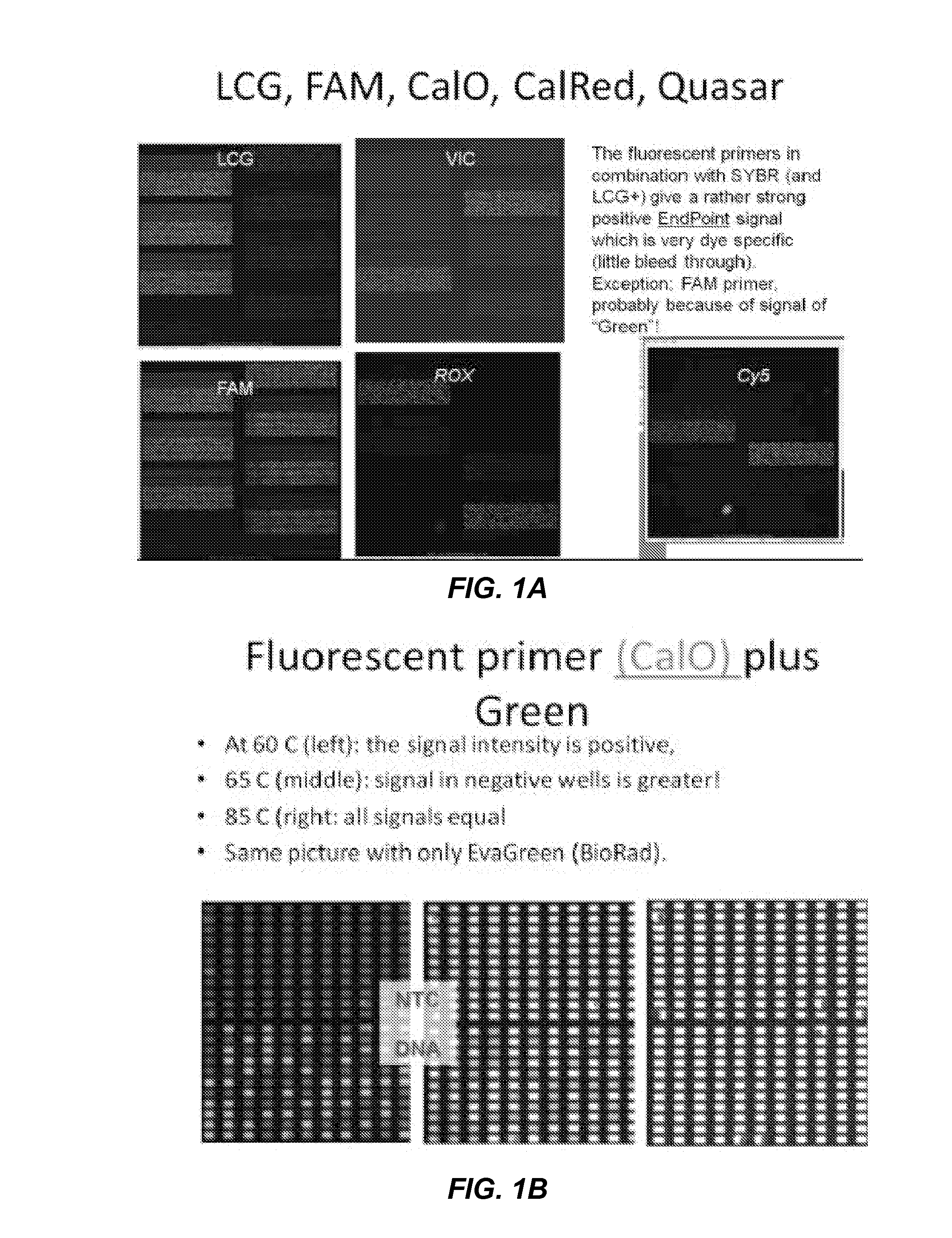
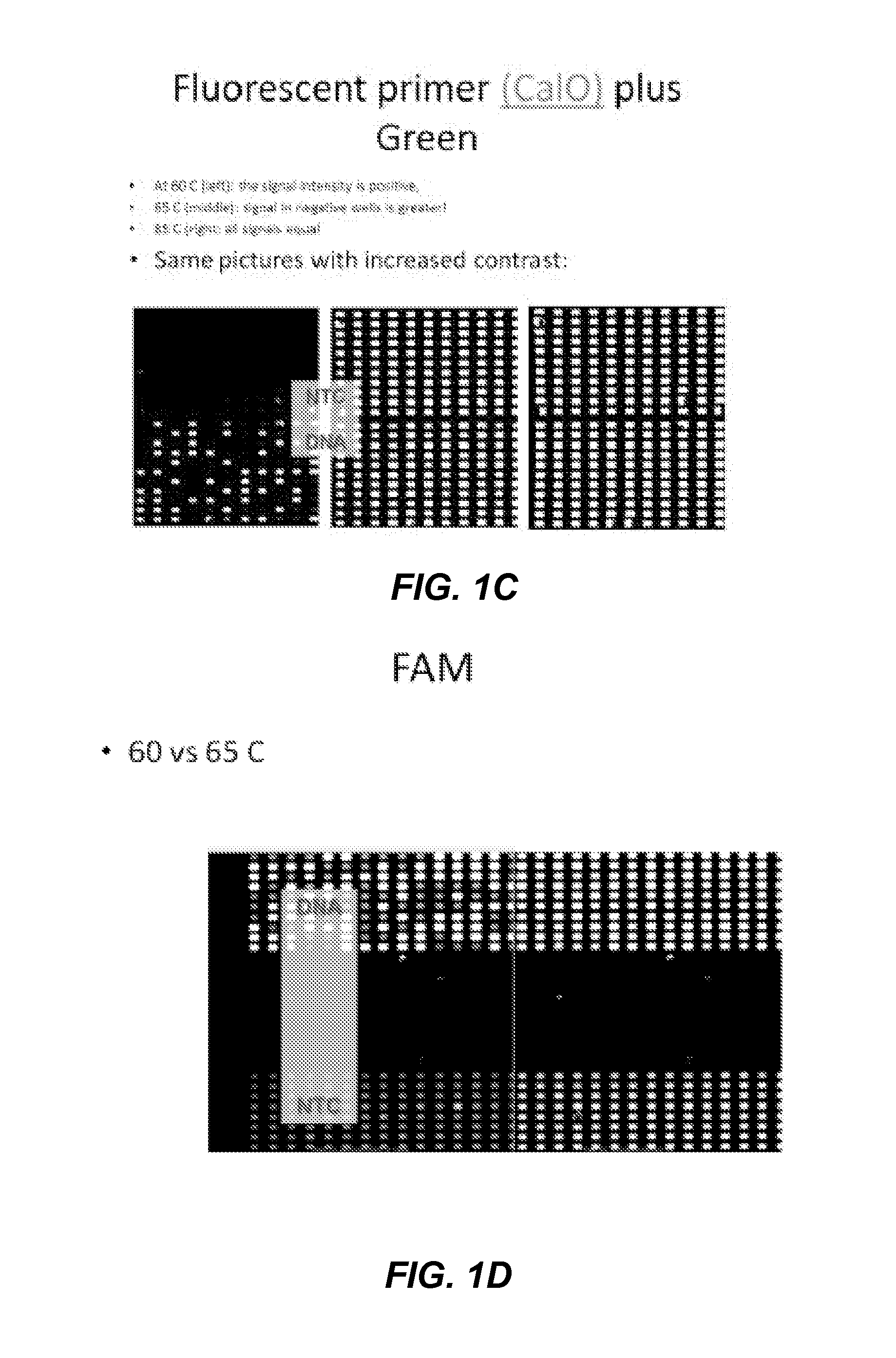
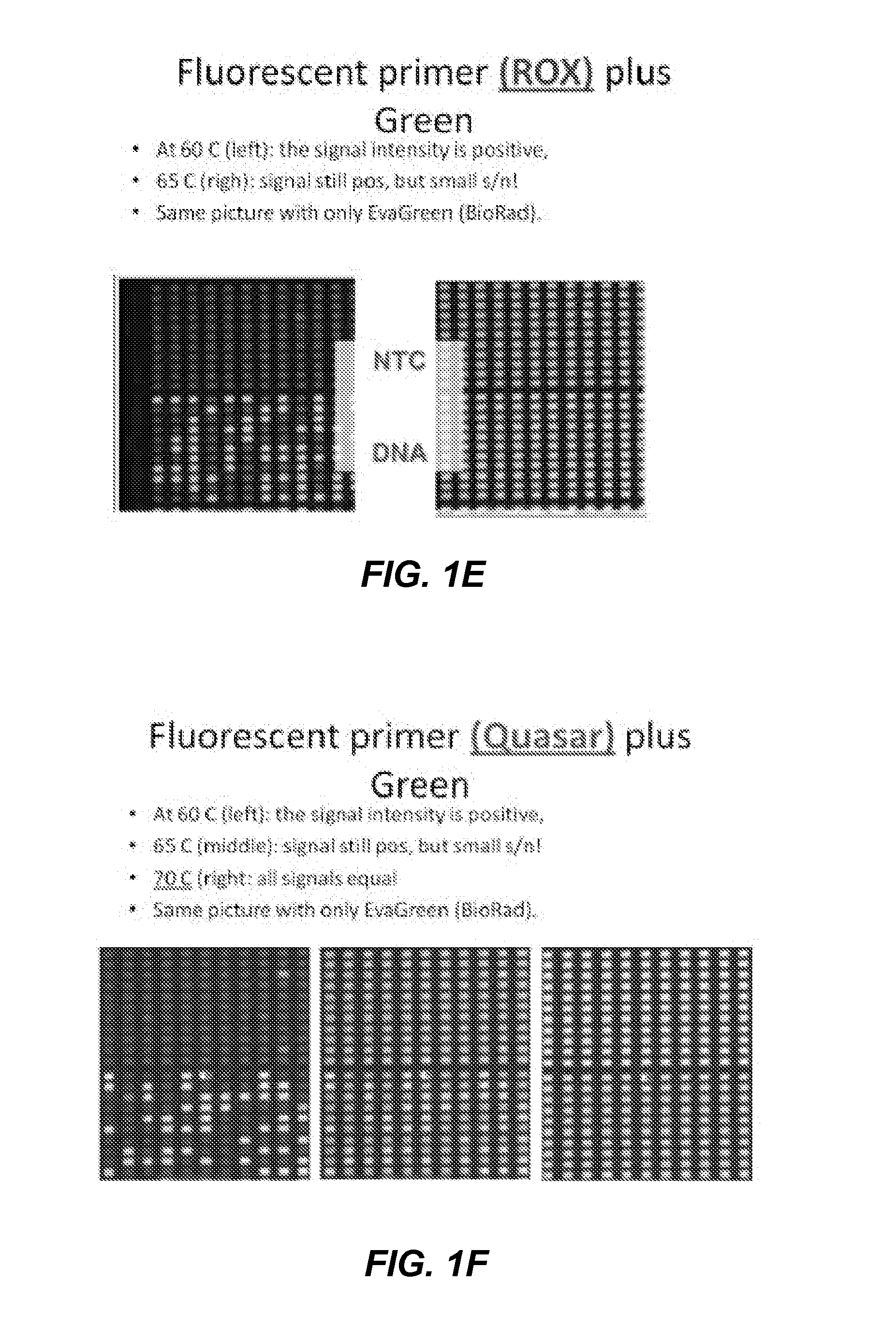
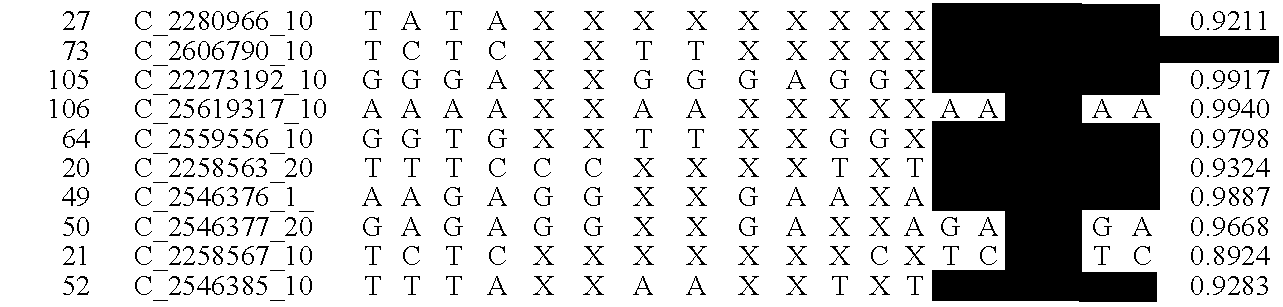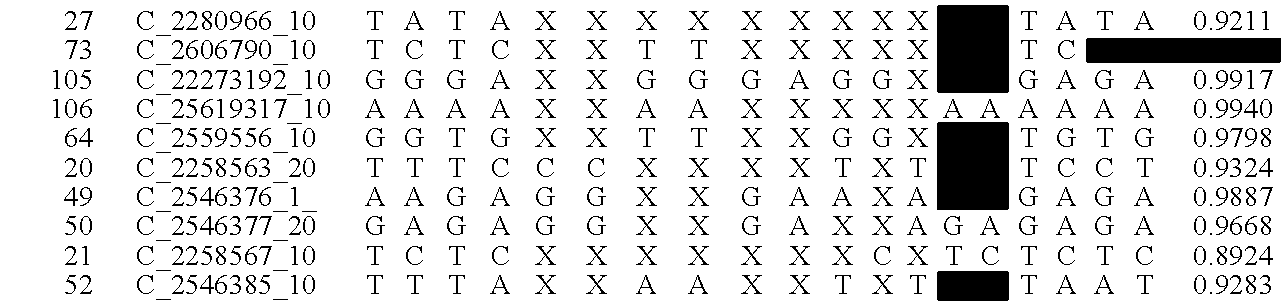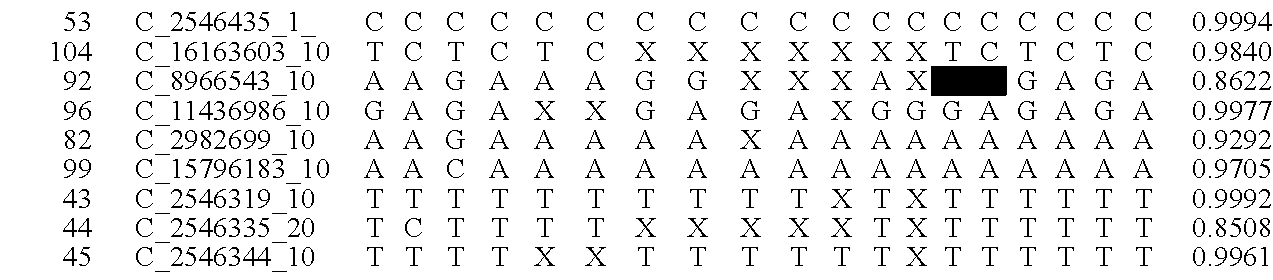Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
158 results about "Aneuploid Cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
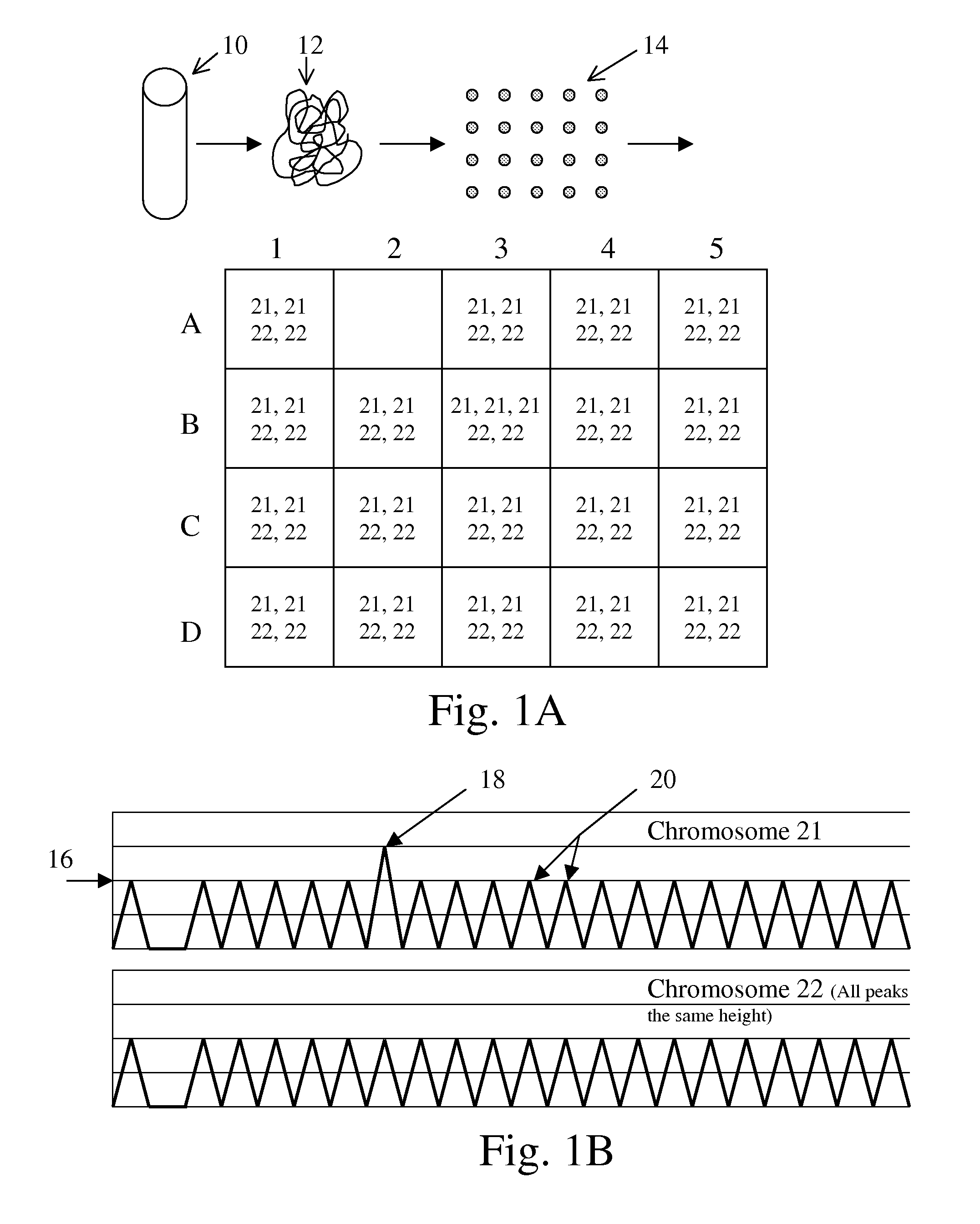
Aneuploidy is the presence of an abnormal number of chromosomes in a cell, for example a human cell having 45 or 47 chromosomes instead of the usual 46. It does not include a difference of one or more complete sets of chromosomes. A cell with any number of complete chromosome sets is called a euploid cell.
Non-invasive fetal genetic screening by digital analysis
ActiveUS20070202525A1Enriching fetal DNAMicrobiological testing/measurementFermentationChorionic villiMassively parallel
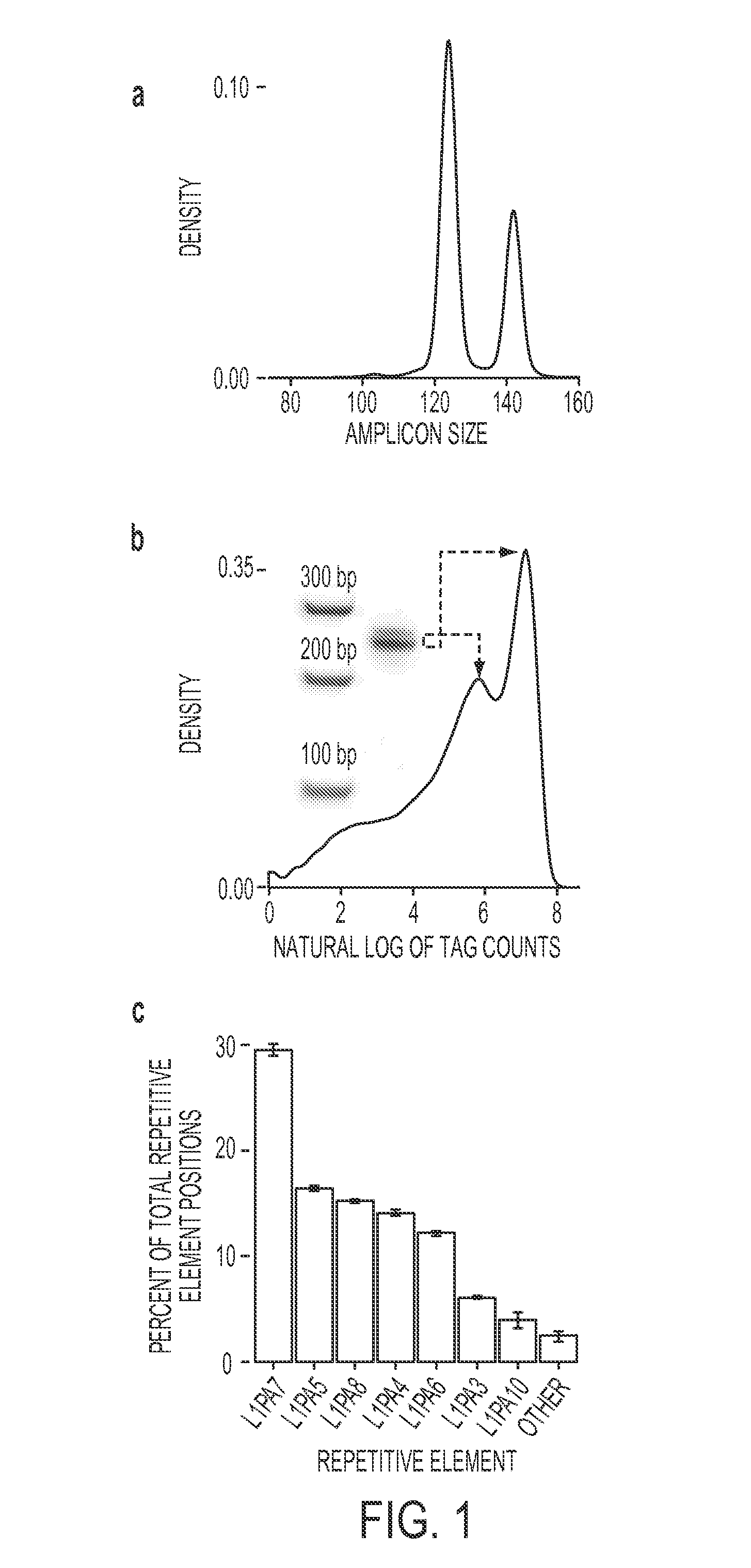
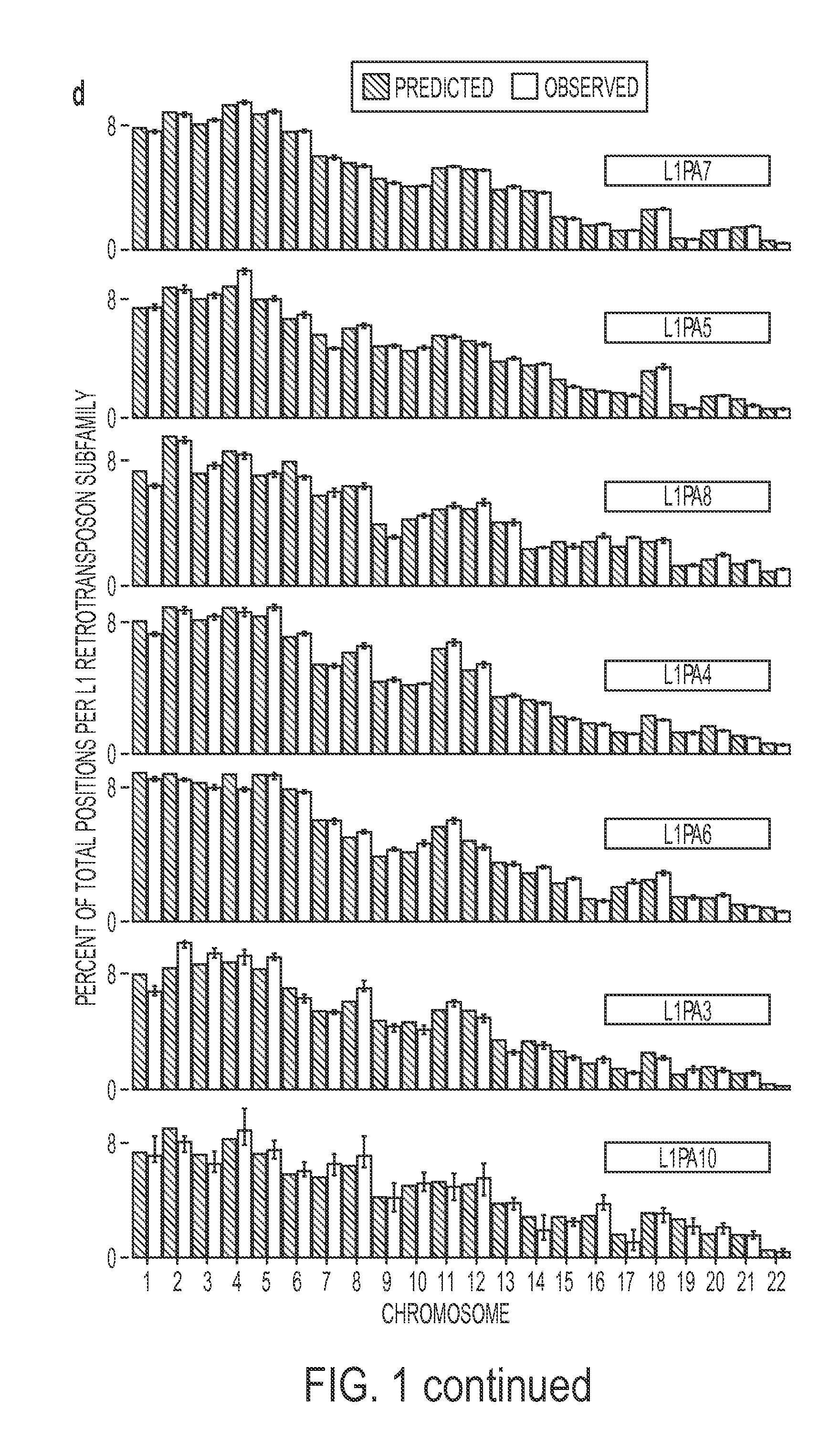
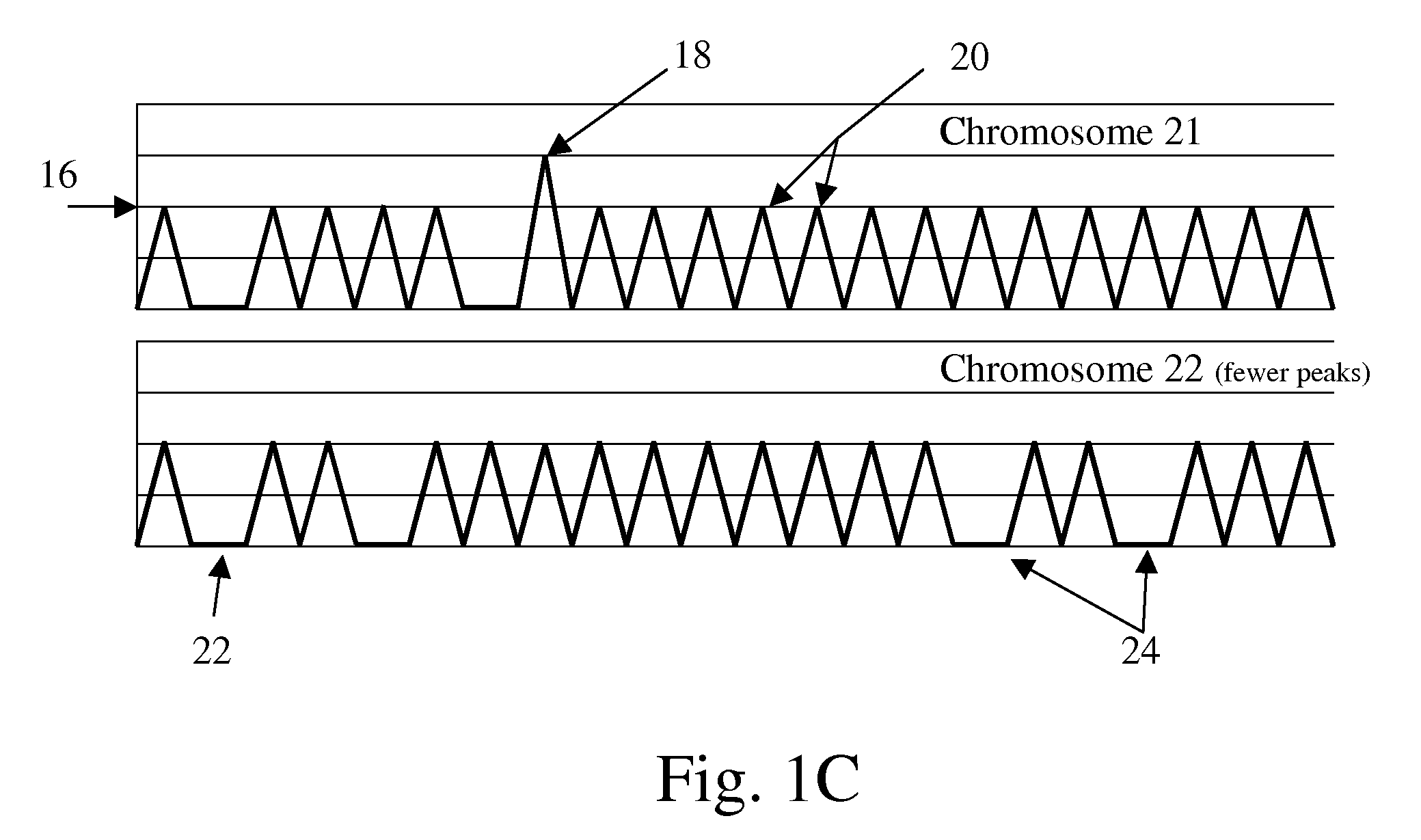
The present methods are exemplified by a process in which maternal blood containing fetal DNA is diluted to a nominal value of approximately 0.5 genome equivalent of DNA per reaction sample. Digital PCR is then be used to detect aneuploidy, such as the trisomy that causes Down Syndrome. Since aneuploidies do not present a mutational change in sequence, and are merely a change in the number of chromosomes, it has not been possible to detect them in a fetus without resorting to invasive techniques such as amniocentesis or chorionic villi sampling. Digital amplification allows the detection of aneuploidy using massively parallel amplification and detection methods, examining, e.g., 10,000 genome equivalents.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Sequencing methods and compositions for prenatal diagnoses
ActiveUS20110201507A1Quality improvementEasy to analyzeMicrobiological testing/measurementLibrary screeningPrenatal diagnosisGenetics
The invention provides methods for determining aneuploidy and / or fetal fraction in maternal samples comprising fetal and maternal cfDNA by massively parallel sequencing. The method comprises a novel protocol for preparing sequencing libraries that unexpectedly improves the quality of library DNA while expediting the process of analysis of samples for prenatal diagnoses.
