus in methods for preventing the sexual transmission of HIV-1, HIV-2, herpesvirus, or an infection caused by Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Haemophilus ducreyi or Treponema pallidum. The tablet which includes CAP as the vaginal medication is vaginally administered to prevent or treat bacterial vaginosis.
Owner:NEW YORK BLOOD CENT
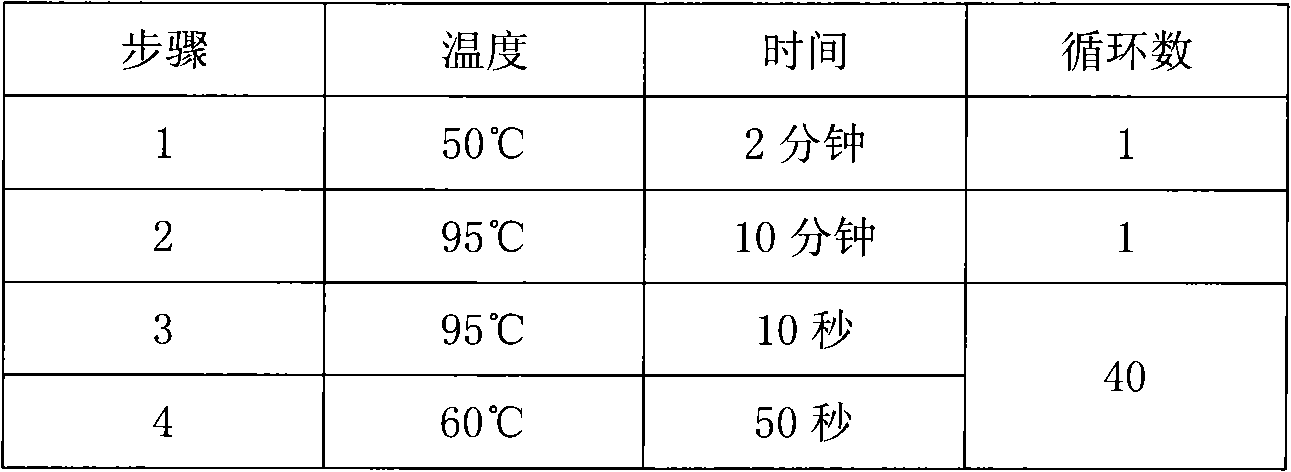
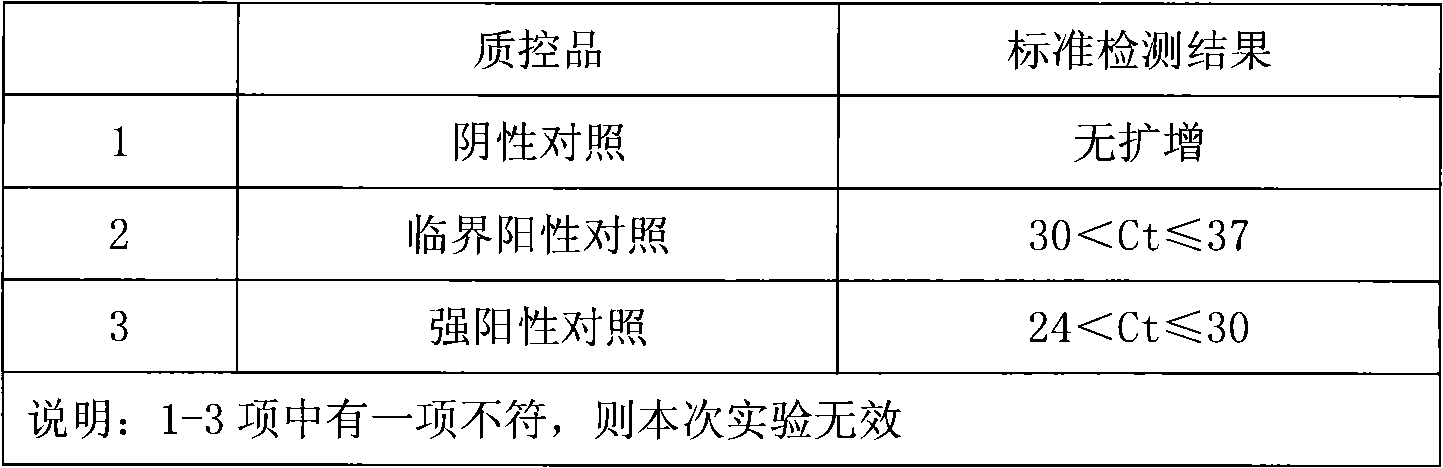
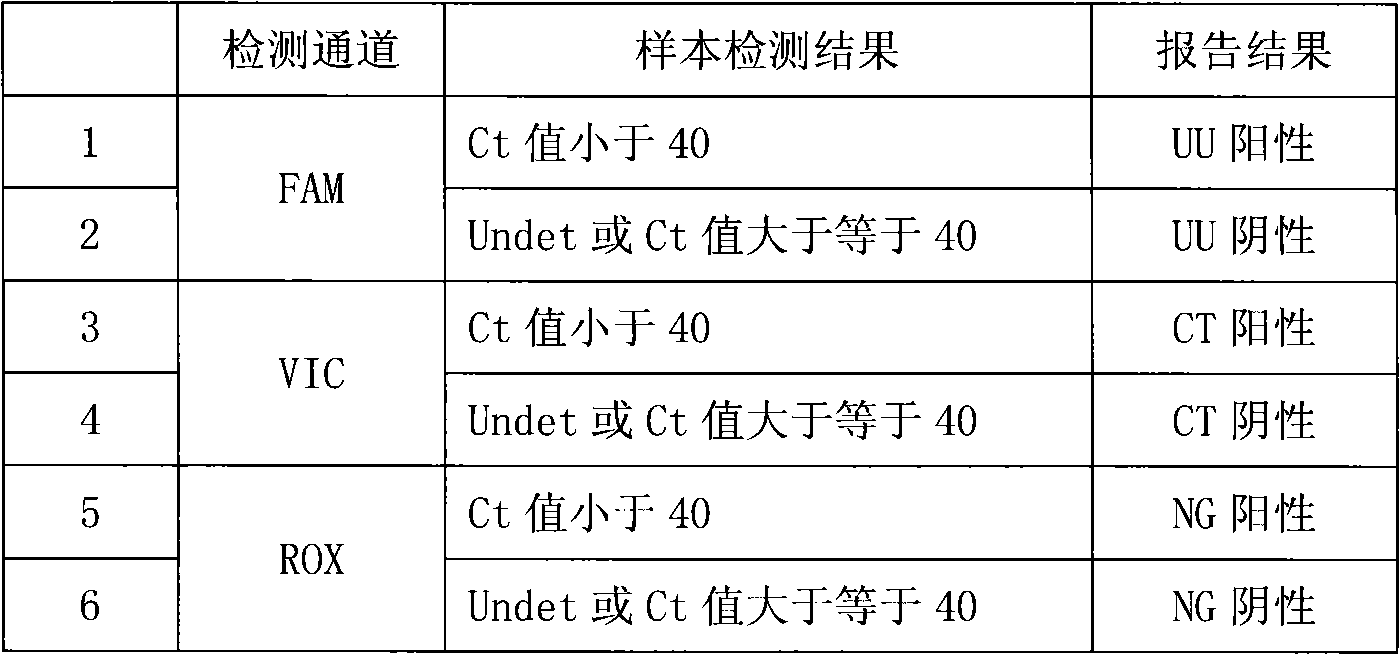
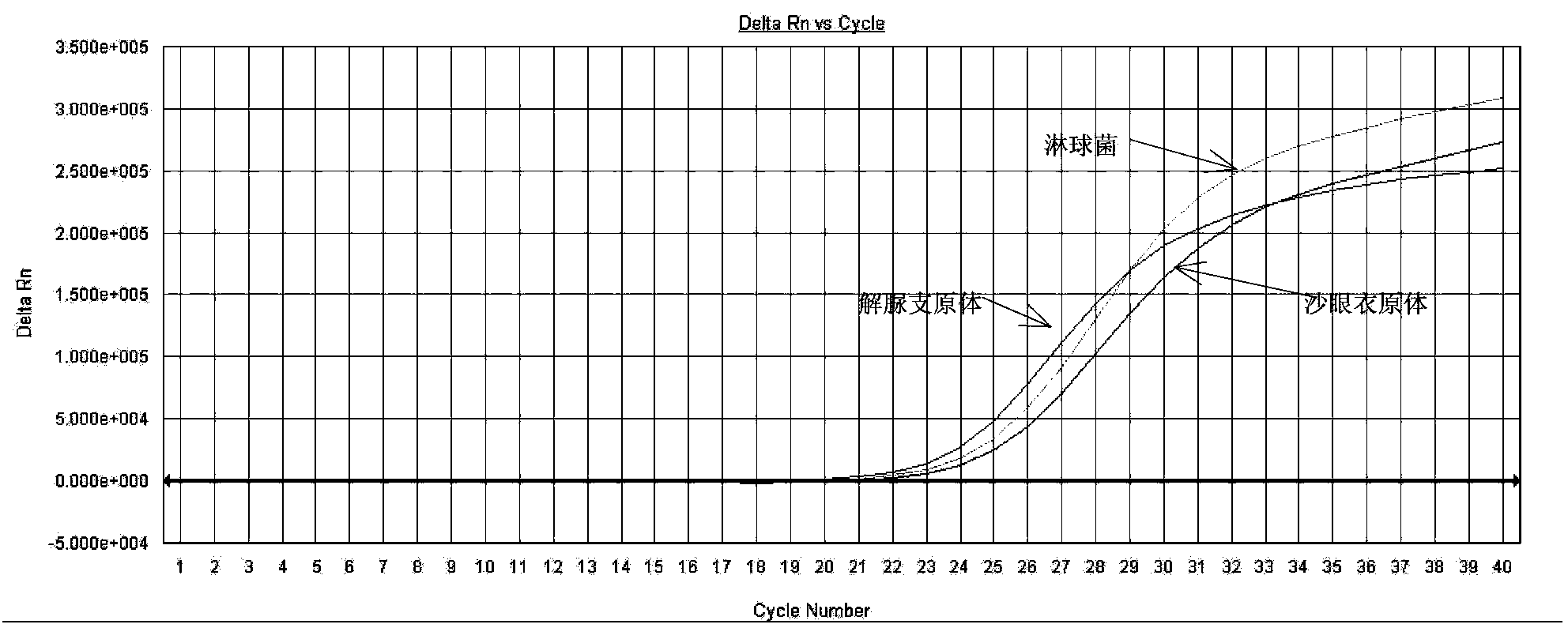
Fluorescence PCR method for diagnosing infection of Chlamydia trachomatis, neisseria gonorrhoeae and ureaplasma urealyticum
ActiveCN101613763AMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
The invention relates to a fluorescence PCR (polymerase chain reaction) detection method for diagnosing infection of Chlamydia trachomatis (CT), neisseria gonorrhoeae (NG) and ureaplasma urealyticum (UU), which belongs to the field of nucleic acid in vitro diagnosis. The method comprises a polymerase chain reaction (PCR) system based on fluorescence PCR technology, contains a forward primer and areverse primer aiming at the CT / the NG / the UU and a fluorescent probe, and can detects DNA of three pathogens such as the CT, the NG, the UU and the like simultaneously in a reaction tub under suitable PCR condition. The method can diagnosing the infection of the CT / the NG / the UU in a clinical sample simply, conveniently and rapidly, has high sensitivity and specificity, and has important clinical value to early control and prevention of relevant genitourinary tract infections, blocking of an infection sources, and infection reduction of related pathogen.
Owner:CITY UNIVERSITY OF HONG KONG
Reagents and methods for detecting Neisseria gonorrhoeae
InactiveUS20050158768A1Quick checkMinimize the numberSugar derivativesMicrobiological testing/measurementNeisseria weaveriCoccidia
This invention provides compositions and methods for detecting Neisseria gonorrhoeae in a sample. This invention also provides related reaction mixtures, kits, systems, and computers.
Owner:ROCHE MOLECULAR SYST INC
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS20030059912A1Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsADAMTS ProteinsPotassium
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-Ile-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Surface protein of Neisseria bacteria
