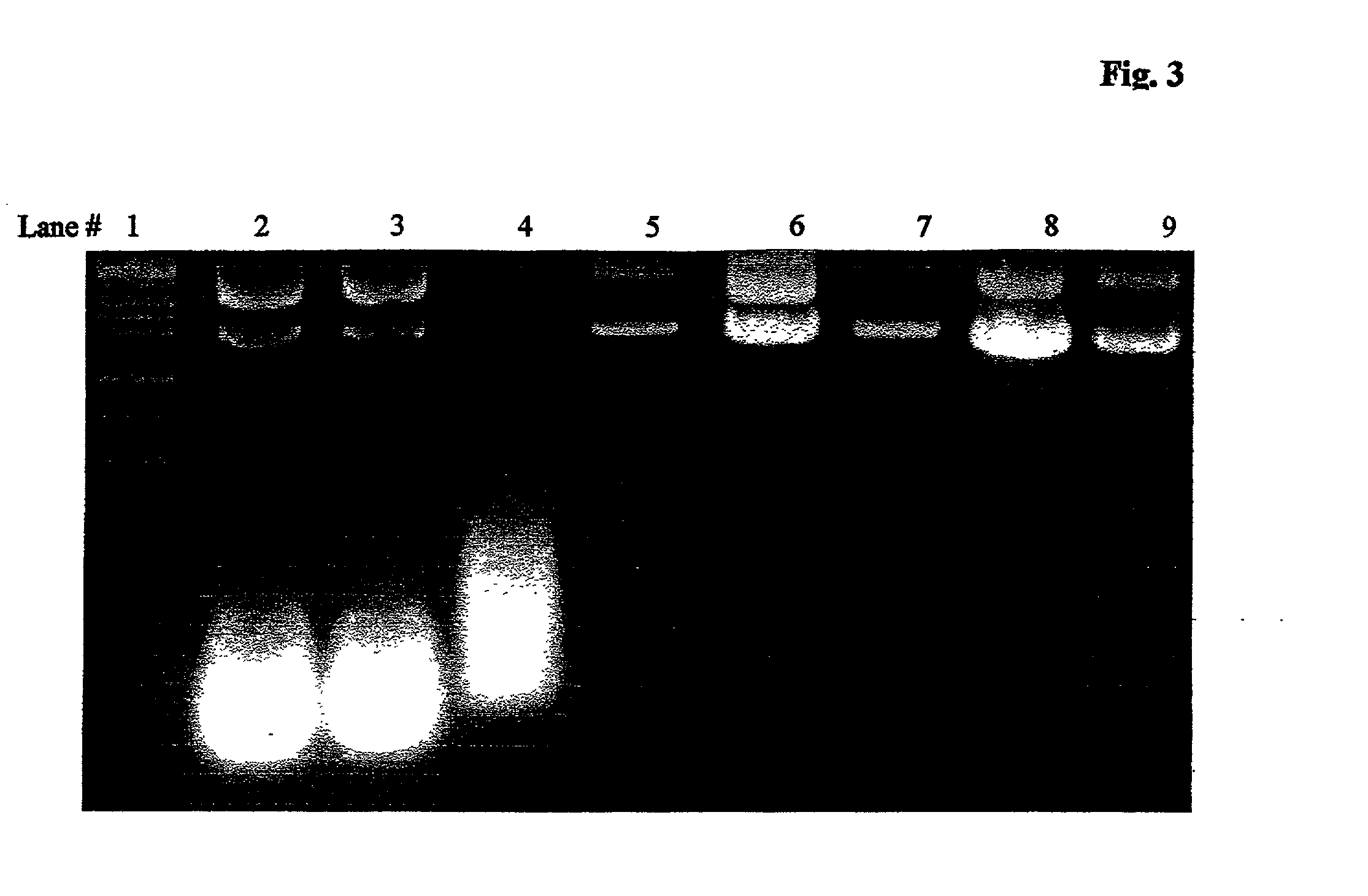
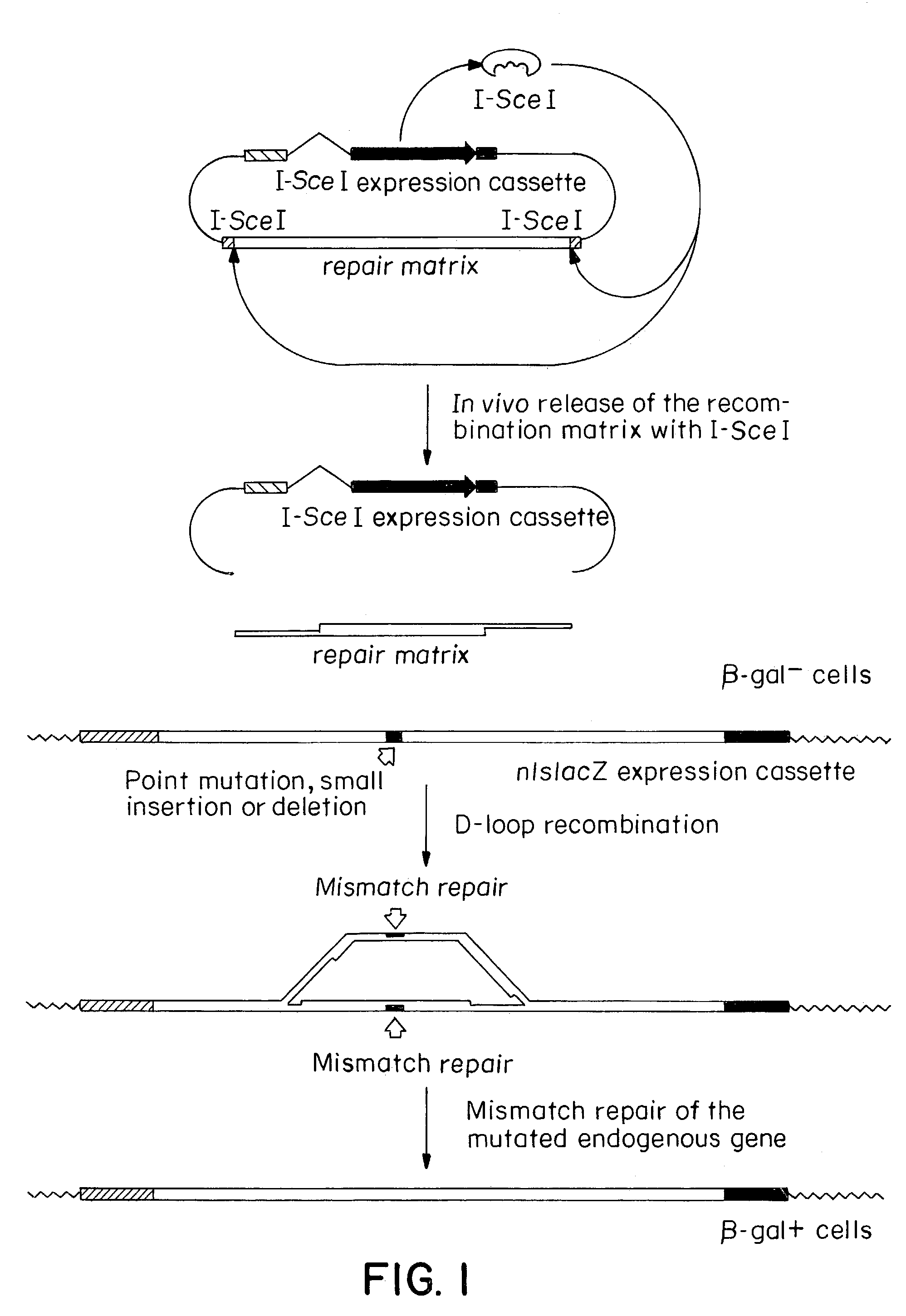
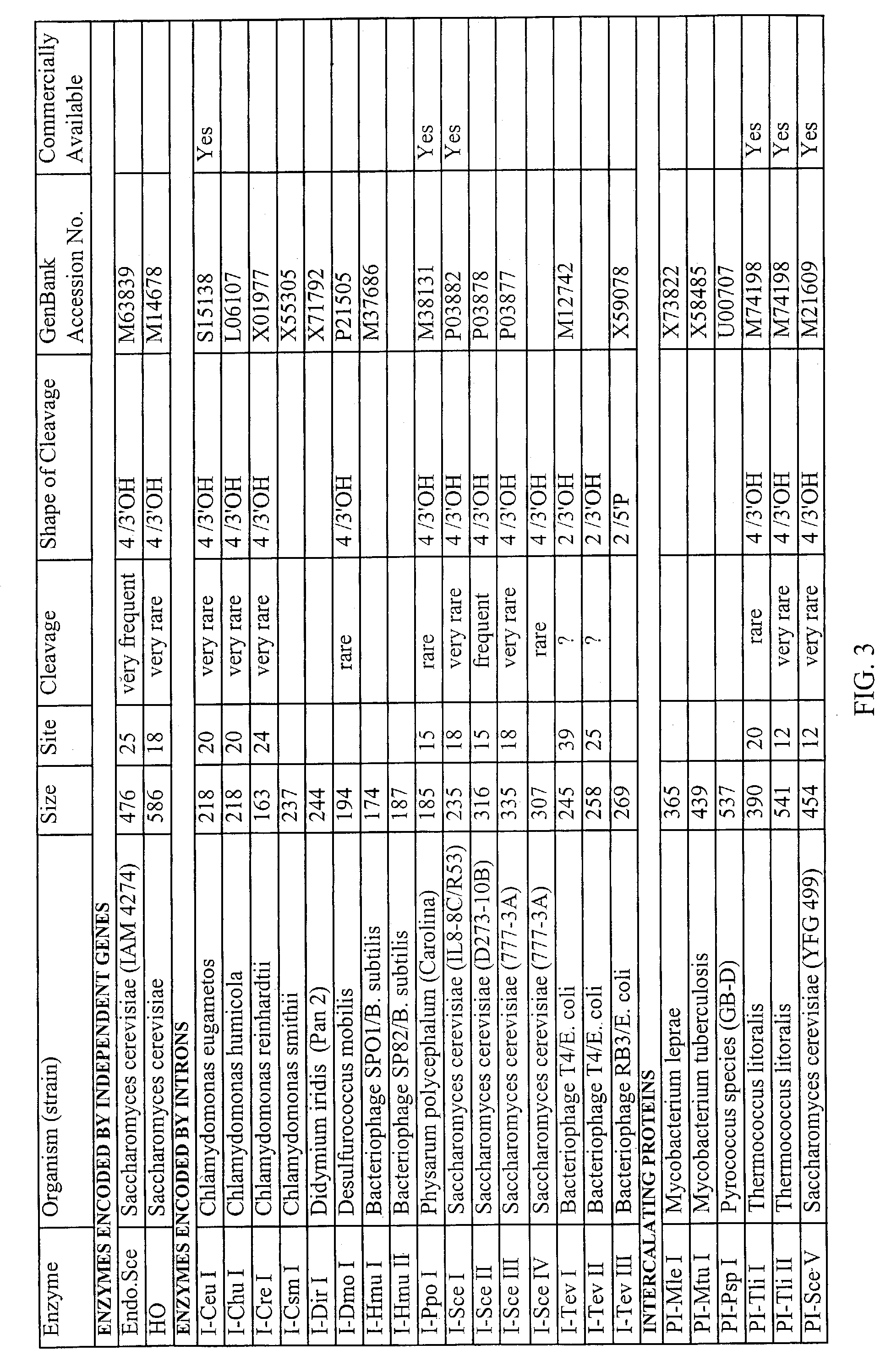
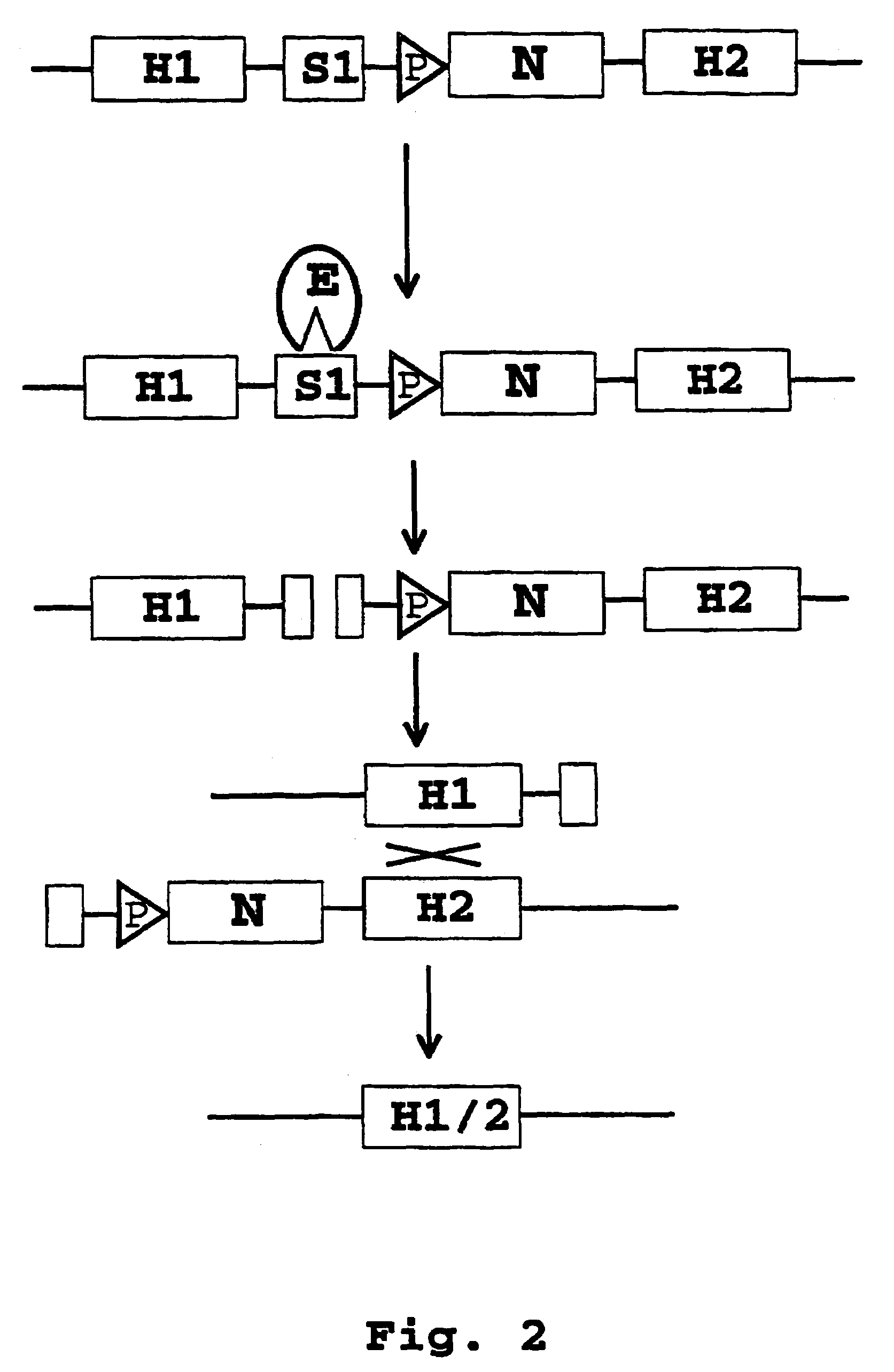
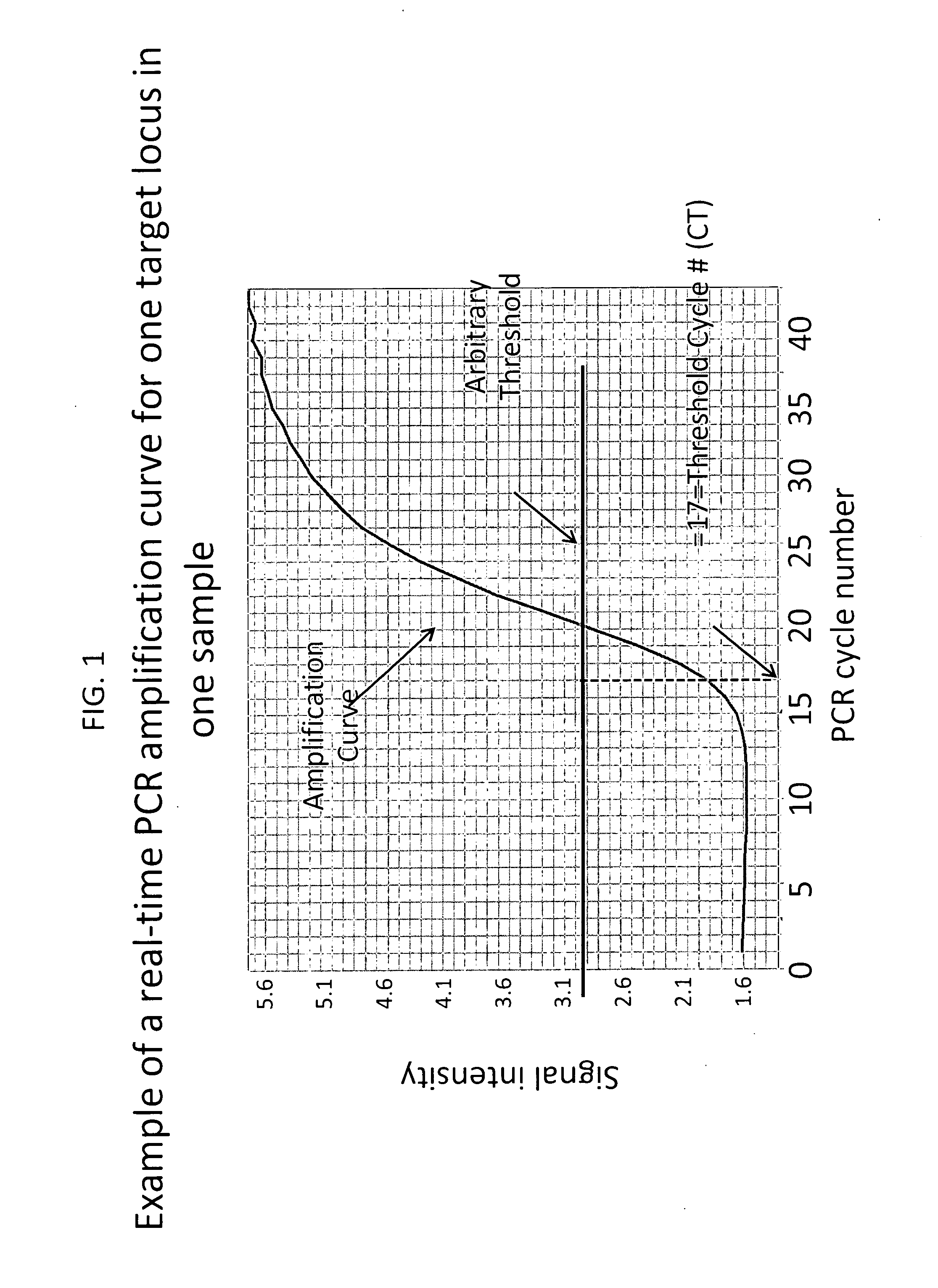
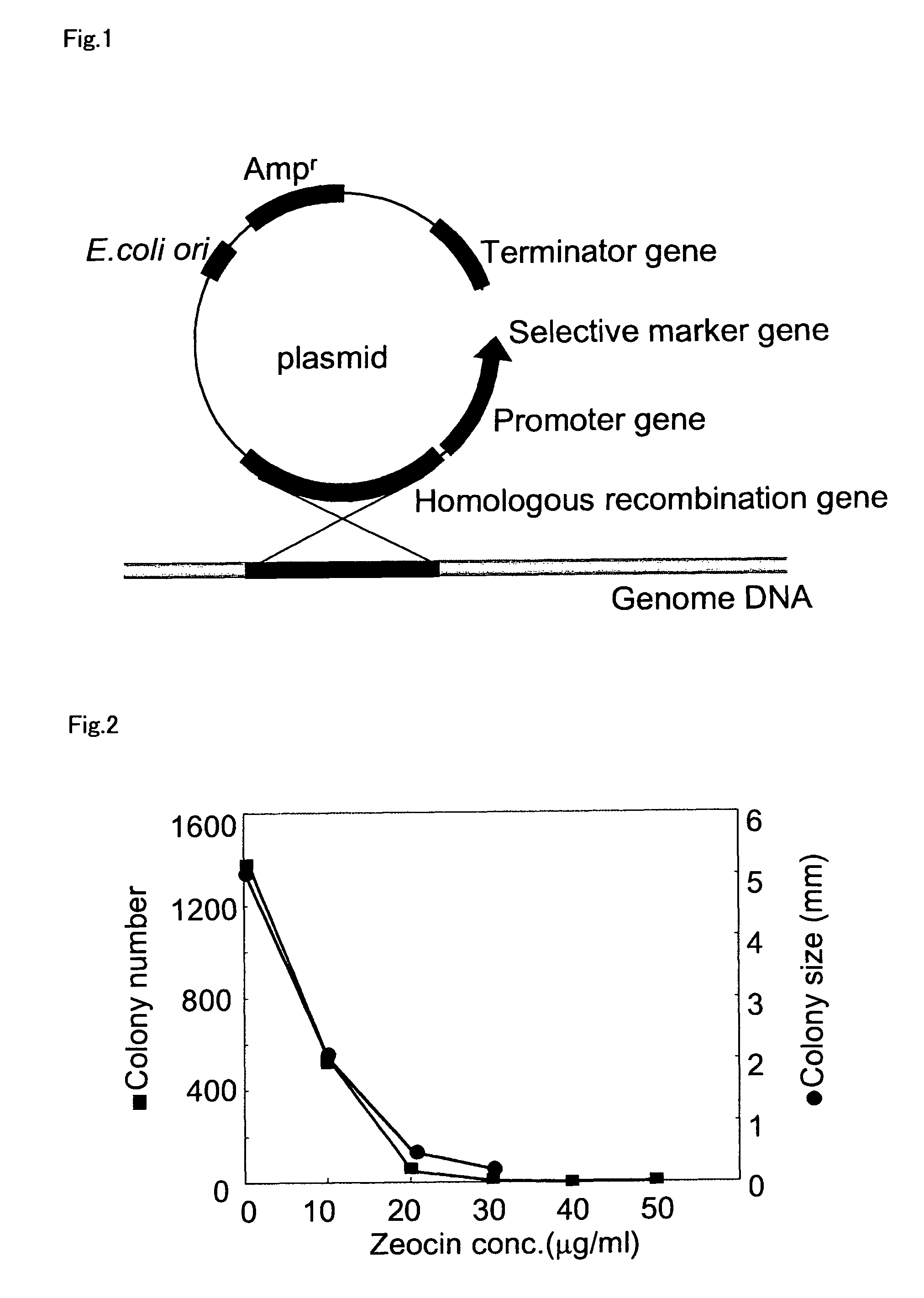
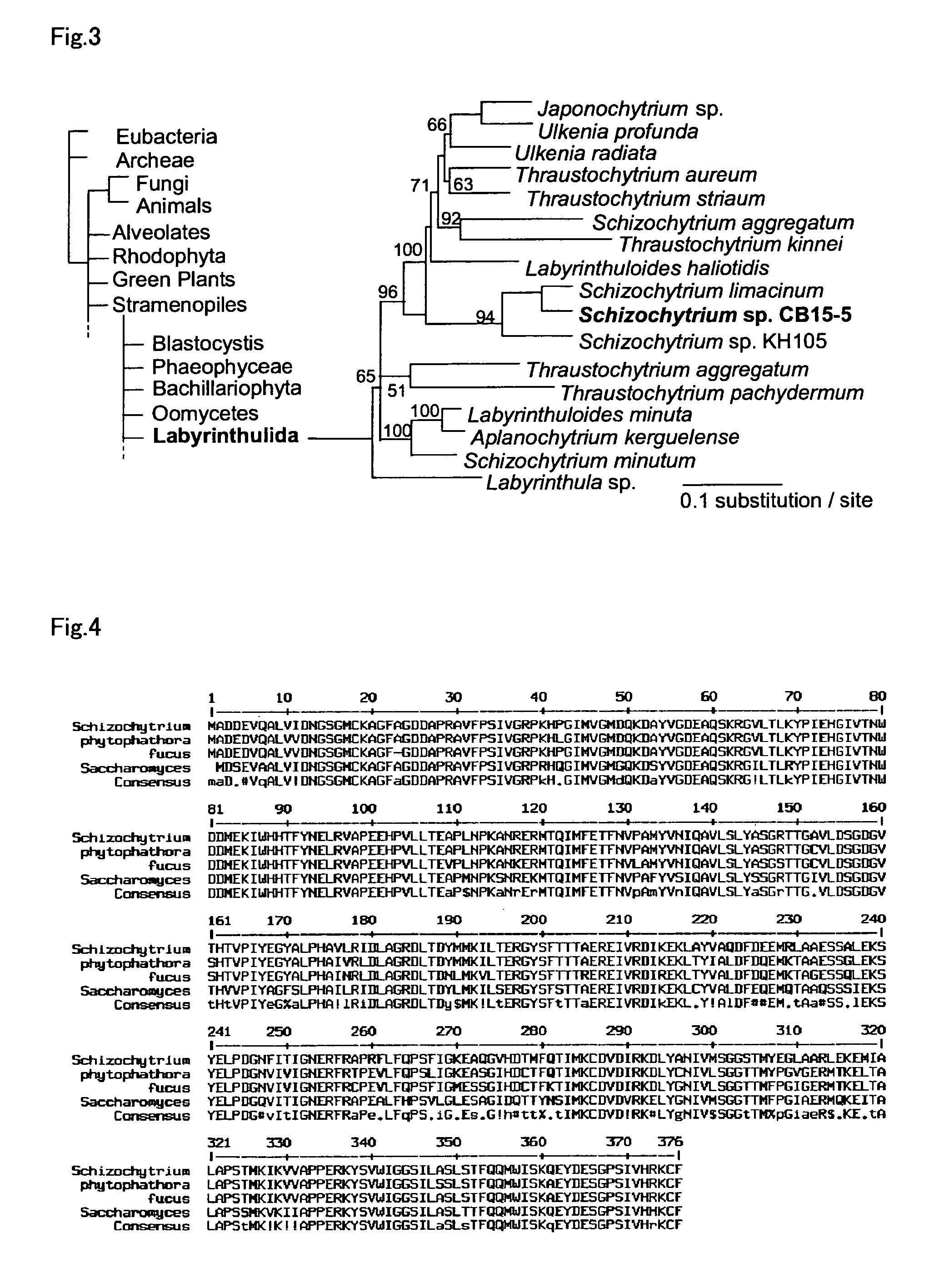
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
177 results about "Chromosomal dna" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In most living organisms, genomic DNA exists as chromosomal DNA. In bacteria, chromosomal DNA freely floats in the cytoplasm while in eukaryotic organisms, they reside inside the nucleus. Chromosomal DNA can be single-stranded or double-stranded. They can also be linear or circular.
