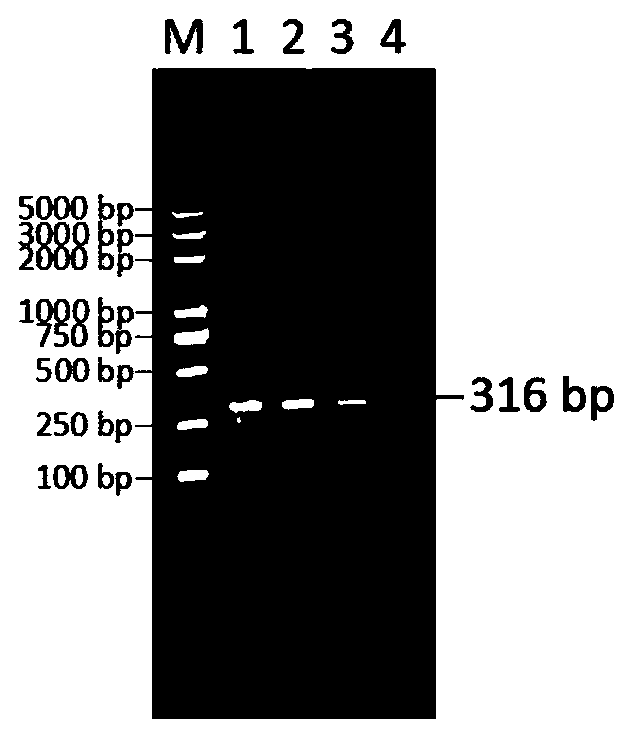
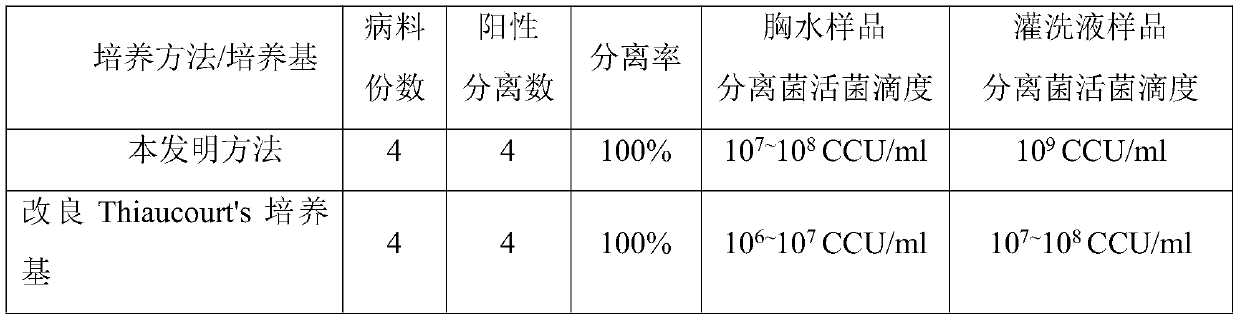
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Allantois" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The allantois (plural allantoides or allantoises) is a hollow sac-like structure filled with clear fluid that forms part of a developing amniote's conceptus (which consists of all embryonic and extra-embryonic tissues). It helps the embryo exchange gases and handle liquid waste.
Method for preparing anti-duck viral hepatitis transfer factor
InactiveCN101953849AFree from infectionSimple manufacturing methodImmunoglobulins against virusesAntiviralsDuck viral hepatitisHepatitis
The invention discloses a method for preparing an anti-duck viral hepatitis transfer factor, which comprises the following steps of: firstly, inoculating duck viral hepatitis in a chick embryo allantoic cavity for multiplication and extracting a virus antigen from the chick embryo allantoic cavity; secondly, immunizing a healthy pig body with the extracted virus antigen; and thirdly, extracting the anti-duck viral hepatitis transfer factor from the spleen of the immune pig. In the invention, specific transfer factor is extracted from duck viral hepatitis viruses and can effectively suppress the duck viral hepatitis viruses and protect the cells of a normal body from being infected with the duck viral hepatitis viruses, so that the duck viral hepatitis is prevented and treated radically. Moreover, the preparation method of the invention is simple and feasible, short in production time and low in cost and has a promising application prospect.
Owner:河南后羿生物工程股份有限公司
Virus production
InactiveUS20050186223A1Increase productionPromote localizationSsRNA viruses negative-senseAnimal cellsChick embryosAllantoic fluid
An improved process for recovery of virus from allantoic fluid of virus-infected chick embryos. Virus associated with granular and fibrous debris in the allantoic fluid can be disassociated from the debris and recovered, thereby increasing viral yield. Dissociation can be achieved by subjecting the virus-debris complex to conditions of increased salt concentrations, e.g., 0.5 M or greater.
Owner:MICROBIX BIOSYSTEMS INC
Preparation methods for duck hepatitis virus immunogen and hyperimmune serum and application of duck hepatitis virus hyperimmune serum
InactiveCN104926939AThe preparation method requires low conditionsEasy to operateSerum immunoglobulinsImmunoglobulins against virusesDuck hepatitis A virusSerum ige
The invention provides preparation methods for duck hepatitis virus immunogen and hyperimmune serum and application of the duck hepatitis virus hyperimmune serum. According to the preparation methods and the application of the duck hepatitis virus hyperimmune serum, the duck hepatitis virus immunogen is obtained through inoculating a serum 1 type duck hepatitis virus CH60 strain DHAV-1 (Duck Hepatitis A Virus type 1) or a serum 3 type duck hepatitis virus CH1 strain DHAV-3 (Duck Hepatitis A Virus type 3) to an allantoic cavity of a chick embryo of 9-10 days old or a duck embryo of 10-12 days old and carrying out proliferation and treatment, and the hyperimmune serum is obtained through mixing the duck hepatitis virus immunogen with a Freund's complete adjuvant or Freund's incomplete adjuvant to prepare solutions of different concentrations, carrying out repeated immunization on immune animals and then sampling and collecting blood and can be applied to the diagnosis and detection on a duck hepatitis virus. The preparation methods provided by the invention have the advantages that the conditional requirements are low, the operation is simple, and the obtained immunogen can meet the requirements on the preparation of specific antiserum.
Owner:SICHUAN AGRI UNIV
Application of H3N2 canine influenza virus CGD1
ActiveCN103223162AReduce severityShorten the detox periodAntiviralsAntibody medical ingredientsTGE VACCINEMicrobiological culture
The invention belongs to the technical field of preparation of virus vaccine, and concretely discloses an application of an H3N2 canine influenza virus CGD1. According to the invention, three self-isolated, identified and preserved strains of H3N2 canine influenza virus CGD1, CGD2 and CGD3 are used to inoculate chick embryo for subcultring, and the CGD1 is found to be good in genetic stability. Therefore, the CGD1 is selected for plaque purification to breed an H3N2 canine influenza virus vaccine strain CIVGDYM1, and the vaccine strain has been preserved in China general microbiological culture collection center, with an accession number being CGMCCNO: 7218. By using the H3N2 canine influenza virus vaccine strain CIVGDYM1 to inoculate the chick embryo for virus breeding, and through gaining allantoic fluid of the chick embryo, inactivating, preparing vaccine and other steps, the safe and effective H3N2 canine influenza virus inactivated vaccine can be prepared.
Owner:SOUTH CHINA AGRI UNIV
Decapeptide inhibiting angiogenesis and use thereof
InactiveCN101597324AEnhanced inhibitory effectSenses disorderPeptide/protein ingredientsDiseaseVascular endothelium
The invention relates to a decapeptide inhibiting angiogenesis and use thereof, belonging to the technical field of biological medicine. The decapeptide inhibiting angiogenesis provided by the invention is deprived from human Homo sapien with an amino acid sequence of YNWNSFGLRF and the molecular weight of 1303.4, and is named as Kp-10. The invention further provides use of the decapeptide inhibiting angiogenesis for preparing medicines for inhibition of angiogenesis, in particular relevant use of Kp-10 for preparing medicines for inhibition of angiogenesis dependent diseases such as cancer, rheumatoid arthritis, psoriasis, diabetes syndrome, and hemangioma. A series of experimental results in VEGF (Vascular Endothelial Growth Factor) induction shows that Kp-10 has obvious inhibition function in the proliferation experiment of human umbilical vein endothelial cells (HUVEC), transfer experiment, micro-tube forming experiment, angiogenesis experiment of chick embryo allantois and mouse cornea experiment, and the decapetide can be used for preparing medicines for inhibiting angiogenesis.