Present invention provides a monoclonal antibody binding to Neisseria bacteria and its target antigen Ag473, which include the sequences of its polynucleotide and its amino acid, wherein the Neisseria bacteria can be Neisseria meningitidis or Neisseria gonorrhoeae; and wherein Ag473 can be made into a vaccine or a diagnostic or therapeutic reagent.
Owner:CENT FOR DISEASE CONTROL DEPT OF HEALTH TAIWAN
Pubes-cleaning disinfection nursing liquid used for pudendum and vagina of human body
InactiveCN101537004AMild and non-irritatingNo pollution in the processAntibacterial agentsHydroxy compound active ingredientsEscherichia coliDisease
The invention provides pubes-cleaning disinfection nursing liquid used for the pudendum and the vagina of a human body, which consists of 'chlorhexidine acetate (or chlorhexidine gluconate), borneol and osthole' added with a proper amount of synergist and the balance of distilled water, wherein the pH value is adjusted to be 4 to 5 by a pH balance factor. The product can kill pathogenic bacteria of HIV, neisseria gonorrhoeae, staphylococcus aureus, escherichia coli, candida albicans and the like and other viruses in one minutes so as to block cross infections of various infectious diseases, eliminate smells and relieve itching.
Owner:北京汉潮联创中药科技有限公司
Antibiotic resistance profile for Neisseria gonorrhoeae and use of same in diagnosis and treatment of gonorrhea
The present invention relates to an antibiotic resistance profile for Neisseria gonorrhoeae by assessing the presence of mutations (e.g., SNP) in antibiotic resistant genes that confer bacterial resistance against antibiotics such as penicillin, tetracycline, fluoroquinolones, cephalosporin, macrolides and spectinomycin. There is provided a method and a kit for generating an antibiotic resistance profile for Neisseria gonorrhoeae by utilizing a multiplex PCR to amplify segments of antibiotic-resistant genes, allele-specific primer extension to detect gene mutation, and detection of such gene mutations with gel electrophoresis, capillary electrophoresis, or DNA microarray. The present method provides useful information to physicians relating the antibiotic susceptibility of Neisseria gonorrhoeae against different classes of antibiotics. Relying on this personalized diagnostic tool, physicians can better inform about antibiotic susceptibility and thereby open up medical intervention avenues for treating Neisseria gonorrhoeae with antibiotics. There is provided a therapeutic application of the antibiotic resistance profile that has advantages of: (i) providing a more effective regime for gonorrhea treatment; and (ii) halting the evolutionary pressures towards antibiotic resistance in the Neisseria gonorrhoeae therapy.
