Acetylisovaleryltylosin tartrate formulation preparation method and prepared acetylisovaleryltylosin tartrate formulation thereof, and pharmaceutical composition
A technology of tivalamectin and composition, which is applied in the field of veterinary drugs, can solve the problems of poor curative effect and low bioavailability of drugs, and achieve the effects of improving clinical treatment effect, delaying release, and prolonging action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
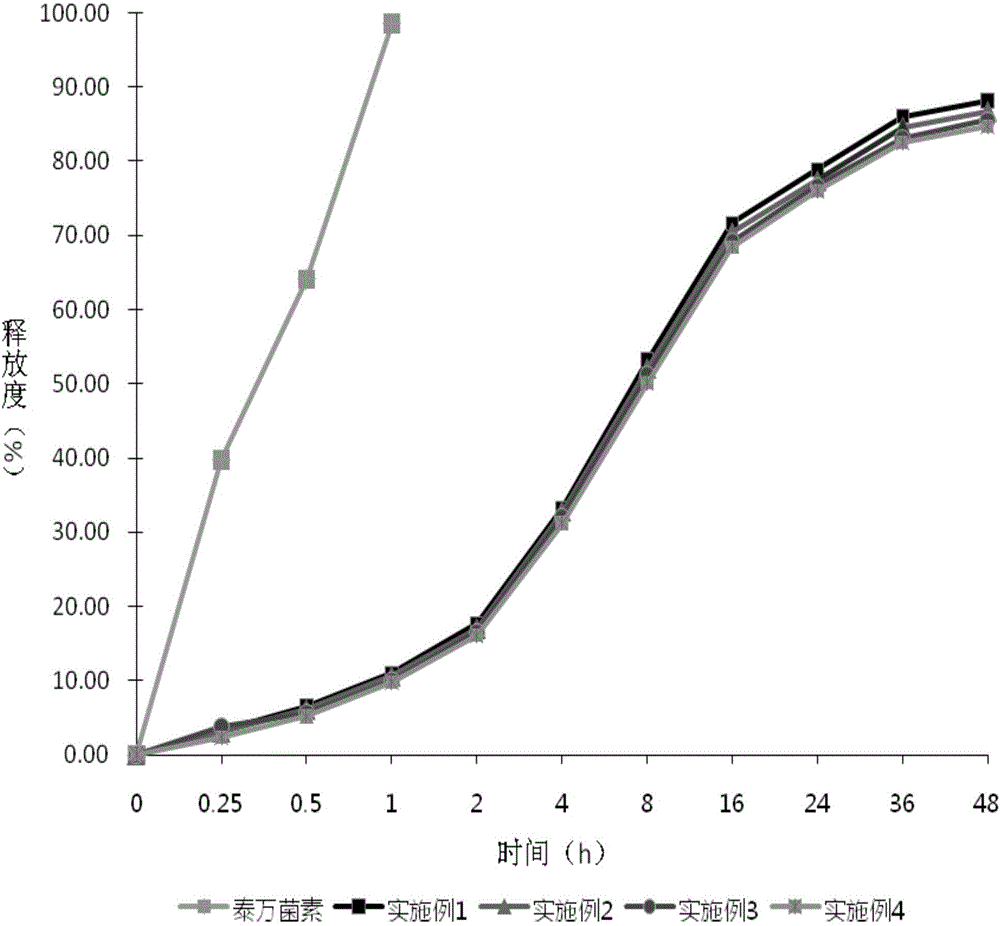
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 tivalamectin preparation of the present invention:
[0030] This example aims to prepare a tyvalancin preparation, including a core material and a wall material, the core material is 100-1000 μm tyvalancin particles in size, the wall material is polylactic acid and gum arabic, the ratio 1:1.
[0031] The preparation method of the tyvanectin preparation comprises the steps of:
[0032] 1. Weigh polylactic acid (molecular weight 5000) and gum arabic wall material and water according to the ratio of 0.05:1 in the ratio of 1:1, dissolve in water, and prepare an adhesive solution;
[0033] 2. Weigh the core material and binder solution according to the ratio of 0.4:1, mix them homogeneously to make a mixture, spray the mixture to dry, the air inlet temperature is 150°C, the outlet air temperature is 60°C, cool , that is, tyvalancin preparations.
Embodiment 2
[0034] The preparation of embodiment 2 tivalamectin preparations of the present invention:
[0035] The purpose of this example is to prepare a kind of tivalamectin preparation, including a core material and a wall material. Gum Arabic in a 1:1 ratio.
[0036] The preparation method of the tyvanectin preparation comprises the steps of:
[0037] 1. Weigh the polylactic acid, gum arabic wall material and water according to the ratio of 0.10:1 in the ratio of 1:1, add it into water to dissolve, and prepare an adhesive solution;
[0038] 2. Weigh the core material and the binder solution according to the ratio of 0.6:1, mix them homogeneously to make a mixture, and spray the mixture to dry (the air inlet temperature is 160°C, and the air outlet temperature is 70°C), After cooling, the tyvalancin preparation is obtained.
Embodiment 3
[0039] The preparation of embodiment 3 tyvanectin preparations of the present invention:
[0040] The purpose of this example is to prepare a tyvalactin preparation, which includes a core material and a wall material, the core material is 100-1000 μm tyvalancin particles in size, the wall material is polylactic acid (molecular weight 30000) and Gum Arabic in a 1:1 ratio.
[0041] The preparation method of the tyvanectin preparation comprises the steps of:
[0042]1. Weigh the polylactic acid, gum arabic wall material and water according to the ratio of 0.10:1 in the ratio of 1:1, add it into water to dissolve, and prepare an adhesive solution;
[0043] 2. Weigh the core material and the binder solution according to the ratio of 0.7:1, mix them homogeneously to make a mixture, and spray the mixture to dry (the air inlet temperature is 170°C, and the air outlet temperature is 80°C), After cooling, the tyvalancin preparation is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com