Owner:EAST CHINA NORMAL UNIV
Anti-tumor biological polysaccharide
The invention discloses an anti-tumor biological polysaccharide; the polysaccharide is obtained from chick embryo allantoic fluid and chick embryo through separating and purifying; the invention further discloses a specific purification technology of the polysaccharide, wherein the technology is to extract the biological polysaccharide by a method combined with salting-out and membrane technology. By adopting the technology disclosed by the invention, the purifying purity and the recovering rate of chick embryo allantoic fluid and chick embryo are greatly enhanced; through preclinical pharmacodynamic experiments, the biological polysaccharide has an exact killing ability to transplant human body tumors such as human primary liver cancer and ovarian cancer in nude mice, and can completely remove the transplant human body tumor cells from the nude mice after being fed with enough dosage and enough time. The polysaccharide disclosed by the invention is a biological active material with no toxicity and low side effect and is very safe; in addition, the polysaccharide is wide in drug administration route, simple in production process and high in recovering rate, and thus being a novel anti-tumor drug capable of providing great social benefit and economic benefit.
Owner:乔民 +1
Novel process for preparing influenza virus split vaccine
ActiveCN102133399AHigh yieldIncrease the effective antigen contentAntiviralsAntibody medical ingredientsHemagglutininPurification methods
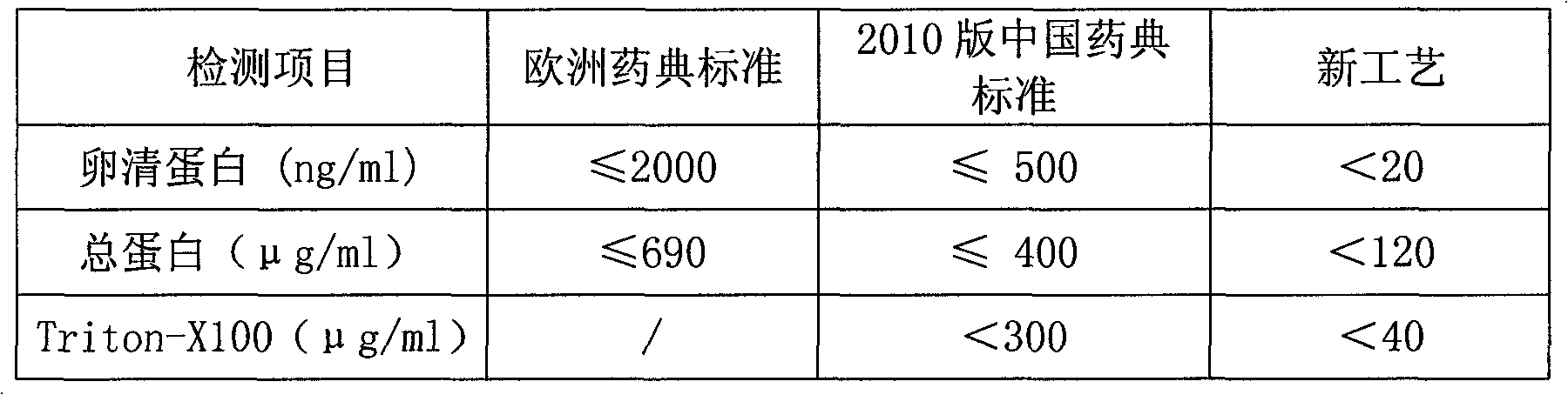
The invention relates to a novel process for preparing influenza virus split vaccine, comprising the following steps of: (1) inoculating and culturing viruses: inoculating working seed lot viruses diluted to virus amount of 2.0-0.6LgEID50 / ml in chick embryo allantois, and culturing at 33-35 DEG C for 48-72h; (2), harvesting and inactivating viruses and concentrating a virus harvest liquid; (3) purifying and splitting viruses: adding TritonX-100 with volume ratio of final concentration of 0.3-0.5% and deoxysodium cholate in a purified virus liquid, mixing evenly and placing at 20-30 DEG C for 90-120min; (4) adding a PB split agent; and (5) sterilizing, filtering and then preparing a semi-finished product: diluting each stock solution to hemagglutinin content of 30micrograms / strain / ml by 0.01mol / L of PBS (Phosphate Buffered Saline) with a pH value of 7.2, and filtering by a filter membrane with aperture of 0.22micron to obtain a semi-finished product. The novel process for preparing influenza virus split vaccine has the beneficial effects that: due to the increase of a novel purification method, high yield can be achieved, effective antigen content can be improved and the contents of residual ovalbumin and split agent causing inoculation reaction can be greatly reduced.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
Inactivated vaccine of cow chlamydia, its preparation and inspection method
ActiveCN1698892AFight infectionInfection fromChlamydiaceae ingredientsAntiinfectivesMicrocosmic saltOil adjuvant
The invention relates to an inactivated vaccine of cow chlamydia, its preparation and the related inspection method during the vaccine preparation. The preparing process comprises diluting the Chlamydia psittaci SX 5 or NX with microcosmic salt buffering liquid or physiological saline, vaccinating to healthy chick embryo hatched at 37 deg. C for 6-7 days, harvesting vitelline membrane and allantois liquid of dead chick embryo after 72 hours as antigens, triturating the antigens, diluting and charging formaldehyde for deactivation, mixing the deactivated antigens with oil adjuvant by the proportion of 1:1, stirring homogeneously, carrying out homogeneous emulsion to obtain the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Anti-H7N9 subtype avian influenza virus monoclonal antibody epitope as well as screening method and application thereof
The invention provides an anti-H7N9 subtype avian influenza virus monoclonal antibody epitope as well as a screening method and application thereof, belonging to the technical field of immunodetection. The screening method comprises the following steps: mixing wild type H7N9 subtype avian influenza virus liquid with a corresponding monoclonal antibody with a neutralizing property for incubation, and inoculating the mixture to an SPF chick embryo, so as to obtain allantoic fluid with a positive hemagglutination titer; and carrying out gradient dilution on the positive allantoic fluid, mixing the positive allantoic fluid with the monoclonal antibody for incubation, inoculating the mixture to the SPF chick embryo, determining a hemagglutination inhibition titer of the monoclonal antibody by selecting the allantoic fluid with the positive hemagglutination titer as an antigen, when the determined hemagglutination inhibition titer is lower than the hemagglutination inhibition titer of a wild type virus by 8log2, determining the positive allantoic fluid as an escape mutant of the wild type H7N9 subtype avian influenza virus, measuring an HA gene sequence of the positive allantoic fluid, and determining the epitope recognized by the monoclonal antibody. By virtue of the method, the specific epitope can be clearly screened; the method is simple, accurate and short in screening period.
Owner:YANGZHOU UNIV
Application of plumbagin in preparing the medicine for preventing the blood vessel from regenerating
InactiveCN101283994AInhibit angiogenesisPrevent proliferationOrganic active ingredientsSenses disorderDiseaseDiabetes retinopathy
The invention relates to a new application of plumbagin, particularly to an application of the plumbagin in preparing drugs for inhibiting tumor angiogenesis, psoriasis, Kaposi's sarcoma, diabetic retinopathy, aleukemic leukemia, rheumatoid arthritis, duodenal ulcer, myeloma, and lymphomata. The experimental results show that the plumbagin can inhibit the proliferation, migration and canalization of endothelial cells, and inhibit the angiogenesis of chick embryo allantoic membrane. Therefore,the plumbagin can be used for preparing drugs for inhibiting the angiogenesis.