Owner:MEDICAL DIAGNOSTIC LAB
Primer and probe sequences for detecting chlamydia trachomatis
ActiveUS20080299567A1Easy to detectSugar derivativesMicrobiological testing/measurementChlamydia trachomatisDisease cause
Owner:ABBOTT MOLECULAR INC
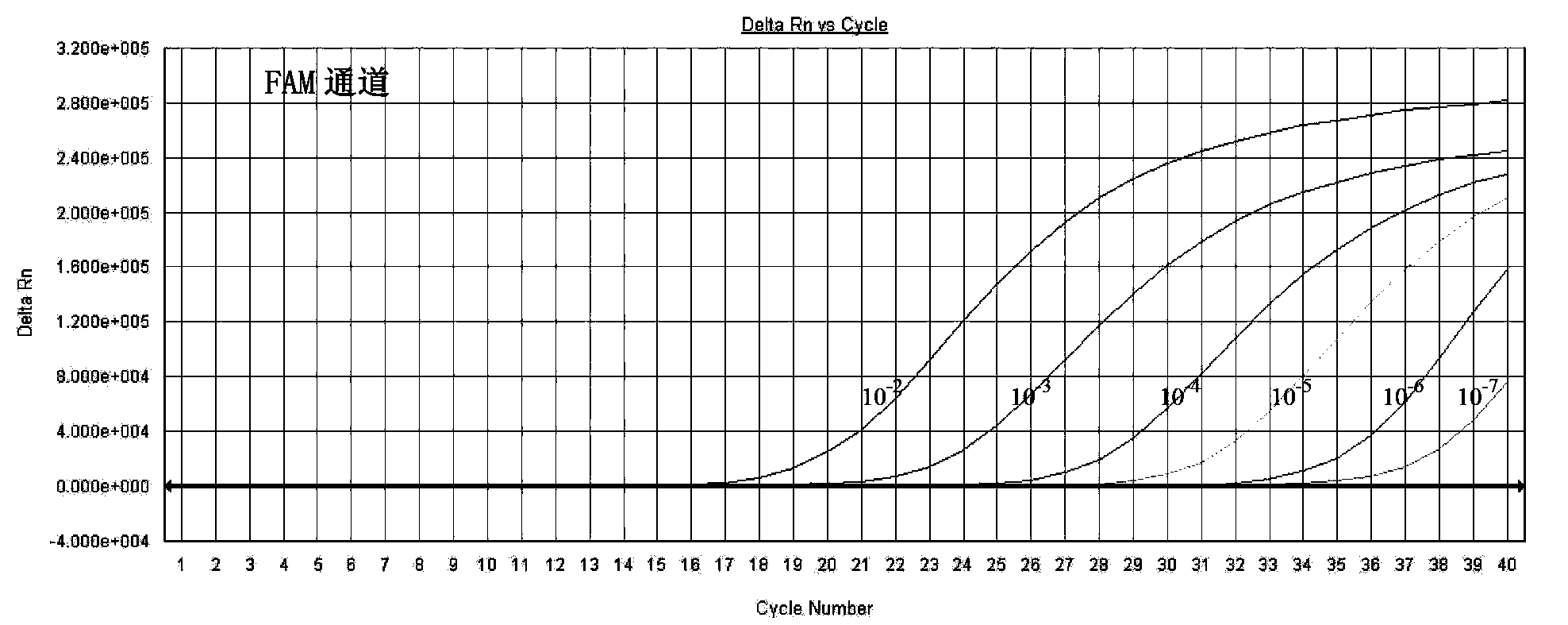
Quintuple fluorescent PCR (polymerase chain reaction) quick and hypersensitive detection kit and application thereof
ActiveCN102888464AGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceMycoplasma hominisPcr method
The invention relates to a real-time PCR (polymerase chain reaction) method for quintuply detecting target nucleic acid in a nucleic acid extracting solution in a single PCR reaction vessel, which is used for detecting Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in a sample.
Owner:苏州华益美生物科技有限公司
Primer, probe and method for detecting human urological genital tract causal agent
InactiveCN101117646AImprove early detection rateSave operating timeMicrobiological testing/measurementDiseaseChlamydia trachomatis
The present invention discloses a guiding object and a probe for testing the human urogenital tract pathogens, and a method for testing the human urogenital tract pathogens using the guiding object and the probe. The present invention can test three different pathogens including Neisseria gonorrhoeae, mycoplasma ureaplasma and chlamydia trachomatis in a same reaction tube simultaneously. The present invention not only can increase the early detection rate of the sexually transmitted diseases, but also can reduce the operating time of the medical personnel, lower the cost, and lighten the economic burden of the patient.
Owner:SHANGHAI SHENYOU JIANHAI BIOLOGICAL TECH LIABILITY
Triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum/ chlamydia trachomatis
ActiveCN103409508ASimple and fast operationAvoid pollutionMicrobiological testing/measurementFluorescence/phosphorescenceSynechococcusPositive control
The invention discloses a triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis. The kit comprises an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reaction liquid, an enzyme mixed liquid, a triple reaction liquid for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis, positive control and negative control. The kit disclosed by the invention overcomes the deficiencies that in the prior art, neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis is poor in specificity, lower in sensitivity and the like, effectively prevents pollution, has the advantages of high sensitivity, good specificity, strong repeatability, quick and objective detection result and the like, and has good application prospect for detecting neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
PCR method of multiple sex propagate pathogene synchronous detection and kit
InactiveCN101935686AThe detection process is fastSimple stepsMicrobiological testing/measurementMicroorganism based processesDiseaseMycoplasma hominis
The invention discloses a single-tube multiple PCR method for synchronous rapid detection of five sex propagate pathogenes (neisseria gonorrhoeae (NG), mycoplasma hominis (MH), mycoplasma genitalium (MG), chlamydia trachomatis (CT) and ureaplasma urealyticum (UU)) and a kit. The method is characterized by amplifying the five pathogenes and performing gel electrophoresis separation and detection by reasonably designing a primer and optimizing the primer concentration combination and PCR condition in the same reaction tube and under the same heat cycle condition. The invention has the characteristics of sensitivity, rapidness, convenience, and the like of the classic PCR, mainly realizes synchronous detection of the five pathogenes, has lower cost, can be used for developing relative kits and is used for clinical diagnosis and epidemiological survey and control.