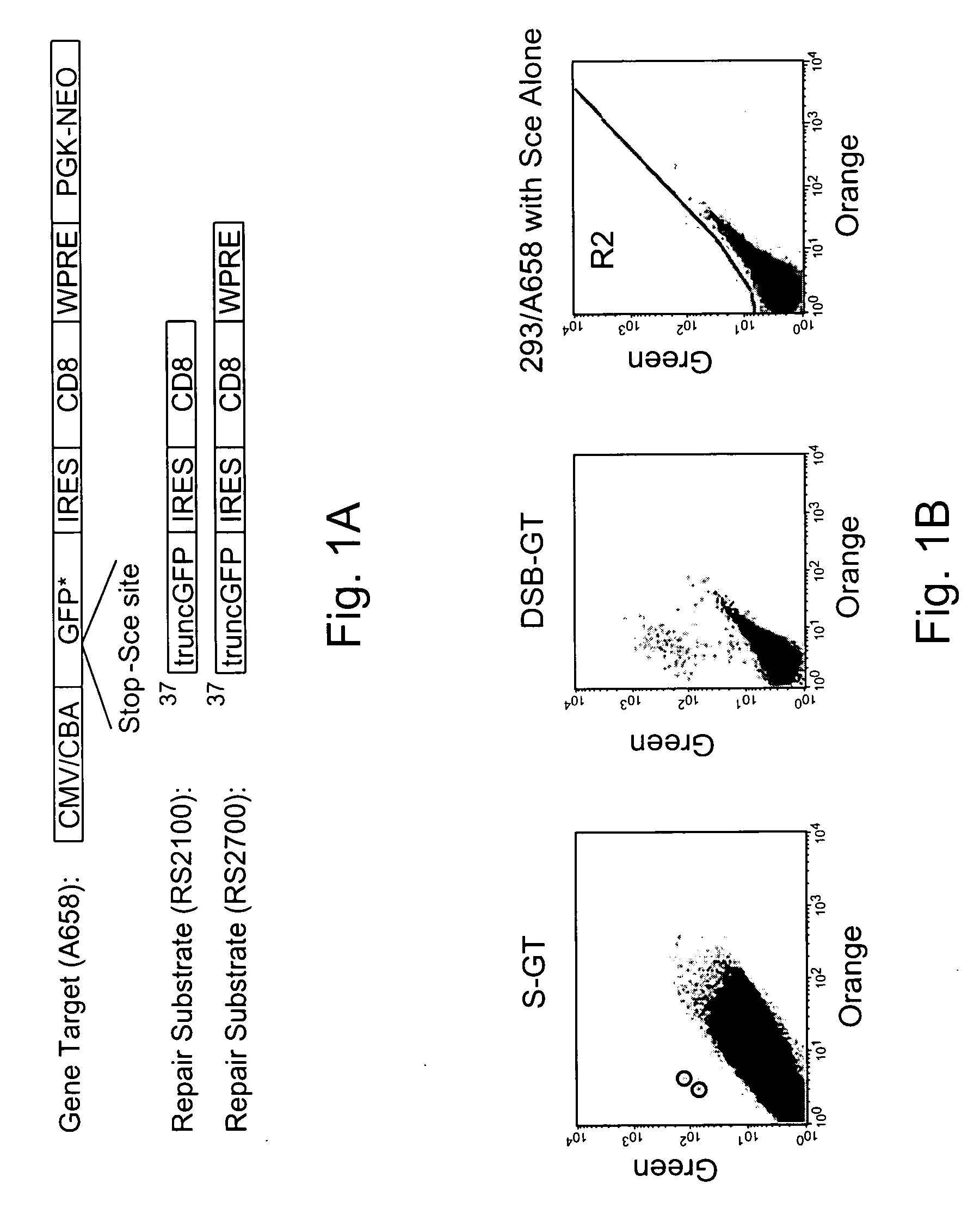
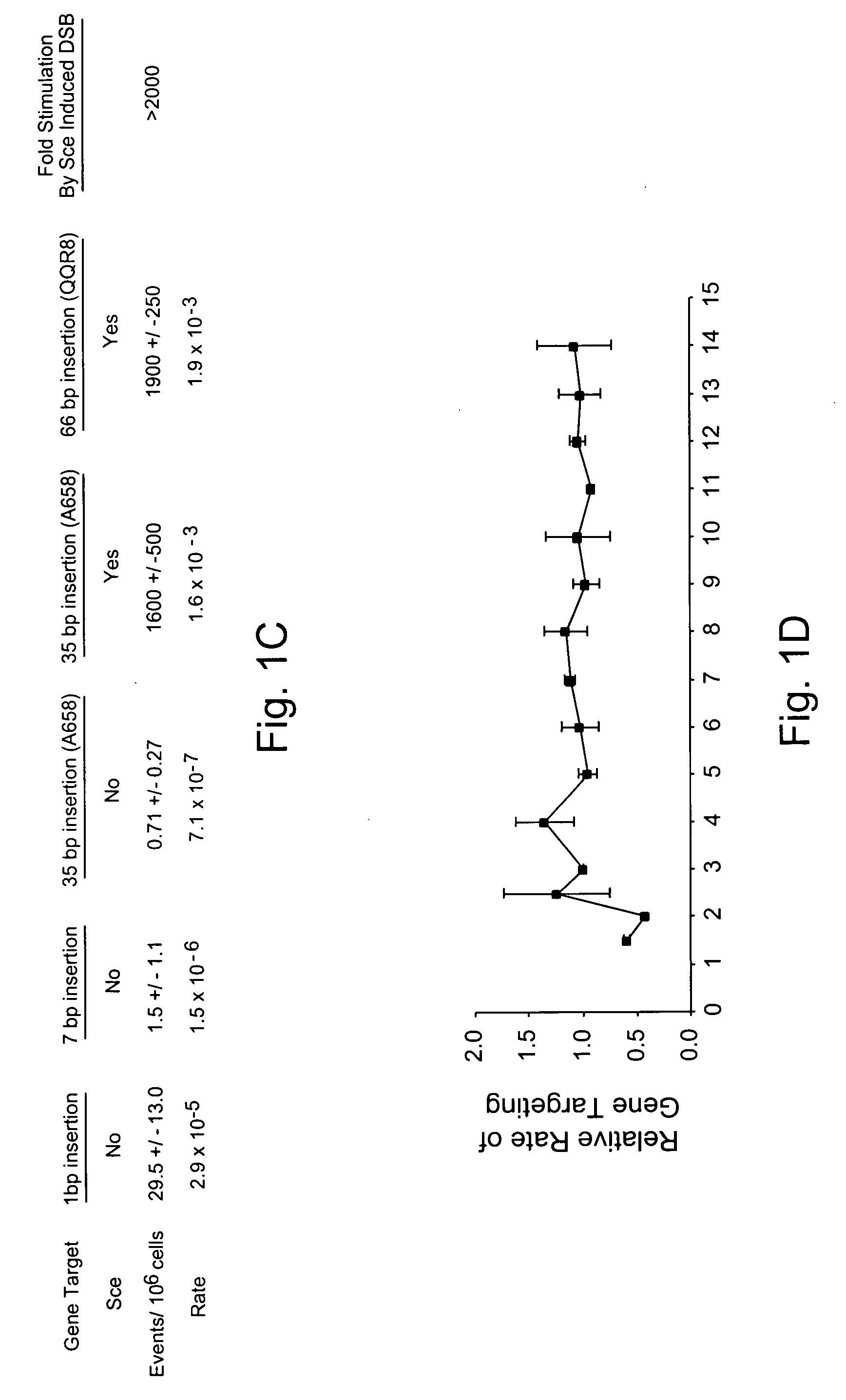
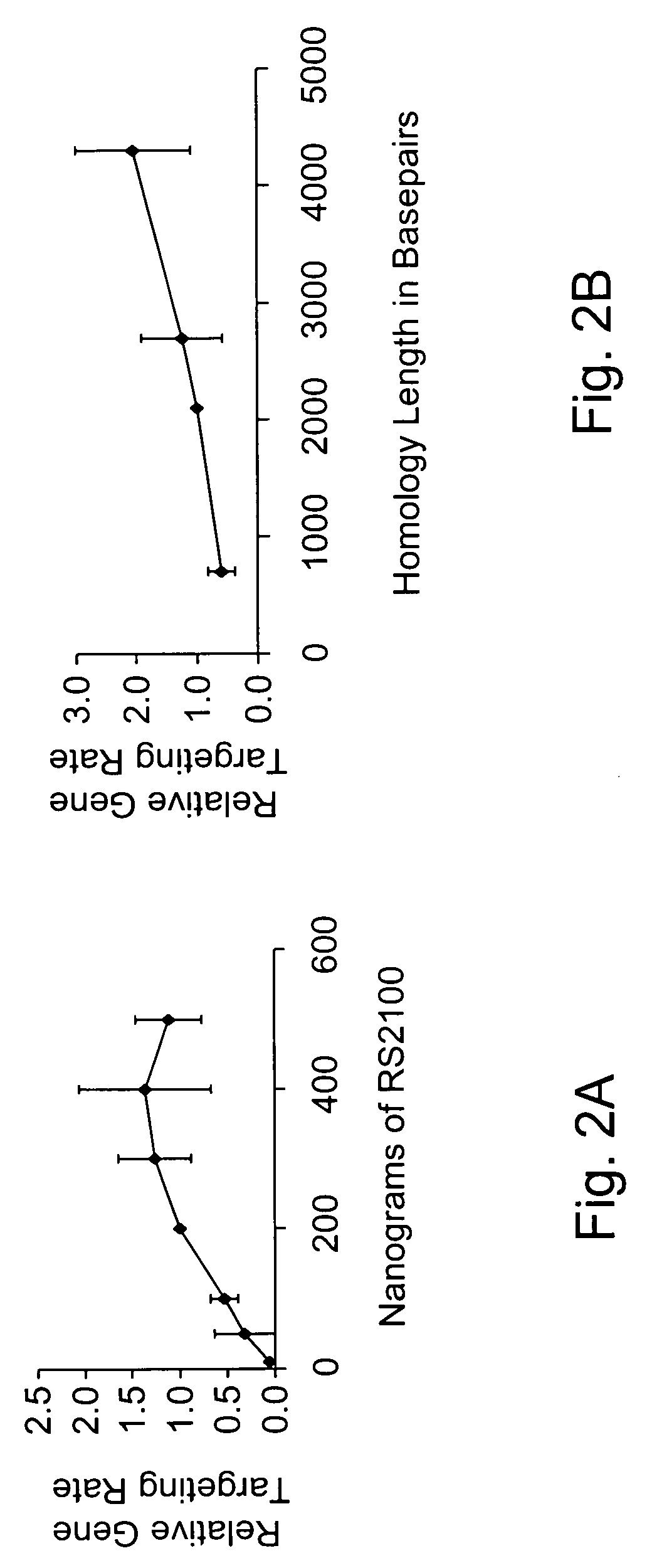
Use of chimeric nucleases to stimulate gene targeting
ActiveUS20050026157A1Ameliorate genetic disorderIncrease productionAntibacterial agentsFusion with DNA-binding domainGene targetsGenetic Change
Gene targeting is a technique to introduce genetic change into one or more specific locations in the genome of a cell. For example, gene targeting can introduce genetic change by modifying, repairing, attenuating or inactivating a target gene or other chromosomal DNA. In one aspect, this disclosure relates to methods and compositions for gene targeting with high efficiency in a cell. This disclosure also relates to methods of treating or preventing a genetic disease in an individual in need thereof. Further disclosed are chimeric nucleases and vectors encoding chimeric nucleases.
Owner:CALIFORNIA INST OF TECH
Methods for prenatal diagnosis of chromosomal abnormalities
ActiveUS20070059707A1Rapid productionAccurate detectionMicrobiological testing/measurementFermentationDiseaseNon invasive
Chromosomal abnormalities are responsible for a significant number of birth defects, including mental retardation. The present invention is related to methods for non-invasive and rapid, prenatal diagnosis of chromosomal abnormalities based on analysis of a maternal blood sample. The invention exploits the differences in DNA between the mother and fetus, for instance differences in their methylation states, as a means to enrich for fetal DNA in maternal plasma sample. The methods described herein can be used to detect chromosomal DNA deletions and duplications. In a preferred embodiment, the methods are used to diagnose chromosomal aneuploidy and related disorders, such as Down's and Turner's Syndrome.
Owner:TRUSTEES OF BOSTON UNIV
Modulation of gene expression by oligomers targeted to chromosomal DNA
Synthesis of a target transcript of a gene is selectively increased in a mammalian cell by contacting the cell with a polynucleotide oligomer of 12-28 bases complementary to a region within a target promoter of the gene under conditions whereby the oligomer selectively increases synthesis of the target transcript.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods for prenatal diagnosis of chromosomal abnormalities
ActiveUS7655399B2Rapid productionAccurate detectionMicrobiological testing/measurementPlasma samplesNon invasive
Chromosomal abnormalities are responsible for a significant number of birth defects, including mental retardation. The present invention is related to methods for non-invasive and rapid, prenatal diagnosis of chromosomal abnormalities based on analysis of a maternal blood sample. The invention exploits the differences in DNA between the mother and fetus, for instance differences in their methylation states, as a means to enrich for fetal DNA in maternal plasma sample. The methods described herein can be used to detect chromosomal DNA deletions and duplications. In a preferred embodiment, the methods are used to diagnose chromosomal aneuploidy and related disorders, such as Down's and Turner's Syndrome.
Owner:TRUSTEES OF BOSTON UNIV
Microdissection-based methods for determining genomic features of single chromosomes
InactiveUS20060211001A1Quick fixPromote associationSugar derivativesMicrobiological testing/measurementGenomic DNAChromosome regions
The present provides a microdissection-based method for identifying a genomic feature present within a visible chromosome region. The method includes steps of: (a) micro-dissecting a single copy of a chromosome to obtain a visible chromosome region; (b) amplifying the visible chromosome region to obtain amplified single chromosome DNA; and (c) subjecting the amplified single chromosome DNA to micro-array analysis whereby such analysis identifies at least one genomic feature present within the visible chromosome region. The method is applicable to determining genomic features including, but not limited to, genomic DNA size, gene content, DNA breakpoint, or DNA polymorphism (e.g., single nucleotide polymorphisms).