Owner:VERINATA HEALTH INC
Simultaneous determination of aneuploidy and fetal fraction
InactiveUS20110224087A1Nucleotide librariesMicrobiological testing/measurementFetal aneuploidyBiology
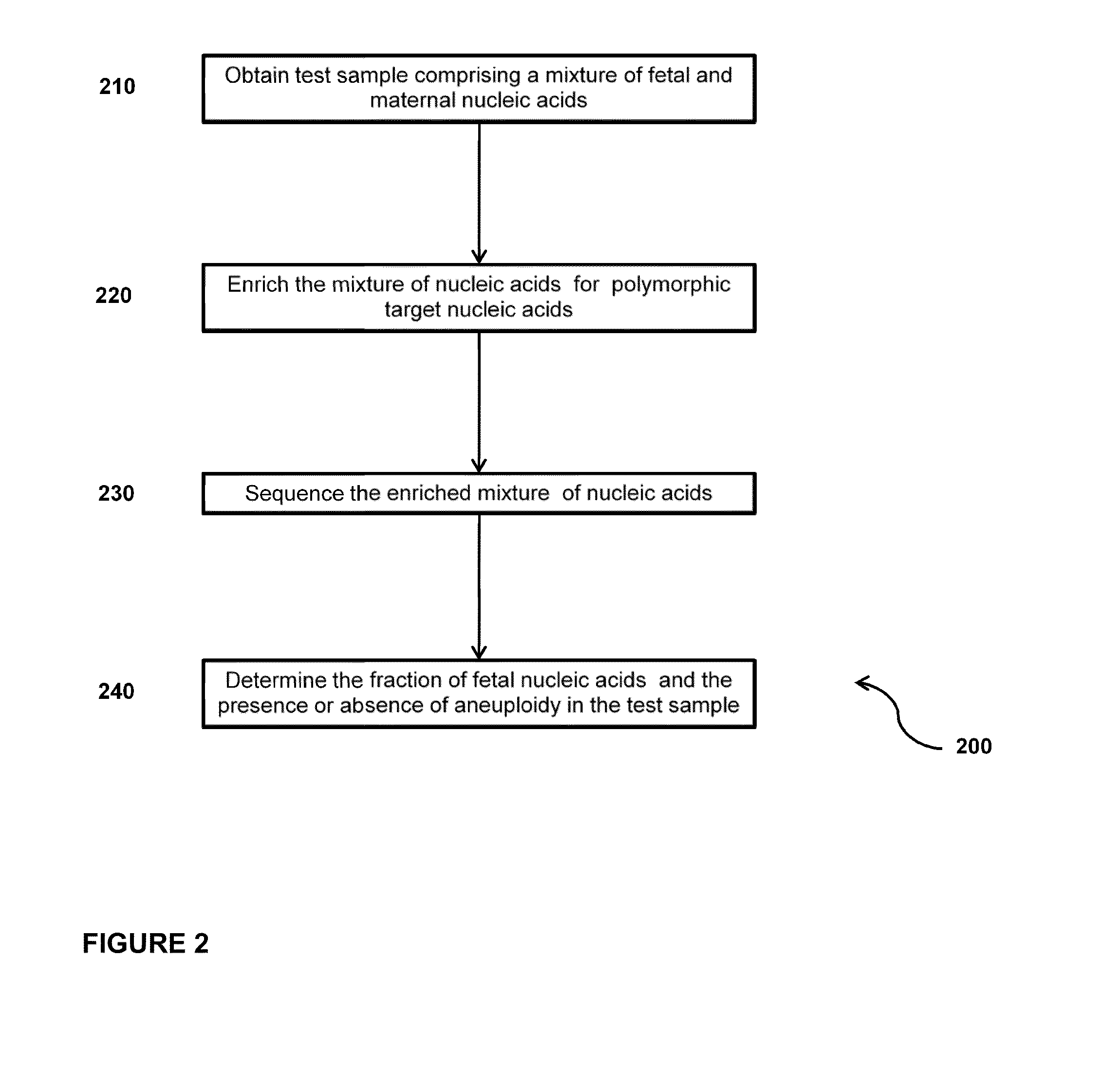
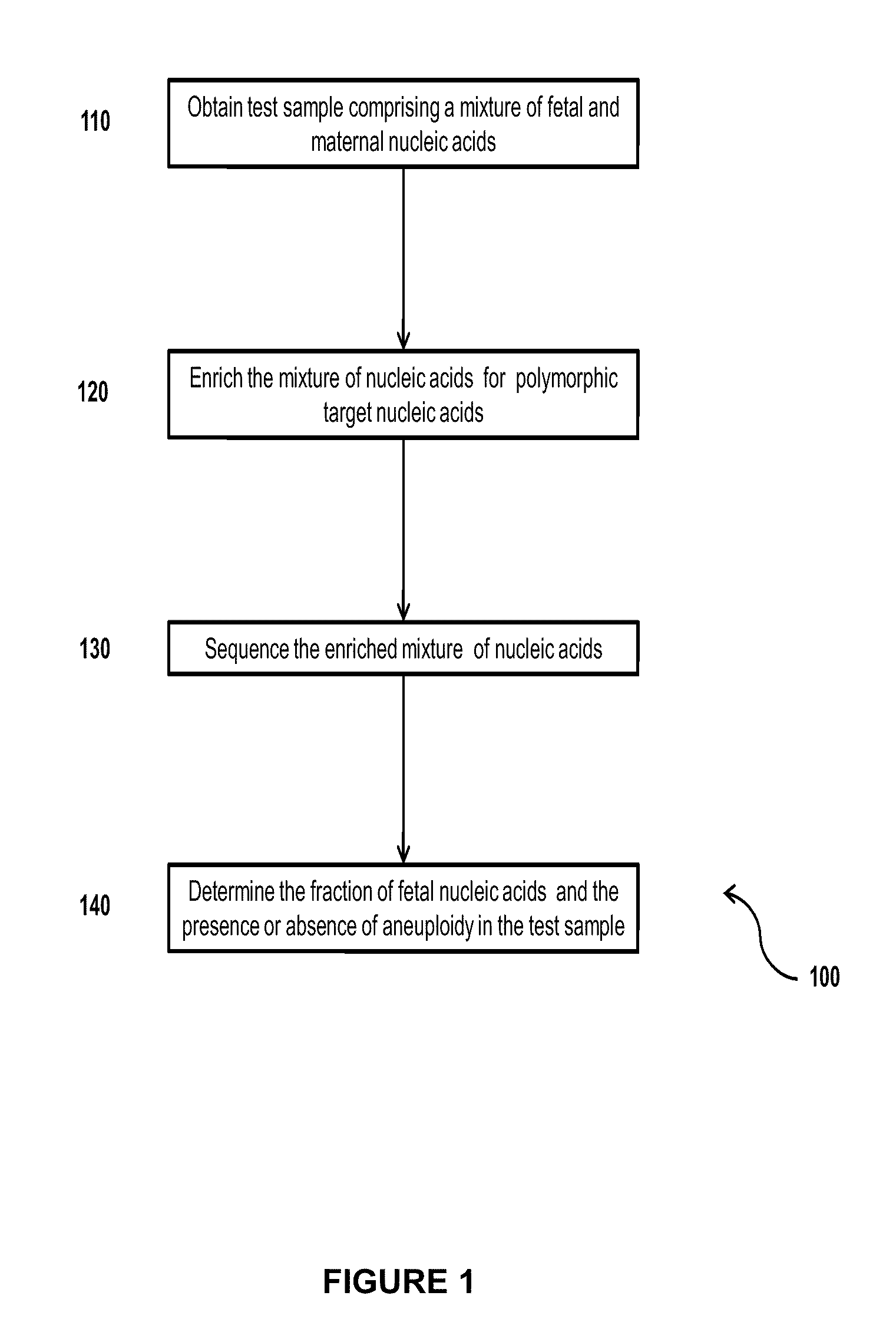
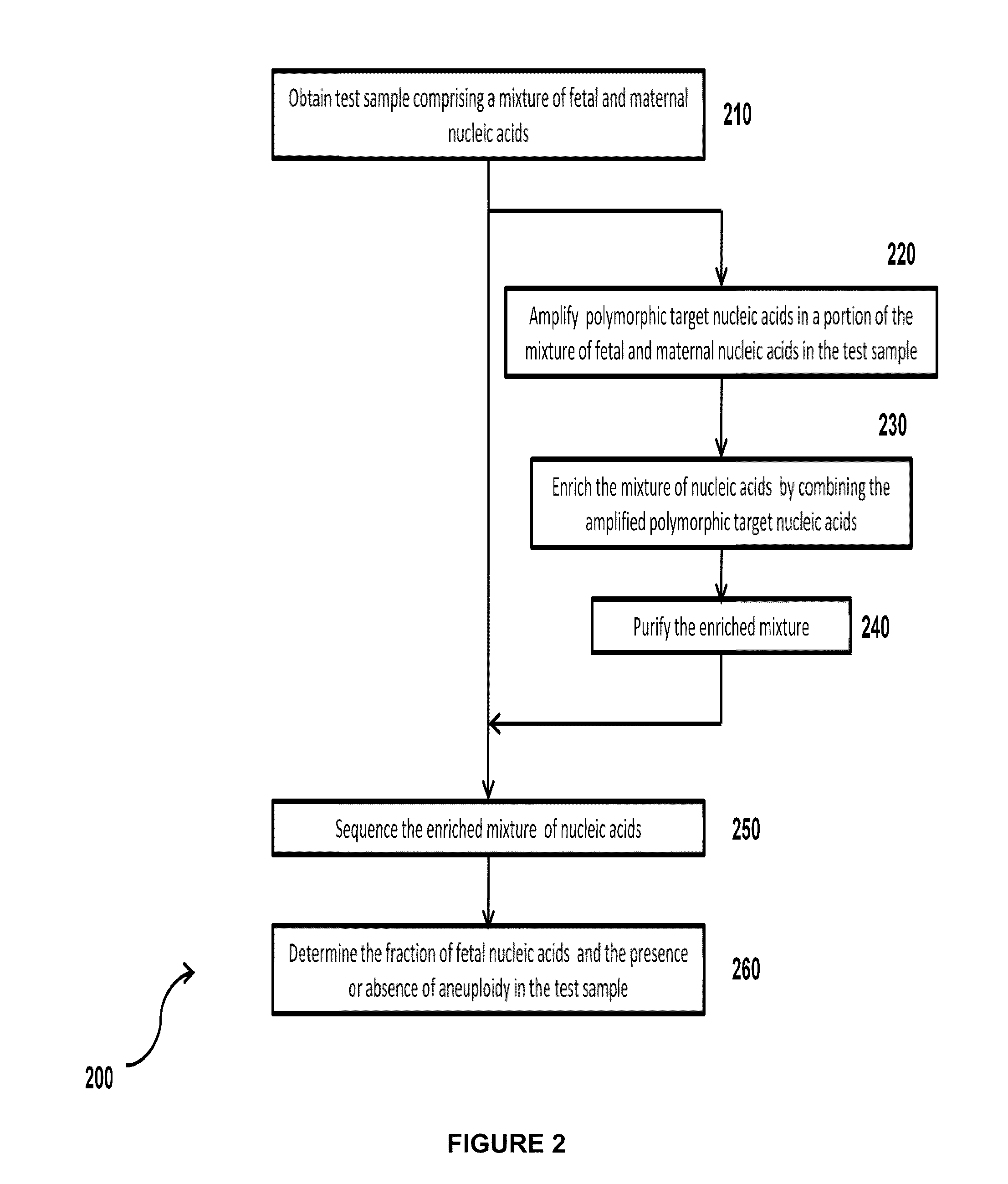
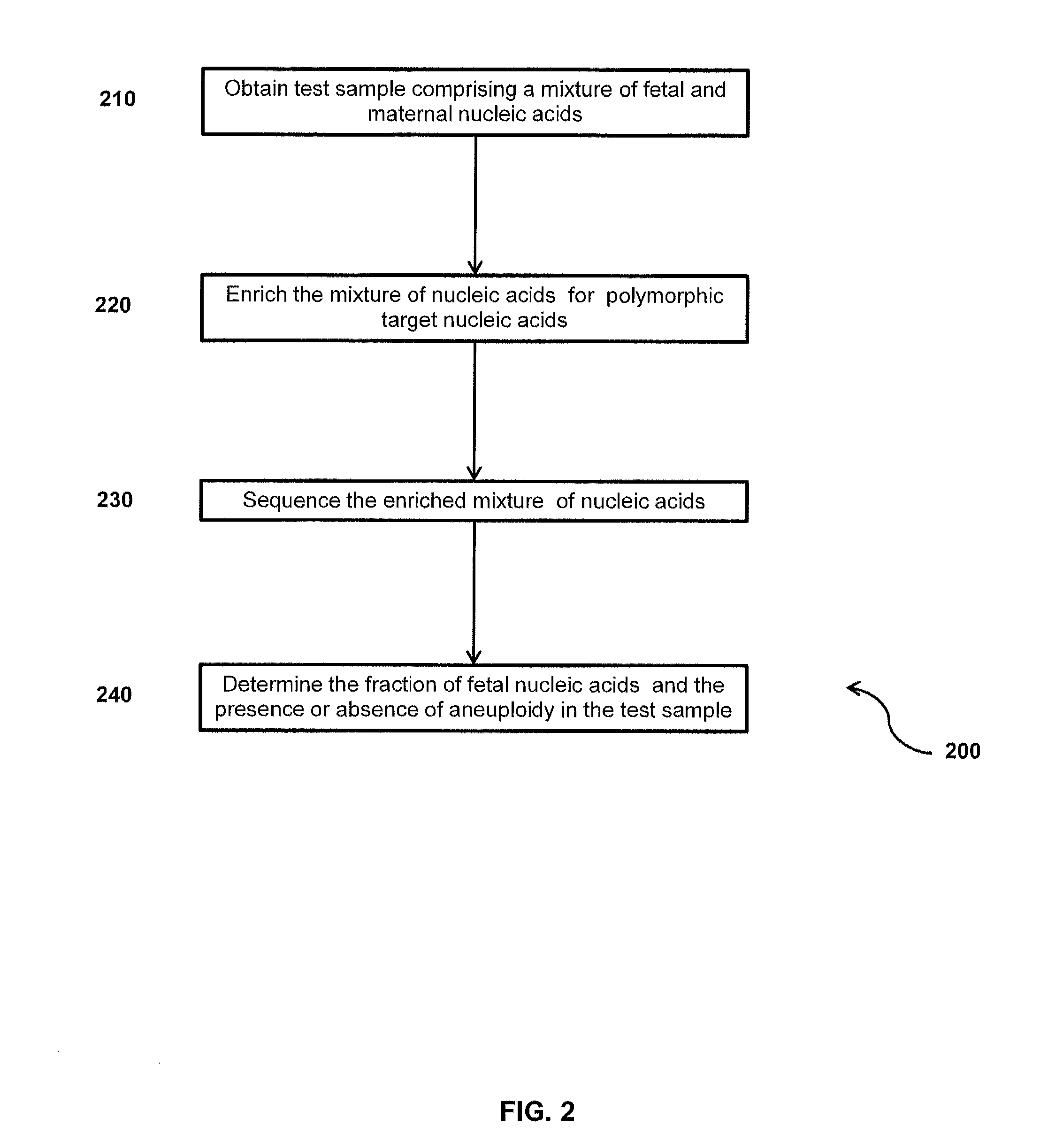
The invention provides compositions and methods for simultaneously determining the presence or absence of fetal aneuploidy and the relative amount of fetal nucleic acids in a sample obtained form a pregnant female. The method encompasses the use of sequencing technologies and exploits the occurrence of polymorphisms to provide a streamlined noninvasive process applicable to the practice of prenatal diagnostics.