Owner:唐文志
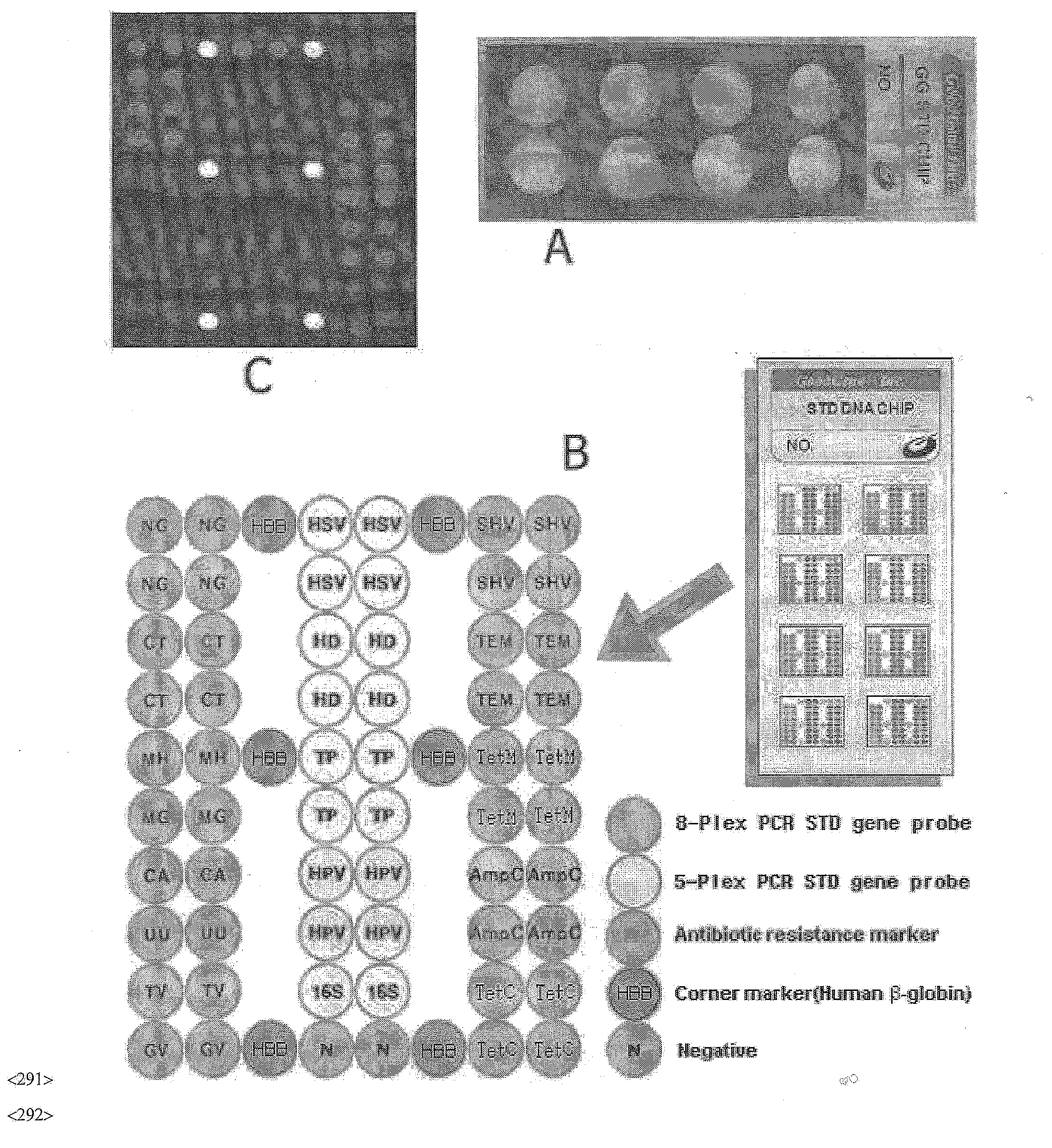
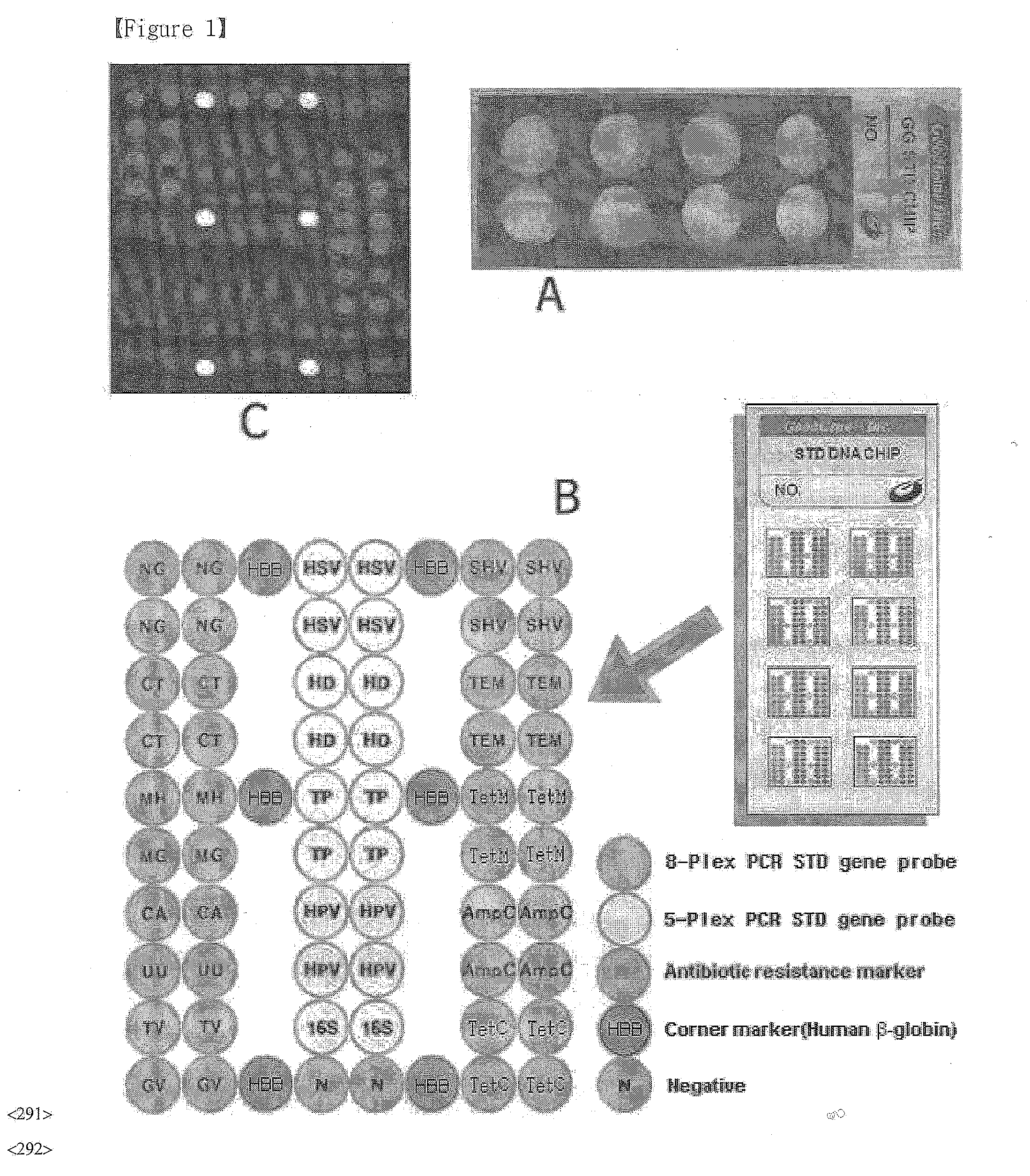
DNA chip, kit for detecting or genotyping bacteria causing sexually transmitted diseases, genotyping antibacterial drug resistance and detecting or genotyping method using the same
InactiveUS20120004113A1Quickly and accurately detectEasy to explainNucleotide librariesMicrobiological testing/measurementDiseaseEscherichia coli
Disclosed are a DNA chip and a kit capable of quickly and accurately detecting or genotyping the highly prevalent and important eleven microbes causing sexually transmitted diseases (STD) Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma genitalium, Mycoplasma hominis, syphilis-causing treponema, pallidum, chancroid-causing Haemophilus ducreyi, genital herpes-causing herpes simplex virus 1 and 2, human papillomavirus (HPV) and Trichomonas vaginalis and three related organisms Candida albicans, Gardnerella vaginalis and coliform bacteria and analyzing antibiotic resistance against tetracycline and lactam antibiotics, and a method for detecting or genotyping using the same. According to the present invention, the presence, genotype and antibiotic resistance of the fourteen organisms can be analyzed quickly and accurately from a DNA sample. With excellent sensitivity, specificity, reproducibility and accuracy of the 14 STD-causing and related microorganisms may be automatically identified quickly and accurately from multiple samples, and selection of antibiotics may be aided.