Owner:WISCONSIN ALUMNI RES FOUND
Modulation of gene expression by oligomers targeted to chromosomal DNA
ActiveUS20070111963A1Increased EF1A mRNA expressionSugar derivativesActivity regulationOligomerFhit gene
Synthesis of a target transcript of a gene is selectively increased in a mammalian cell by contacting the cell with a polynucleotide oligomer of 12-28 bases complementary to a region within a target promoter of the gene under conditions whereby the oligomer selectively increases synthesis of the target transcript.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for introducing a gene into Labyrinthulomycota
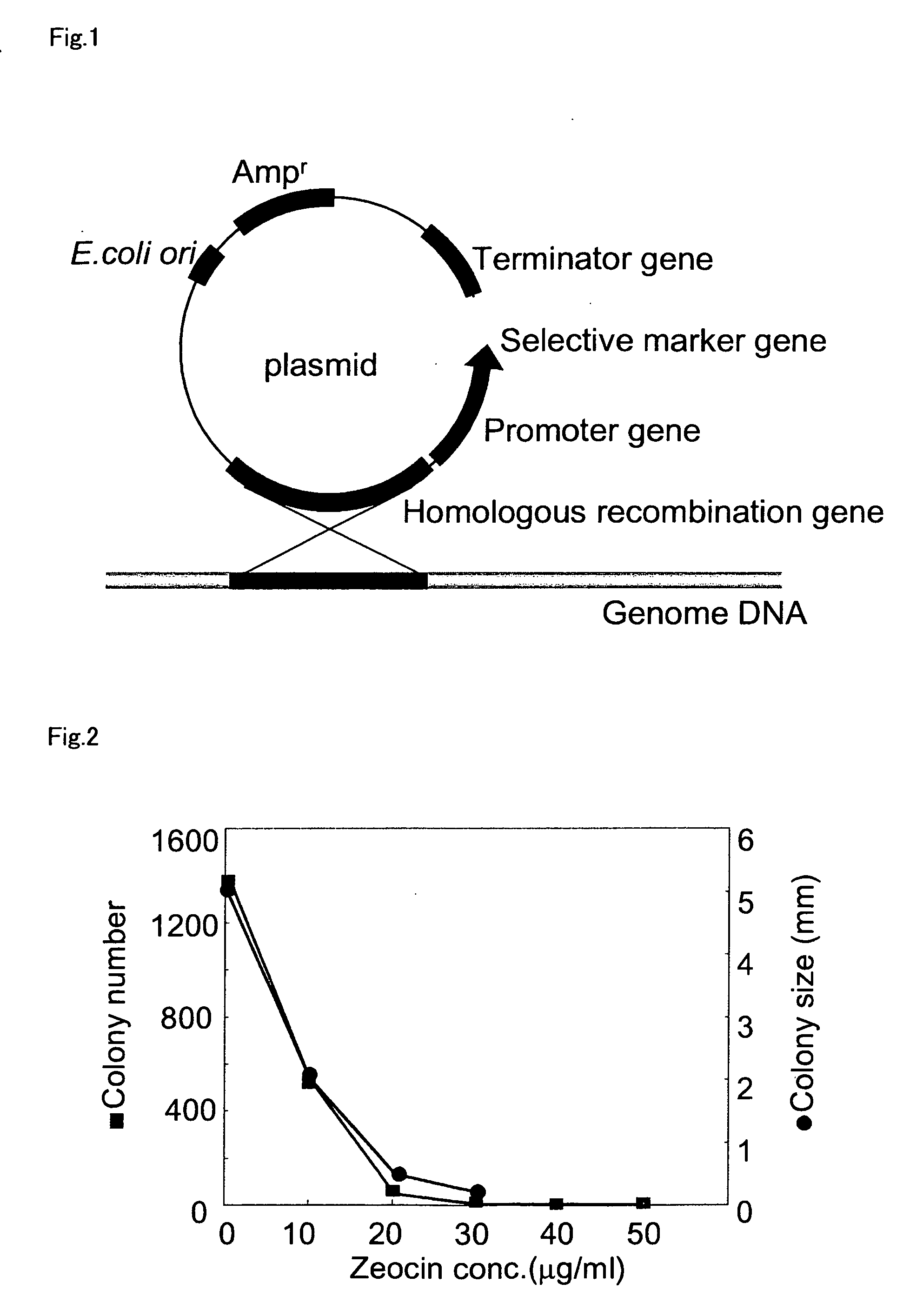
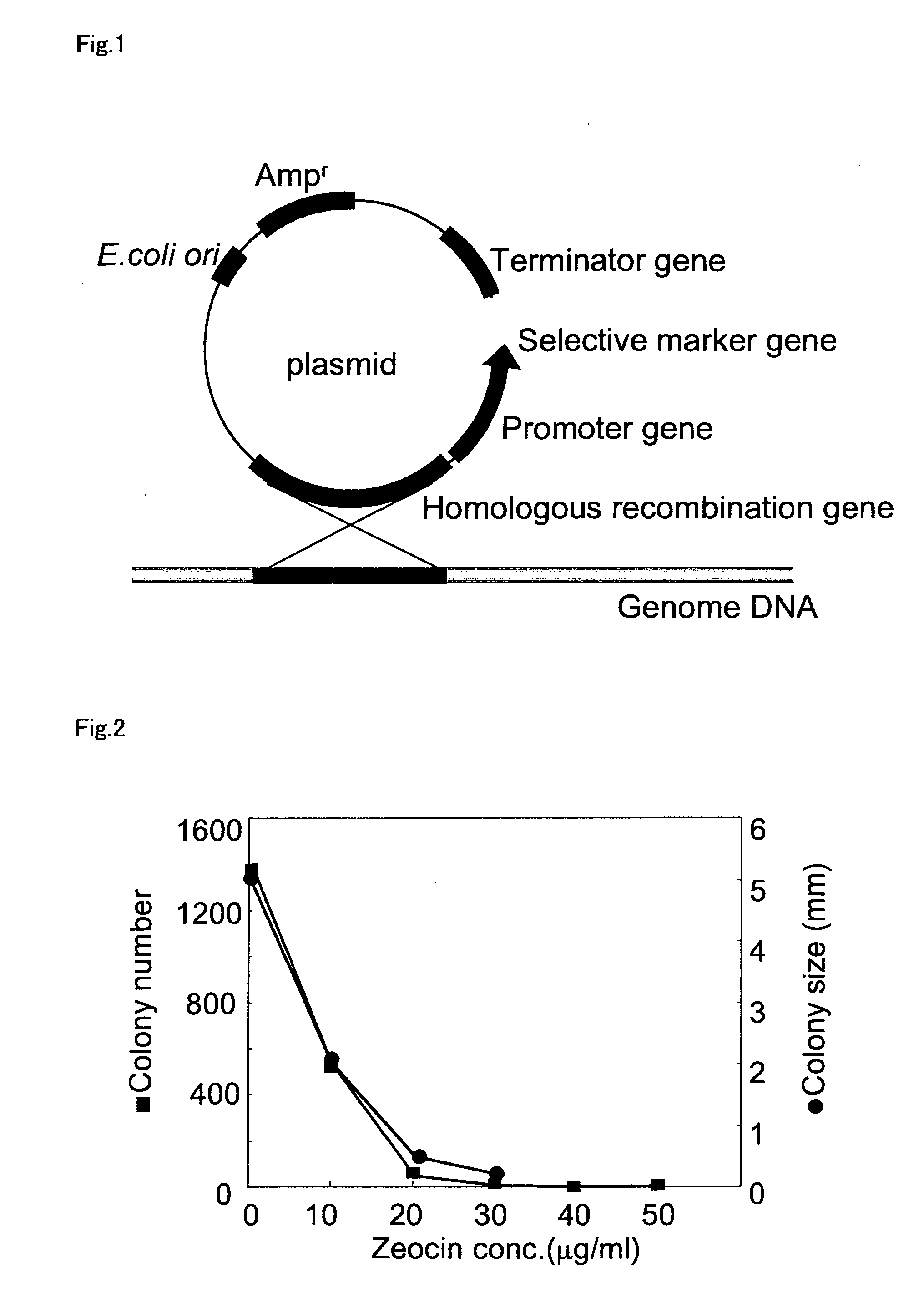
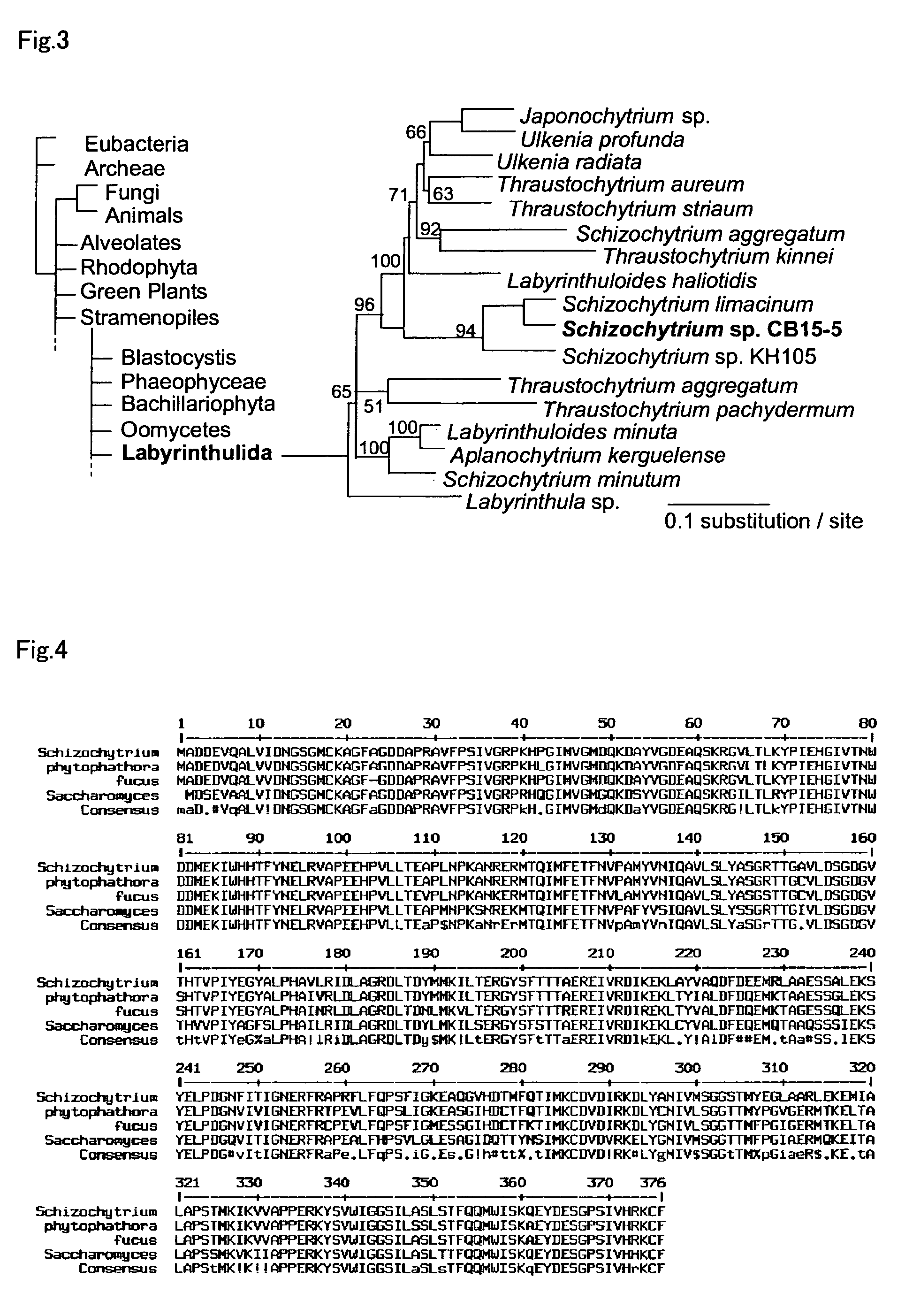
An object of the present invention is to provide a transformation system for Labyrinthulomycota that allows the elucidation of biosynthetic mechanisms of lipids such as PUFA and carotenoids as well as for the construction of a high production system and the design and development of novel functional lipid molecules by the control of the mechanisms. The present invention provides a method for introducing a transgene into a cell of Labyrinthulomycota, which comprises introducing into a cell of Labyrinthulomycota a recombinant vector comprising a transgene and a nucleotide sequence which is homologous to a part of chromosomal DNA of Labyrinthulomycota and is capable of homologous recombination with the chromosomal DNA, and then inducing homologous recombination in this homologous nucleotide sequence.
Owner:HIROSHIMA UNIVERSITY +1
Recombination systems and methods for eliminating nucleic acid sequences from the genome of eukaryotic organisms
The invention relates to recombination systems and methods for eliminating nucleic acid sequences from the chromosomal DNA of eukaryotic organisms, and to transgenic organisms—preferably plants—which comprise these systems or were generated using these methods.
Owner:SUNGENE +1
Vector capable for transformation of labyrinthulomycota
An object of the present invention is to provide a transformation system for Labyrinthulomycota that allows the elucidation of biosynthetic mechanisms of lipids such as PUFA and carotenoids as well as for the construction of a high production system and the design and development of novel functional lipid molecules by the control of the mechanisms. The present invention provides a vector for the transformation of Labyrinthulomycota with a transgene, which comprises at least (1) a nucleotide sequence which is homologous to a part of chromosomal DNA of Labyrinthulomycota and is capable of homologous recombination with the chromosomal DNA, (2) a selection marker gene having a promoter sequence located upstream and a terminator sequence located downstream, and (3) a cloning site for transgene insertion having a promoter sequence located upstream and a terminator sequence located downstream.