Owner:VERINATA HEALTH INC
Diagnosing Fetal Chromosomal Aneuploidy Using Genomic Sequencing With Enrichment
PendingUS20100112590A1High sensitivityMore accurateMicrobiological testing/measurementProteomicsGenomic sequencingDNA fragmentation
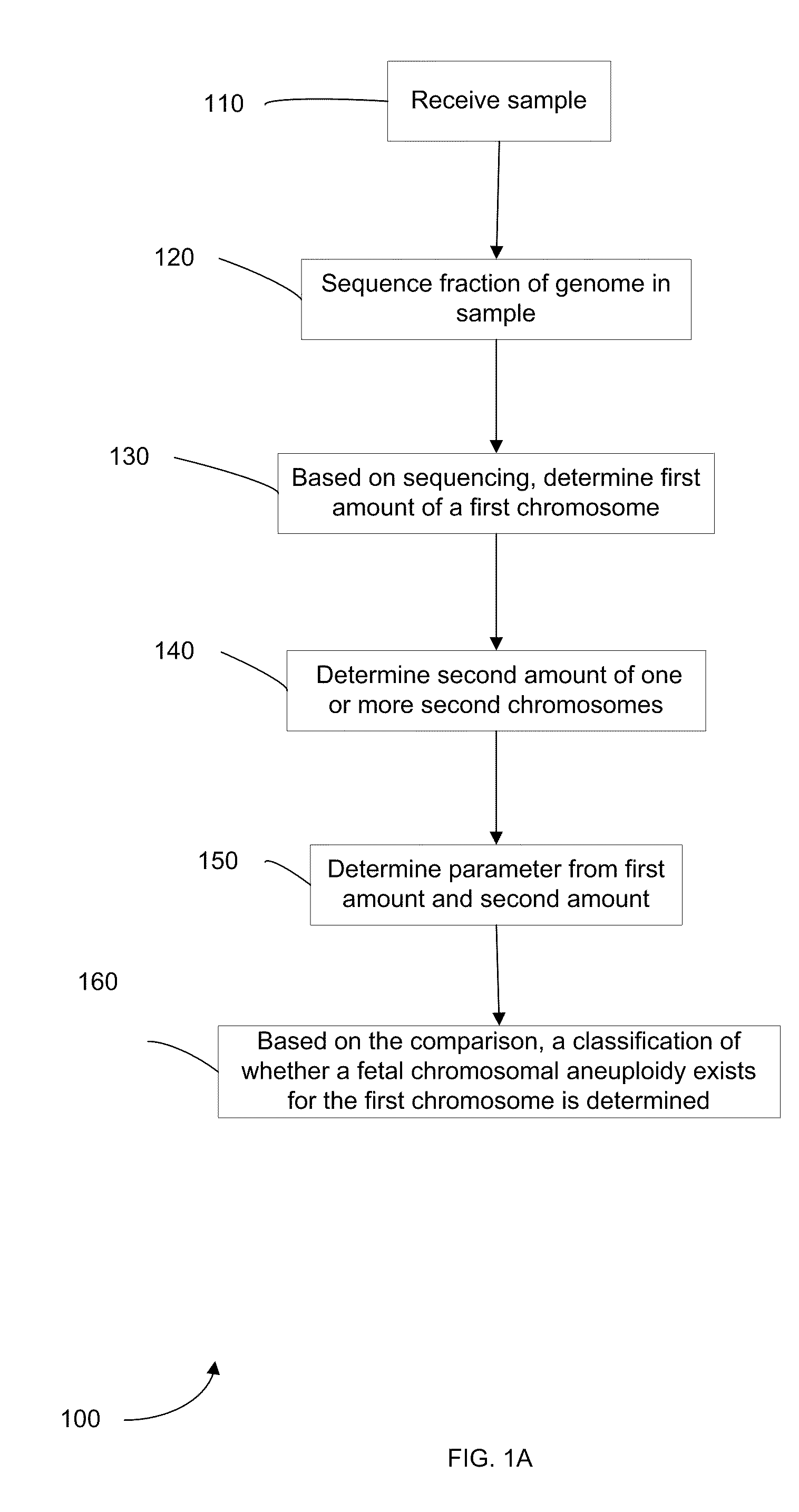
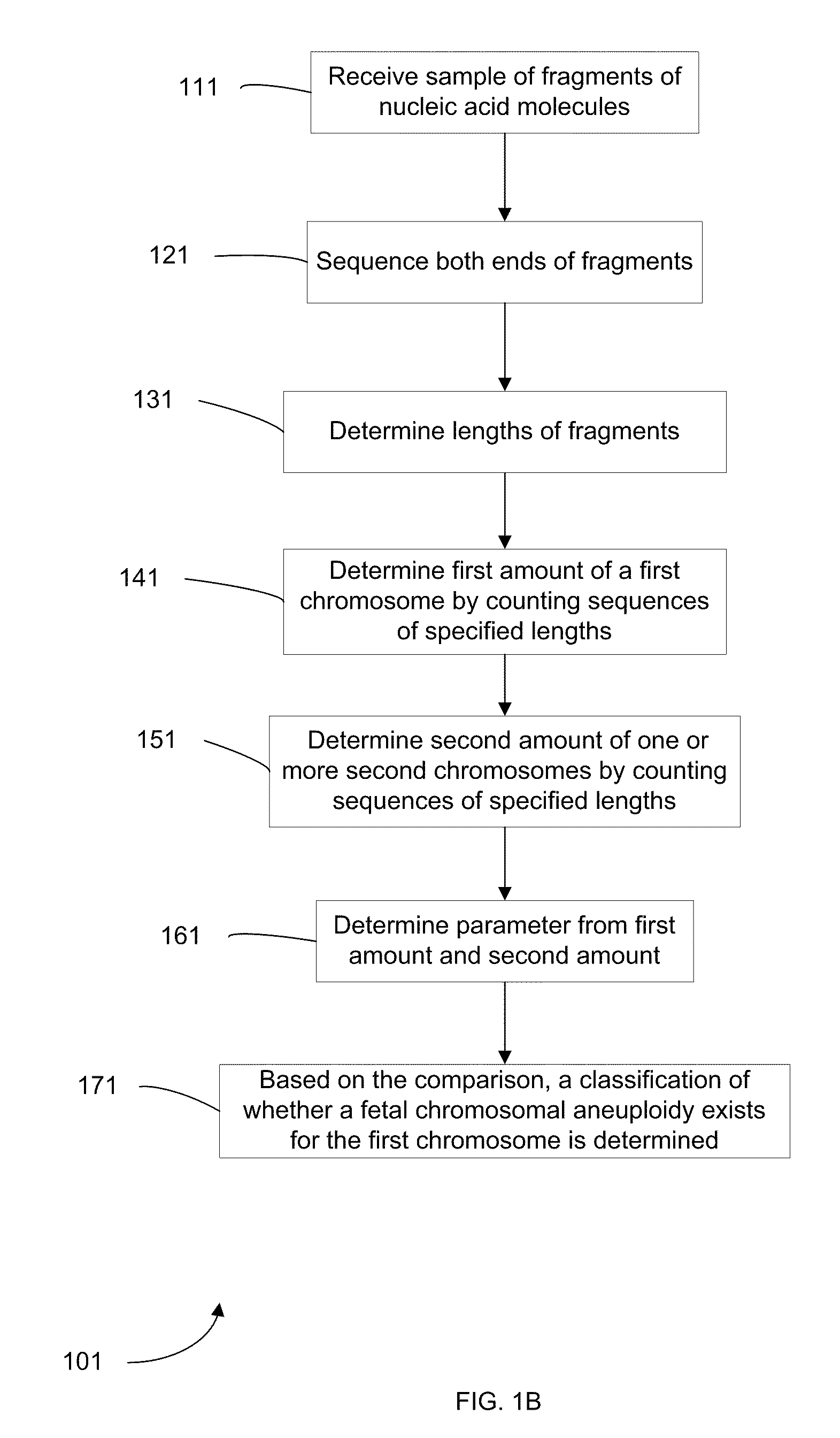
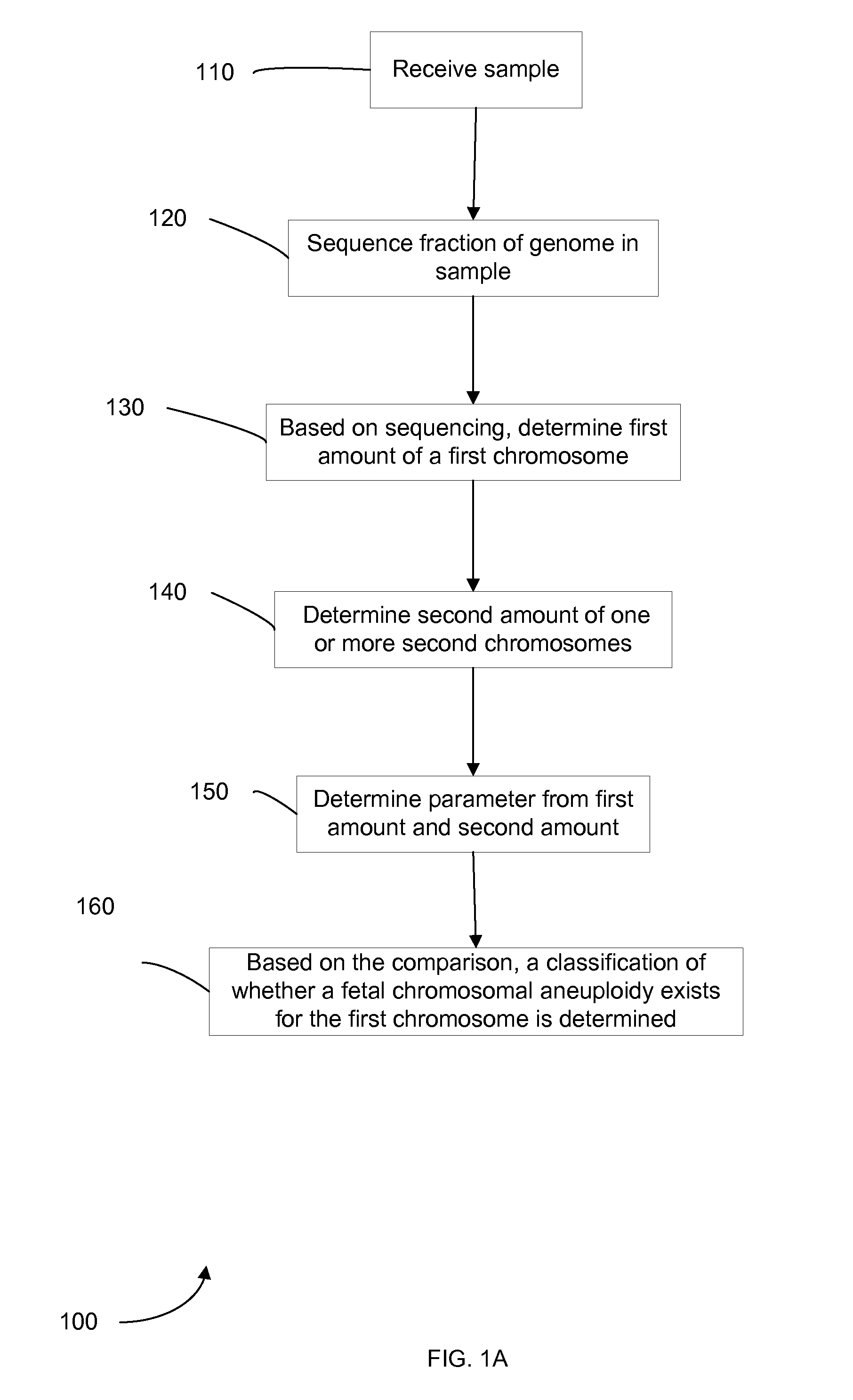
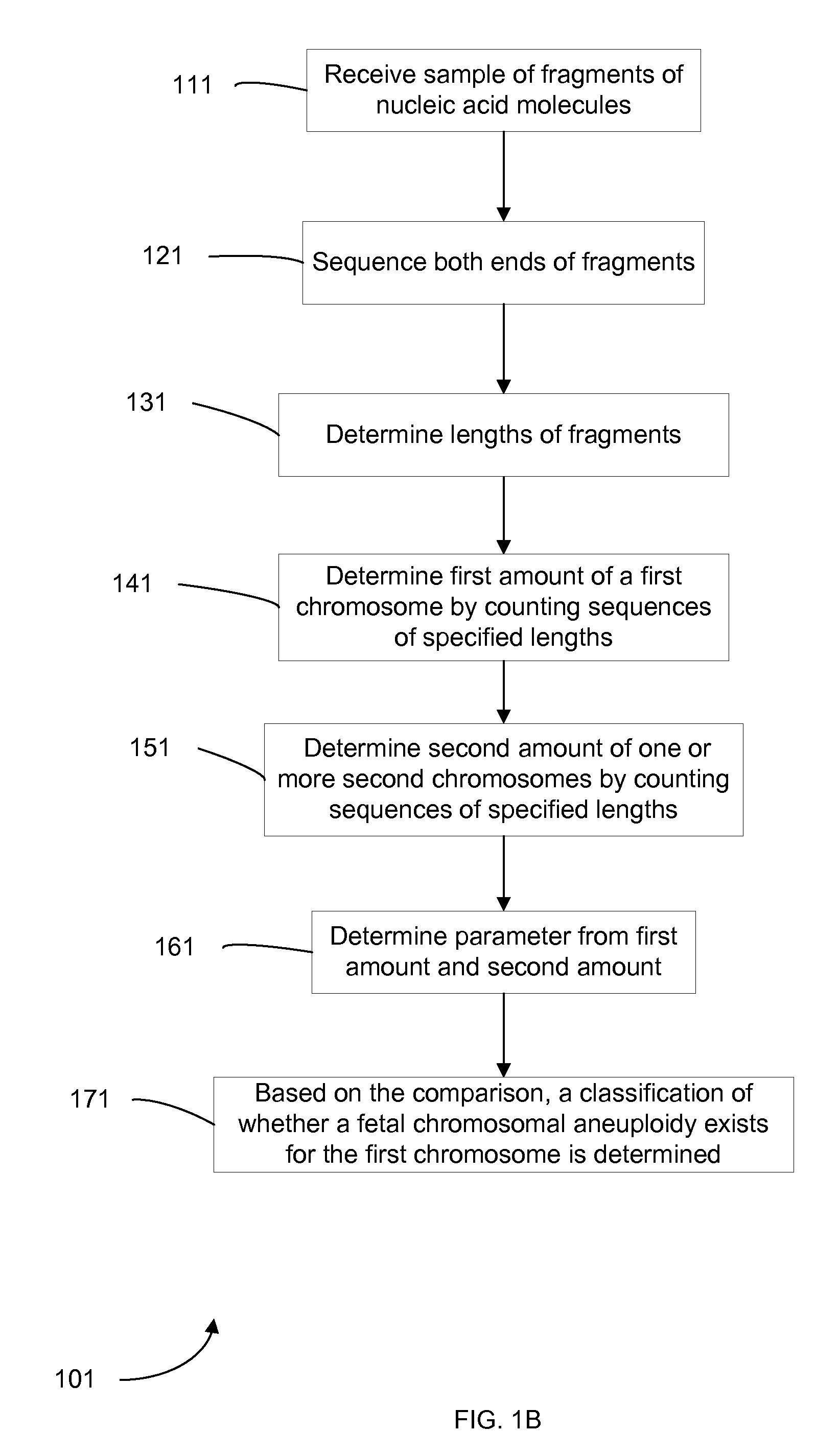
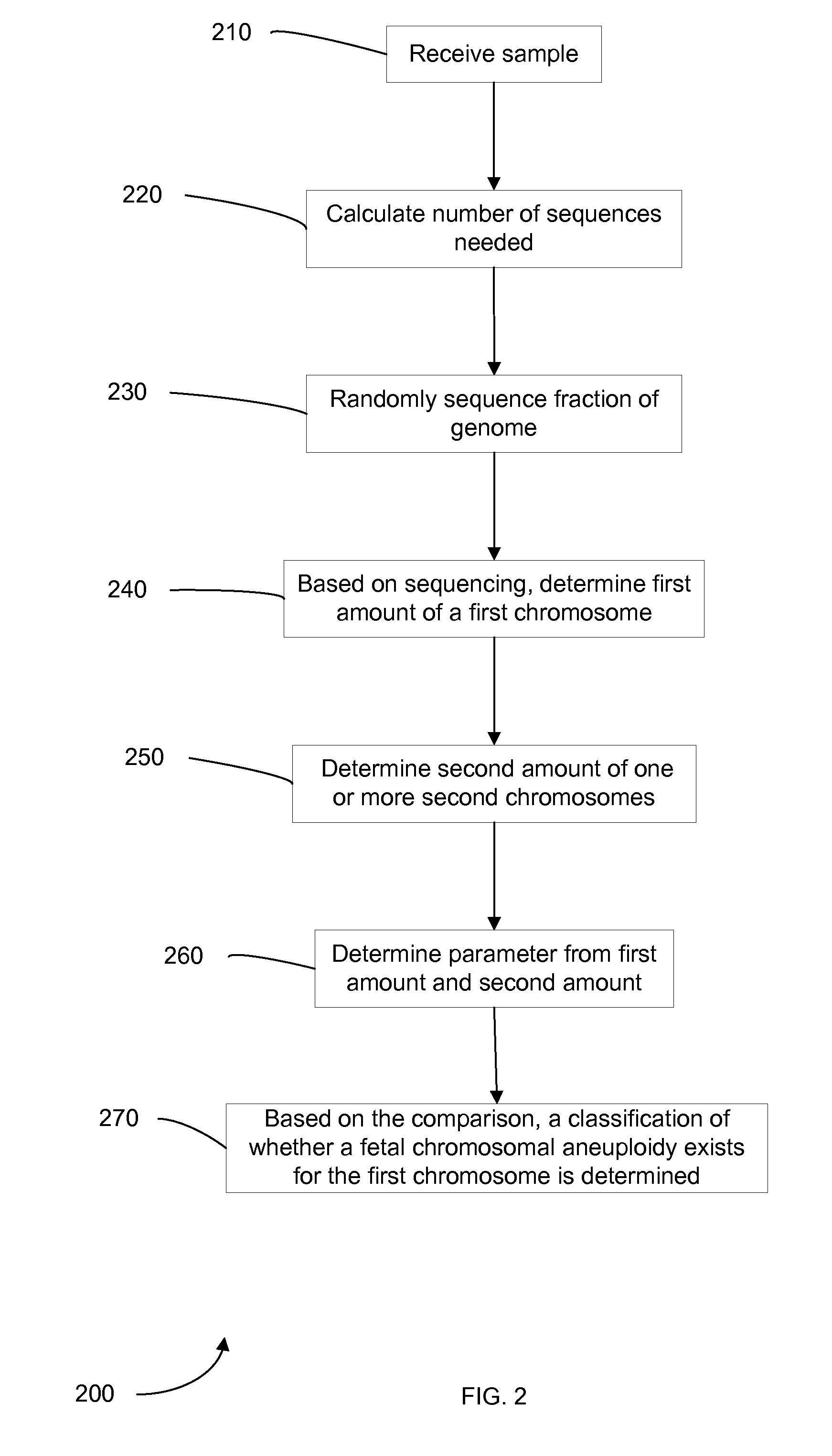
Embodiments of this invention provide methods, systems, and apparatus for determining whether a fetal chromosomal aneuploidy exists from a biological sample obtained from a pregnant female. Nucleic acid molecules of the biological sample are sequenced, such that a fraction of the genome is sequenced. Respective amounts of a clinically-relevant chromosome and of background chromosomes are determined from results of the sequencing. The determination of the relative amounts may count sequences of only certain length. A parameter derived from these amounts (e.g. a ratio) is compared to one or more cutoff values, thereby determining a classification of whether a fetal chromosomal aneuploidy exists. Prior to sequencing, the biological sample may be enriched for DNA fragments of a particular sizes.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Rare cell analysis using sample splitting and DNA tags
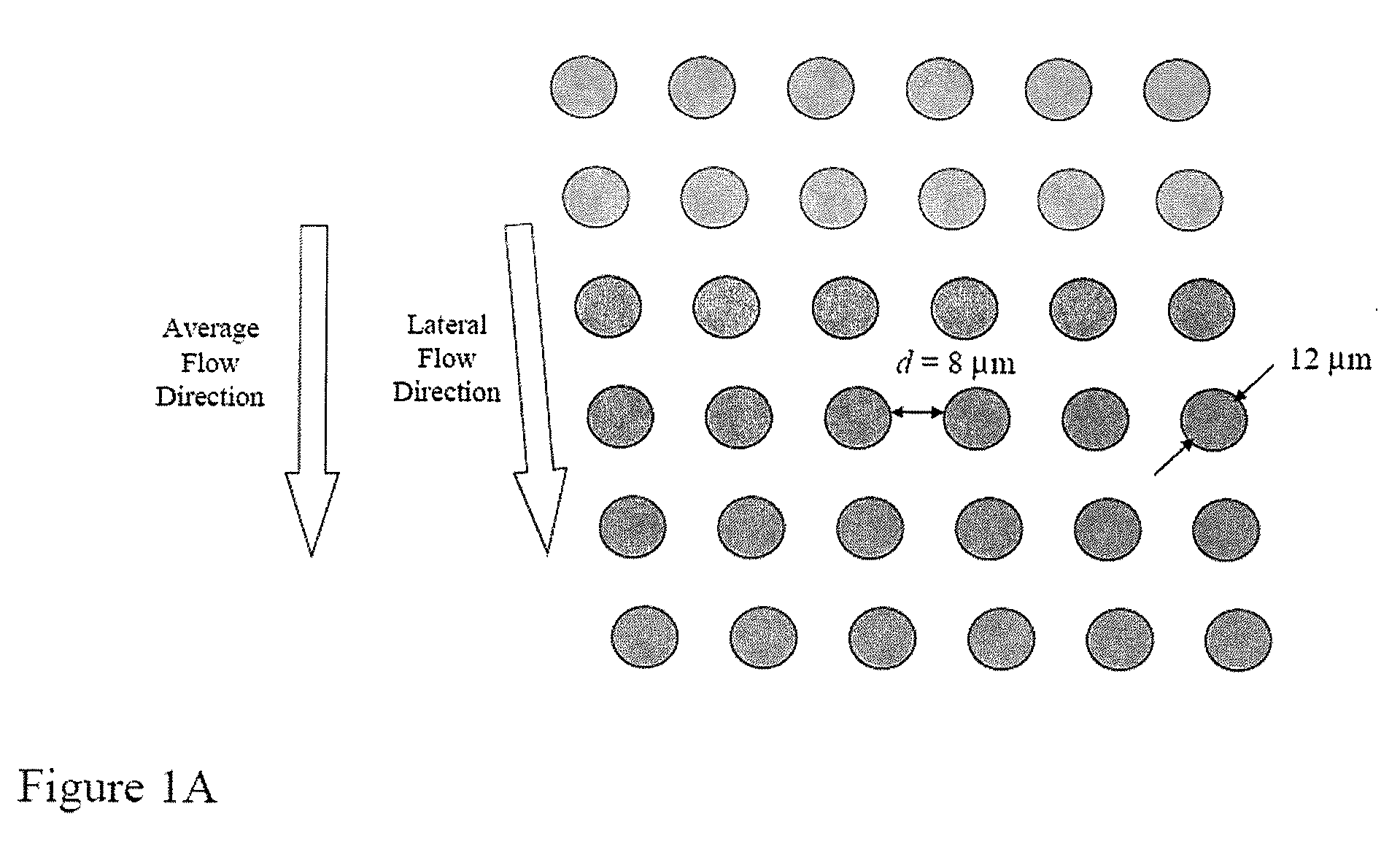
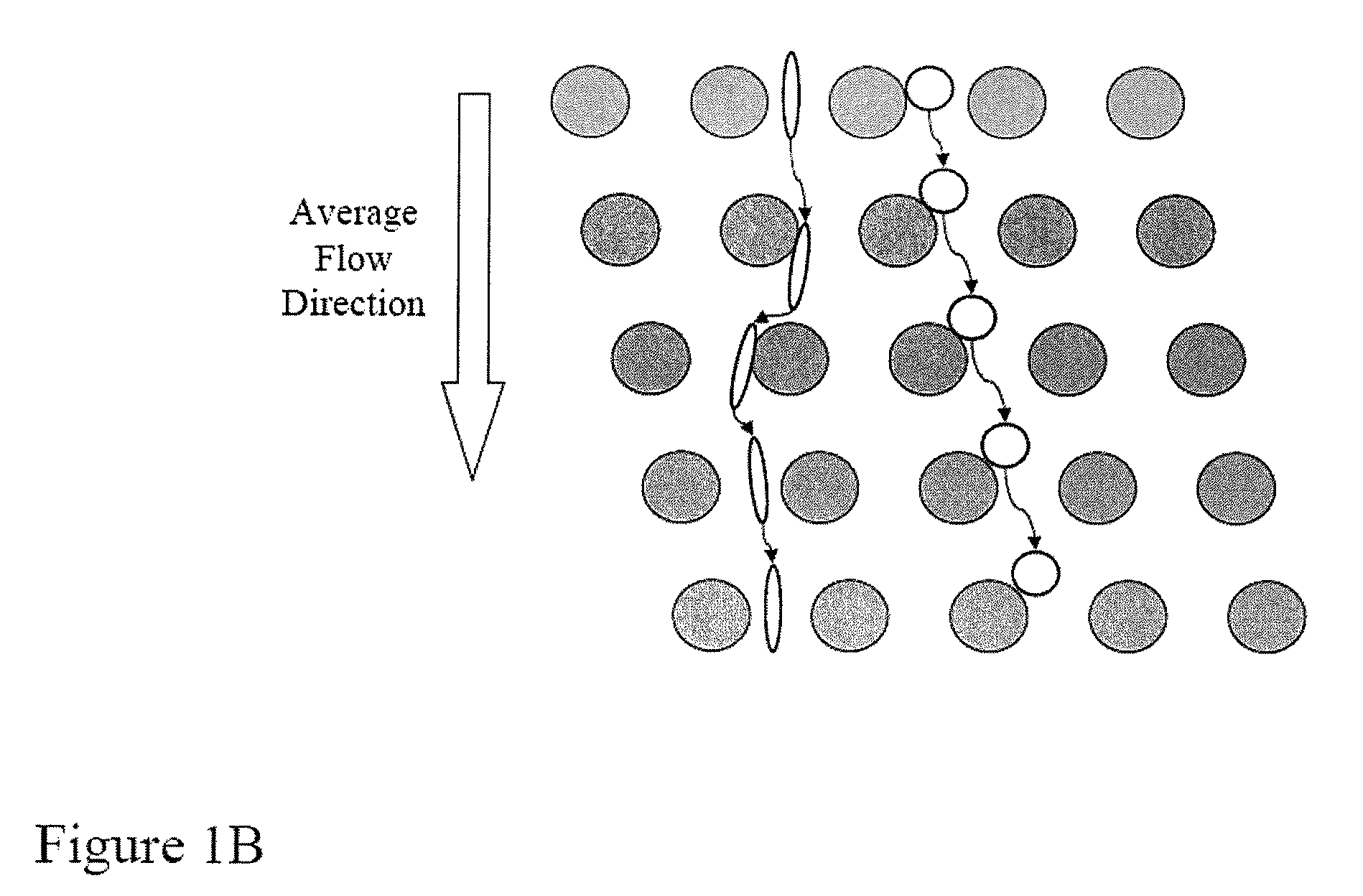
ActiveUS20080220422A1Microbiological testing/measurementDead animal preservationFetal abnormalityRare cell
The present invention provides systems, apparatuses, and methods to detect the presence of fetal cells when mixed with a population of maternal cells in a sample and to test fetal abnormalities, e.g. aneuploidy. The present invention involves labeling regions of genomic DNA in each cell in said mixed sample with different labels wherein each label is specific to each cell and quantifying the labeled regions of genomic DNA from each cell in the mixed sample. More particularly the invention involves quantifying labeled DNA polymorphisms from each cell in the mixed sample.
Owner:VERINATA HEALTH INC +2
Method for the detection of chromosomal aneuploidies
ActiveUS7645576B2High sensitivityIncreased riskSugar derivativesMicrobiological testing/measurementAlleleNon invasive
Owner:TRUSTEES OF BOSTON UNIV +1
Method for sample analysis of aneuploidies in maternal samples
InactiveUS20120270739A1Decreases sequencing biasQuality improvementMicrobiological testing/measurementLibrary member identificationPrenatal diagnosisAnalysis method
The invention provides methods for determining aneuploidy and / or fetal fraction in maternal samples comprising fetal and maternal cfDNA by massively parallel sequencing. The method comprises a novel protocol for preparing sequencing libraries that unexpectedly improves the quality of library DNA while expediting the process of analysis of samples for prenatal diagnoses. The novel protocol can be performed in solution or on a solid surface.