Owner:GOODGENE
Real-Time Multiplex Detection of Three Bacterial Species Responsible for Sexually Transmitted Diseases
The invention relates to the detection of three different bacterial species which are responsible for sexually-transmitted diseases, i.e., Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG) and Mycoplasma genitalium (MG). The invention more particularly relates to the detection of these three species in real-time PCR, in multiplex PCR and in real-time multiplex PCR. The invention provides reference templates sequences, which are especially adapted to the design of primers and probes, which can be used together in the same tube to detect CT and / or MG and / or NG by real-time multiplex amplification.
Owner:BIO RAD EURO GMBH
Proteinase K resistant surface protein of neisseria meningitidis
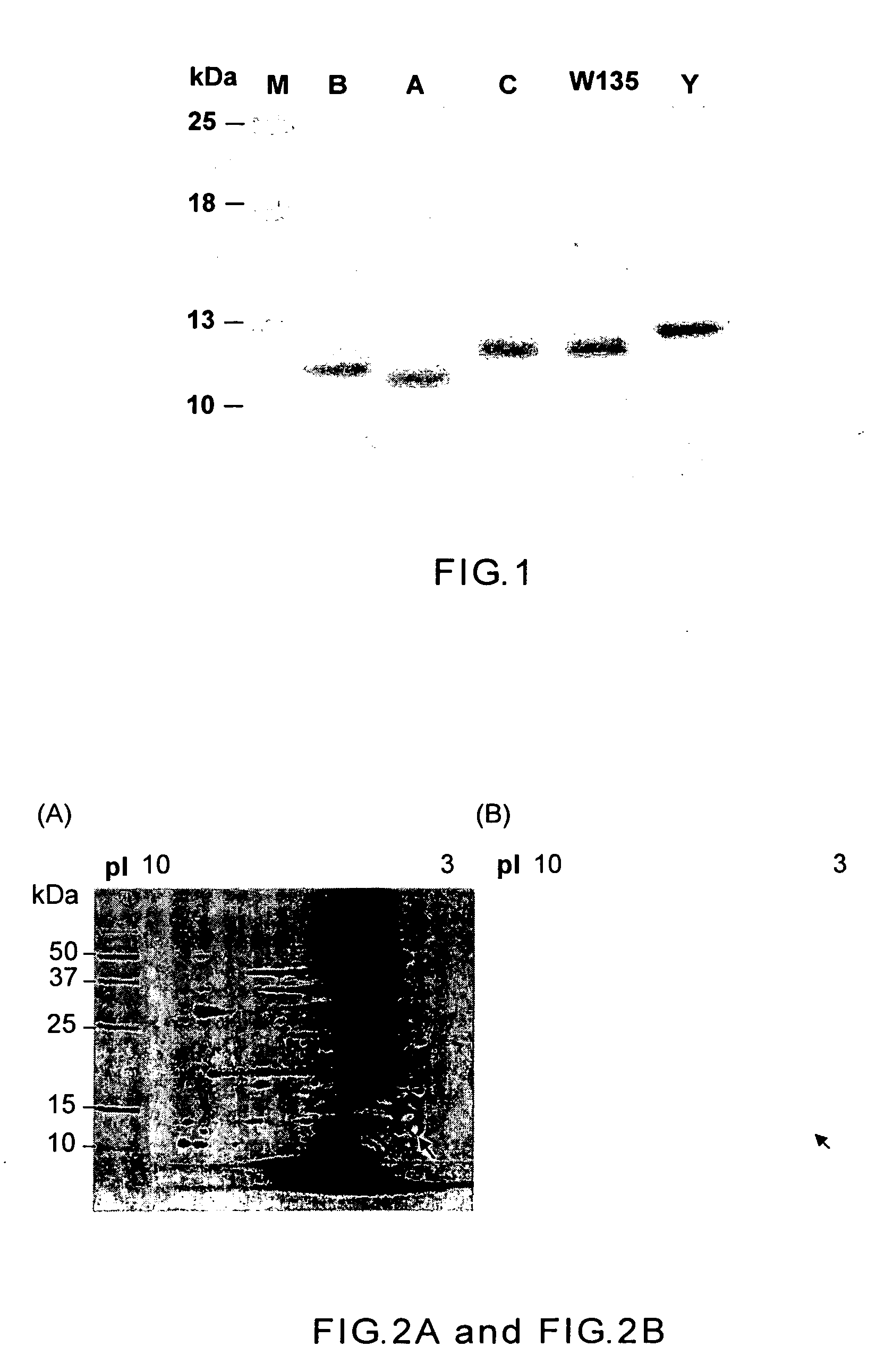
The identification of a highly conserved, immunologically accessible antigen at the surface of Neisseria facilitates treatment, prophylaxis, and diagnosis of Neisseria diseases. This antigen is highly resistant to Proteinase K and has an apparent molecular weight of 22 kDa on SDS-PAGE. Specific polynucleotides encoding proteins of this class have been isolated from three Neisseria meningitidis strains and from one Neisseria gonorrhoeae strain. These polynucleotides have been sequenced, and the corresponding full-length amino acid sequences of the encoded polypeptides have been deduced. Recombinant DNA methods for the production of the Neisseria surface protein, and antibodies that bind to this protein are also disclosed.
Owner:ID BIOMEDICAL
Methods of detecting chlamydia and gonorrhea and of screening for infection/inflammation based on genomic copy number
ActiveUS20160257998A1Microbiological testing/measurementAntiinfectivesNucleic acid sequencingChlamydiae
Compositions and methods for detecting Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are provided. The present invention also provides methods and compositions for screening for infection / inflammation based on genomic copy number. Described herein is a method that entails assaying a sample obtained from the urogenital tract of the mammal for an indicator of genomic copy number, wherein a genomic copy number level that is higher than a control genomic copy number level is indicative of the presence of infection or inflammation of the urogenital tract. Also described in a kit of the invention that includes a primer and / or probe for detecting or sequencing an indicator of genomic copy number, wherein the indicator of genomic copy number comprises a nucleic acid sequence that is expected to be present in the genome of the mammal in one or two copies; and a primer and / or probe for detecting or sequencing a nucleic acid sequence that is indicative of a pathogen that infects the urogenital tract or a miRNA correlated with inflammation.
Owner:CEPHEID INC
85kDa neisserial antigen
An 85 kDa antigen from Neisseria meningitidis and Neisseria gonorrhoeae has been cloned, sequenced and expressed. The antigen is common to diverse strains, serogroups and serotypes of N. meningitidis, and also to N. gonorrhoeae, N. polysaccharia and N. lactamica. The protein sequences of N. meningitidis (serogroups A and B) and N. gonorrhoeae are highly homologous.