Owner:EAST CHINA NORMAL UNIV
Duck embryo early sex identification method and special kit
ActiveCN104698173ADoes not affect normal developmentImprove welfareMaterial analysisDiseaseAnimal science
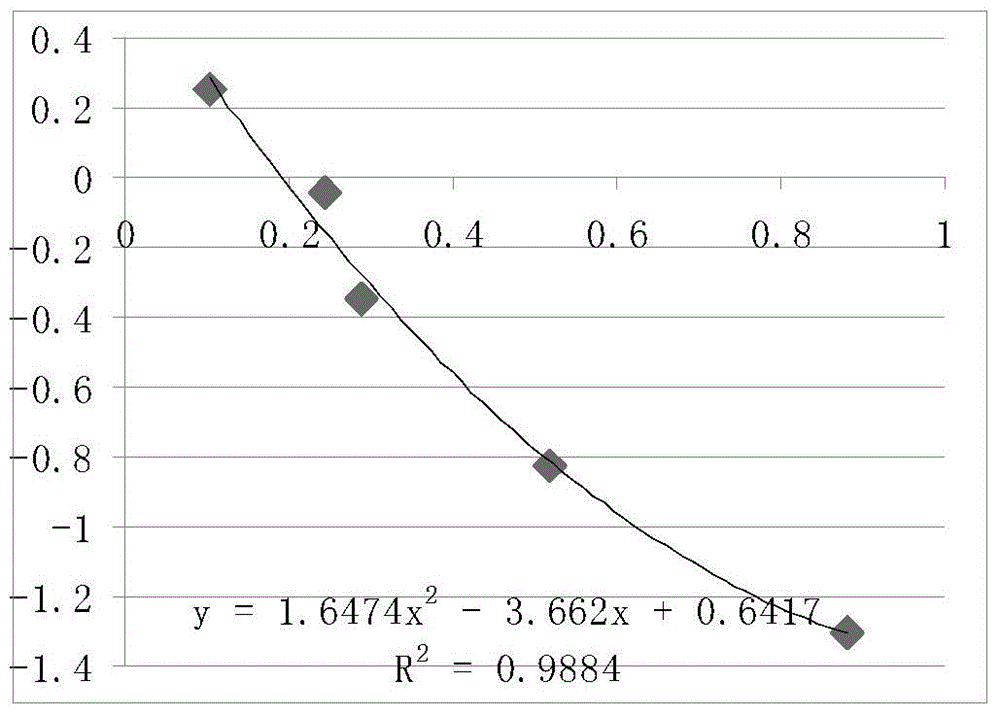
The invention belongs to the technical field of poultry sex detection, and in particular relates to a duck embryo early sex identification method and a special kit. The duck embryo early sex identification method is established by collecting a hatched early duck embryo allantoic fluid, making use of a monoclonal antibody for estradiol detection and carrying out antigen-antibody reaction. Male and female sexes are judged according to a characteristic that the content of estradiol in a female embryo is higher than 1ng / ml and the content of the estradiol in a male embryo is lower than 0.6ng / ml. The identification method disclosed by the invention can identify the female and male sexes in an early hatching period without an influence on the further development of the embryo; and the identification method is conducive to animal welfare and relieving the transmission of vertical diseases.
Owner:武汉赛维尔生物科技有限公司
Anti-H3N8 subtype equine influenza virus monoclonal antibody and application thereof
InactiveCN101892200ANo cross reactionMicroorganism based processesImmunoglobulins against virusesSerodiagnosesEquine infectious anemia
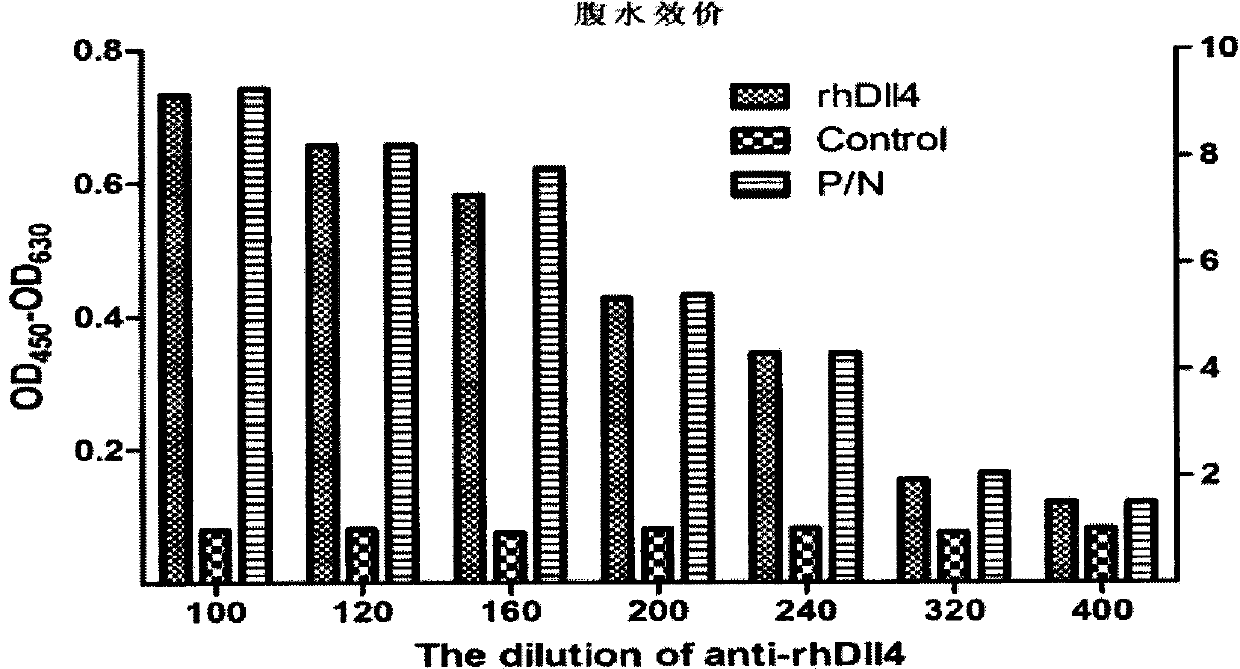
The invention discloses an anti-H3N8 subtype equine influenza virus monoclonal antibody and an application thereof. The anti-H3N8 subtype equine influenza virus monoclonal antibody is secreted by hybridoma cell strains with the microbial collection number of CGMCC No.3543. EL1SA, and Western-bolt methods prove that the monoclonal antibody of the invention has no cross reaction with equine infectious anemia virus, equine arteritis virus and normal chick embryo allantois fluid; and the abdominal dropsy ELISA valence of the H3N8 / 10 of the invention is 1:1.6*104. The anti-H3N8 subtype equine influenza virus monoclonal antibody of the invention can be applied to antigenicity analysis on A-type equine influenza virus, serodiagnosis, vaccine quality monitoring, epidemiological survey and other aspects.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Pigeon paramyxo virus type 1 PPMV-1/BJ-C strain and application thereof
InactiveCN112063596AImproving immunogenicityGood cultivation characteristicsSsRNA viruses negative-senseViral antigen ingredientsAdjuvantVirus type
The invention provides a pigeon paramyxo virus type 1 PPMV-1 / BJ-C strain and application thereof. The attenuated strain PPMV-1 / BJ-C is constructed by mutating an F protein cleavage site of the attenuated strain PPMV-1 / BJ-C into a La Sota strain corresponding site on the basis of a pigeon plague virus virulent strain PPMV-1 / BJ. PPMV-1 / BJ-C has the advantages of good immunogenicity, good culture characteristics, stable titer and the like, the pigeon plague virus PPMV-1 / BJ-C strain is inoculated to 9-11-day-old SPF chick embryos through an allantoic cavity, allantoic fluid of infected chick embryos is harvested and inactivated with diethyleneimine, and then an aluminum hydroxide adjuvant is added to prepare a pigeon plague virus inactivated vaccine. The inactivated vaccine prepared from the PPMV-1 / BJ-C strain can generate a relatively high antibody, can prevent ND caused by pigeon plague virus, and the vaccine has the advantages of safety, quick response, lasting immune period and the like, and has wide application prospects.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
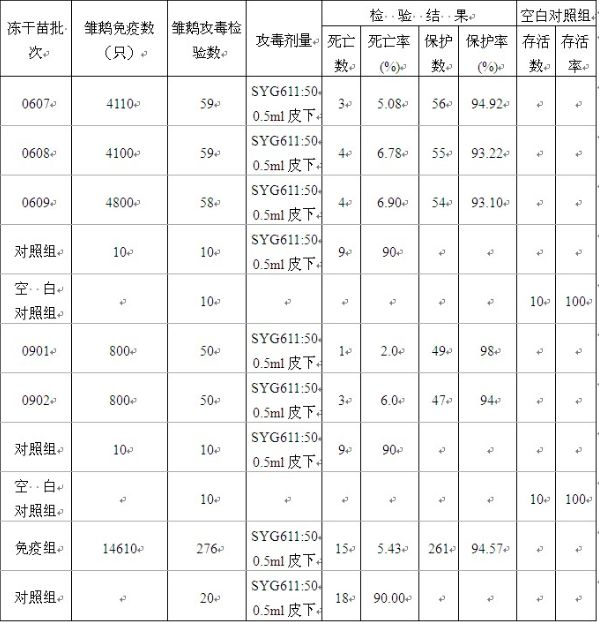
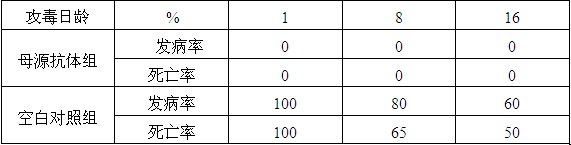
Preparation method of live vaccine for gosling plague and live vaccine prepared therefrom
InactiveCN101972473AIncrease productionImprove stabilityAntiviralsAntibody medical ingredientsPenicillinDisinfectant