Owner:VERINATA HEALTH INC
Method for the detection of chromosomal aneuploidies
ActiveUS20060252071A1High sensitivityIncreased riskSugar derivativesMicrobiological testing/measurementAlleleNon invasive
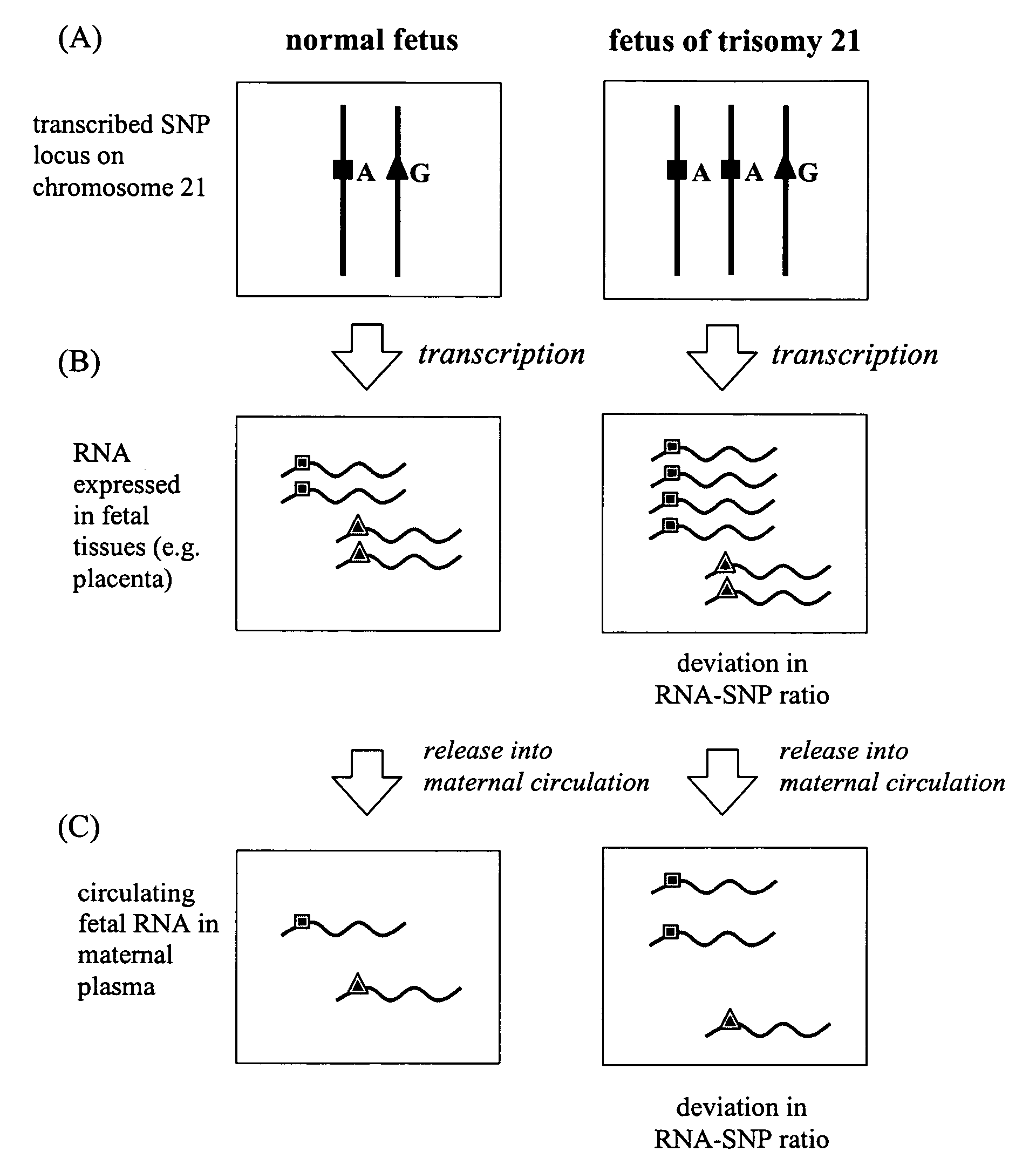
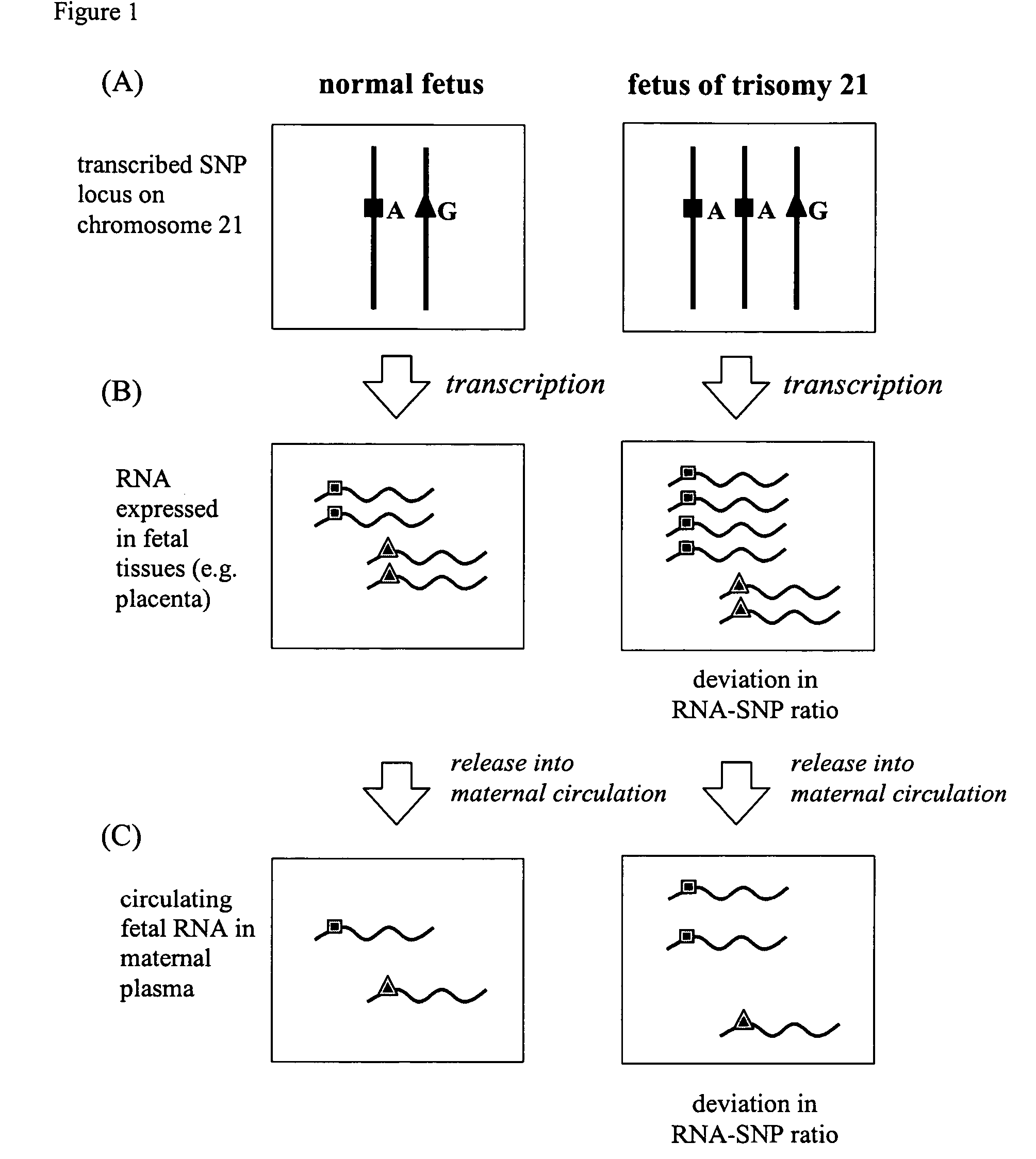
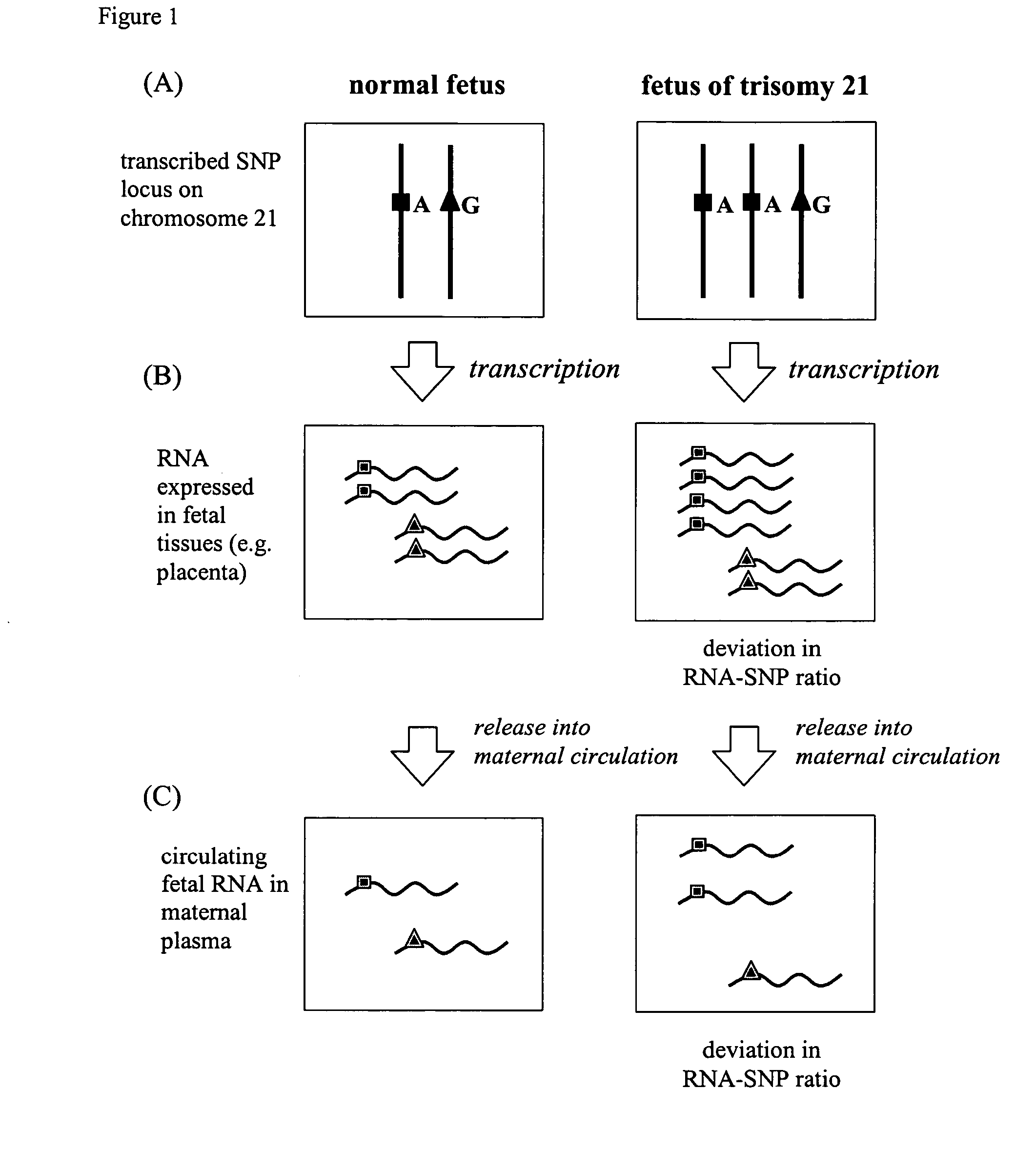
The non-invasive detection of fetal chromosomal aneuploidies is demonstrated. Alleles of fetal RNA-SNPs present in a biological sample (e.g. maternal blood) containing fetal RNA are detected and quantified in order to determine the ratio of the alleles. This ratio is compared to a standard control consisting of euploid fetuses. Deviation of allele ratio indicates the presence of chromosomal aneuploidy.
Owner:TRUSTEES OF BOSTON UNIV +1
System and method for cleaning noisy genetic data and determining chromosome copy number
ActiveUS20080243398A1Significant resultImprove fidelityData processing applicationsMicrobiological testing/measurementGenetic MaterialsEmbryo
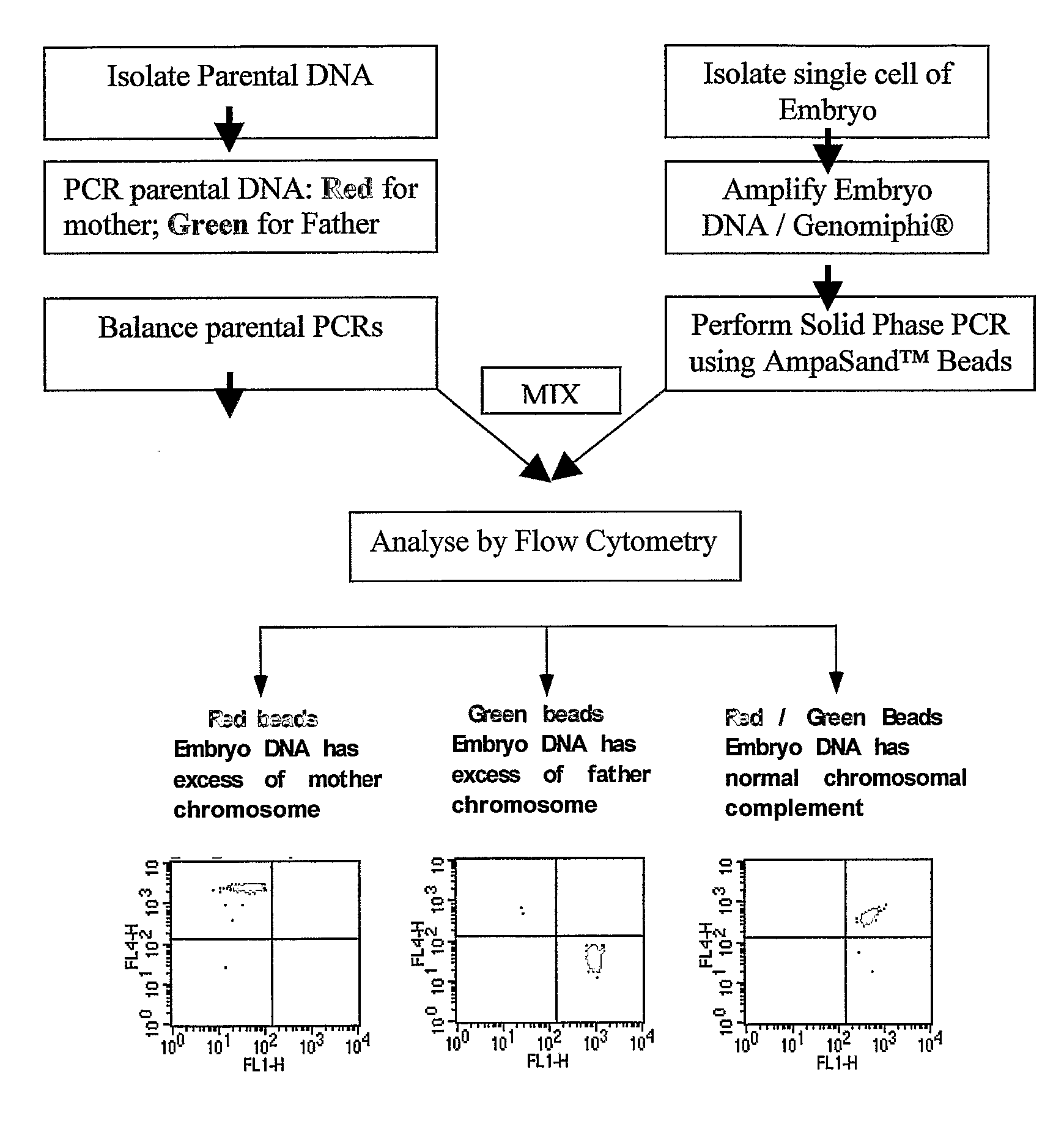
Disclosed herein is a system and method for increasing the fidelity of measured genetic data, for making allele calls, and for determining the state of aneuploidy, in one or a small set of cells, or from fragmentary DNA, where a limited quantity of genetic data is available. Genetic material from the target individual is acquired, amplified and the genetic data is measured using known methods. Poorly or incorrectly measured base pairs, missing alleles and missing regions are reconstructed using expected similarities between the target genome and the genome of genetically related individuals. In accordance with one embodiment of the invention, incomplete genetic data from an embryonic cell are reconstructed at a plurality of loci using the more complete genetic data from a larger sample of diploid cells from one or both parents, with or without haploid genetic data from one or both parents. In another embodiment of the invention, the chromosome copy number can be determined from the measured genetic data of a single or small number of cells, with or without genetic information from one or both parents. In another embodiment of the invention, these determinations are made for the purpose of embryo selection in the context of in-vitro fertilization. In another embodiment of the invention, the genetic data can be reconstructed for the purposes of making phenotypic predictions.
Owner:NATERA
Methods for prenatal diagnosis of chromosomal abnormalities
ActiveUS20070059707A1Rapid productionAccurate detectionMicrobiological testing/measurementFermentationDiseaseNon invasive
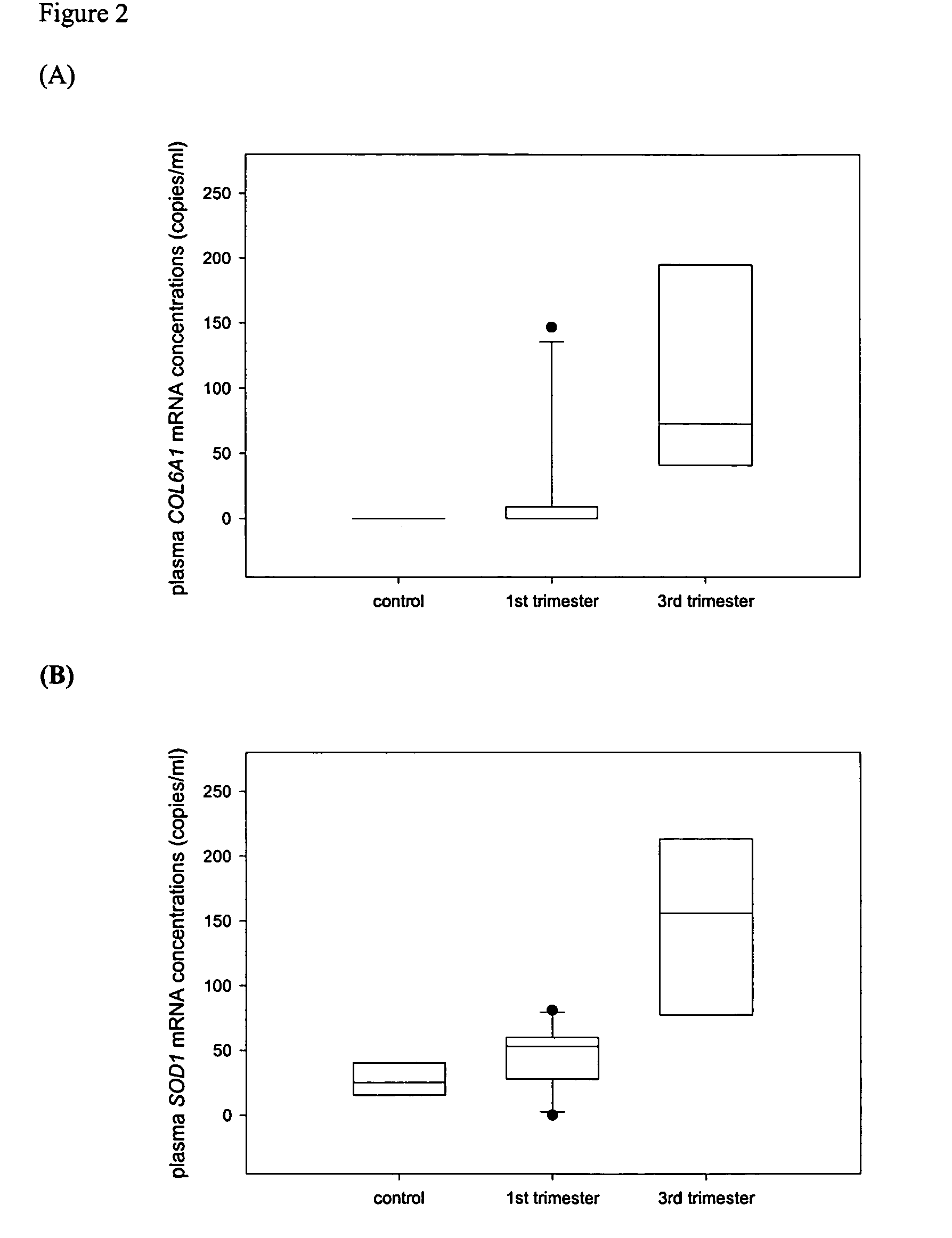
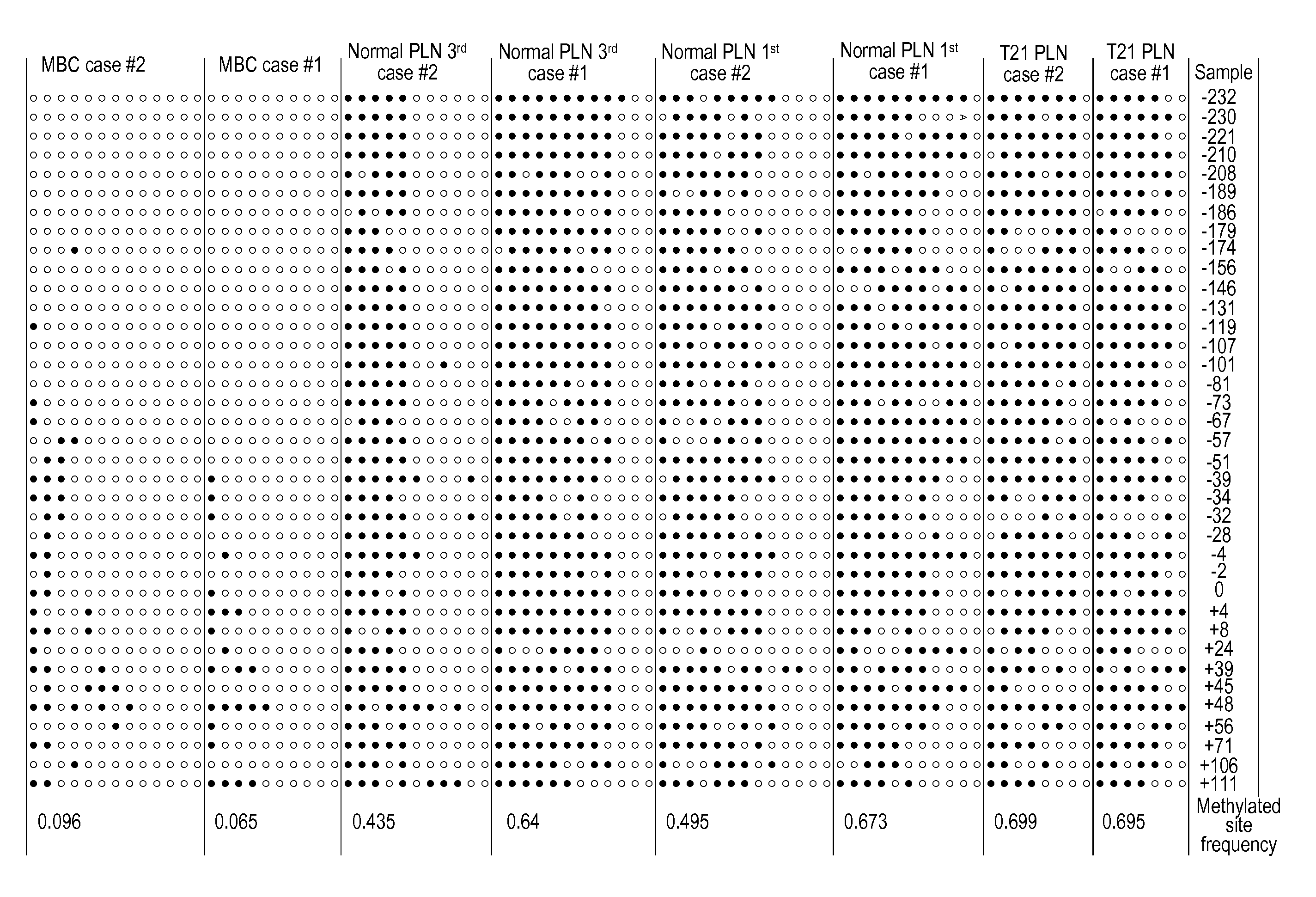
Chromosomal abnormalities are responsible for a significant number of birth defects, including mental retardation. The present invention is related to methods for non-invasive and rapid, prenatal diagnosis of chromosomal abnormalities based on analysis of a maternal blood sample. The invention exploits the differences in DNA between the mother and fetus, for instance differences in their methylation states, as a means to enrich for fetal DNA in maternal plasma sample. The methods described herein can be used to detect chromosomal DNA deletions and duplications. In a preferred embodiment, the methods are used to diagnose chromosomal aneuploidy and related disorders, such as Down's and Turner's Syndrome.