Owner:STATENS INST FOR FOLKEHELSE +1
Nano silver clean-keeping sterilized shower cream and manufacture method thereof
InactiveCN101416924AImprove the bactericidal effectImprove repair effectCosmetic preparationsToilet preparationsShower gelChemistry
The invention discloses a nano-silver cleaning sterilizing shower gel which is prepared by the following materials according to weight percentage: 15 percent to 25 percent of surfactant (AES), 8 percent to 12 percent of mild surfactant, 8 percent to 12 percent of amphoteric surfactant, 0.001 percent to 0.01 percent of nano-silver, 0.1 percent to 0.3 percent of chelating agent, 0.1 percent to 0.2 percent of citric acid (Acd), 0.2 percent to 1 percent of flavor, 50 percent to 70 percent of distilled water, 0.1 percent to 0.2 percent of preservative and 1 percent to 3 percent of thickener. The preparation method is as follows: the nano-silver particles are dissolved in the distilled water and uniformly mixed with the chelating agent after floating particles are filtrated; then the mixture is heated to the constant temperature of 80 DEG C and the surfactant is added into the mixture and uniformly mixed; after the mild surfactant is added into the mixture and uniformly mixed, the amphoteric surfactant is added into the mixture and the mixture is cooled to 45 DEG C; the citric acid is added into the mixture and uniformly mixed, and the pH value is adjusted to be between 5.5 and 7.0; during the cooling, the flavor, the preservative, the thickener and pigments are added in sequence and blended while being added until the temperature drops to the room temperature. The shower gel has strong bactericidal function to chlamydia trachomatis and the neisseria gonorrhoeae of sexually transmitted diseases.
Owner:卓鸿思
Surface protein of Neisseria bacteria
Present invention provides a monoclonal antibody binding to Neisseria bacteria and its target antigen Ag473, which include the sequences of its polynucleotide and its amino acid, wherein the Neisseria bacteria can be Neisseria meningitidis or Neisseria gonorrhoeae; and wherein Ag473 can be made into a vaccine or a diagnostic or therapeutic reagent.
Owner:CENT FOR DISEASE CONTROL DEPT OF HEALTH TAIWAN
Beta-glucan modified meningitis polysaccharide conjugate vaccine and preparation method thereof
ActiveCN104548090AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsCarrier-bound antigen/hapten ingredientsCyanogen bromideMeningococcal meningitis
