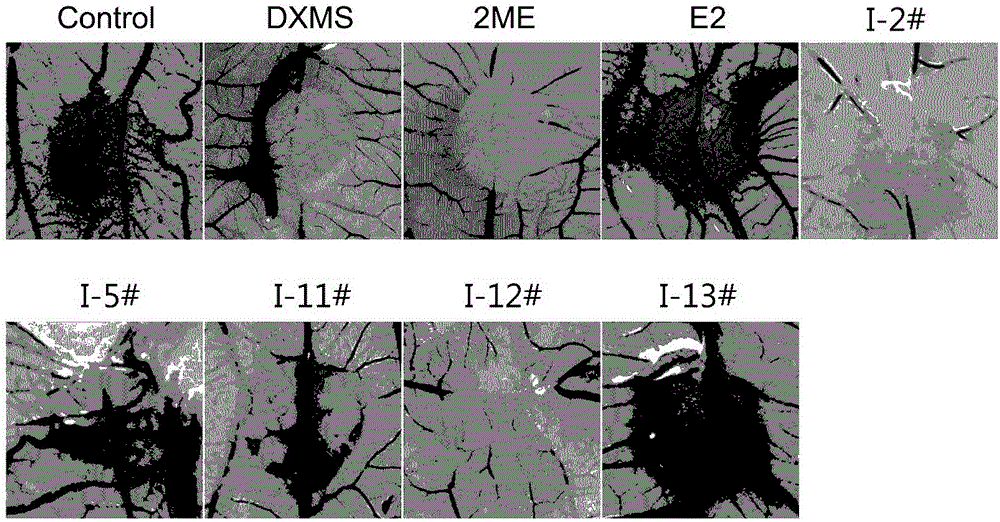
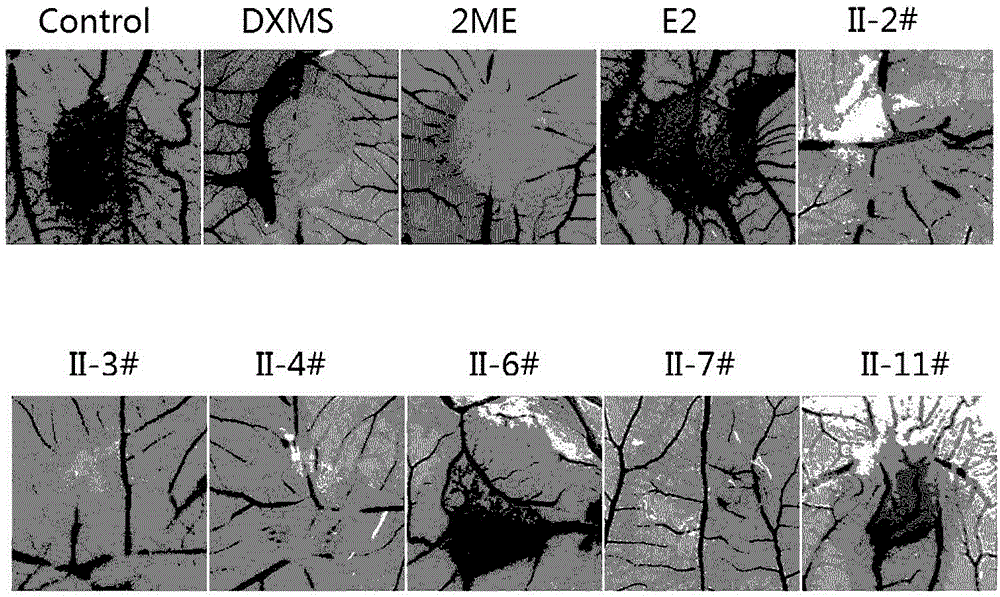
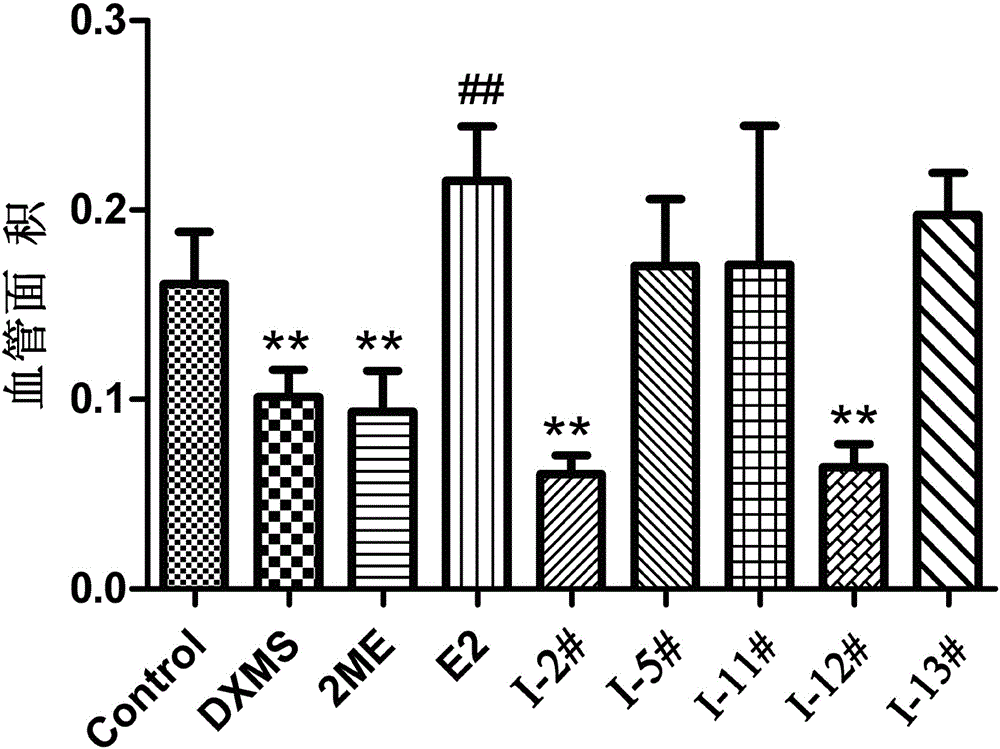
2/16-site-substituted chalcone derivative taking estrogen as mother nucleus and preparation method and application of derivative
A technology of chalcone derivatives and estrogen, applied in the field of chalcone derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] Preparation of Example 1 Series I Estrogen Nucleus Raw Material
[0088] (1) Preparation of 2-acetylestradiol-17β-ethyl ester (A2)
[0089] References Steroids, 2002, 67:1065–1070 method, weigh 30.0g (111.65mmol) of estradiol and transfer it to a 1000ml round bottom flask, add 300ml of anhydrous pyridine, fully stir and dissolve, then slowly add 75ml (396.7mmol) of acetic anhydride was stirred at room temperature under dark conditions. After 15 hours, the reaction was basically completed. In order to ensure the complete reaction, the stirring was continued for 1 hour to detect no significant change, and it was determined that the reaction was complete. Treatment: Stir and cool the reaction mixture in an ice bath, quickly pour it into a container filled with a large amount of ice water, continue to stir, and a white flocculent precipitate precipitates, let it stand for 30 minutes, vacuum filter, and wash the filter cake with water until there is no pyridine smell , vac...
Embodiment 32
[0106] The preparation of embodiment 32-phenylacryloyl estradiol (I-1)