Owner:TRUSTEES OF BOSTON UNIV
Direct Molecular Diagnosis of Fetal Aneuploidy
ActiveUS20110151442A1Addressing slow performanceSugar derivativesMicrobiological testing/measurementSpecific chromosomeFetal tissues
Methods and materials for detection of aneuploidy and other chromosomal abnormalities using fetal tissue are disclosed. Results can be obtained rapidly, without cell culture. The method uses digital PCR for amplification and detection of single target sequences, allowing an accurate count of a specific chromosome or chromosomal region. Specific polynucleic acid primers and probes are disclosed for chromosomes 1, 13, 18, 21, X and Y. These polynucleic acid sequences are chosen to be essentially invariant between individuals, so the test is not dependent on sequence differences between fetus and mother.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Detection of chromosomal disorders
InactiveUS20050250111A1Improve accuracyFast and accurate and simple and inexpensive detectionMicrobiological testing/measurementFermentationDiseaseGenomic DNA
Methods for detecting in a single assay any one of multiple chromosomal disorders that result from aneuploidy or certain mutations, particularly microdeletions, and kits for use therein. A polymerase chain reaction (PCR) is carried out to amplify eukaryotic genomic DNA using a plurality of primer oligonucleotide pairs wherein one primer of each pair has a detectable label attached 5′ thereto. A plurality of the primer pairs are targeted to DNA segments of different chromosomes of interest which are indicative of potential chromosomal disorders, and one pair is targeted for a control gene. The amplified PCR products are purified, and single-stranded DNA having the detectable labels is obtained therefrom and hybridized with spots on a microarray that each contain DNA oligonucleotide probes having nucleotide sequences complementary to a nucleotide sequence of one strand of each segment. The microarray is imaged for presence of labels on its respective spots, and the absence or presence of chromosomal disorders as indicated by one or more of the targeted DNA segments of interest is diagnosed by first comparing the imaging results to the imaging of spots specific to the control gene and then to results obtained from imaging normal DNA.
Owner:NOVARTIS AG
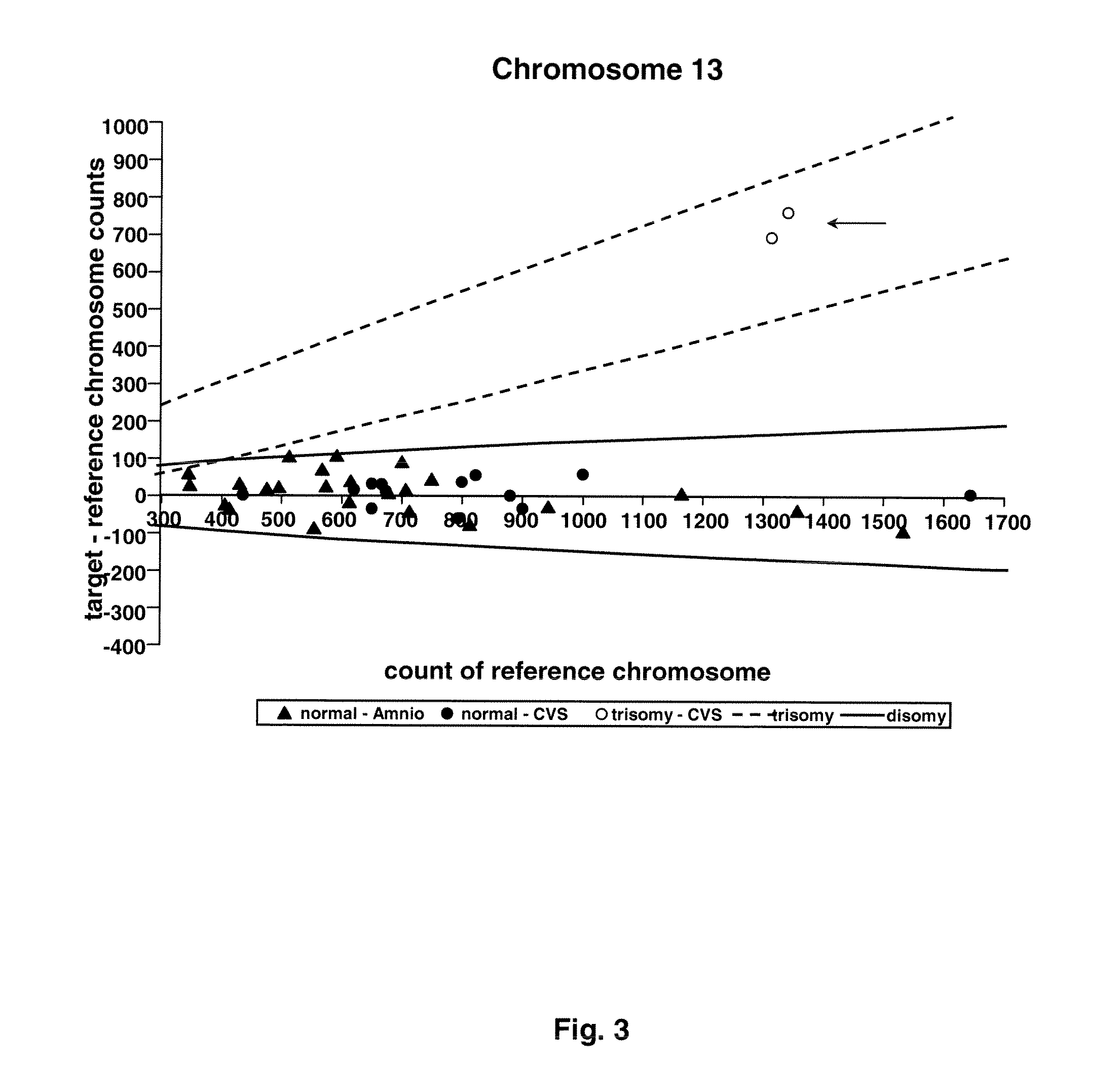
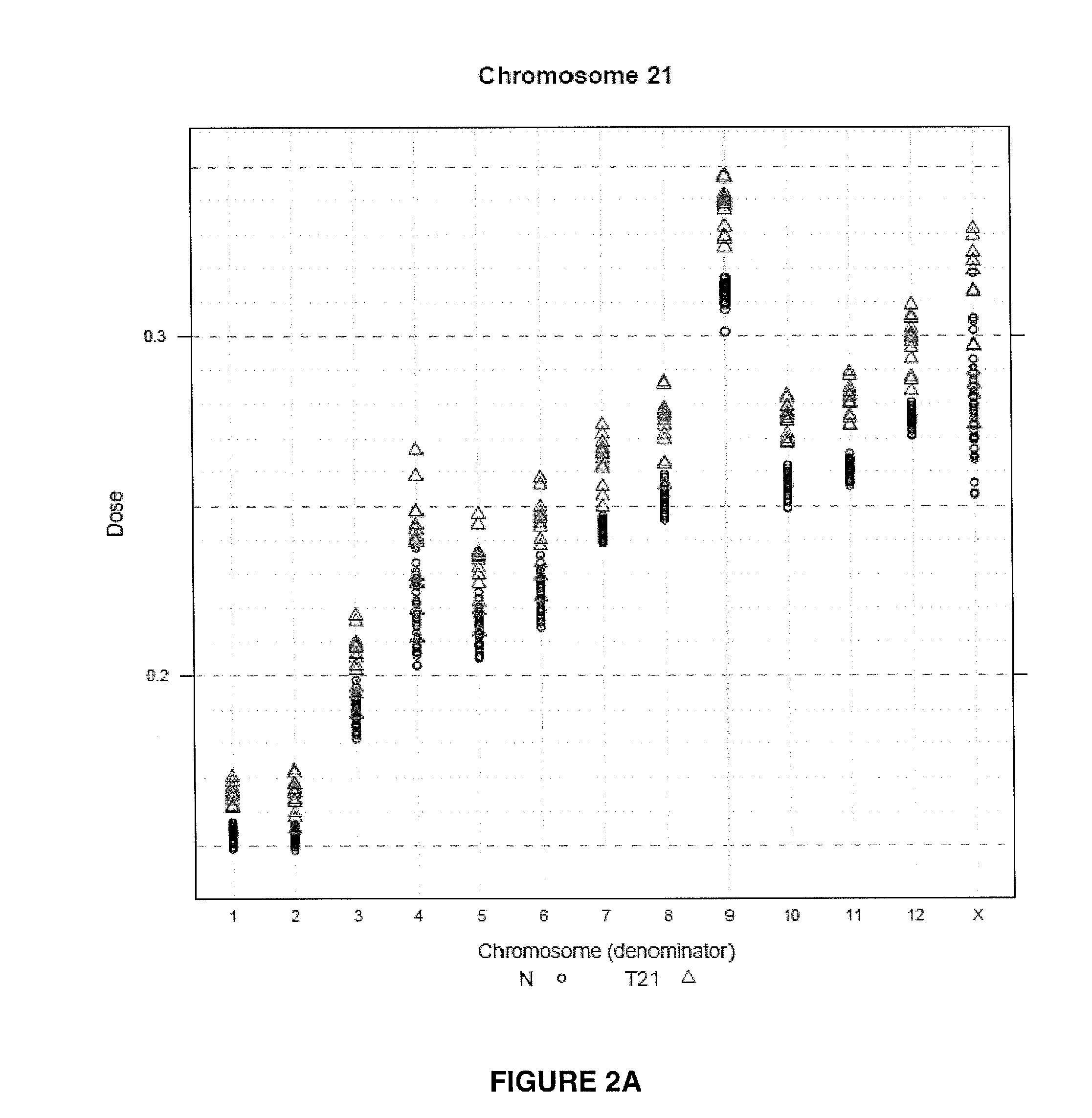
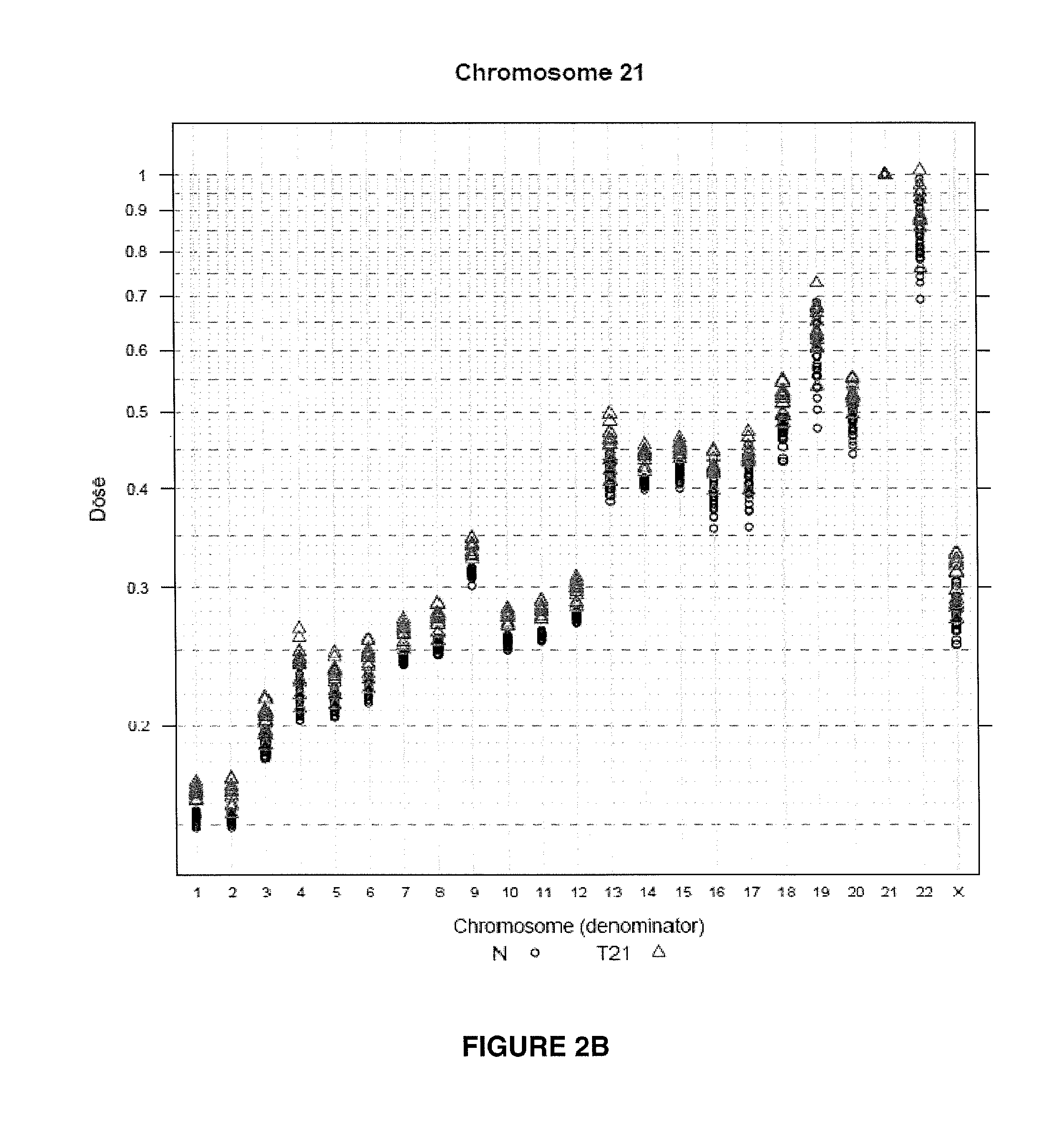
Method for determining copy number variations
The invention provides a method for determining copy number variations (CNV) of a sequence of interest in a test sample that comprises a mixture of nucleic acids that are known or are suspected to differ in the amount of one or more sequence of interest. The method comprises a statistical approach that accounts for accrued variability stemming from process-related, interchromosomal and inter-sequencing variability. The method is applicable to determining CNV of any fetal aneuploidy, and CNVs known or suspected to be associated with a variety of medical conditions. CNV that can be determined according to the present method include trisomies and monosomies of any one or more of chromosomes 1-22, X and Y, other chromosomal polysomies, and deletions and / or duplications of segments of any one or more of the chromosomes, which can be detected by sequencing only once the nucleic acids of a test sample. Any aneuploidy can be determined from sequencing information that is obtained by sequencing only once the nucleic acids of a test sample.
Owner:VERINATA HEALTH INC
Rapid aneuploidy detection
ActiveUS20150051085A1Assessing genomic copy number sensitively and rapidlyNucleotide librariesMicrobiological testing/measurementCell freeTrisomy
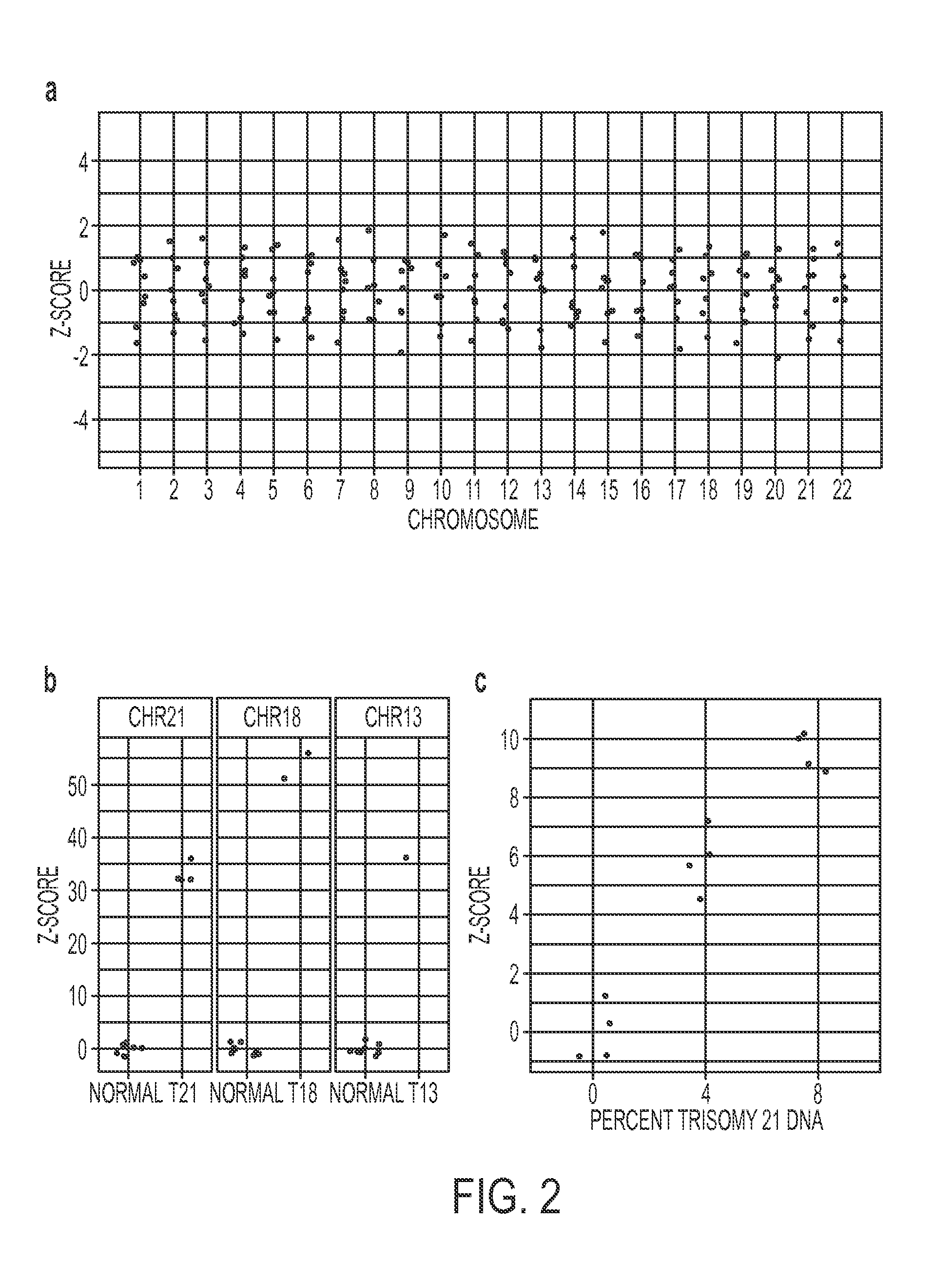
Massively parallel sequencing of cell-free, maternal plasma DNA was recently demonstrated to be a safe and effective screening method for fetal chromosomal aneuploidies. Here, we report an improved sequencing method achieving significantly increased throughput and decreased cost by replacing laborious sequencing library preparation steps with PCR employing a single primer pair. Using this approach, samples containing as little as 4% trisomy 21 DNA could be readily distinguished from euploid samples.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for detecting chromosomal aneuploidy
The present invention relates to a new, non-invasive method for detecting chromosomal aneuploidy by analyzing a sample from a pregnant woman. The detection is based on the ratio between the amount of a fetal methylation marker located on a chromosome relevant to the aneuploidy and the amount of a fetal genetic marker located on a reference chromosome, offering improved accuracy.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Method for genetic testing of human embryos for chromosome abnormalities, segregating genetic disorders with or without a known mutation and mitochondrial disorders following in vitro fertilization (IVF), embryo culture and embryo biopsy
InactiveUS20080085836A1Reduce significant riskImprove the level ofLibrary screeningLibrary member identificationLess invasiveContamination
We describe a method for interrogating the content and primary structure of DNA by microarray analyses and to provide comprehensive genetic screening and diagnostics prior to embryo transfer within an IVF setting. We will accomplish this by the following claims: 1) an optimized embryo grading system, 2) a less invasive embryo biopsy with reduced cellular contamination, 3) an optimized DNA amplification protocol for single cells, 4) identify aneuploidy and structural chromosome abnormalities using microarrays, 5) identifying sub-telomeric chromosome rearrangements, 6) a modified DNA fingerprinting protocol, 7) determine imprinting and epigenetic changes in developing embryos, 8) performing genome-wide scans to clarify / diagnose multi-factorial genetic disease and to determine genotype / haplotype patterns that may predict future disease, 9) determining single gene disorders with or without a known DNA mutation, 10) determining mtDNA mutations and / or the combination of mtDNA and genomic (nuclear) DNA aberrations that cause genetic disease.