Owner:FUJIFILM CORP +1
Method for providing hyaluronic acid
InactiveUSRE37336E1Facilitate easeAbility to controlBacteriaSugar derivativesEscherichia coliRecombinant DNA
Disclosed are DNA segments encoding <DEL-S DATE="20010821" ID="DEL-S-00001">hyaluronic acid synthase which are employed to construct recombinant cells useful in the production of hyaluronate synthase or hyaluronic acid (HA)<DEL-E ID="DEL-S-00001"> <INS-S DATE="20010821" ID="INS-S-00001">the recombinant DNA segment identified in FIG. 5. <INS-E ID="INS-S-00001">In preferred aspects, chromosomal DNA from Streptococcus equisimilis is partially digested with EcoRI and the resultant fragments are ligated to form recombinant vectors. These vectors are useful in the transformation of host cells such as E. coli and or Streptococcal hosts. <DEL-S DATE="20010821" ID="DEL-S-00002">Resultant transformants are screened by the novel screening assays to identify colonies which have incorporated HA synthase DNA in a form that is being actively transcribed into the corresponding HA synthase enzyme. These colonies may be selected and employed in the production of the enzyme itself or its product, HA.<DEL-E ID="DEL-S-00002"> <INS-S DATE="20010821" ID="INS-S-00002">The recombinant DNA segment identified in FIG. 5 is then inserted into a recombinant Streptococcal host for the production of hyaluronic acid (HA).<INS-E ID="INS-S-00002">
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
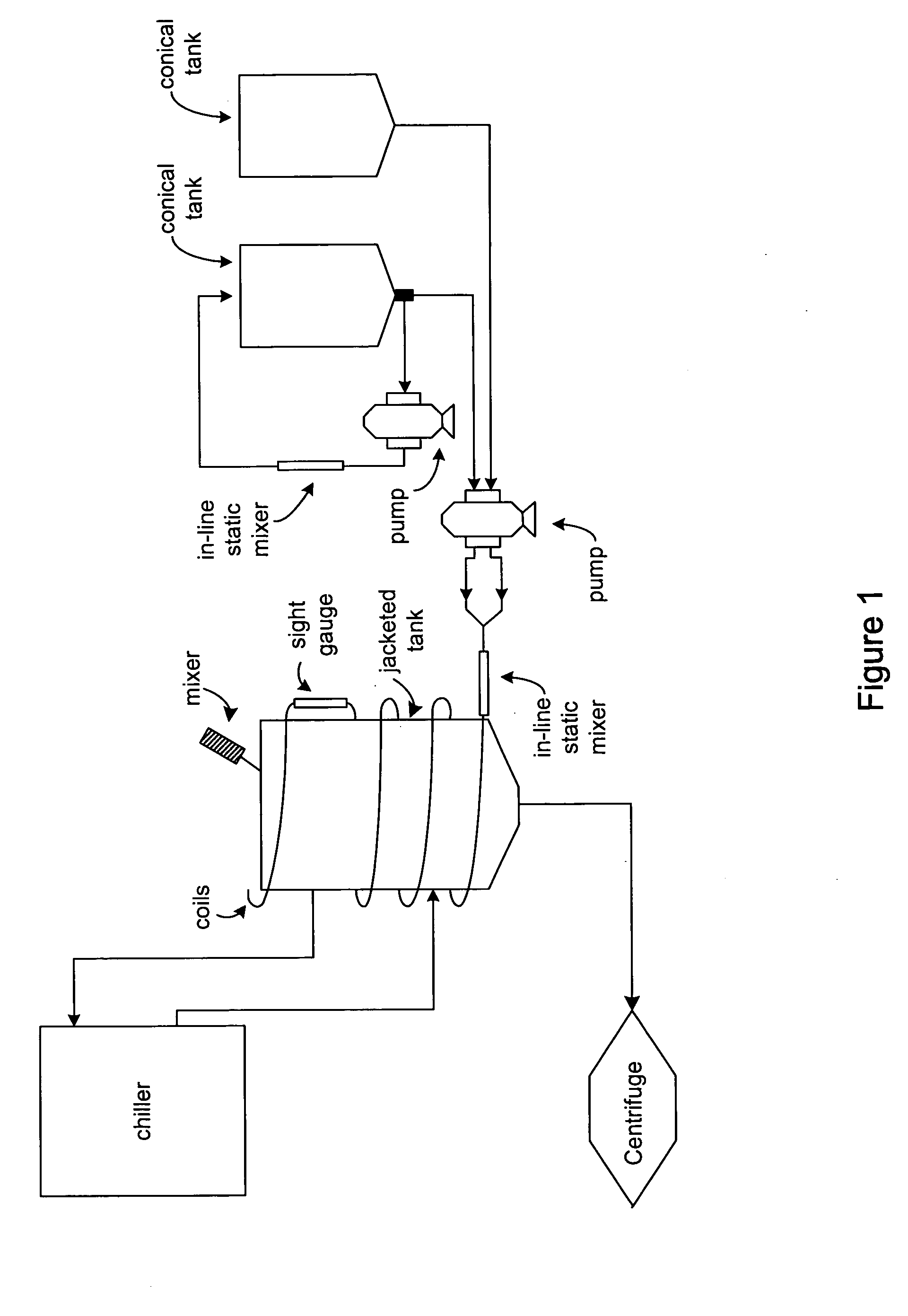
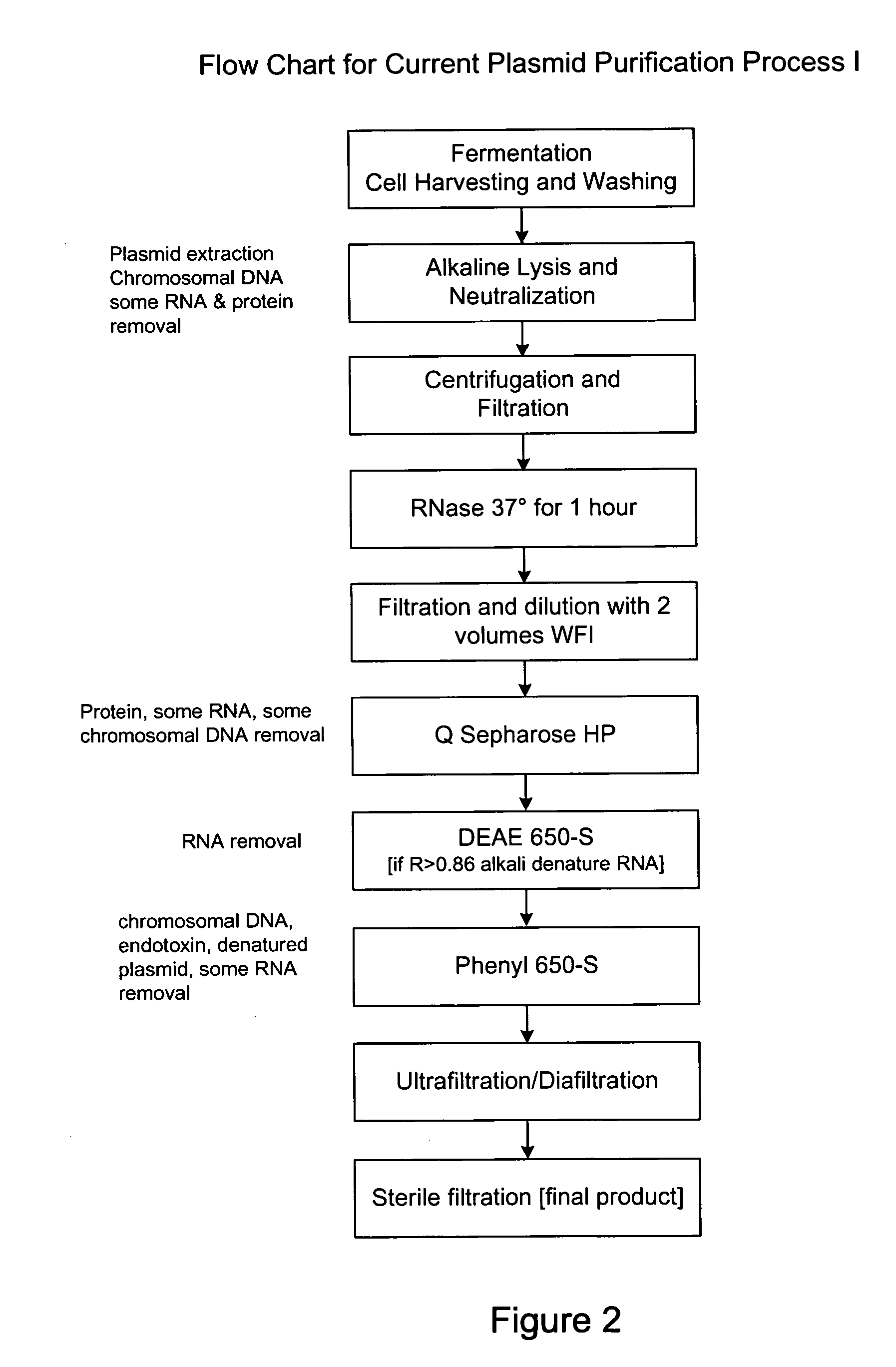
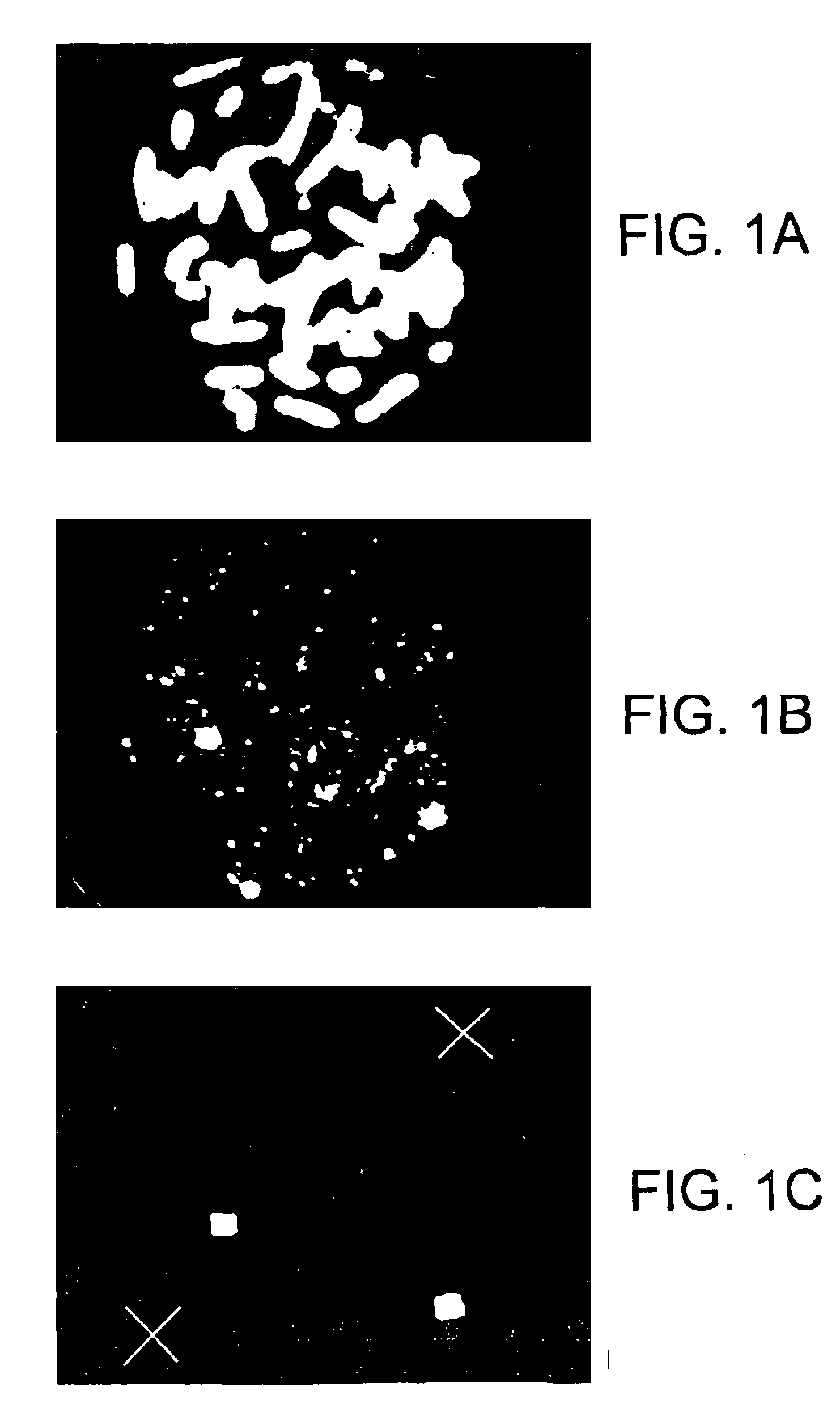
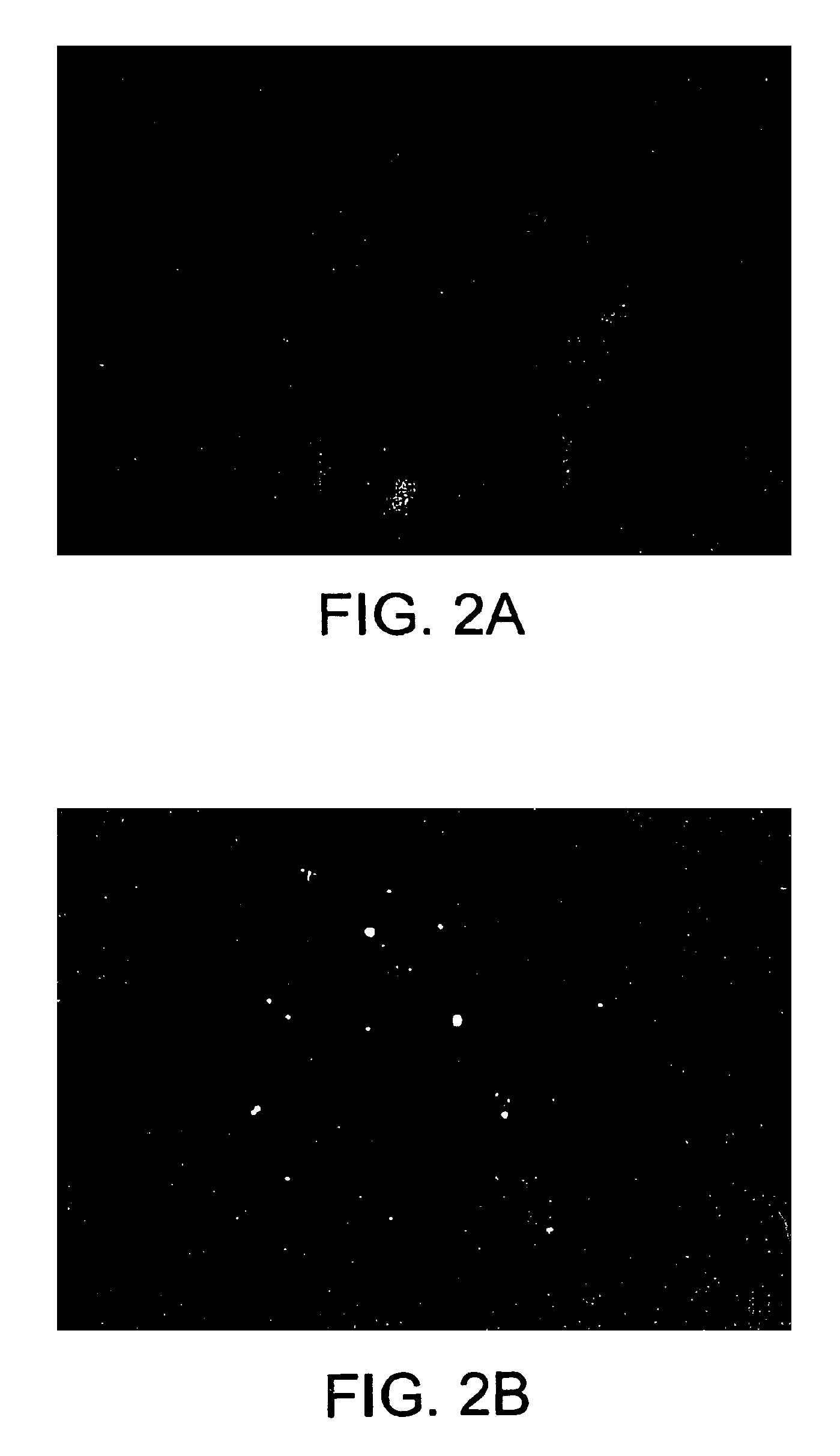
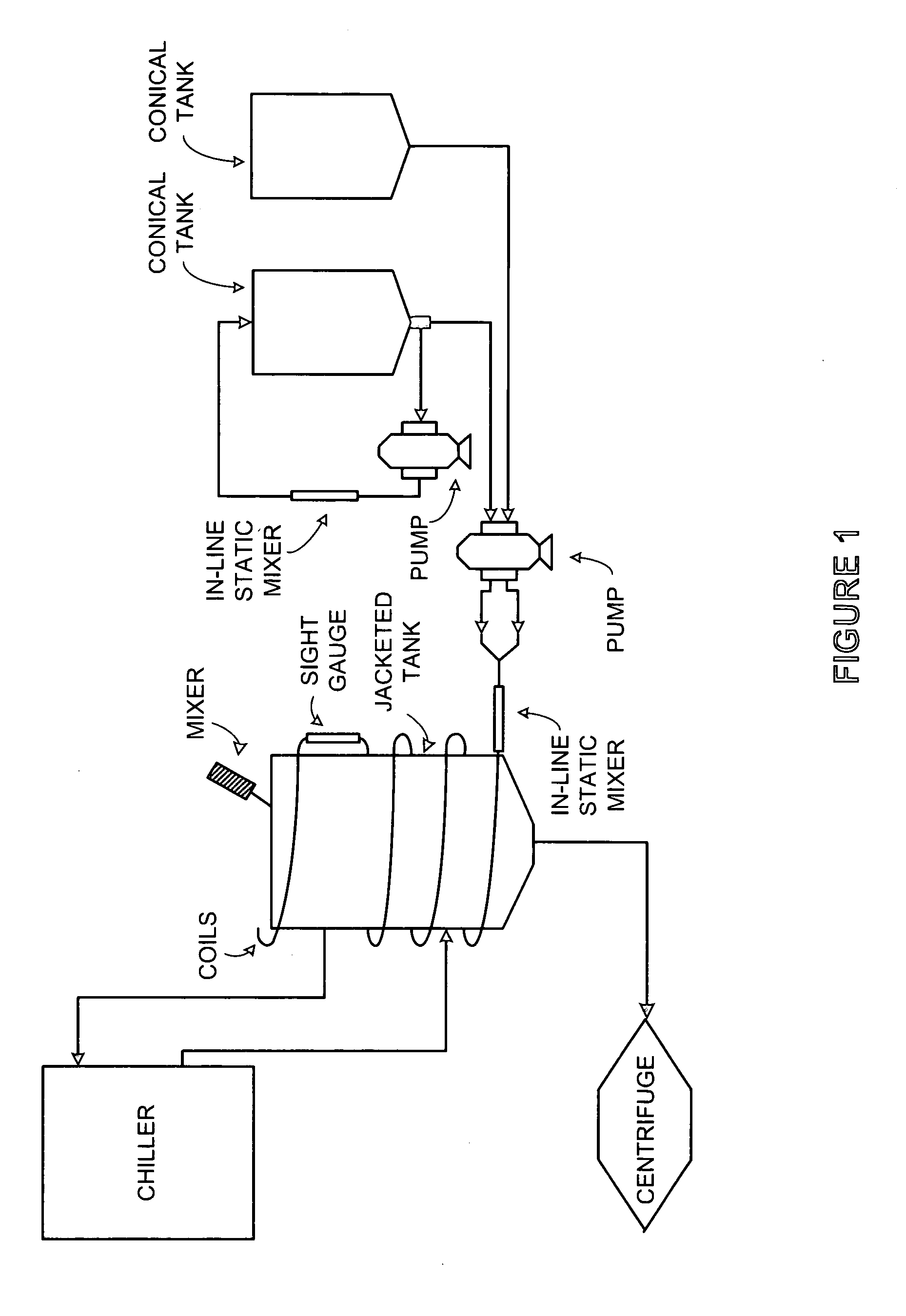
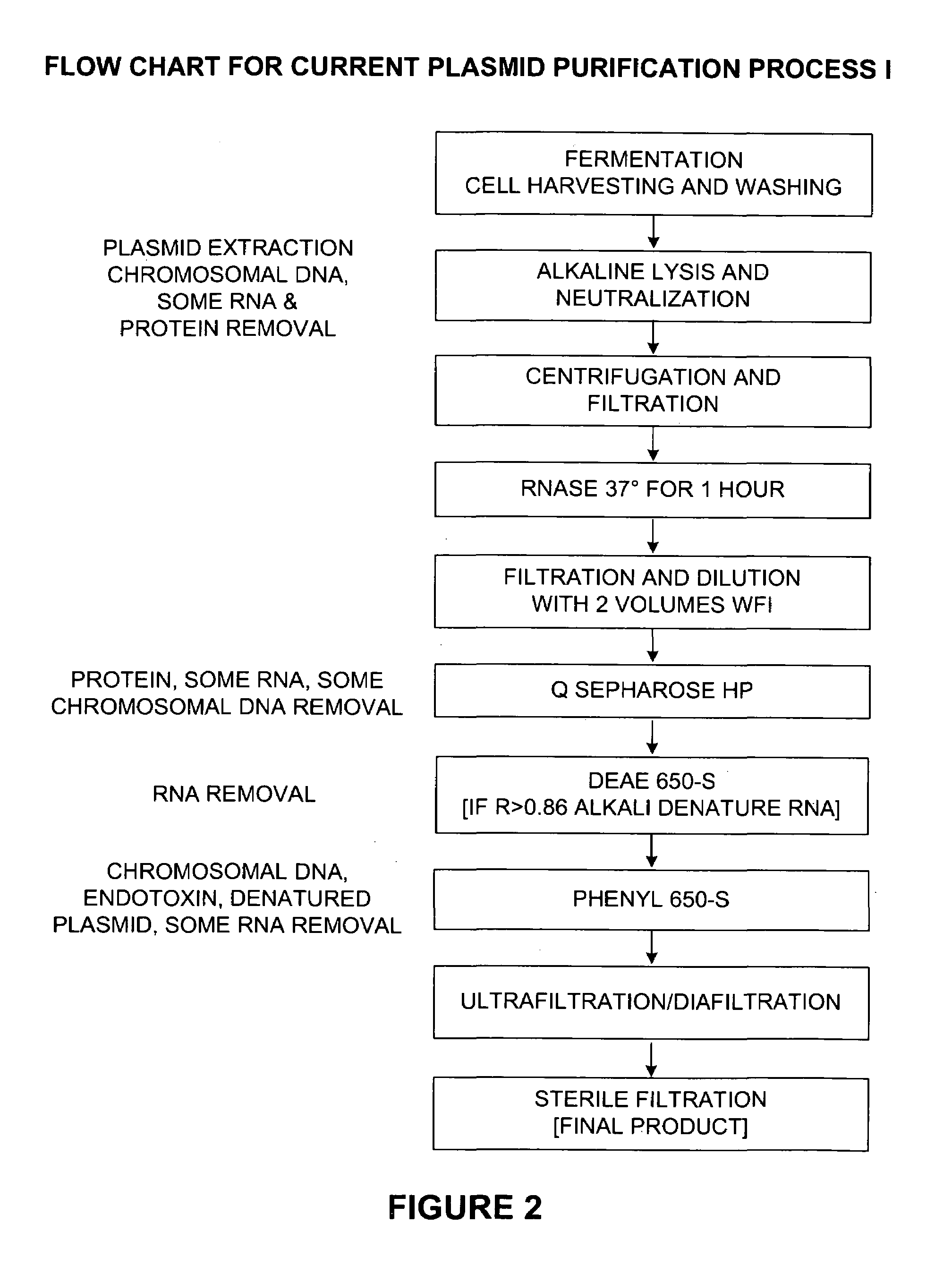
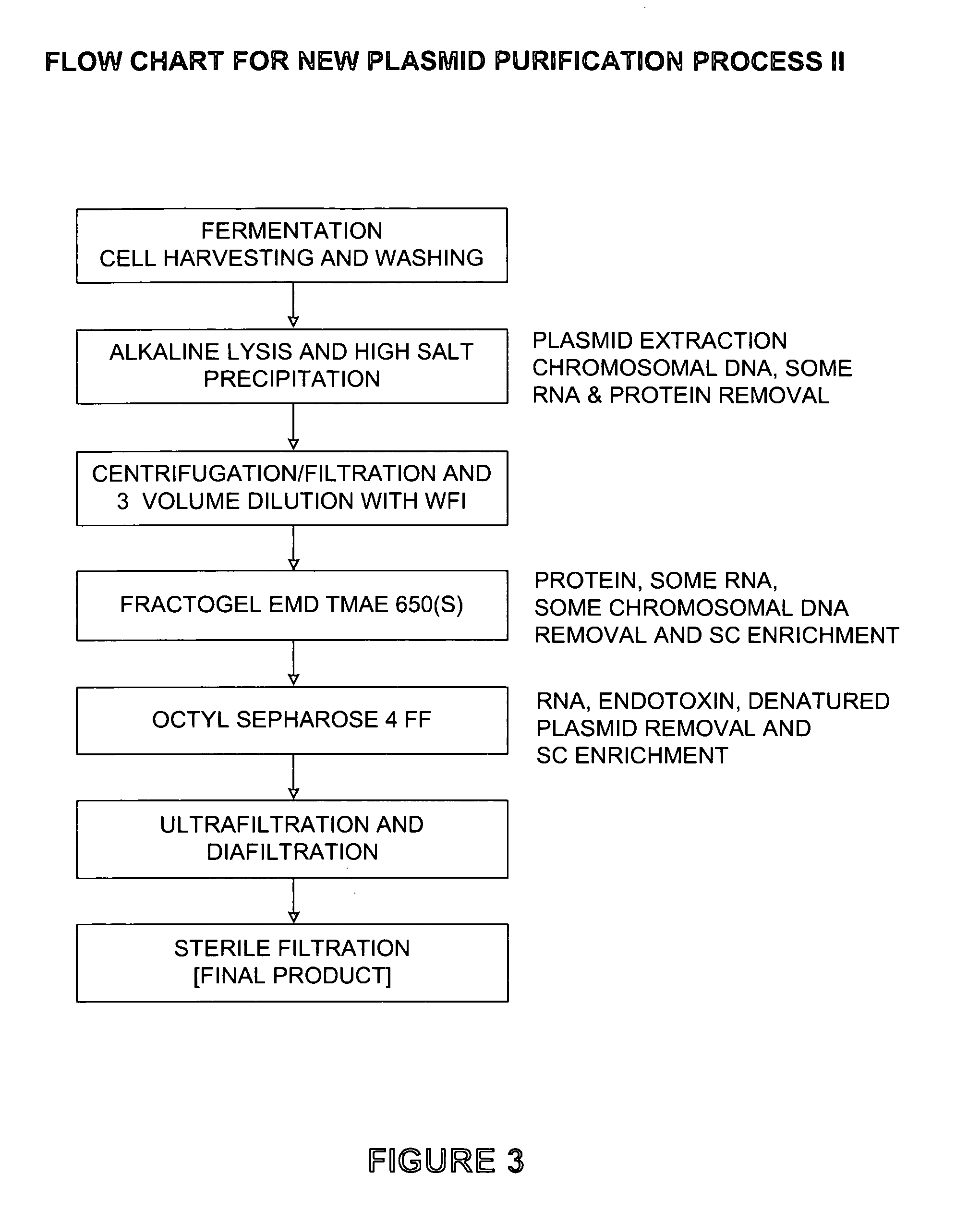
Process and equipment for plasmid purification
InactiveUS8236495B2Easy to operateConsistent levelCation exchanger materialsOrganic anion exchangersEscherichia coliLysis
A scalable alkaline lysis process, including procedures and devices for the isolation of large quantities (grams and kilograms) of plasmid DNA from recombinant E. coli cells. Effective, controllable, and economical operation, and consistent low level of host chromosomal DNA in the final plasmid product. Involves a series of new unit operations and devices for cell resuspension, cell lysis, and neutralization.