[0107] Dissolve 2-acetylestradiol-17β-ethyl ester (A2, 200mg, 0.562mmol) in 5ml of 95% ethanol solution, add 5ml of 10% aqueous sodium hydroxide solution, and stir on a stirrer until dissolved; dropwise at room temperature 5ml of 95% ethanol solution of benzaldehyde (69.17mg, 1.2eq) was added dropwise, and the system turned orange red. After the dropwise addition, it was transferred to an oil bath at 45°C, and after four hours of reflux, TLC detection was carried out. The agent is ethyl acetate:petroleum ether=1:2, the reaction is complete. Stop heating, after the temperature drops, add 10% hydrochloric acid under ice bath, adjust the pH to about 5, filter with suction, and pass through the column with ethyl acetate:petroleum ether=1:3 to obtain the compound 2-phenylacryloylestradiol ( Ⅰ-1) Yellow powder 183 mg, yield 83%. 1HNMR (400MHz, CDCl3) δ12.60(s, 1H), 7.90(d, J=15.5HZ, 1H), 7.79(s, 1H), 7.67(dd,...
Embodiment 4
[0108] Preparation of Example 42-(3',4',5'-trimethoxyphenylacryloyl)estradiol (I-2)
[0109]Dissolve 2-acetylestradiol (A1, 177mg, 0.562mmol) in 10ml of 95% ethanol solution, stir on a stirrer, add 5ml of 8% sodium hydroxide aqueous solution and stir until dissolved, then slowly drop into 10ml3,4 , 95% ethanol solution of 5-trimethoxybenzaldehyde (132.3mg, 1.2eq), the system turns orange, after the dropwise addition, it is transferred to an oil bath at 60°C, and after two hours of reflux, TLC detection , The developer is ethyl acetate:petroleum ether=1:2, the reaction is complete. Stop heating, and after the temperature drops, add 10% hydrochloric acid under ice bath, adjust the pH to about 5, filter with suction, and pass through the column with ethyl acetate:petroleum ether=1:3. 235 mg of compound 2-(3',4',5'-trimethoxyphenylacryloyl)estradiol (I-2) yellow powder was obtained with a yield of 85%. 1 HNMR (400MHz, CDCl 3 )δ12.58(s,1H),7.81(d,J=15.4Hz,1H),7.76(s,1H),7.47(d,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com