Owner:KEARNS WILLIAM G +1
Selection of cells using biomarkers
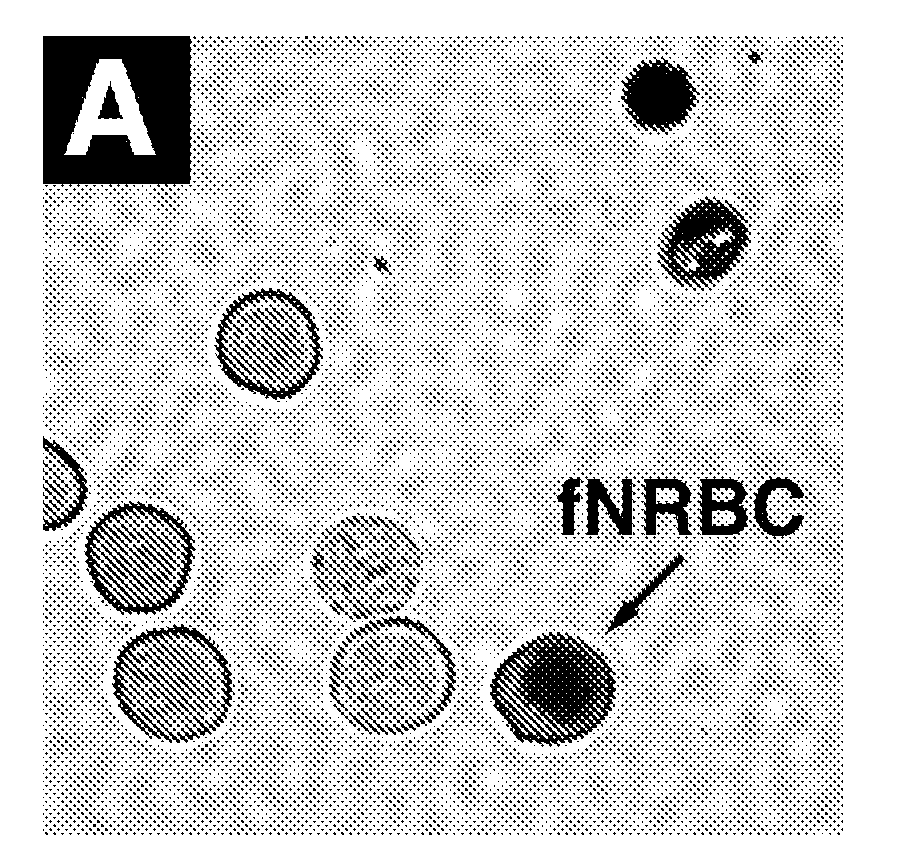
InactiveUS20080113358A1Easy to separateIncrease the number ofMicrobiological testing/measurementMaterial analysisFetal abnormalityFetal cell
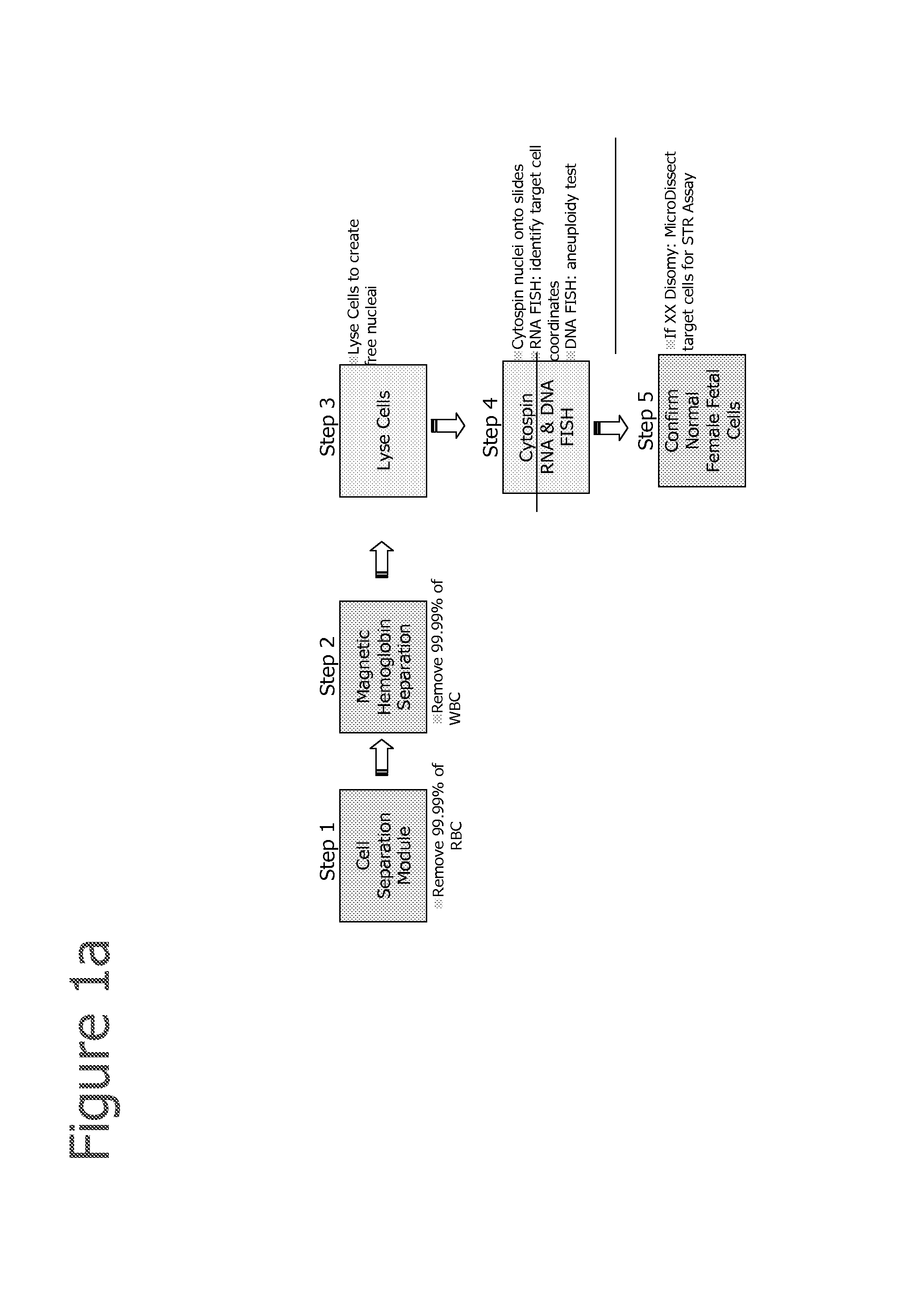
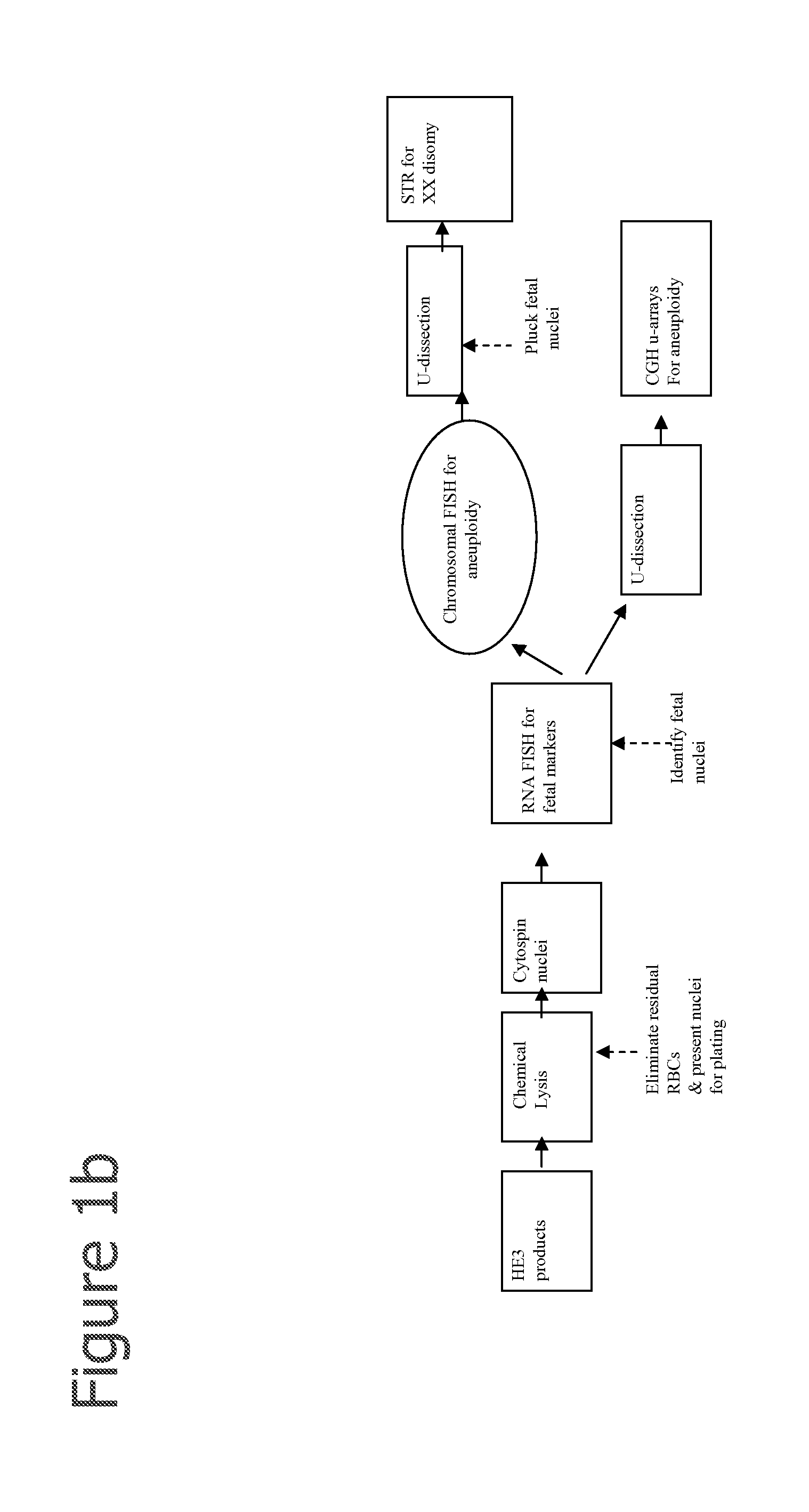
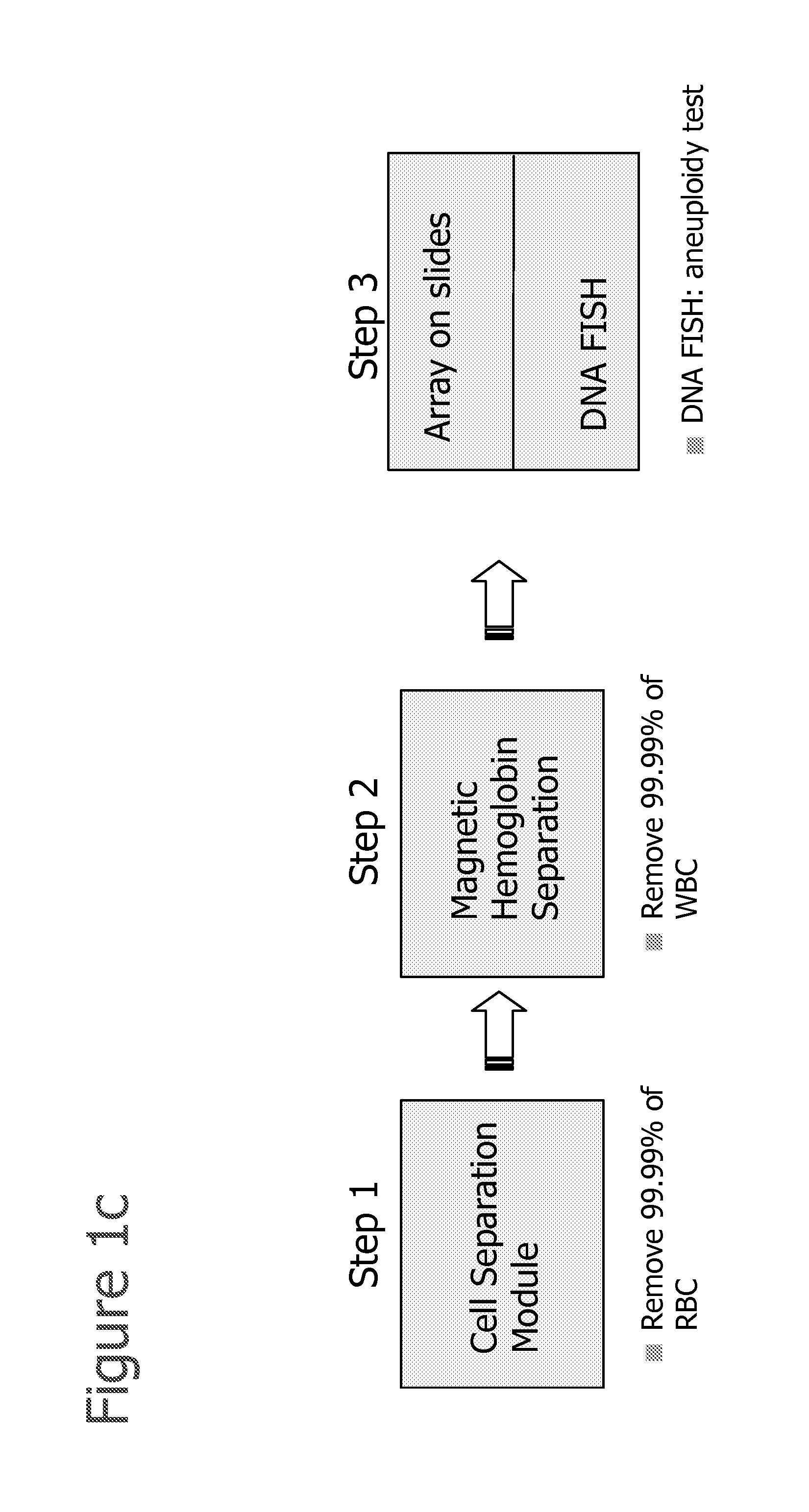
The present invention provides systems, apparatuses, and methods to isolate, select or detect the presence of a target cell (e.g., fetal cells) in a sample comprising mixed populations of cells that vastly outnumber the target cells. Target cells include fetal cells, such as nucleated red blood cells, and methods of selecting such cells include diagnosis of fetal abnormalities, i.e., aneuploidy. Furthermore, methods comprise utilizing fetal biomarkers to select fetal cells in a sample comprising fetal and adult cells.
Owner:THE GENERAL HOSPITAL CORP +2
Methods for prenatal diagnosis of chromosomal abnormalities
ActiveUS7655399B2Rapid productionAccurate detectionMicrobiological testing/measurementPlasma samplesNon invasive
Chromosomal abnormalities are responsible for a significant number of birth defects, including mental retardation. The present invention is related to methods for non-invasive and rapid, prenatal diagnosis of chromosomal abnormalities based on analysis of a maternal blood sample. The invention exploits the differences in DNA between the mother and fetus, for instance differences in their methylation states, as a means to enrich for fetal DNA in maternal plasma sample. The methods described herein can be used to detect chromosomal DNA deletions and duplications. In a preferred embodiment, the methods are used to diagnose chromosomal aneuploidy and related disorders, such as Down's and Turner's Syndrome.
Owner:TRUSTEES OF BOSTON UNIV
System and method for cleaning noisy genetic data and determining chromosome copy number
ActiveUS8515679B2Improve fidelityData processing applicationsMicrobiological testing/measurementGenetic MaterialsEmbryo
Disclosed herein is a system and method for increasing the fidelity of measured genetic data, for making allele calls, and for determining the state of aneuploidy, in one or a small set of cells, or from fragmentary DNA, where a limited quantity of genetic data is available. Genetic material from the target individual is acquired, amplified and the genetic data is measured using known methods. Poorly or incorrectly measured base pairs, missing alleles and missing regions are reconstructed using expected similarities between the target genome and the genome of genetically related individuals. In accordance with one embodiment of the invention, incomplete genetic data from an embryonic cell are reconstructed at a plurality of loci using the more complete genetic data from a larger sample of diploid cells from one or both parents, with or without haploid genetic data from one or both parents. In another embodiment of the invention, the chromosome copy number can be determined from the measured genetic data of a single or small number of cells, with or without genetic information from one or both parents. In another embodiment of the invention, these determinations are made for the purpose of embryo selection in the context of in-vitro fertilization. In another embodiment of the invention, the genetic data can be reconstructed for the purposes of making phenotypic predictions.
Owner:NATERA
Noninvasive Diagnosis of Fetal Aneuploidy by Sequencing
ActiveUS20110246083A1Maximum resultMicrobiological testing/measurementDisease diagnosisMassive parallel sequencingFetal aneuploidy
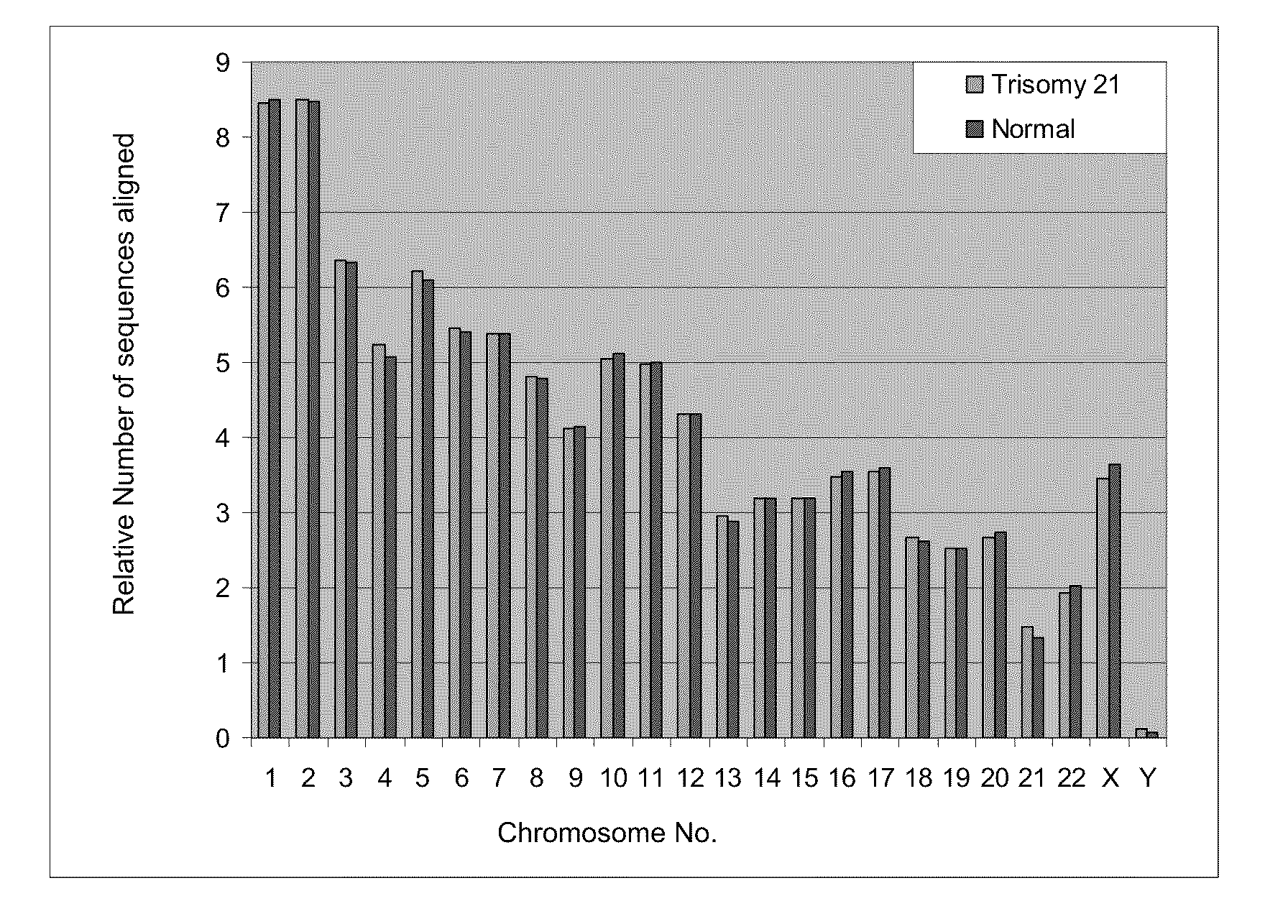
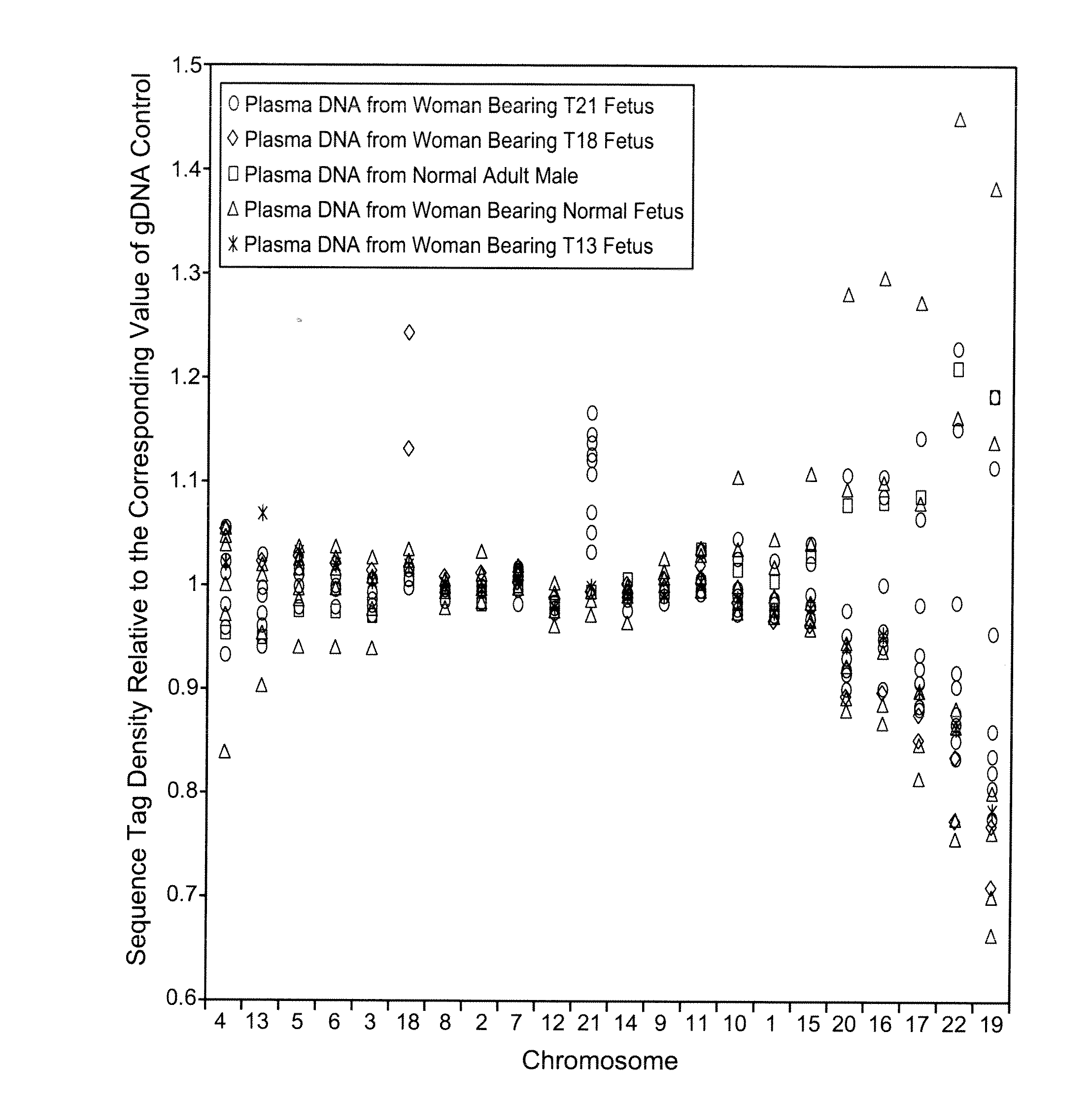
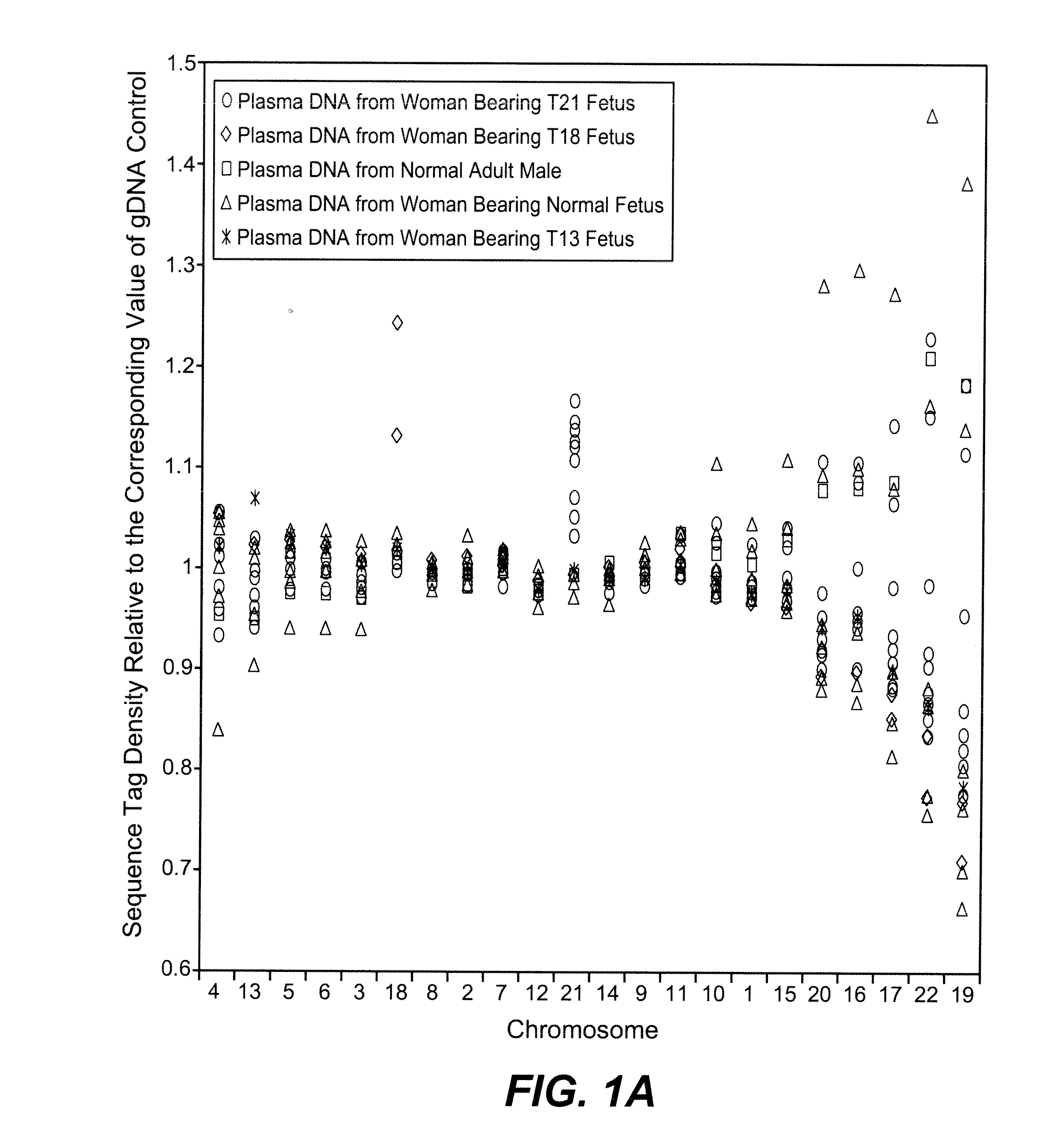
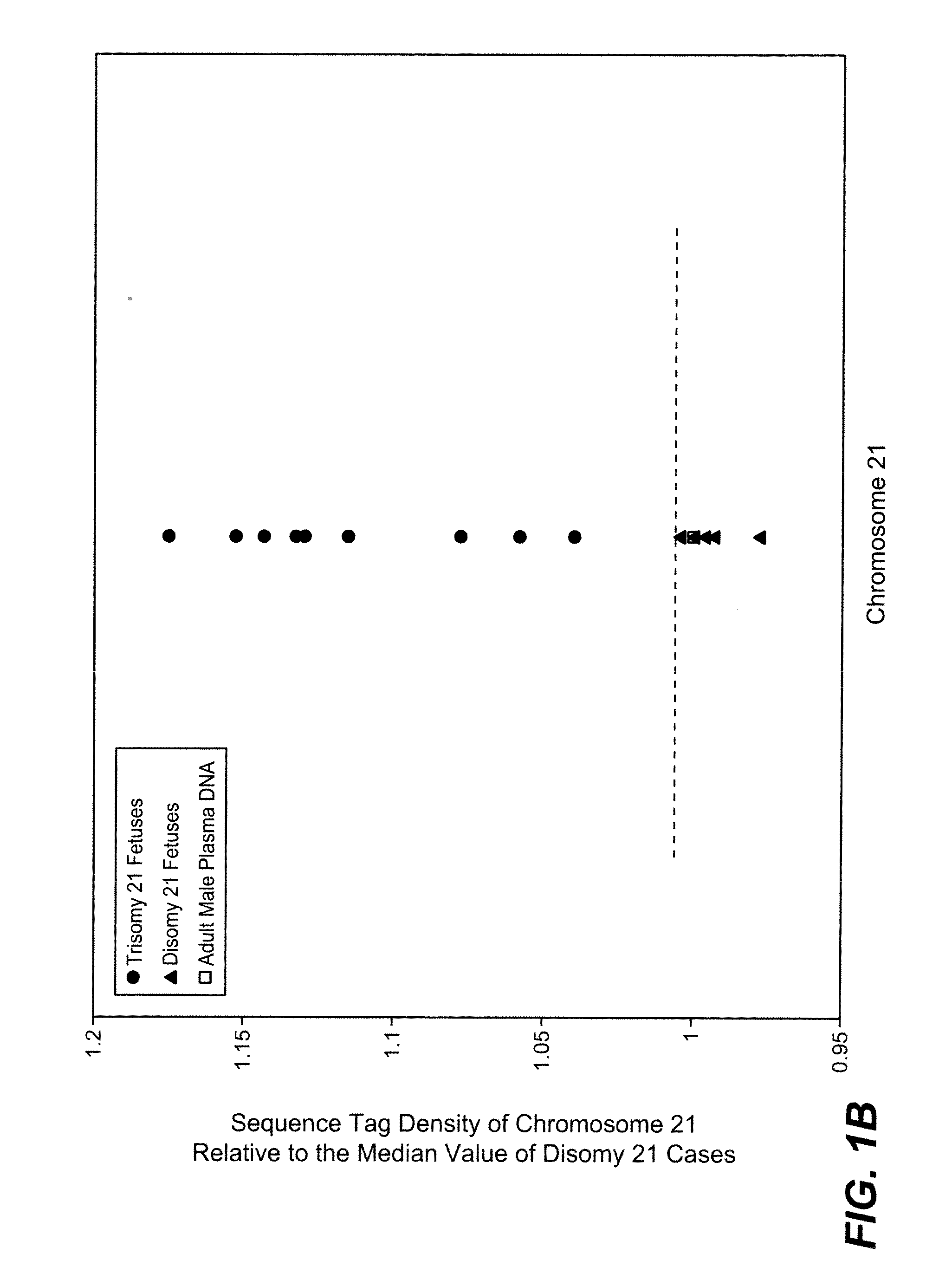
Disclosed is a method to achieve digital quantification of DNA (i.e., counting differences between identical sequences) using direct shotgun sequencing followed by mapping to the chromosome of origin and enumeration of fragments per chromosome. The preferred method uses massively parallel sequencing, which can produce tens of millions of short sequence tags in a single run and enabling a sampling that can be statistically evaluated. By counting the number of sequence tags mapped to a predefined window in each chromosome, the over- or under-representation of any chromosome in maternal plasma DNA contributed by an aneuploid fetus can be detected. This method does not require the differentiation of fetal versus maternal DNA. The median count of autosomal values is used as a normalization constant to account for differences in total number of sequence tags is used for comparison between samples and between chromosomes.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method Of Detecting Aneuploidy
InactiveUS20080102455A1Quick checkCheap methodMicrobiological testing/measurementBiological bodyIvf treatment
The present invention provides a method for detecting aneuploidy in a subject. This method has applications for the detection of aneuploidy in single cells, embryos and complete organisms. The present invention has particular application for the detection of aneuploidy in human and other animal embryos generated by in-vitro fertilization. Pre-implantation screening for aneuploidy has the potential to significantly increase the rate of successful carriage to term after IVF treatment, and significantly reduce the incidence of birth defects in children conceived with the assistance of IVF treatment. Kits for the detection of aneuploidy are also provided.