The invention discloses a preparation method of a live vaccine for gosling plague and a live vaccine prepared therefrom. The preparation method of the live vaccine for the gosling plague comprises the steps of screening the breeding virus SYG61 of the gosling plague; diluting the breeding virus of the gosling plague viruses by utilizing sterilizing PBS (Phosphate Buffer Solution) in the ratio of 1 to 100; inoculating a well-developed goose embryo with 12 days, wherein each embryo flocking urine cavity is 0.2ml; the goose embryo dying in 48 to 144 hours is placed into a refrigerator with 2 to 8 DEG C for 4 to 24 hours; soaking the cooled goose embryo into a 5% bromo-geramine disinfectant for 5 to 10 minutes; placing into a sterile chamber after wiping drily; disinfecting a gas chamber eggshell part by utilizing 5% iodine tincture; opening the eggshell in a sterile procedure; eliminating an egg membrane and tearing a flocking urine membrane; sucking allantoic fluid and amniotic fluid; adding 5% sucrose skimmed milk as a stabilizing agent; simultaneously adding 1000 units / ml of penicillin and streptomycin respectively; fully agitating; quantitatively subpackaging; and rapidly refrigerating and drying in a vacuum state after subpackaging. The preparation method of the live vaccine for the gosling plague of the invention has the advantages of fewer steps and easy operation. Moreover, the prepared live vaccine for the gosling plague has the advantages of high yield, good stability, low cost and good immunity protection effect.
Owner:SINOPHARM YANGZHOU VAC BIOLOGICAL ENG CO LTD
2/16-site-substituted chalcone derivative taking estrogen as mother nucleus and preparation method and application of derivative
The invention belongs to the field of medicinal chemicals, and relates to a 2 / 16-site-substituted chalcone derivative taking estrogen as a mother nucleus and a preparation method and application of the derivative. The preparation method comprises the following step: combining chalcone structural fragments on an estrogen body in parallel, thereby keeping or improving the activity of anti-tumor cells and inhibiting activity of tumor cell angiogenesis. The structural formula of the 2 / 16-site-substituted chalcone derivative is as shown in the specification. The in-vitro anti-tumor cell proliferation activity test and the chick embryo allantois test show that the derivative has certain anti-tumor cell proliferation activity and inhibiting activity of tumor cell angiogenesis; and the dose-effect relationship study shows that the tumor cell inhibition function of the derivative is prior to that of 2-methoxy estradiol with broad-spectrum anti-tumor activity, and moreover the half-life period can be prolonged.
Owner:ZHENGZHOU UNIV
Eggshell precise hole cutting device for chicken embryo experiment
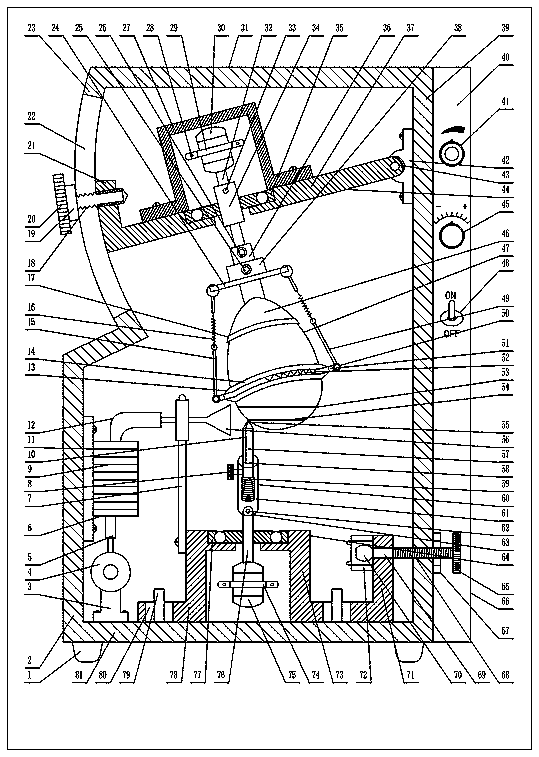
The invention provides an egg shell precise hole cutting device for a chicken embryo experiment. A rotating assembly mounted in a rigid shell with a shock absorbing foot pad drives a clamping assemblywith position adjustable and a to-be-cut eggshell to rotate, a rigid shell base plate is provided with a displacement assembly with an elastic drill bit for cutting the to-be-cut eggshell, and when aball head handle installed in a right shifting screw hole on the right side of the rigid shell is screwed in and screwed out, the shifting assembly and the elastic drill bit are driven to move left and right to cut holes on the to-be-cut eggshell; an absorption assembly with an umbrella-shaped suction opening installed on the left side of the rigid shell can adsorb eggshell debris through a negative pressure fan and a filter box body; and a rotation speed knob, a drill speed knob and a power switch which are mounted on the right side control panel of the rigid shell can adjust the rotation and cutting speed and start cutting. The device has the beneficial effects that the eggshell of the chicken embryo experiment can be subjected to undamaged inner membrane and fine and accurate hole cutting operation with standard round holes, and standardized egg-type chicken embryo urinary bladder membrane exposure scientific research and application can be stably and safely achieved.