Owner:URIGEN PHARMA INC
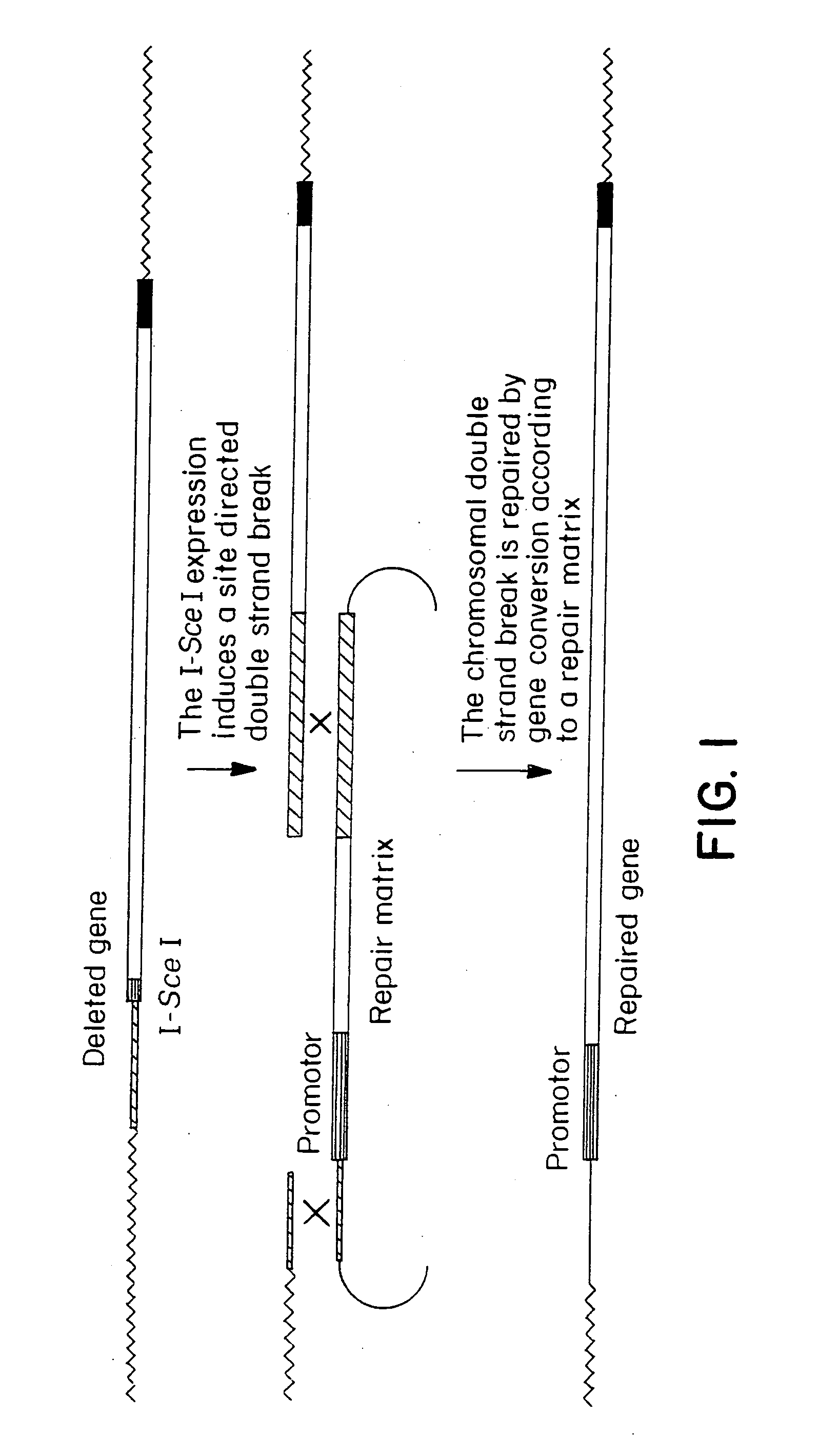
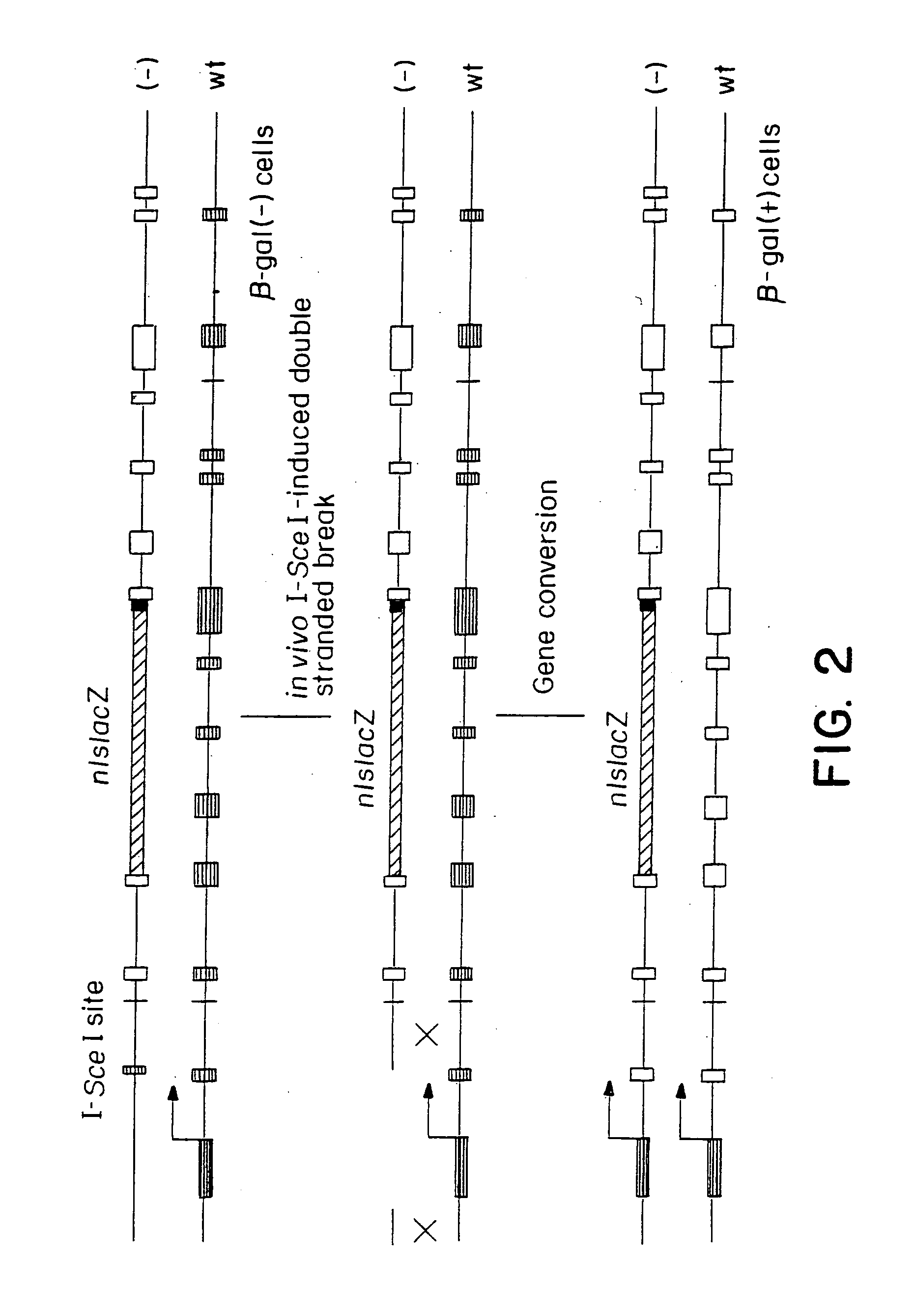
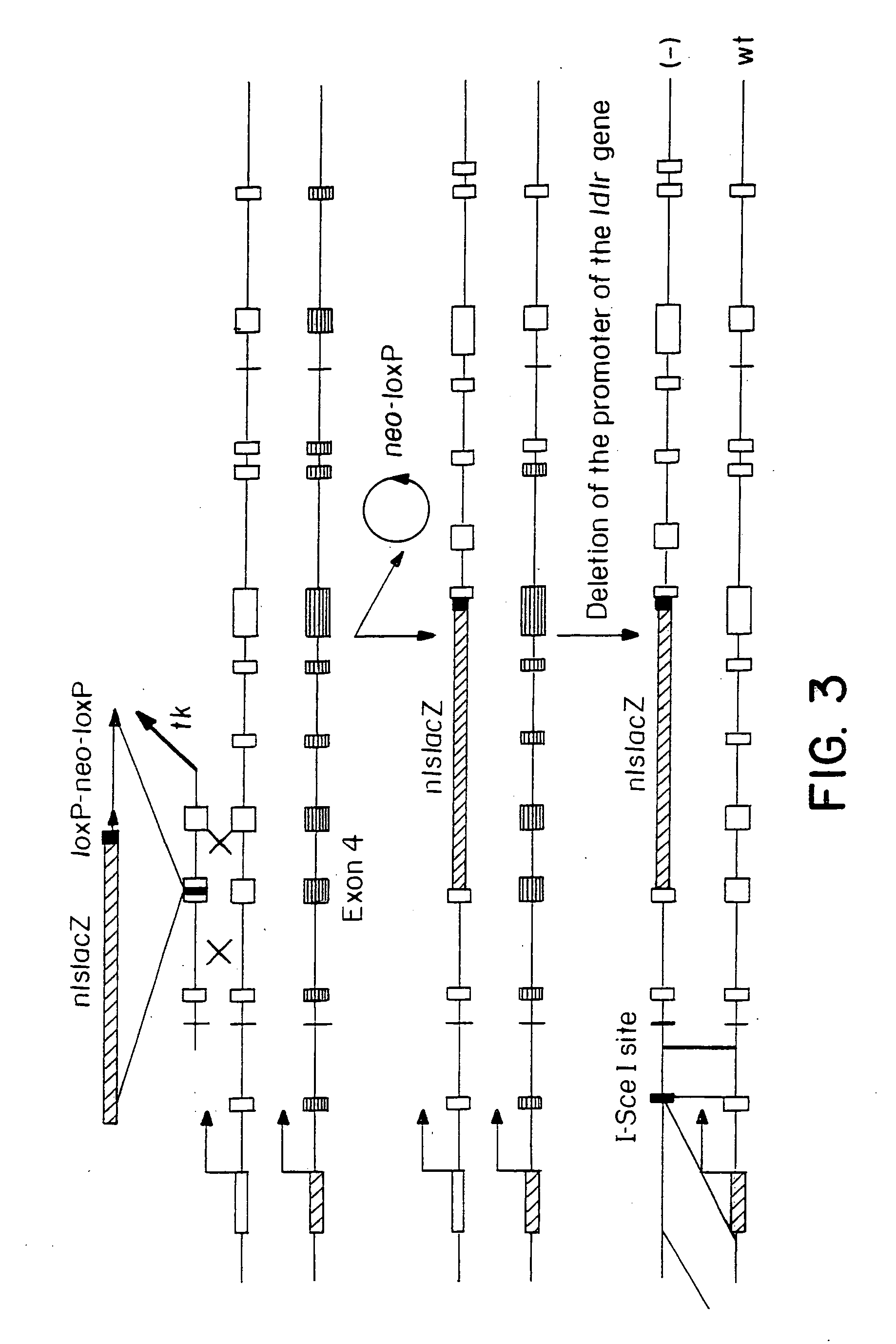
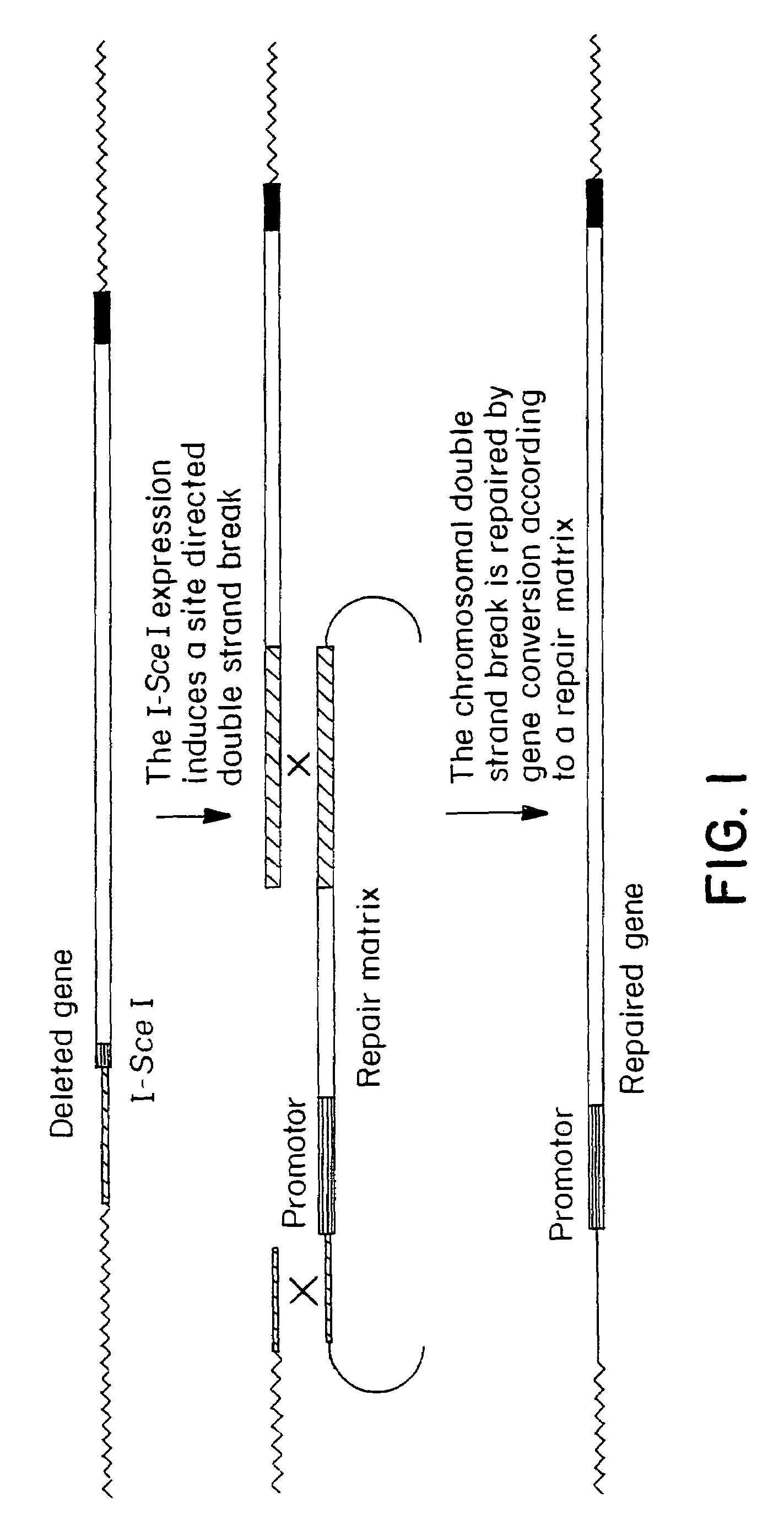
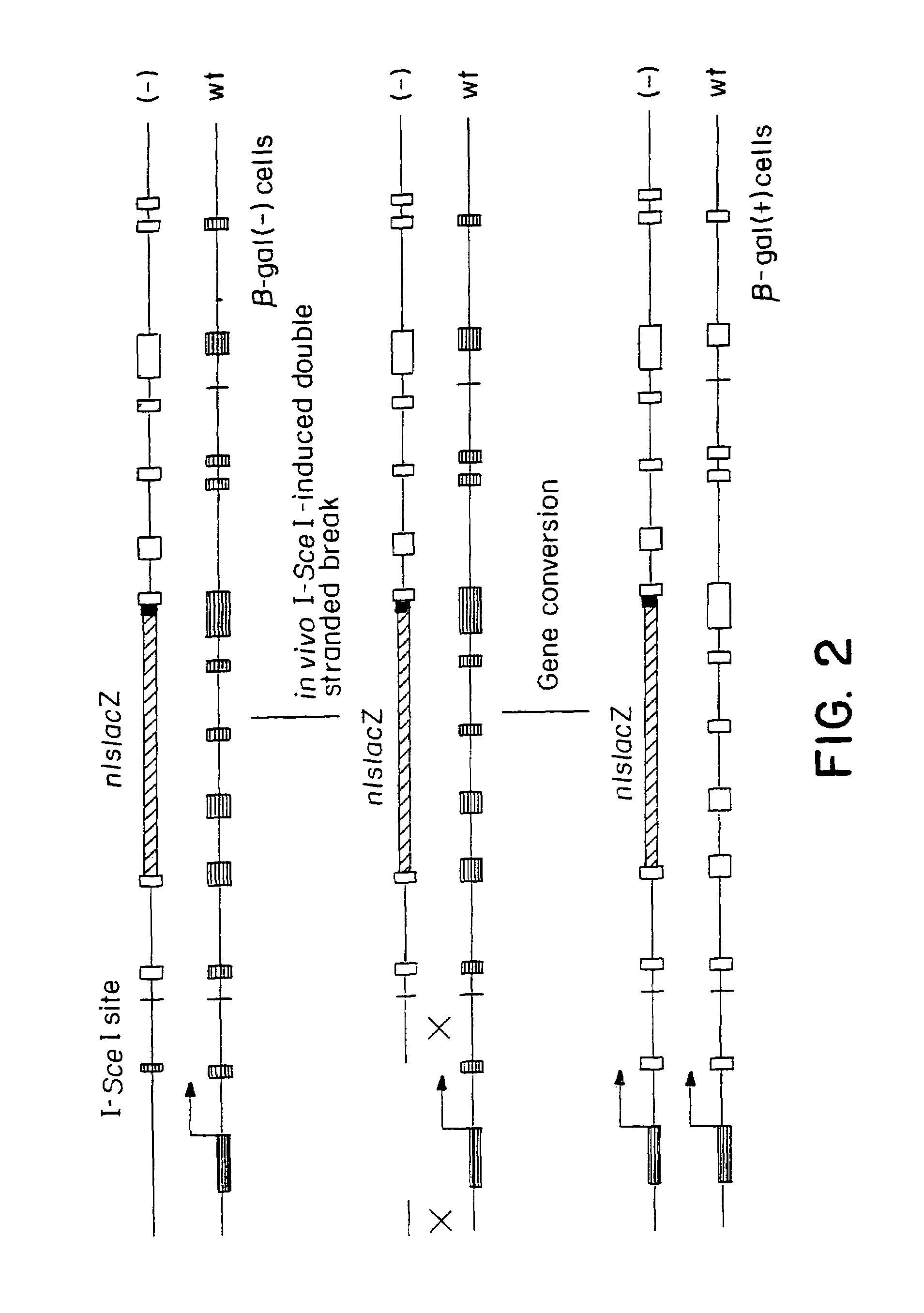
Gene repair involving the induction of double-stranded DNA cleavage at a chromosomal target site
InactiveUS20070141038A1Highly efficient recombinational eventEfficient introductionOrganic active ingredientsBiocideDouble strandLiving cell
Methods of modifying, repairing, attenuating and inactivating a gene or other chromosomal DNA in a cell are disclosed. Also disclosed are methods of treating or prophylaxis of a genetic disease in an individual in need thereof. Further disclosed are chimeric restriction endonucleases.