Owner:GENERA BIOSYSTEMS LTD
System and method for cleaning noisy genetic data and determining chromosome copy number
ActiveUS20140032128A1Significant resultImprove fidelityMicrobiological testing/measurementBiostatisticsDiploid cellsEmbryo
Disclosed herein is a system and method for increasing the fidelity of measured genetic data, for making allele calls, and for determining the state of aneuploidy, in one or a small set of cells, or from fragmentary DNA, where a limited quantity of genetic data is available. Poorly or incorrectly measured base pairs, missing alleles and missing regions are reconstructed using expected similarities between the target genome and the genome of genetically related individuals. In accordance with one embodiment, incomplete genetic data from an embryonic cell are reconstructed at a plurality of loci using the more complete genetic data from a larger sample of diploid cells from one or both parents, with or without haploid genetic data from one or both parents. In another embodiment, the chromosome copy number can be determined from the measured genetic data, with or without genetic information from one or both parents.
Owner:NATERA
Diagnosing fetal chromosomal aneuploidy using massively parallel genomic sequencing
PendingUS20110318734A1High sensitivityMore accurateMicrobiological testing/measurementProteomicsGenomic sequencingDNA fragmentation
Embodiments of this invention provide methods, systems, and apparatus for determining whether a fetal chromosomal aneuploidy exists from a biological sample obtained from a pregnant female. Nucleic acid molecules of the biological sample are sequenced, such that a fraction of the genome is sequenced. Respective amounts of a clinically-relevant chromosome and of background chromosomes are determined from results of the sequencing. The determination of the relative amounts may count sequences of only certain length. A parameter derived from these amounts (e.g. a ratio) is compared to one or more cutoff values, thereby determining a classification of whether a fetal chromosomal aneuploidy exists. Prior to sequencing, the biological sample may be enriched for DNA fragments of a particular sizes.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Proteomic analysis of biological fluids
ActiveUS20070161125A1Eliminate needMicrobiological testing/measurementAnalogue computers for chemical processesDiseaseGynecology
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns a comprehensive proteomic analysis of human amniotic fluid (AF) and cervical vaginal fluid (CVF), and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, pre-term labor, and / or chromosomal defects. The invention further concerns the identification of biomarkers and groups of biomarkers that can be used for non-invasive diagnosis of various pregnancy-related disorders, and diagnostic assays using such biomarkers.
Owner:HOLOGIC INC
System and method for cleaning noisy genetic data and determining chromosome copy number
ActiveUS10083273B2Improve fidelityData processing applicationsMicrobiological testing/measurementDiploid cellsEmbryo
Disclosed herein is a system and method for increasing the fidelity of measured genetic data, for making allele calls, and for determining the state of aneuploidy, in one or a small set of cells, or from fragmentary DNA, where a limited quantity of genetic data is available. Poorly or incorrectly measured base pairs, missing alleles and missing regions are reconstructed using expected similarities between the target genome and the genome of genetically related individuals. In accordance with one embodiment, incomplete genetic data from an embryonic cell are reconstructed at a plurality of loci using the more complete genetic data from a larger sample of diploid cells from one or both parents, with or without haploid genetic data from one or both parents. In another embodiment, the chromosome copy number can be determined from the measured genetic data, with or without genetic information from one or both parents.