Owner:TIANJIN HOPE IND & TRADE
Chicken embryo culture method of Portunus tritubereulatus reovirus
InactiveCN102628030AWide range of sensitivityEasy accessMicroorganism based processesViruses/bacteriophagesAmniotic fluidViral culture
The invention discloses a chicken embryo culture method of Portunus tritubereulatus reovirus and is characterized by comprising the following steps of: using SPF chicken embryo as an in vitro culture system of Portunus tritubereulatus reovirus, selecting healthy chicken embryo of 3 and 4 days-old, inoculating a prepared Portunus tritubereulatus reovirus inoculated solution onto a chorioallantoic membrane of the chicken embryo, continuously culturing until the chicken embryo is dead, collecting dead chicken embryo cultured for 3-10 days, separating the chorioallantoic membrane and amniotic fluid, mixing the chorioallantoic membrane and the amniotic fluid, homogenizing the mixture, centrifuging and filtering to obtain a supernatant of Portunus tritubereulatus reovirus. The invention has the following advantages: a more efficient and adaptive Portunus tritubereulatus reovirus in vitro culture method is provided for rapid and effective in vitro subcultring of the virus; and the method overcomes the defect that there is no adaptive culture system during the culture process of Portunus tritubereulatus reovirus, fills the blank of chicken embryo culture system of crab virus and has laid a foundation for further research and application.
Owner:NINGBO UNIV
Application of butein in preparing the medicine for preventing the blood vessel from regenerating
InactiveCN101336909AEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsDiseaseMyeloid leukemia
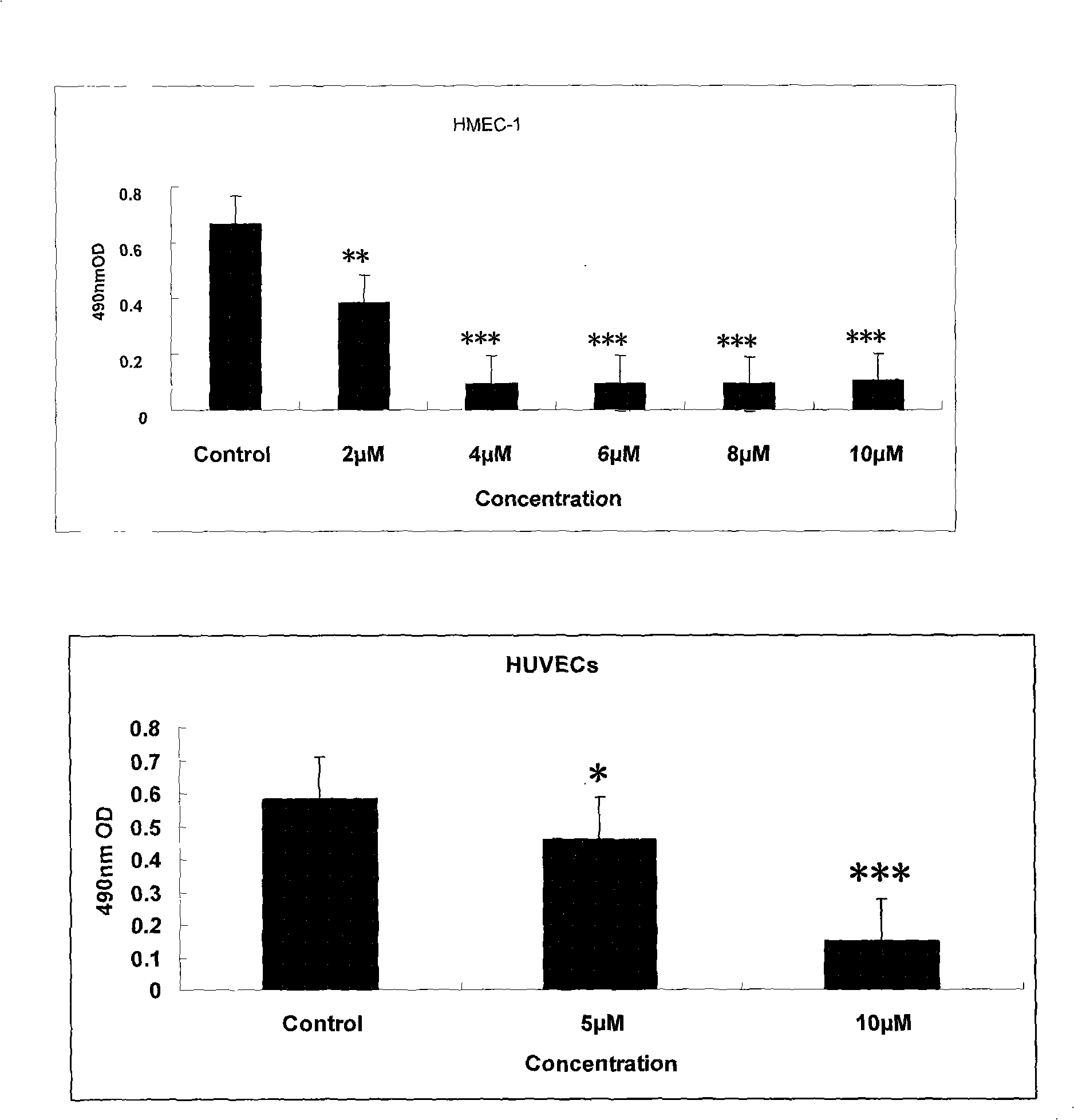
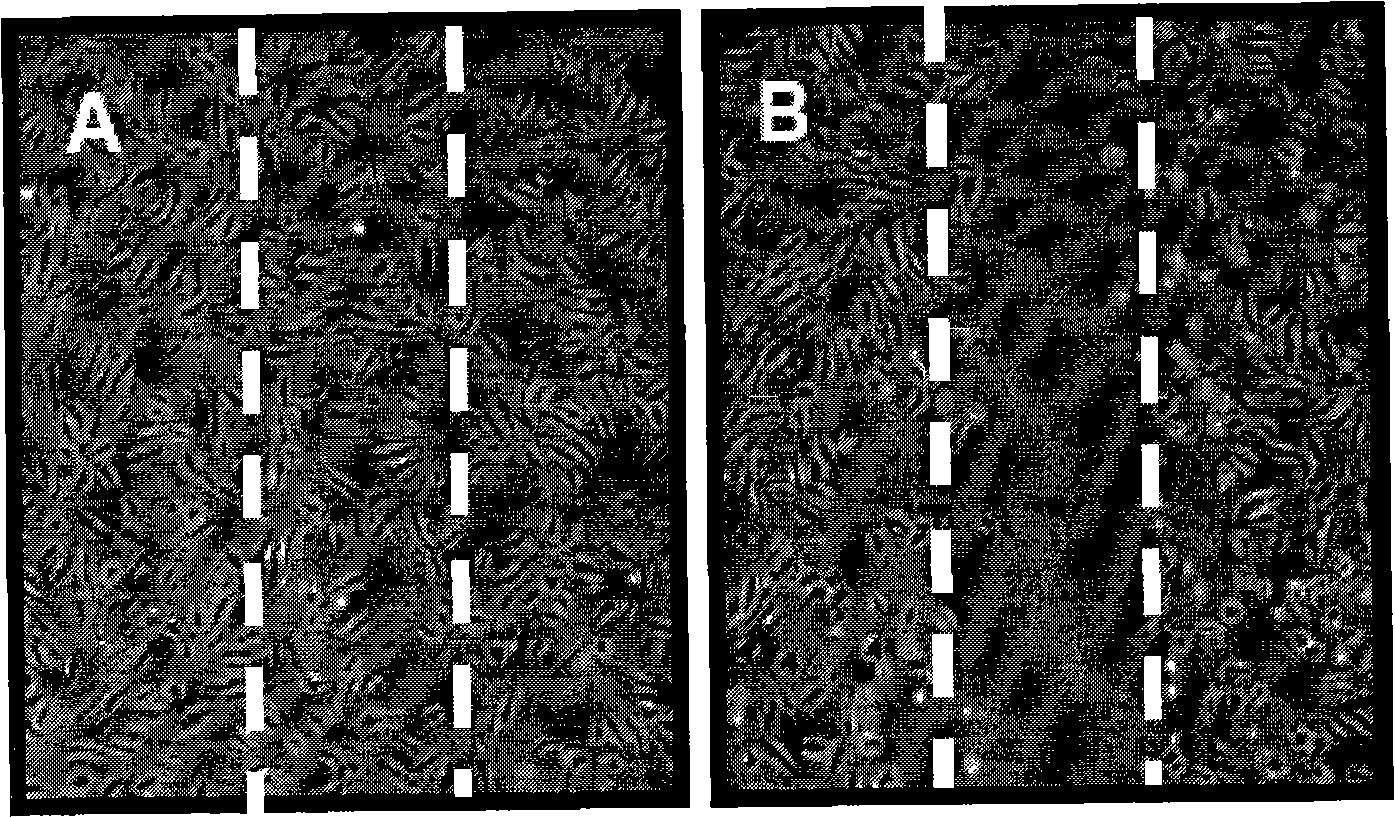
The invention relates to a new application of butein, particularly to an application of butein in preparing angiogenesis inhibitory drugs for treating angiogenesis-dependent diseases such as breast cancer, colon cancer, lymphomata, acute myeloid leukemia, rheumatoid arthritis, psoriasis, diabetes syndrome, hemangioma, etc. The butein has distinct inhibitory action in both human umbilical vein endothelial cells (HUVEC) proliferation experiment, HUVEC migration experiment, HUVEC invasion experiment, human microvascular endothelial cells (HMEC-1) canalization experiment, chick embryo allantois angiogenesis, etc.; and can be used in preparing angiogenesis inhibitory drugs.