The invention relates to a beta-glucan modified meningitis polysaccharide conjugate vaccine and a preparation method thereof. The preparation method of the beta-glucan modified meningitis polysaccharide conjugate vaccine comprises the following steps: (1) activating meningococcus polysaccharide by cyanogen bromide, and then deriving by adopting adipic dihydrazide; (2) combining a derived meningococcus polysaccharide derivative with carrier protein; (3) activating beta-glucan; and (4) modifying polysaccharide-protein conjugate by the activated beta-glucan. By virtue of the steps, a novel and efficient meningitis polysaccharide conjugate vaccine can be prepared and can be used for preventing infection caused by epidemic cerebrospinal meningitis Neisseria gonorrhoeae.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Neisseria gonorrhoeae, Chlamydia trachomatis and Ureaplasma urealyticum detection kits
ActiveCN102277429AWill not affect the results of the hybridizationGood synchronizationMicrobiological testing/measurementBiotinA-DNA
The invention discloses a gonococcus, chlamydia trachomatis and ureaplasma urealyticum detection kit. The detection kit comprises a gene chip (1) and various primers (2), wherein the gene chip carries (i) nucleotide probes of different pathogens, (ii) a biotin point-labeled DNA (deoxyribonucleic acid) sequence and (iii) a DNA sequence carrying beta-globulin encoding part; the nucleotide probes are a sequence of SEQ ID Nos:1-3 or a sequence complementary to SEQ ID Nos:1-3; the biotin point-labeled DNA sequence is SEQ ID No.4; the DNA sequence carrying beta-globulin encoding part serving as an internal control point is SEQ ID No.5; and various primers are used for amplifying the DNA sequences in a clinical sample, and have DNA sequences of SEQ ID Nos.6-11. The kit can quickly and accuratelydetect infections of gonococcus, chlamydia trachomatis and ureaplasma urealyticum, and has magnificent meaning to pathogen detection.
Owner:GUANGDONG HYBRIBIO BIOTECH CO LTD
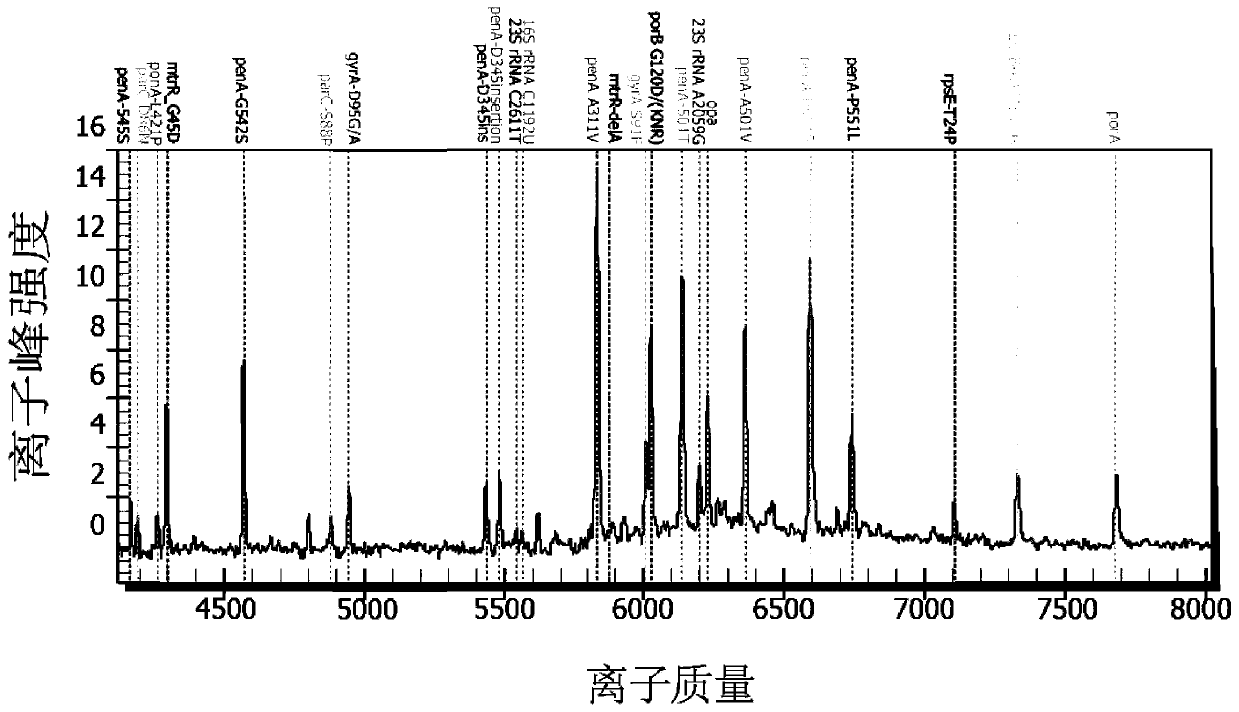
Multi-detection method for drug-resistant sites of neisseria gonorrhoeae
PendingCN111394438AMicrobiological testing/measurementDNA/RNA fragmentationMultiplexMass Spectrometry-Mass Spectrometry
The invention provides a method for detecting mutation of drug-resistant sites of 19 neisseria gonorrhoeae. The method utilizes a multiplex PCR-mass spectrometry method to detect the mutation of the drug-resistant sites of the 19 neisseria gonorrhoeae. The detection method comprises the following steps: 1) designing primers for different drug-resistant sites; 2) carrying out multiplex PCR amplification reaction; 3) treating shrimp alkaline phosphatase; 4) carrying out single base extension reaction; 5) desalting and purifying resin; and 6) carrying out mass spectrometric detection.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