Owner:NATERA
Non-Invasive Fetal Genetic Screening by Digital Analysis
The present methods are exemplified by a process in which maternal blood containing fetal DNA is diluted to a nominal value of approximately 0.5 genome equivalent of DNA per reaction sample. Digital PCR is then be used to detect aneuploidy, such as the trisomy that causes Down Syndrome. Since aneuploidies do not present a mutational change in sequence, and are merely a change in the number of chromosomes, it has not been possible to detect them in a fetus without resorting to invasive techniques such as amniocentesis or chorionic villi sampling. Digital amplification allows the detection of aneuploidy using massively parallel amplification and detection methods, examining, e.g., 10,000 genome equivalents.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
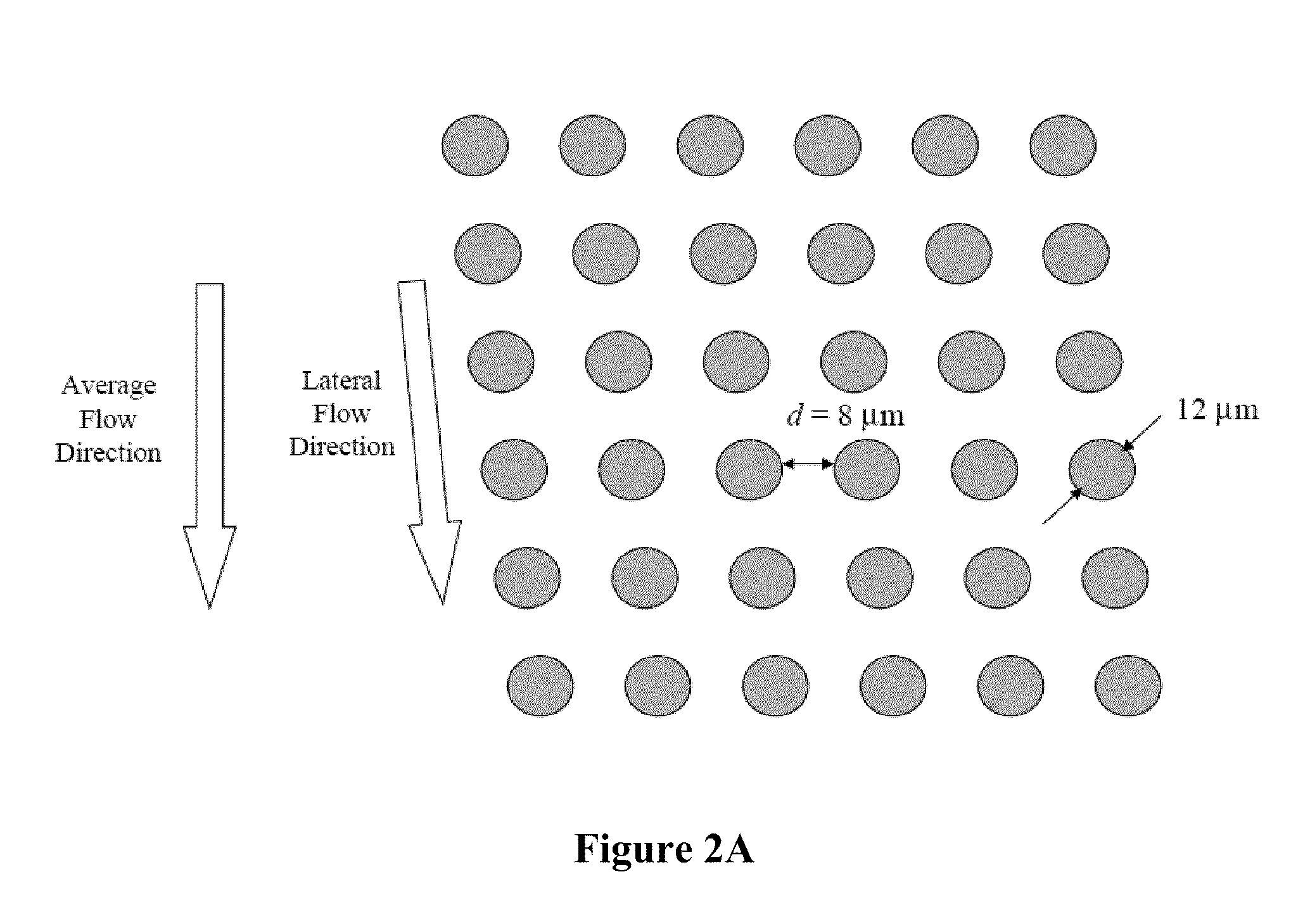
Rare cell analysis using sample splitting and DNA tags
ActiveUS20100136529A1Microbiological testing/measurementLaboratory glasswaresFetal abnormalityRare cell
The present invention provides systems, apparatuses, and methods to detect the presence of fetal cells when mixed with a population of maternal cells in a sample and to test fetal abnormalities, e.g. aneuploidy. The present invention involves labeling regions of genomic DNA in each cell in said mixed sample with different labels wherein each label is specific to each cell and quantifying the labeled regions of genomic DNA from each cell in the mixed sample. More particularly the invention involves quantifying labeled DNA polymorphisms from each cell in the mixed sample.
Owner:VERINATA HEALTH INC +2
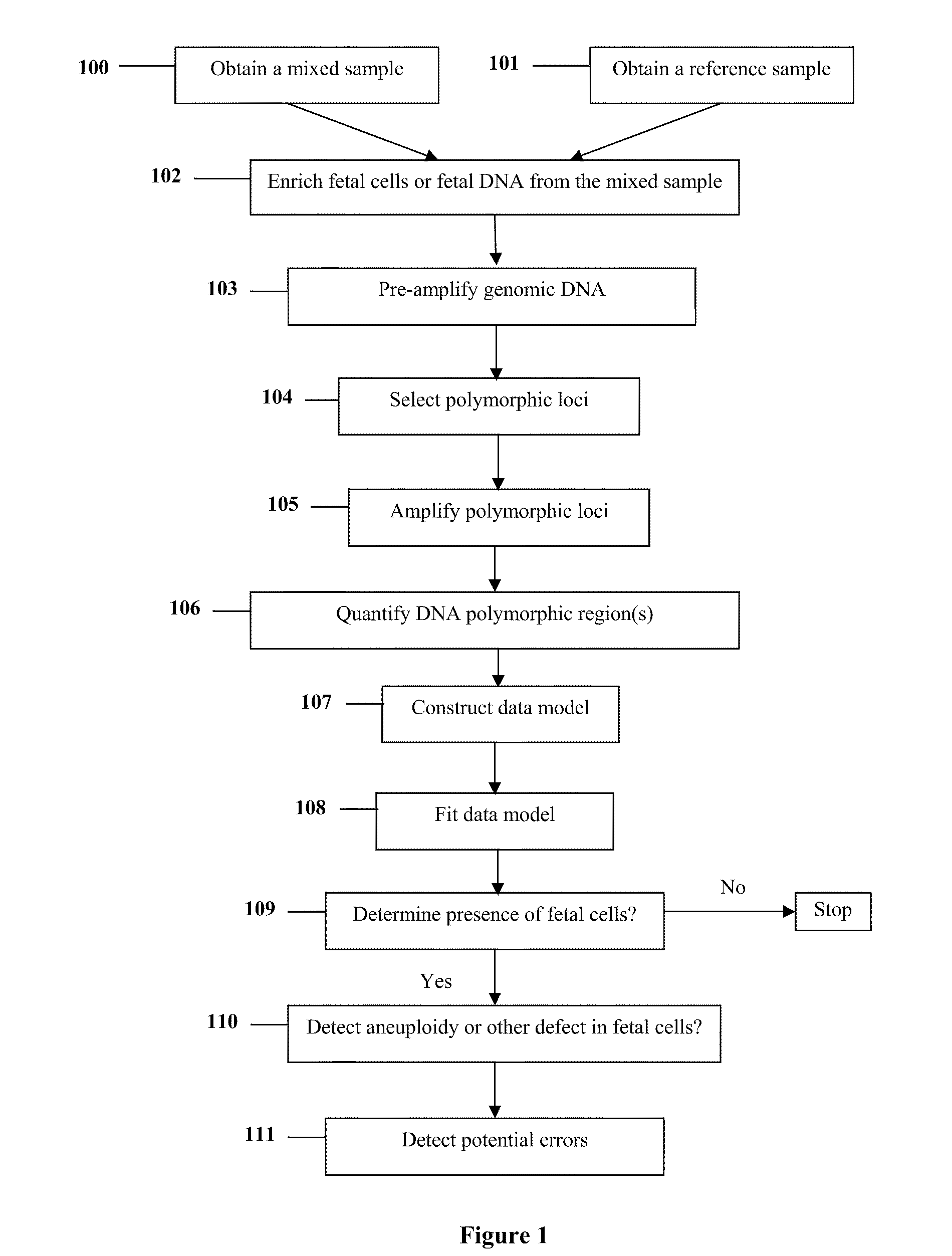
Diagnosis of fetal abnormalities using polymorphisms including short tandem repeats
InactiveUS20090280492A1Microbiological testing/measurementPreparing sample for investigationFetal abnormalityGenomic DNA
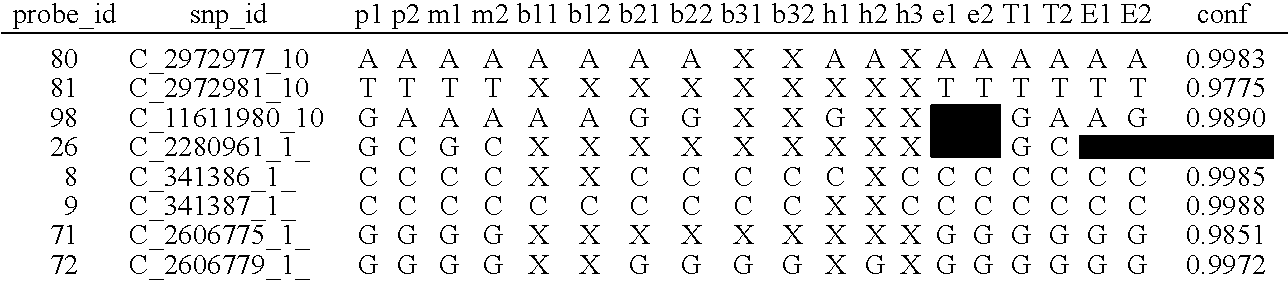
The present invention provides systems, apparatuses, and methods to detect the presence of fetal cells when mixed with a population of maternal cells in a sample and to test fetal abnormalities, i.e. aneuploidy. In addition, the present invention provides methods to determine when there are insufficient fetal cells for a determination and report a non-informative case. The present invention involves quantifying regions of genomic DNA from a mixed sample. More particularly the invention involves quantifying DNA polymorphisms from the mixed sample.
Owner:STOUGHTON ROLAND +2
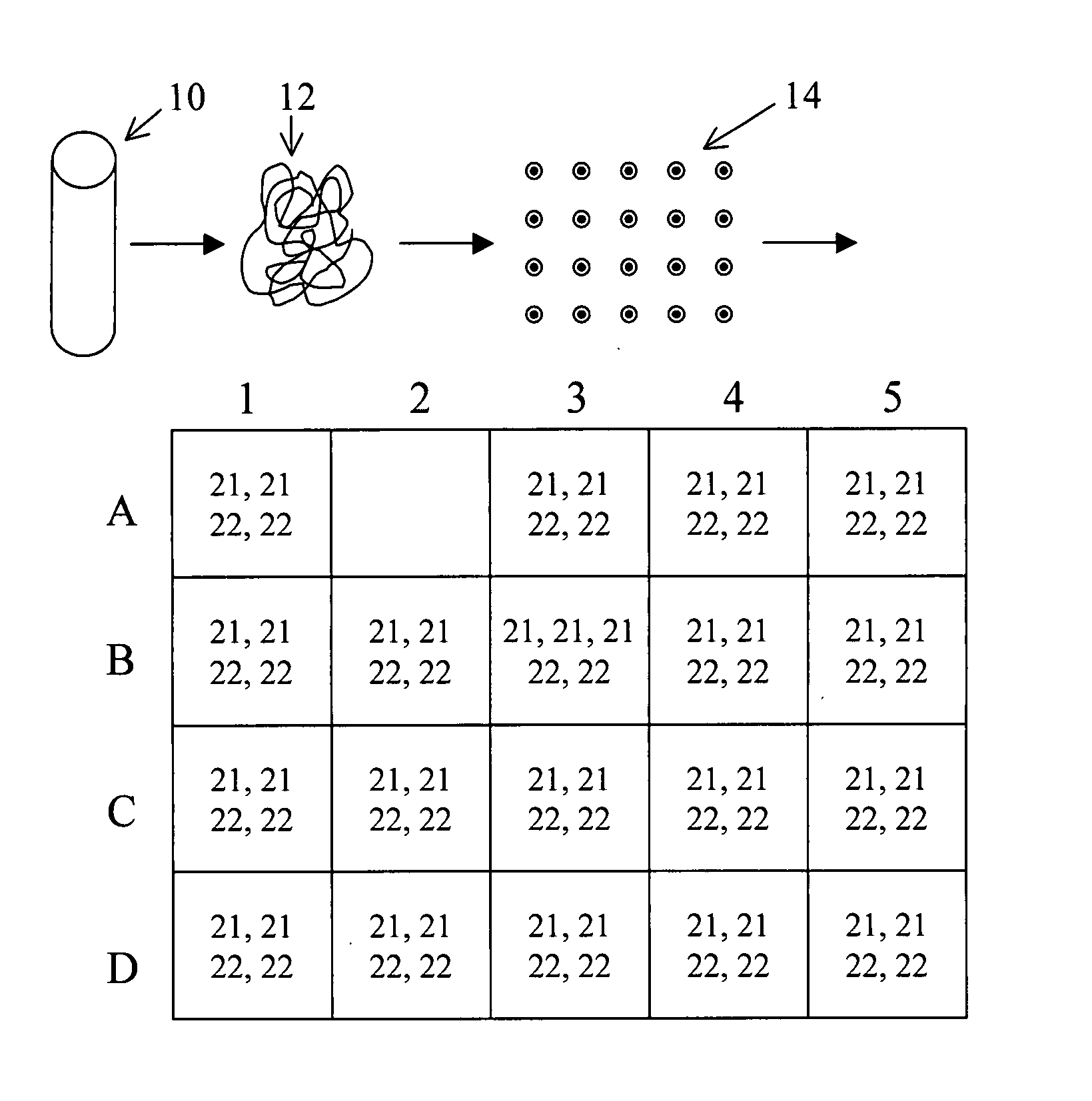
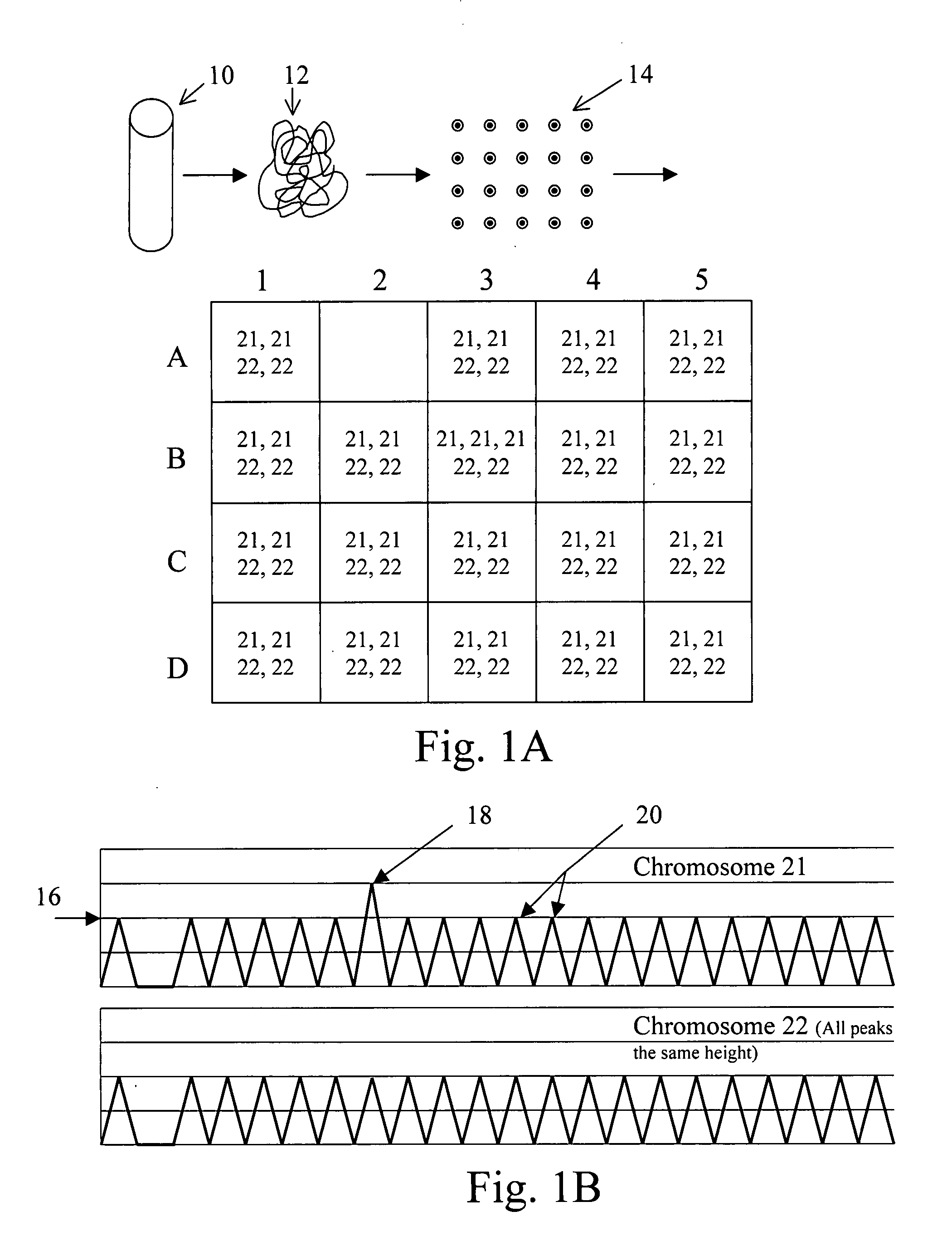
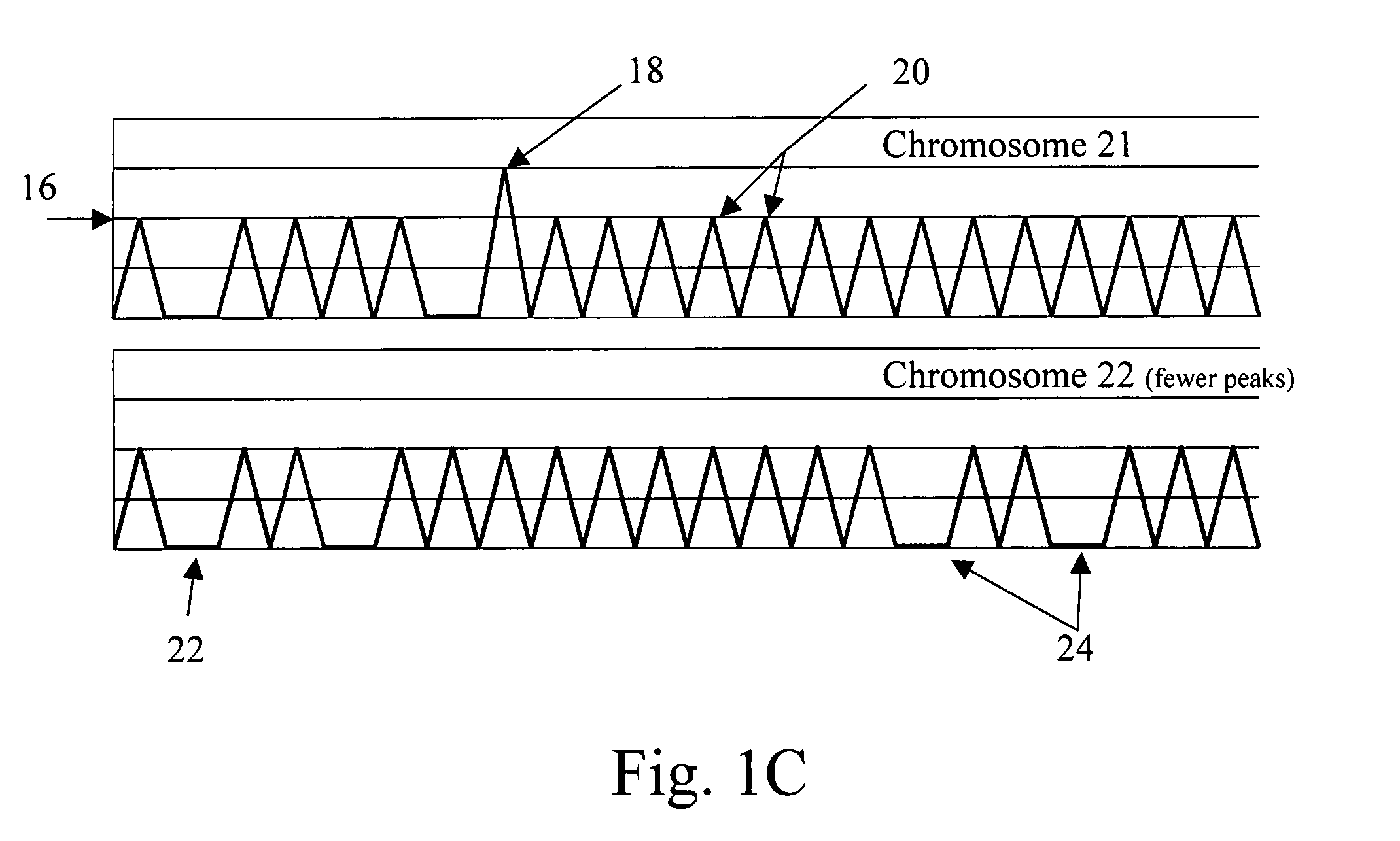
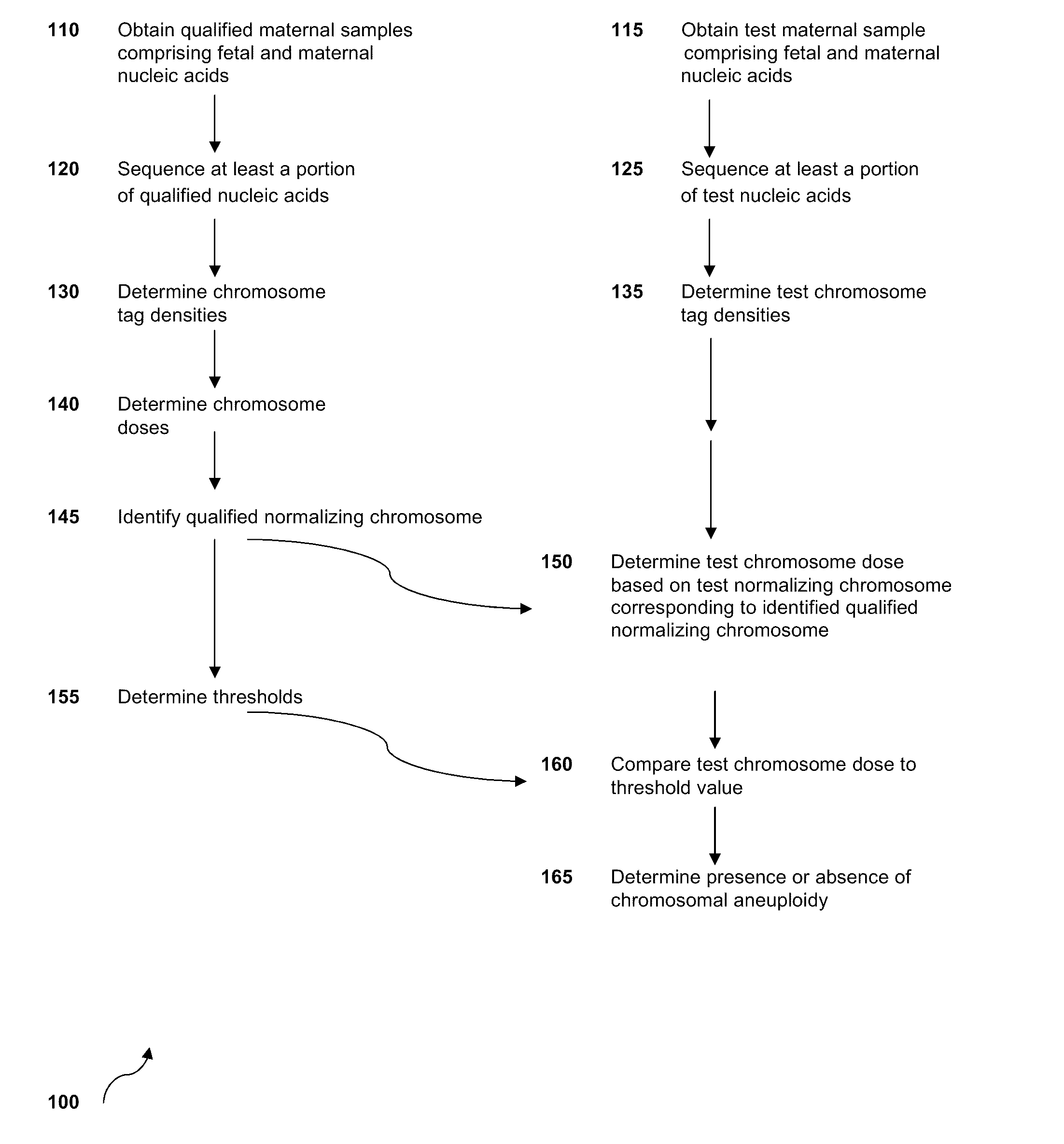
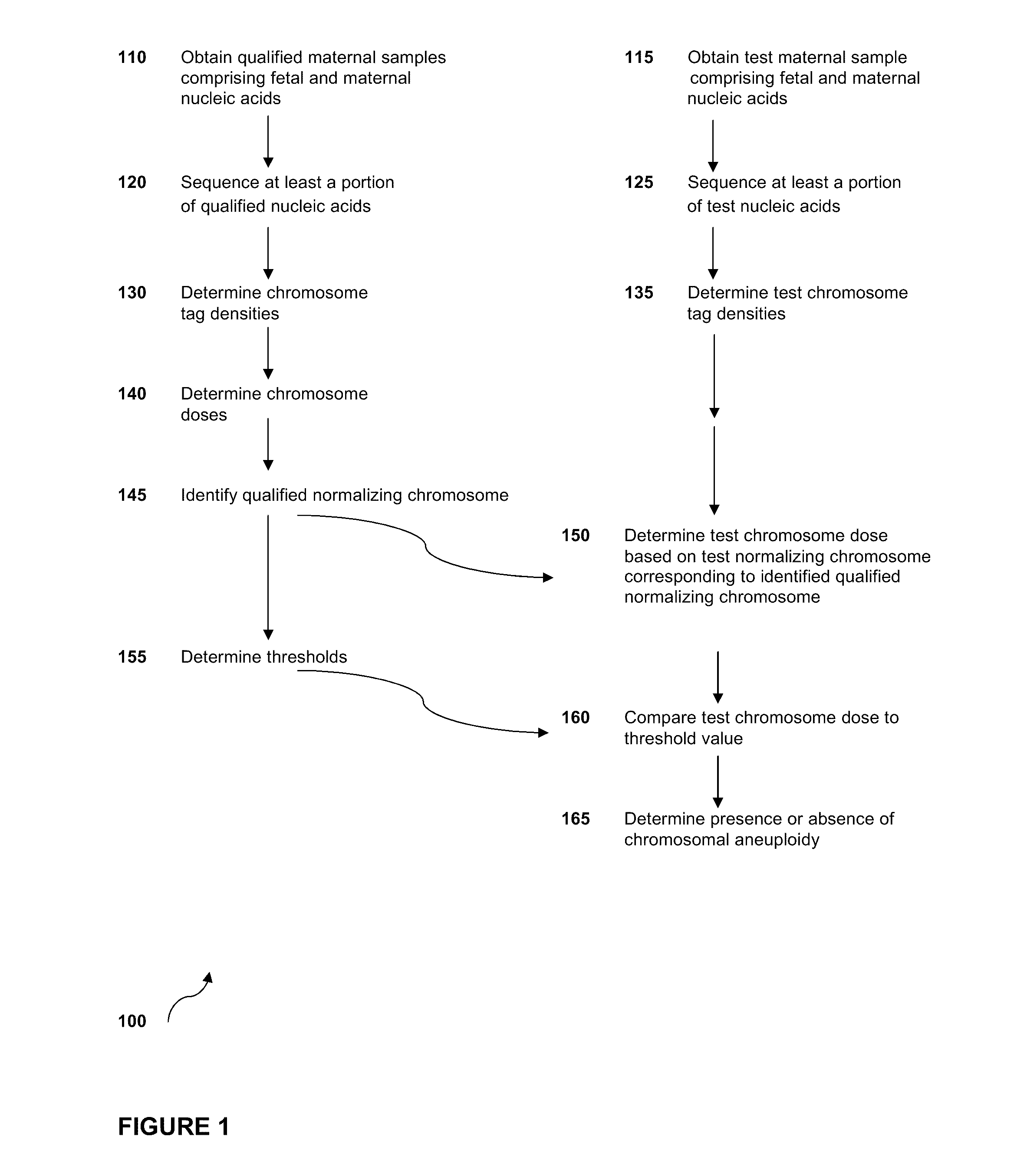
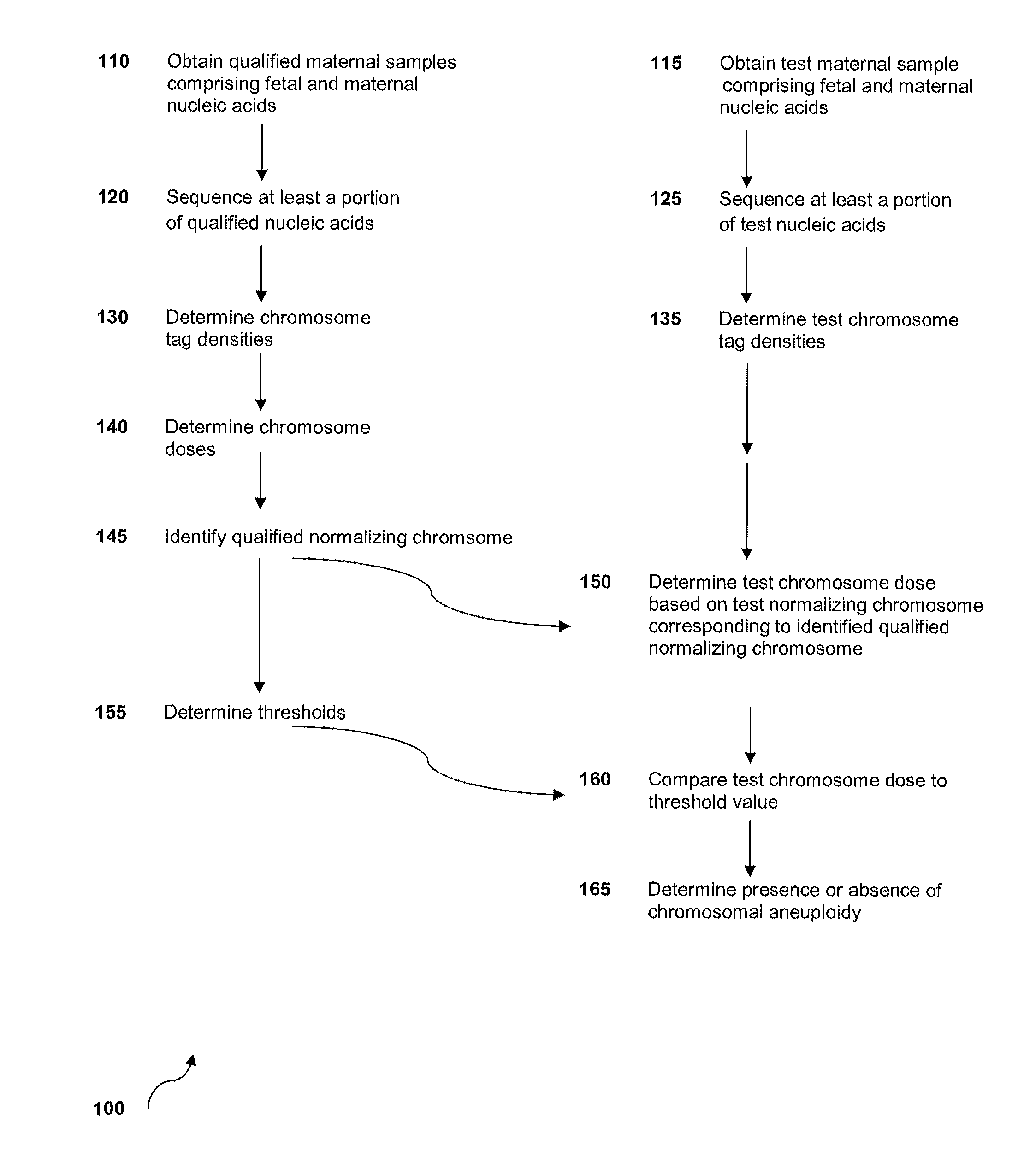
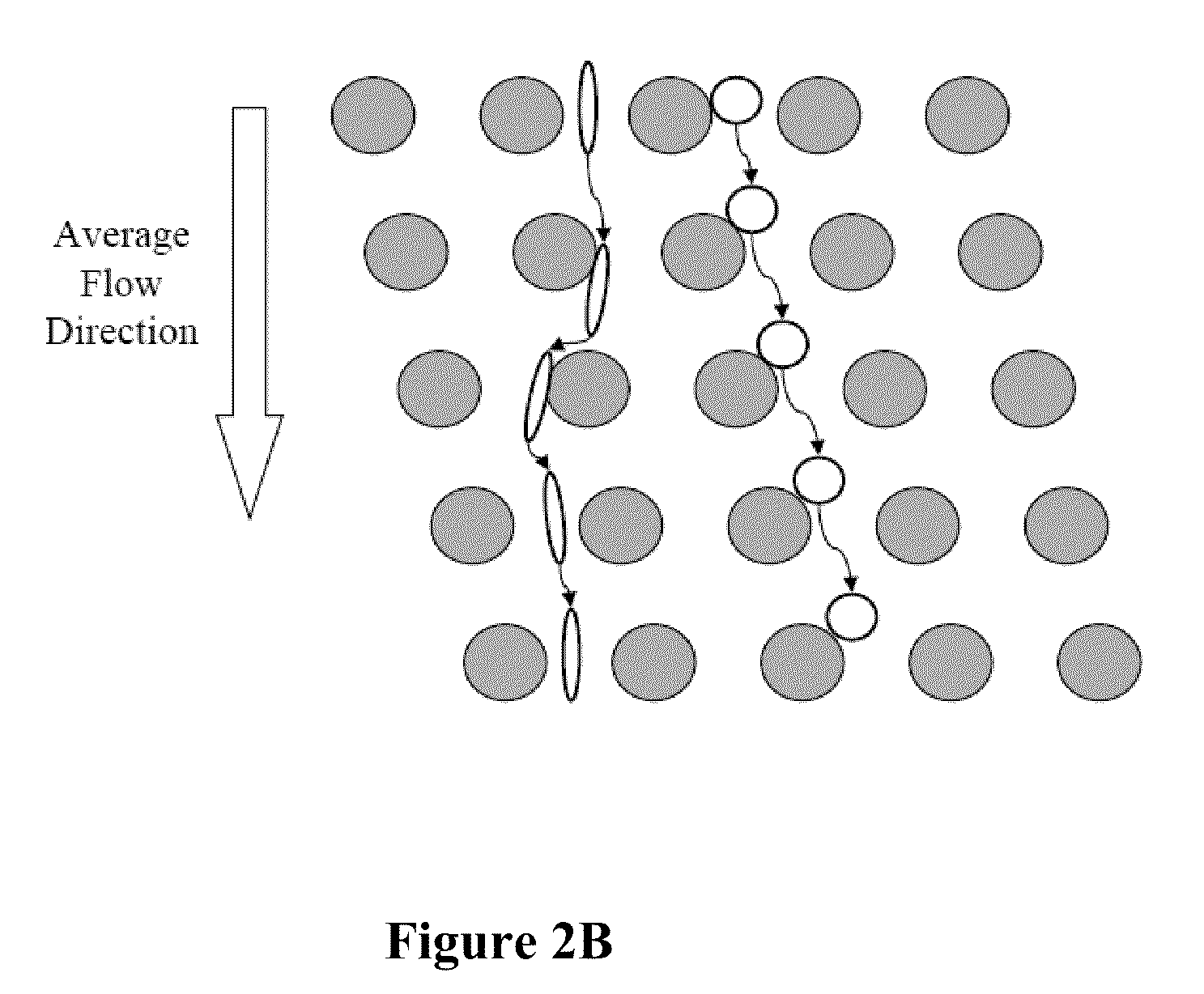
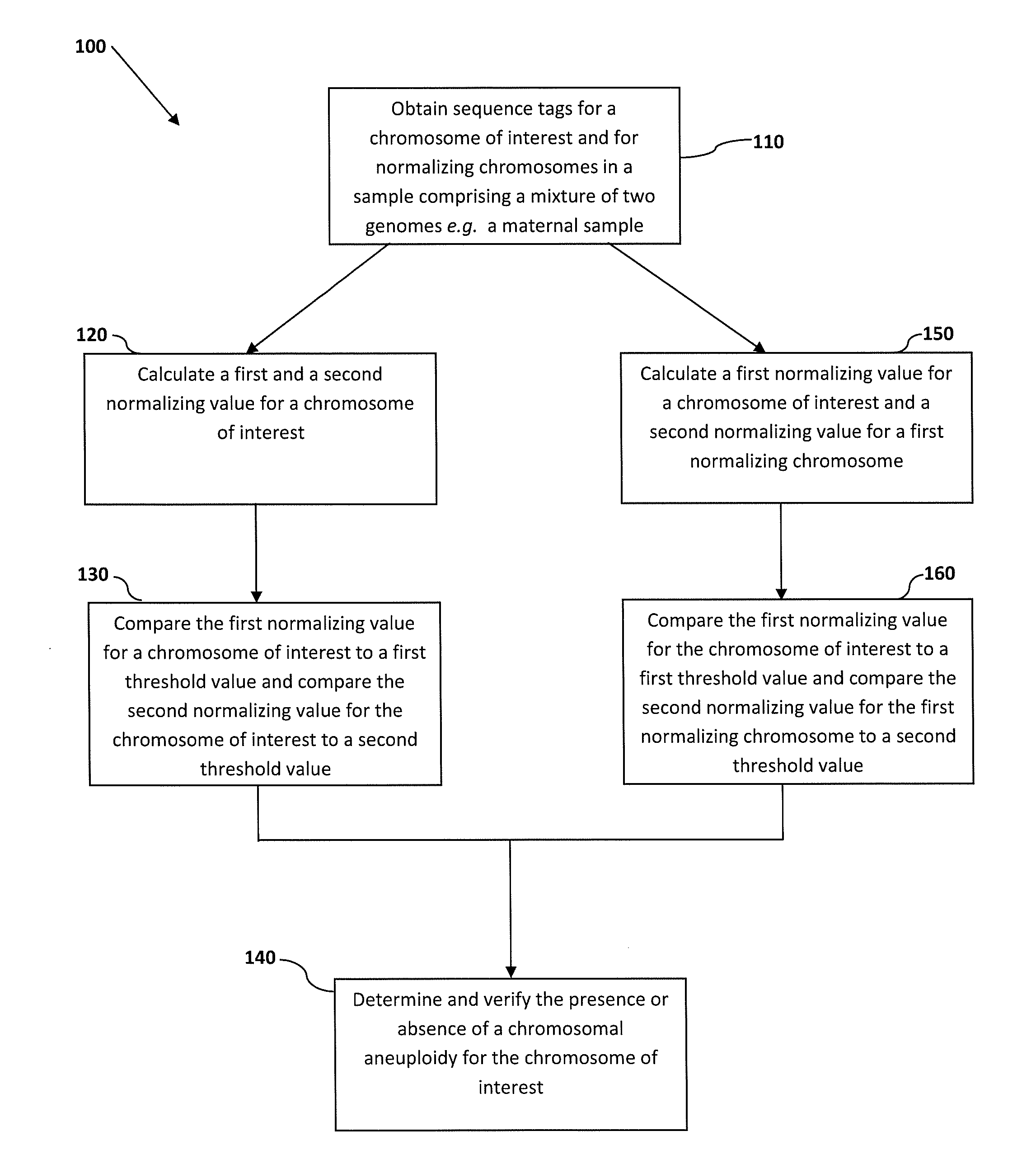
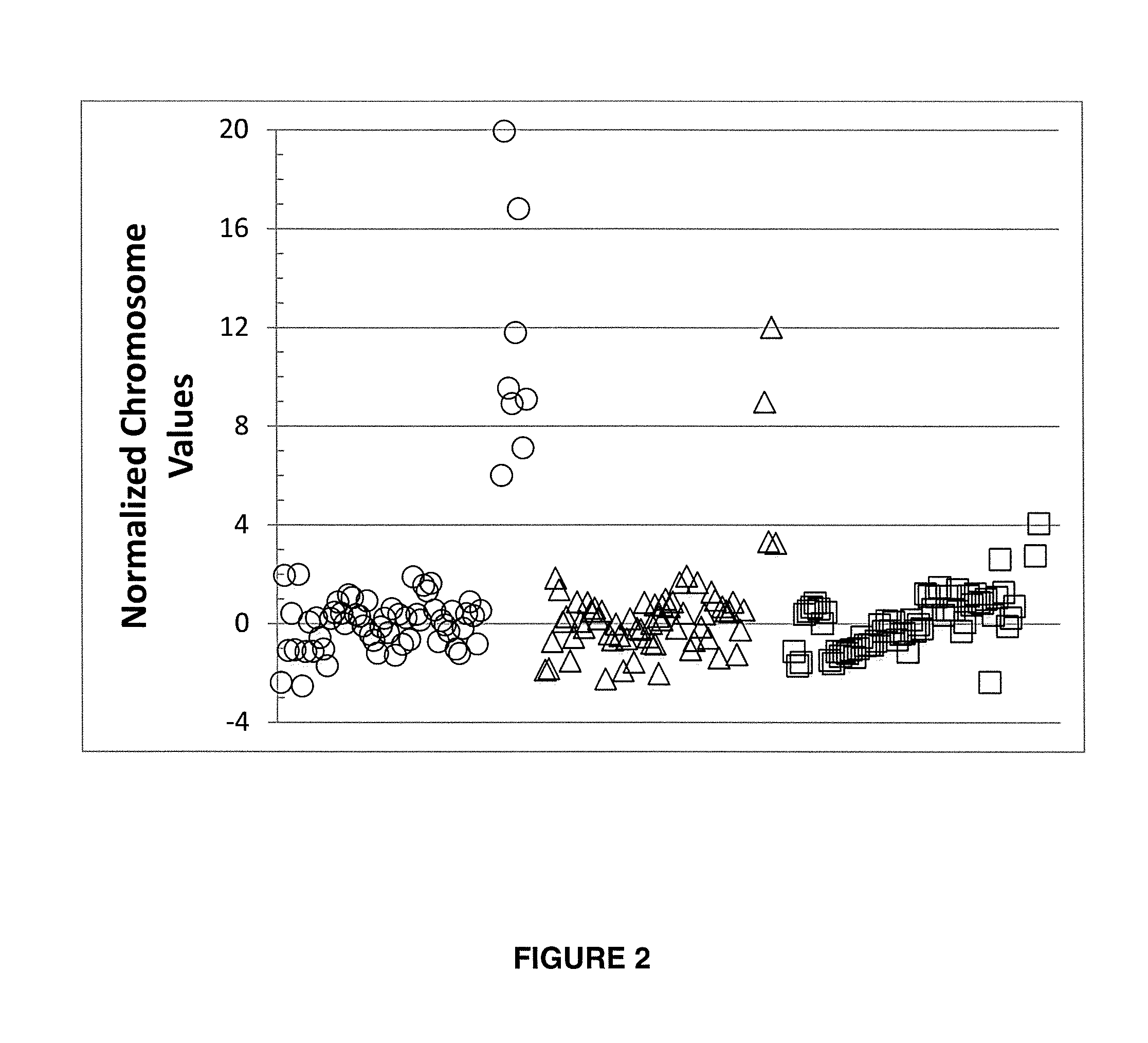
Normalizing chromosomes for the determination and verification of common and rare chromosomal aneuploidies
Owner:VERINATA HEALTH INC
Assays for the detection of genotype, mutations, and/or aneuploidy
InactiveUS20140186827A1Increase percentageMicrobiological testing/measurementDisease diagnosisAssayGenotype
The present invention provides amplification-based methods for detection of genotype, mutations, and / or aneuploidy. These methods have broad applicability, but are particularly well-suited to detecting and quantifying target nucleic acids in free fetal DNA present in a maternal bodily fluid sample.
Owner:FLUIDIGM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com