Owner:EAST CHINA NORMAL UNIV
Anti-human Delta like4 monoclonal antibody
InactiveCN103804497AEffectively perform biological functionsGenetic material ingredientsImmunoglobulins against animals/humansHeavy chainNucleotide
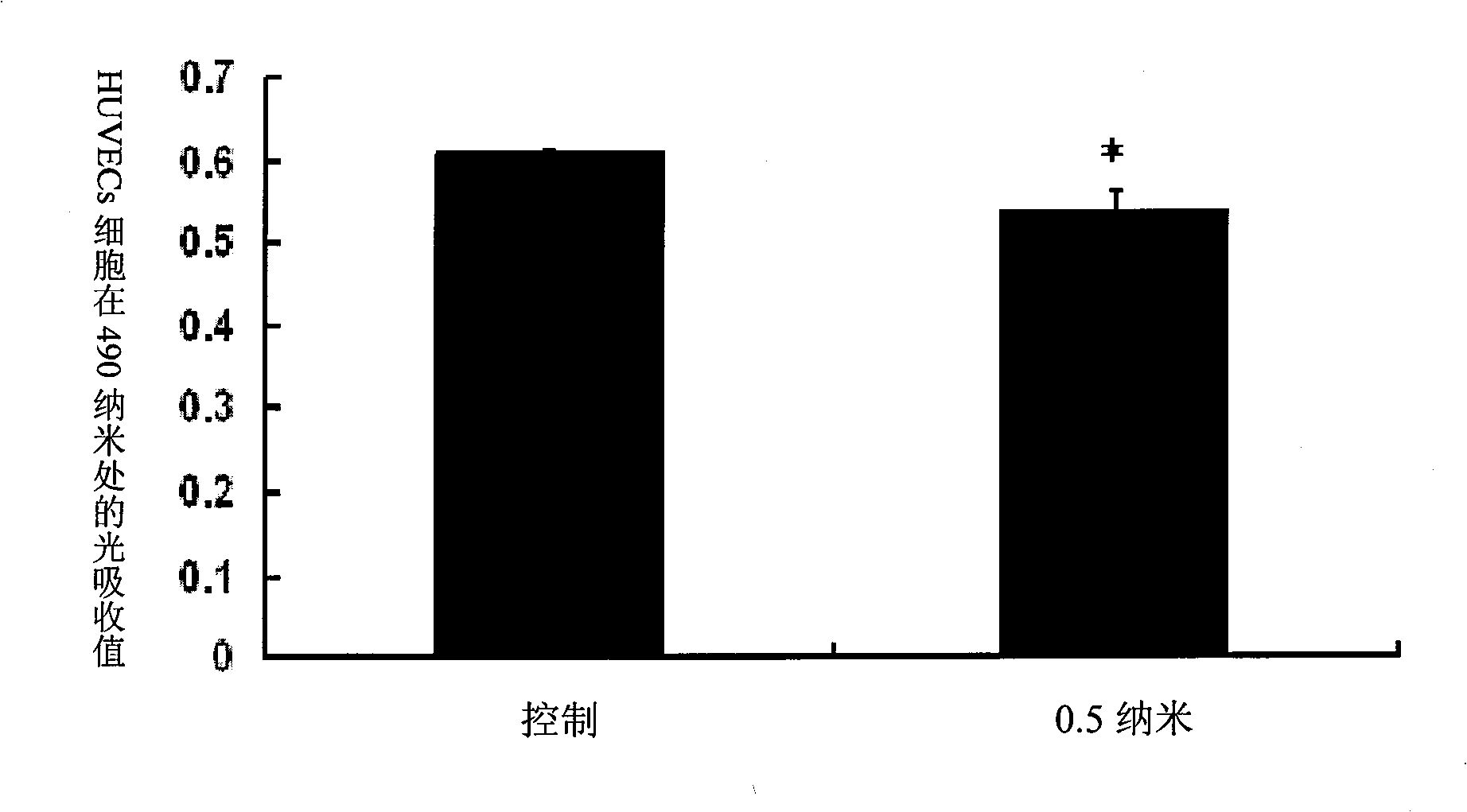
The invention relates to an anti-human Delta like4 (hD114) monoclonal antibody. The monoclonal antibody or a segment thereof is characterized by being capable of specifically combining with hD114, blocking off the inhibition of D114 on the proliferation of human umbilical vascular endothelial cells (HUVEC), and promoting the proliferation of the blood capillary in a chick embryo allantois membrane model. The invention specifically discloses a screening method and preparation method of the anti-human Delta like4 monoclonal antibody. The invention further discloses nucleotide and amino acid sequences, including nucleotide and amino acid sequences corresponding to complementary determining regions CDR1, CDR2 and CDR3, of heavy chain variable regions and light chain variable regions of the anti-human Delta like4 monoclonal antibody.
Owner:CHINA PHARM UNIV
Preparation method of standard sample of H9N2 subtype avian influenza virus
The invention discloses a preparation method of a standard sample of a H9N2 subtype avian influenza virus. The preparation method comprises the following steps: continuously diluting the H9N2 subtype avian influenza virus with normal saline ten times to the concentration of 10<8>, inoculating five 10-days-old SPF (specific pathogen free) embryonated eggs into each dilution of H9N2 subtype avian influenza virus, wherein each embryonated egg is 0.1ml, continuously hatching the embryonated eggs at the temperature of 37 DEG C after the inoculation, and lighting the embryonated eggs everyday; b, gaining inoculated embryonated eggs dying after 72-120 hours and allantoic fluid of the embryonated eggs living for 120 hours one by one, respectively measuring HA(hyaluronic acid) value of the allantoic fluid of each embryonated egg, and taking the allantoic fluid in one embryonated egg infected by a highly diluted virus; C. orderly repeating the step a and the step b twice, wherein the H9N2 subtype avian influenza virus of the step a is a transferred inoculant produced by the step b in the repetitive process; and d. finally diluting the transferred inoculant produced by the step b with normal saline ten times, inoculating ten-times diluted solution to the inside of an allantoic cavities of the 10 to 11-days-old SPF embryonated eggs, continuously hatching the SPF embryonated eggs, lighting the embryonated eggs everyday to obtain inoculated embryonated egg allantoic fluid dying after 72 to 120 hours, subpackaging resultant with ampoule, and storing the ampoules at the temperature of minus 80 DEG C.