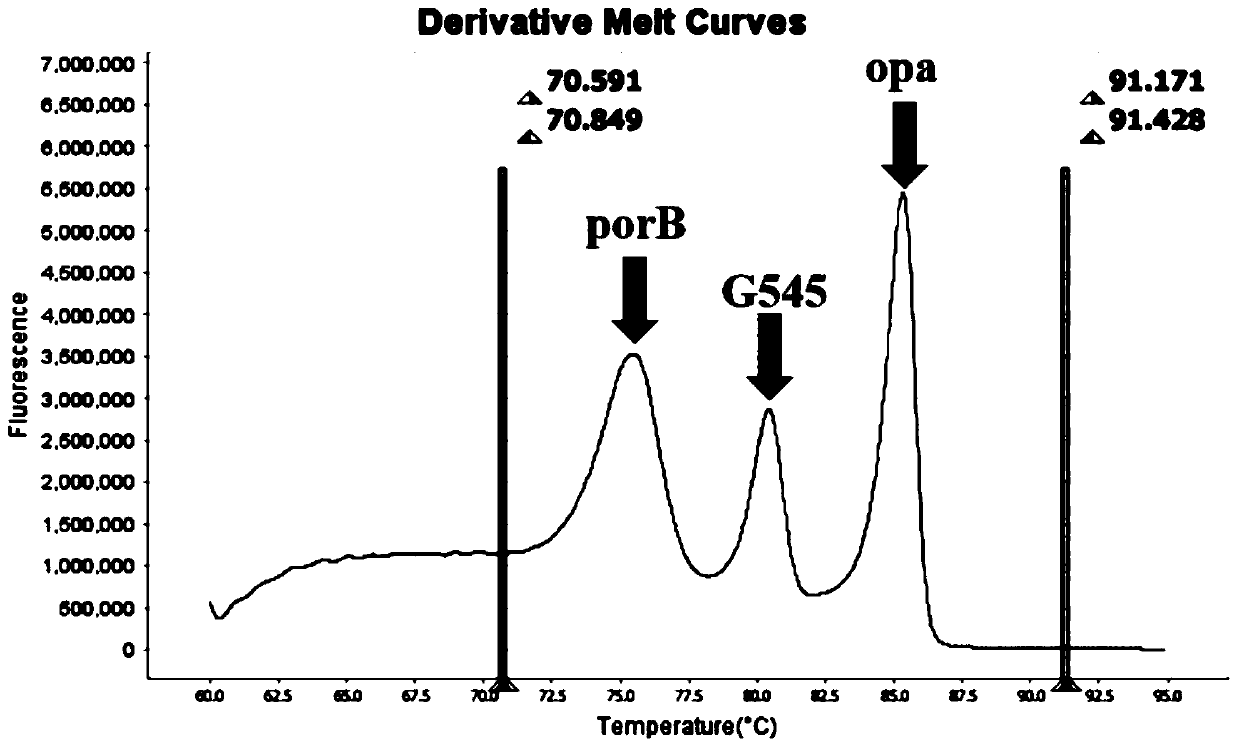
Method and kit for multiple detection of drug resistance sites of neisseria gonorrhoeae
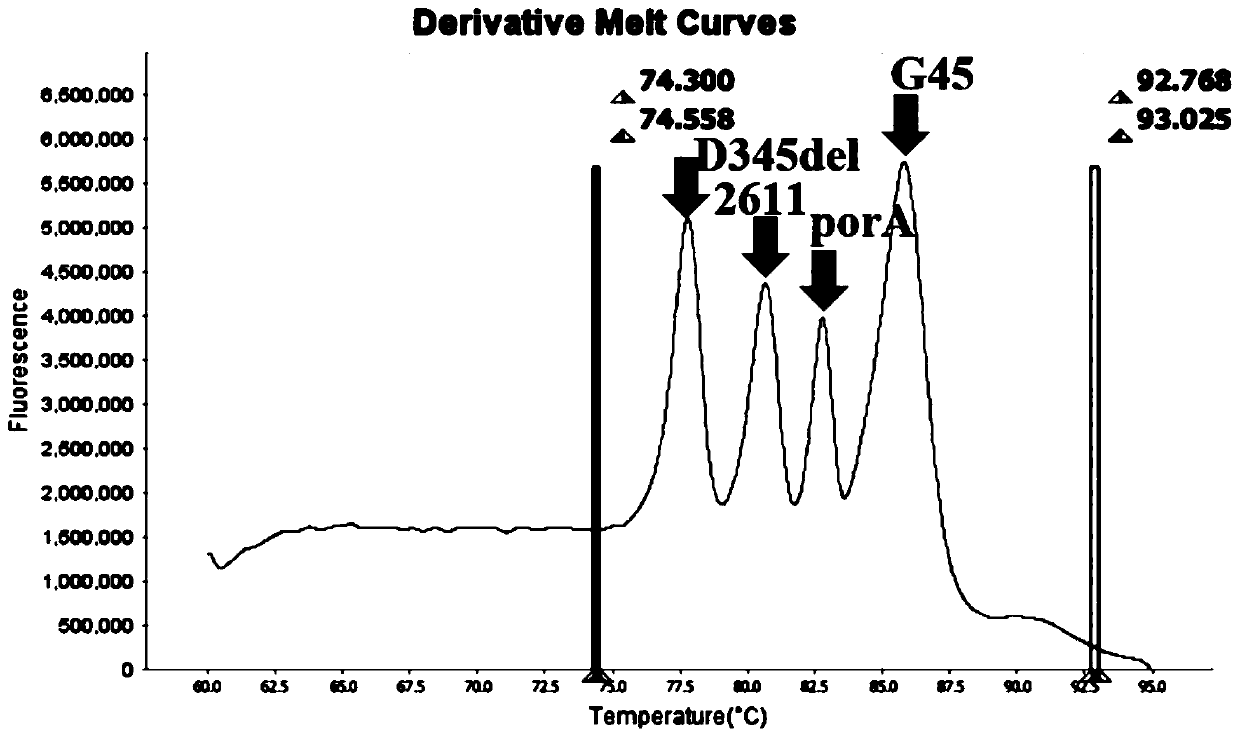
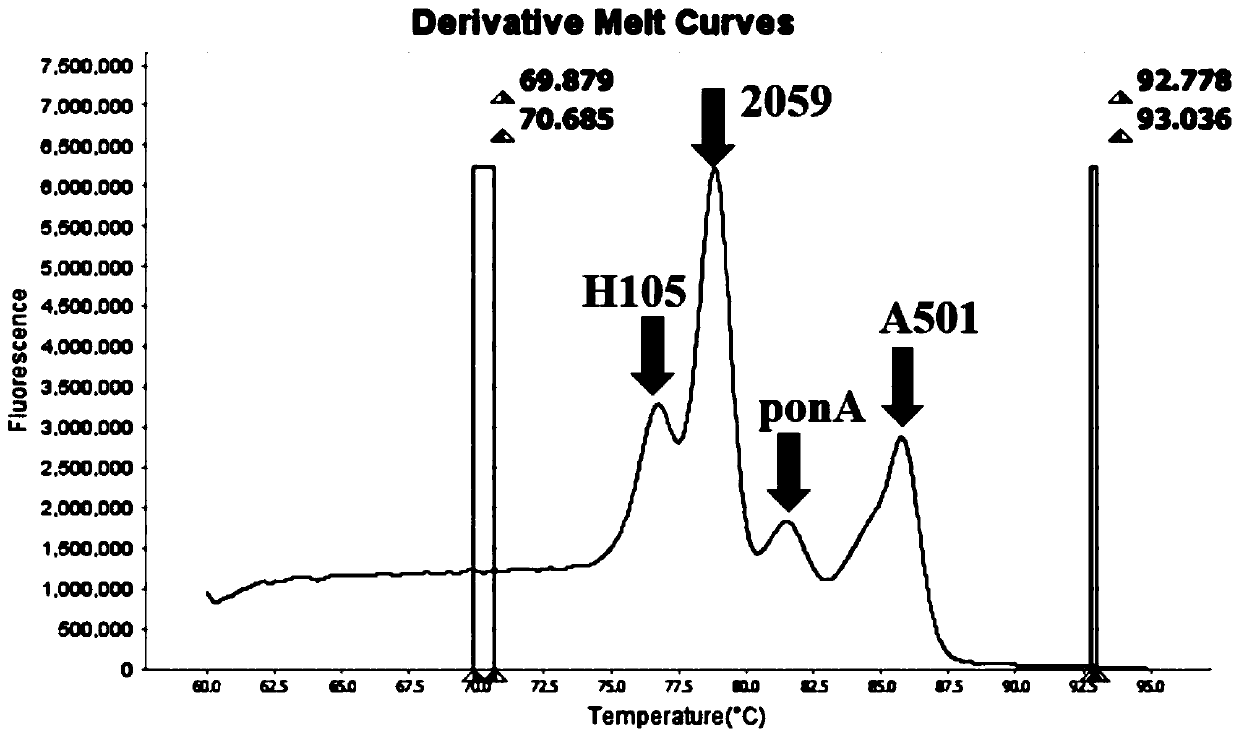
PendingCN110643722AFast and Sensitive AnalysisImprove accuracyMicrobiological testing/measurementMicroorganism based processesHigh Resolution Melt AnalysisRelated gene
The invention belongs to the technical field of molecular biology detection, and relates to a detection method for drug resistance sites, in particular to a method and a kit for multiple detection ofthe drug resistance sites of neisseria gonorrhoeae. A provided specific primer for the multiple detection of the drug resistance sites of the neisseria gonorrhoeae selects drug-resistance-related genes of two drug combinations (ceftriaxone and azithromycin) as target genes for detection, and includes penA, ponA, porB, mtrR, and 23S rRNA. Based on high resolution melting curve analysis technique, the DNA demulsification process is monitored in real time through high resolution melting of PCR products, the mutation condition of the genes is analyzed according to the characteristic change of a melting curve, and thus a basis is provided for determining the drug-resistant condition of the neisseria gonorrhoeae.
Owner:USTAR BIOTECHNOLOGIES (HANGZHOU) CO LTD
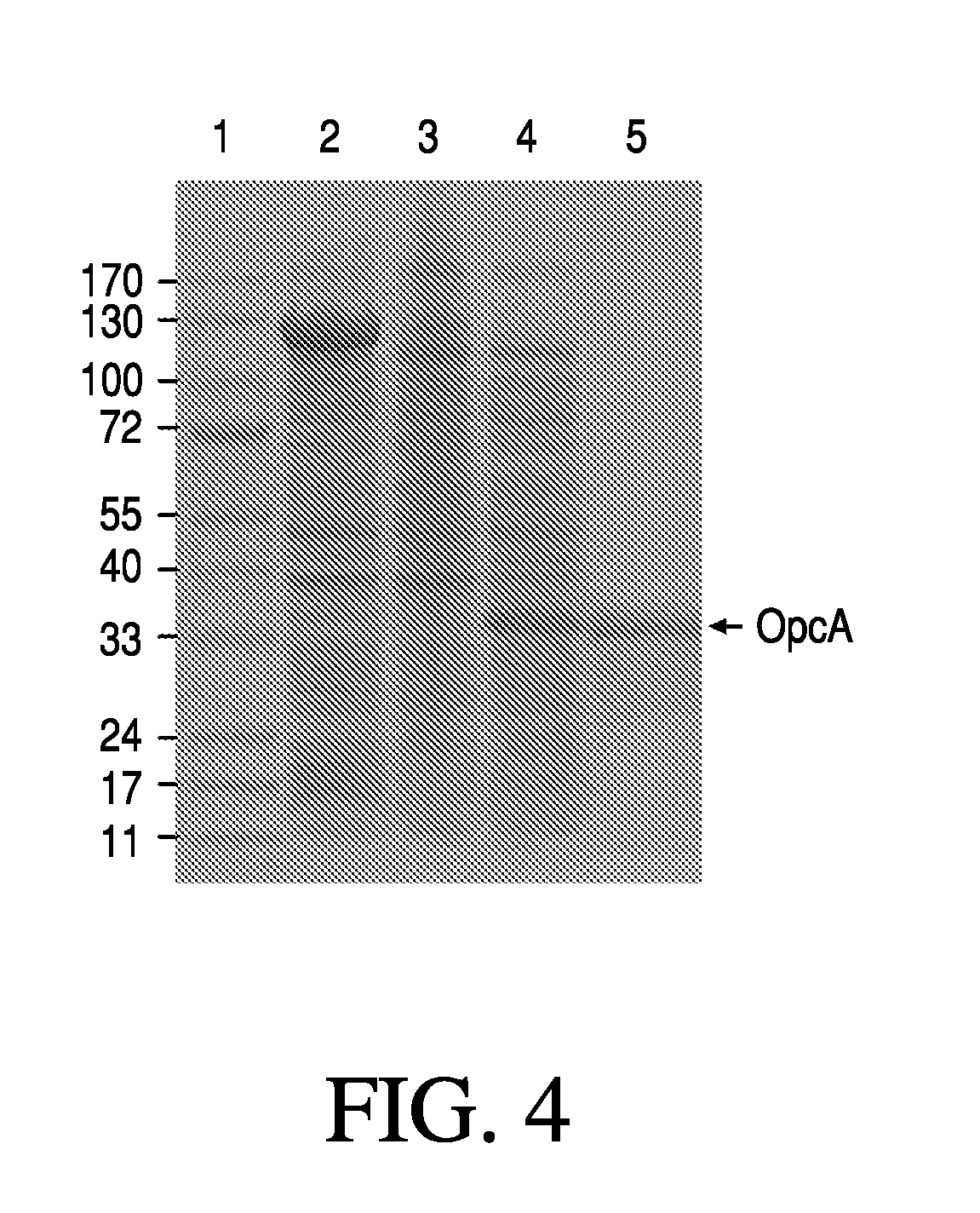
Gonococcal vaccines
The present invention relates to immunogenic compositions comprising a recombinant Neisseria gonorrhoeae OpcA and methods of eliciting an immune response in a mammal by administering a formulation comprising N. gonorrhoeae OpcA or a portion or fragment of N. gonorrhoeae OpcA. The invention also provides for methods and kits for diagnosing N. gonorrhoeae infection using said recombinant N. gonorrhoeae OpcA.
Owner:CREATV MICROTECH
Antigenic neisserial peptides
InactiveUS20070053926A1Efficient transportImprove hydrophobicityAntibacterial agentsNervous disorderAntigenADAMTS Proteins
WO99 / 24578 discloses a large number of proteins from Neisseria meningitidis and Neisseria gonorrhoeae. The present invention relates to fragments of those proteins which comprise at least one antigenic determinant. Homologous sequences and proteins comprising these fragments are also disclosed.
Owner:CHIRON CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com