Owner:DALIAN NATIONALITIES UNIVERSITY
IBV-III antigen production process
The invention supplies a manufacturing technique of IBV-HI antigen, the preparation process of antigen is that: the virus fluid of containing the protein virus is isolated from chicken embryo allantois fluid of containing respiratory type IBV the virus and blending with clostridium filtrate of rabbits A type wei surname as a culture medium, after two hours water bath action with 37deg.C, put the fluid into the 4deg.C fridge for 48 hours, through freeze drying to get antigen, the related centrifuge divided into two centrifugal, first the mixed fluid through high speed centrifugation, choose the supernatant after the centrifugation; then it progress the two centrifugation as ultracentrifugation, the time is 2-3h, the rotation rate of centrifugation is 25000-35000g, abandon the supernatant after the centrifugation, and use deposit suspension to obtain virus fluid. Using this invention method, infectious bronchitis HI antigen of respiratory system has relatively low cost, type specificity, high sensitivity, stability feature, it makes HI method to detect respiratory infectious bronchitis feasible, and enhance the credibility and maneuverability of its detection.
Owner:秦卓明 +2
Chick embryo culture method for White spot syndrome virus
The invention discloses a chick embryo culture method for White spot syndrome virus, which is characterized in that a SPF chick embryo is employed as a in vitro culture system of the White spot syndrome virus, a healthy chick embryo precultured for 6-7 days is selected, the prepared degermd White spot syndrome virus is inoculated to a chick embryo allantois membrane, and the death chick embryo with the culture time of 5-10 days is collected for homogenate and centrifugation to obtain a supernatant containing White spot syndrome virus, the culture method provided by the invention has the advantage that a WSSV in vitro culture method with high efficiency and effective application is provided for rapidly and effectively in vitro subcultring on WSSV, the problems of no appropriate culture system during WSSV culture process and difficult virus subcultring in a current culture method can be overcome, thereby the application of the chick embryo culture method in WSSV can supply the blank of the White spot syndrome virus chick embryo culture system and establish a base for further research and application.
Owner:NINGBO UNIV
Preparation method of serum-free mycoplasma ovipneumoniae antigen
PendingCN110452855AEasy to operateAntibacterial agentsBacterial antigen ingredientsSerum freeMycoplasma antigen
The invention provides a preparation method of serum-free mycoplasma ovipneumoniae antigen. The method comprises the following steps: (1) preparation of chicken embryos: preparing the healthy SPF chicken embryos at 7-8 days of age, the embryos are vigorous, clear in blood vessels, and cultured in an incubator; (2) preparation of mycoplasma ovipneumoniae inoculum; and (3) chicken embryo inoculation, culture and harvest: inoculating the healthy SPF chicken embryos of 7-8 days old in step (1) of the mycoplasma ovipneumoniae inoculum, 0.2ml of the inoculum is inoculated for each chicken embryo, After inoculation, the incubator is further cultured for 5 days, and the allantoic fluid of dead chicken embryos and / or the allantoic fluid of chicken embryos that do not died after 5 days are aseptically obtained to obtain the serum-free mycoplasma ovipneumoniae antigen. In the present invention, the mycoplasma ovipneumoniae is subcultured in chicken embryos, and the allantoic fluid of chicken embryos is collected to obtain the serum-free mycoplasma ovipneumoniae antigen. The method of the present invention does not use serum, and can avoid many problems in using serum.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for cloning complete sequence of muscovy duck origin goose parvovirus genome encoding area
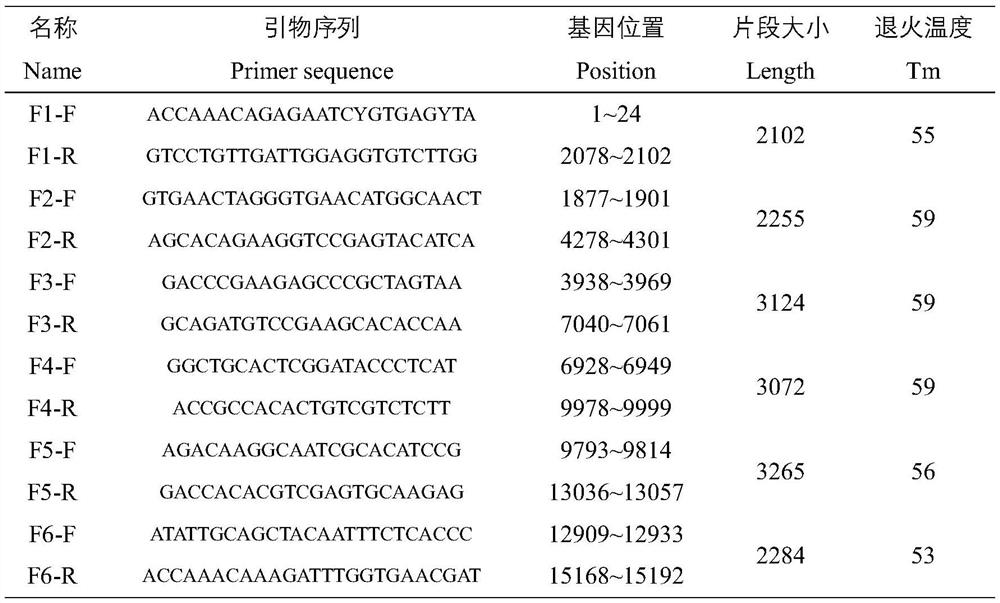
InactiveCN104232672AEasy accessReduce workloadMicroorganism based processesVector-based foreign material introductionAllantoisCoding region
The invention discloses a method for cloning a complete sequence of a muscovy duck origin goose parvovirus genome encoding area. A viral genome DNA is extracted from muscovy duck embryo allantoic fluid containing a muscovy duck origin goose parvovirus; with the viral genome DNA as a template, a gene segment containing the complete sequence of the encoding area is amplified by virtue of designed specific primers; a target gene segment is connected with a carrier; and positive colonies are selected to be subjected to sequencing, so as to directly obtain the complete sequence of the muscovy duck origin goose parvovirus genome encoding area. Compared with a traditional method, the method disclosed by the invention has the characteristics of long amplified target segment, small experimental workload, low cost and high efficiency.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Gosling plague virus originated from muscovy ducks
ActiveCN105907728AImprove securityAvoid infectionViral antigen ingredientsMicroorganism based processesUltrafiltrationOil emulsion
The invention aims at providing a gosling plague virus originated from muscovy ducks. The gosling plague virus YBGPV-M strain originated from muscovy ducks is preserved in China Center for Type Culture Collection (CCTCC) on March 31st, 2016 with the preservation serial number of CCTCC NO: V201620. The screened gosling plague virus originated from muscovy ducks is used preparing a vaccine. The gosling plague virus YBGPV-M strain which is originated from muscovy duck and is high in virus content and good in immunogenicity is selected and then is inoculated to duck embryos, infected embryos and allantois liquid are collected and subjected to homogenation, ultrafiltration and concentration and inactivation with a formaldehyde solution, an oil emulsion is added and mixed for emulsion, and the vaccine is prepared. The prepared vaccine has the advantages of being efficient and high in safety.
Owner:YEBIO BIOENG OF QINGDAO
Competitive particle immunoassay methods utilizing fluorescence microscopy
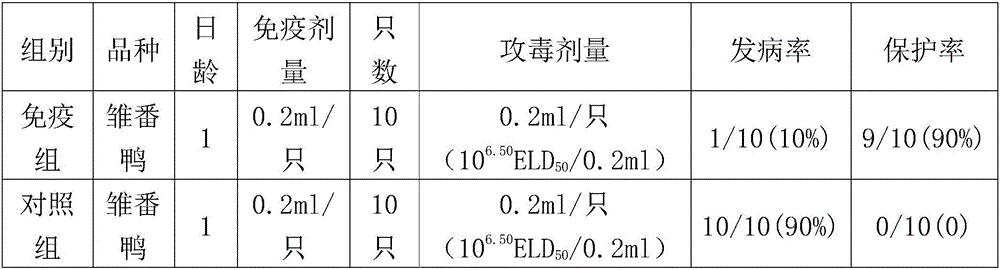
The present invention provides a method for measuring an analyte in a sample by a competitive immunoassay using fluorescence microscopy. In a specific embodiment, the present invention provides a method for determining the sex of an avian embryo in ovo by determining the presence of an estrogenic steroid compound in a sample of embryonic fluid (eg, allantoic fluid or blood) of an avian egg (Figure 1).
Owner:EMBREX INC
Isolated culture method of Mycoplasma capricolum subsp.Capripneumoniae (Mccp)
PendingCN110551634AOvercome many problems caused by usingLow costBacteriaMicroorganism based processesAntigenUrine
The invention provides an isolated culture method of Mycoplasma capricolum subsp.Capripneumoniae (Mccp). The method comprises the following steps: (1), treatment of a pathological material: a treatedsample solution is obtained; (2), sample inoculation: the treated sample solution is diluted by 0.01 M of sterile PBS (phosphate buffer solution) with pH of 7.2, 7-8-day-old healthy SPF (specific pathogen free) chick embryos are inoculated with the diluted sample solution, and after the inoculation, the SPF chick embryos are put in an incubator and incubated continuously, wherein the temperature of the incubator is 37.0-37.5 DEG C and the humidity is 50%; and (3), harvesting: the survival condition of the chick embryos is observed, dead chick embryos after the inoculation are preserved at 4 DEG C for 12-24 h, and chick embryo allantoic fluid is collected. The method can be applied to isolated culture of the Mccp conveniently and rapidly, a Mccp antigen containing no animal serum can be obtained conveniently and rapidly, and various problems caused by application of serum can be solved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Antigen harvesting device for infectious bronchitis
PendingCN111763611AReduce cloggingQuick drawBiological material testing proceduresSpecific use bioreactors/fermentersYolkAntigen
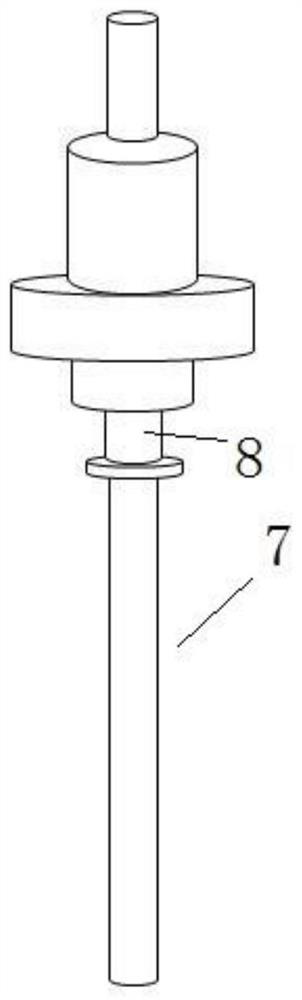
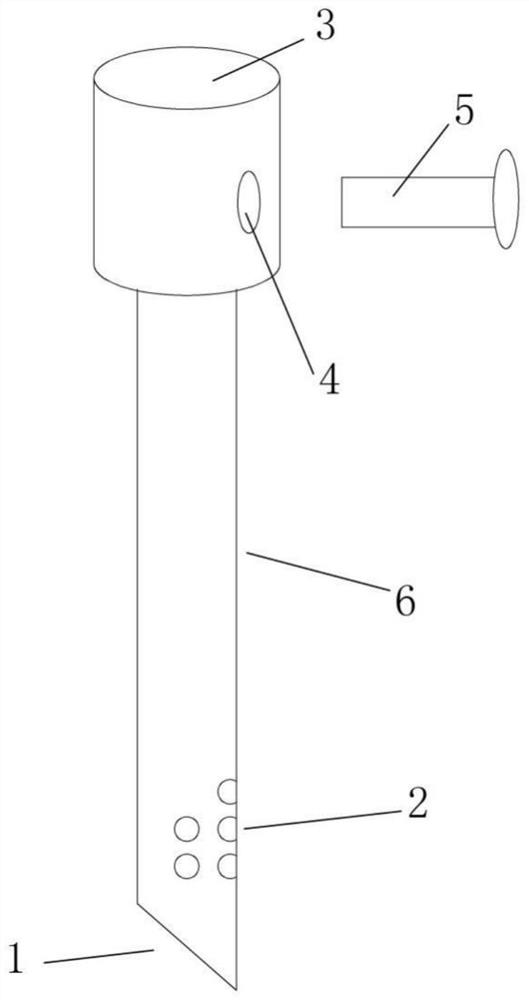
The invention relates to the technical field of antigen harvesting, and especially relates to an antigen harvesting device for infectious bronchitis. The harvesting device includes a harvesting head;the harvesting head includes a liquid suction rod (7) and a coat; the coat is formed by a liquid suction rod inserting head (3) and a liquid suction rod coat (6) in successive arrangement from top tobottom through connection; the lower end of the liquid suction rod coat (6) is a beveled sharp head (1), and the included angle between the inclined plane of the beveled sharp head (1) and a horizontal plane is 30-45 degrees; and the sidewall, on the same side with the tip of the beveled sharp head (1), of the liquid suction rod coat (6) is provided with multiple liquid suction holes (2). The beveled sharp head of the device is only in contact with the eggshell edge of chicken embryo during harvesting, and the incline plane can effectively avoid the yolk when going down; and the harvesting head has more liquid suction holes at the top part and less liquid suction holes at the bottom part, so that liquid suction rod can be further shortened, therefore, blocking can be effectively avoided, allantoic fluid can be quickly sucked, and thorough suction can be achieved.
Owner:兆丰华生物科技(南京)有限公司
Cream for treating belt channel stasis and removing proud flesh
InactiveCN108837107AImprove siltingNo side effectsMetabolism disorderAntipyreticButtocksANGELICA ROOT EXTRACT
The invention discloses a cream for treating belt channel stasis and removing proud flesh. The cream comprises the following components: butanediol, allantois, disodium EDTA, polydimethylsiloxane, glyceryl stearate, PEG-100 stearate, PEG-40 hydrogenated castor oil, shea butter, sorbitan stearate, caprylic / capric triglyceride, cetostearyl alcohol, tocopherol acetate, bitter orange flower oil, wildsoybean seed extract, yam root extract, danshen root extract, Chinese angelica root extract, common burreed rhizome extract, safflower extract, capsicum extract, and the like. The cream provided in the invention is rich in various active extracts, can be applied to the belt channel (waist and abdomen position) for a moment, can immediately relieve the belt channel meridians, dredge channels, promote blood circulation, accelerate the circulation of qi and blood in the meridians, improve the stasis of the belt channel, and enhance intestinal peristalsis, has a good laxative effect for constipation patients, is beneficial to fat metabolism, reduces the generation of waist, abdomen and buttock proud fleshes, and eliminates waist and abdomen 'swimming rings' and 'mother's buttock'.
Owner:江西登云健康美业互联有限公司
Extraction method of goose parvovirus DNA (deoxyribonucleic acid)
InactiveCN102250886AImprove securityWide variety of sourcesMicroorganism based processesDNA preparationChloroformAllantois
The invention relates to an extraction method of goose parvovirus DNA (deoxyribonucleic acid), belonging to the field of biotechnology. The extraction method comprises the following steps: adding equal-volume of chloroform to a goose embryo allantoic fluid containing goose parvovirus, vibrating at room temperature for 15-20 minutes, centrifuging at 3500 rpm for 10 minutes, sucking supernatant, then adding equal-volume of chloroform, vibrating, centrifuging, repeating three times, sucking the supernatant, adding the supernatant into an EP (electropolished) tube, boiling in boiling water for 3-5 minutes, centrifuging at 8000-10000 rpm for 5 minutes, and sucking the supernatant to obtain the goose parvovirus DNA. In the extraction method provided by the invention, only chloroform is used as the reagent, and the virus is split to the allantoic fluid and fat and protein large particles are removed by repeated freezing and thawing, boiling and centrifuging; and the extraction method is convenient for operation, has low cost and high efficiency and is convenient for laboratory operation.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com