Owner:INST PASTEUR +1
Process and equipment for plasmid purfication
InactiveUS20060106208A1Easy to operateConsistent levelCation exchanger materialsIon-exchanger regenerationLysisGram
A scalable alkaline lysis process, including procedures and devices for the isolation of large quantities (grams and kilograms) of plasmid DNA from recombinant E. coli cells. Effective, controllable, and economical operation, and consistent low level of host chromosomal DNA in the final plasmid product. Involves a series of new unit operations and devices for cell resuspension, cell lysis, and neutralization.
Owner:URIGEN PHARMA INC
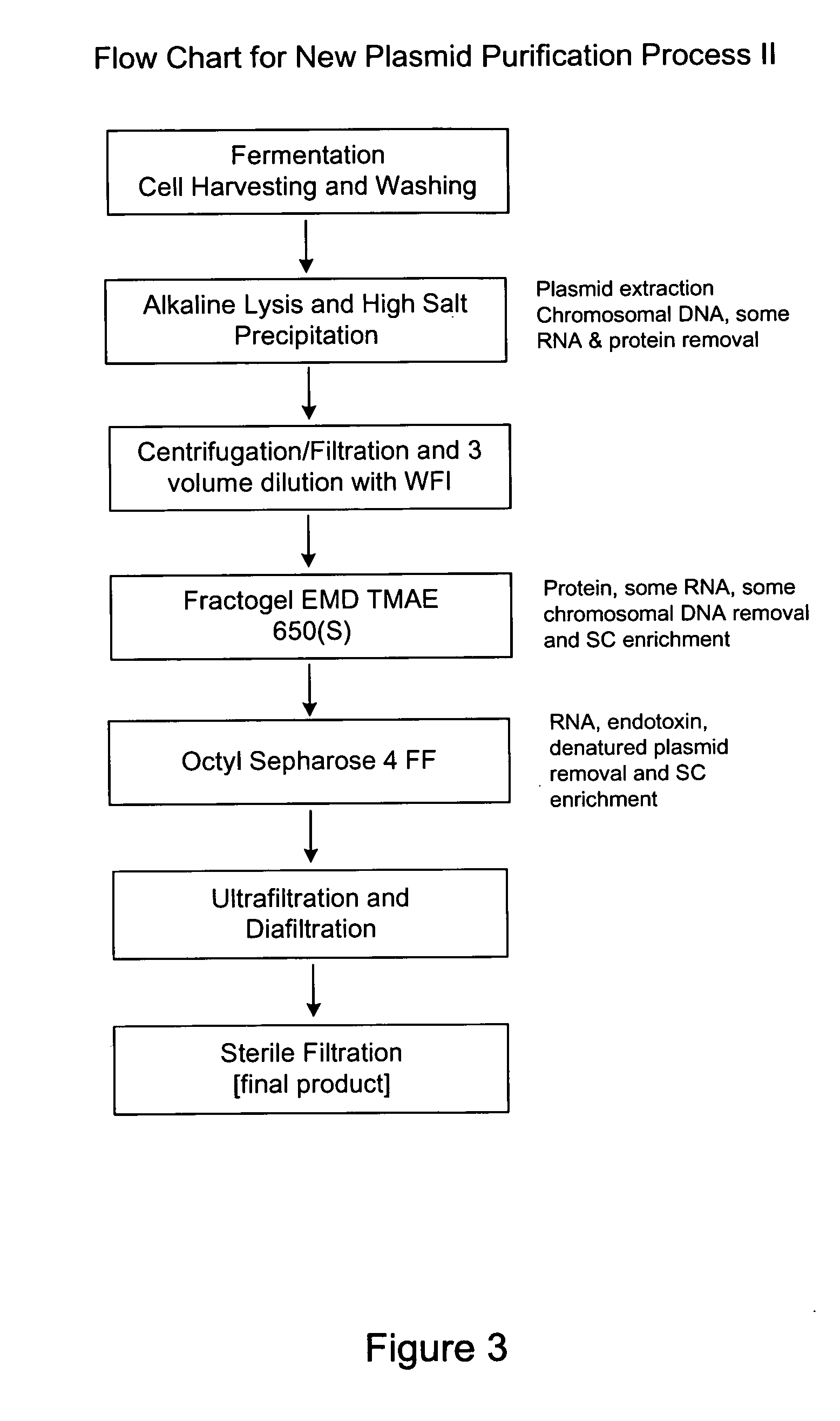
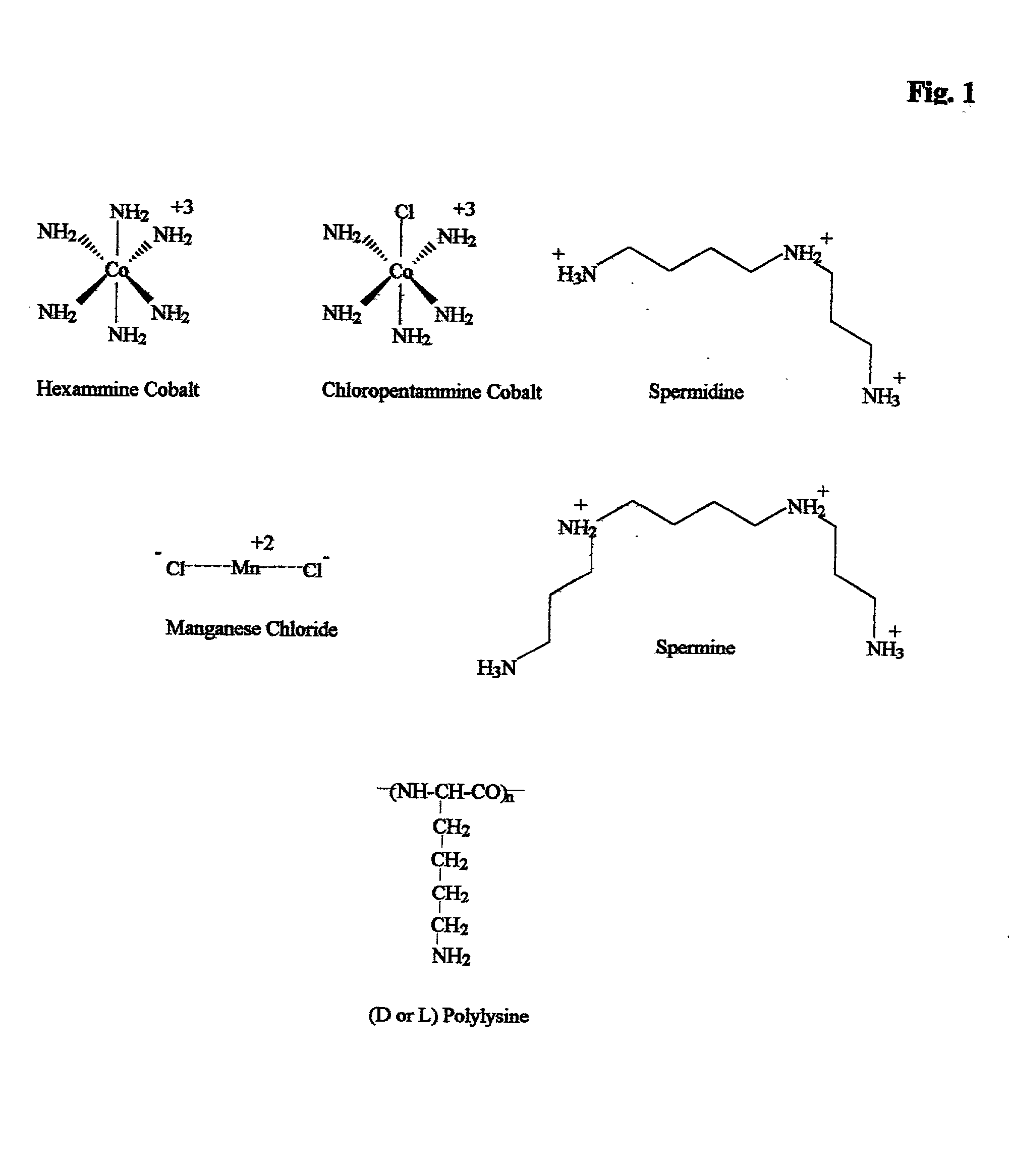
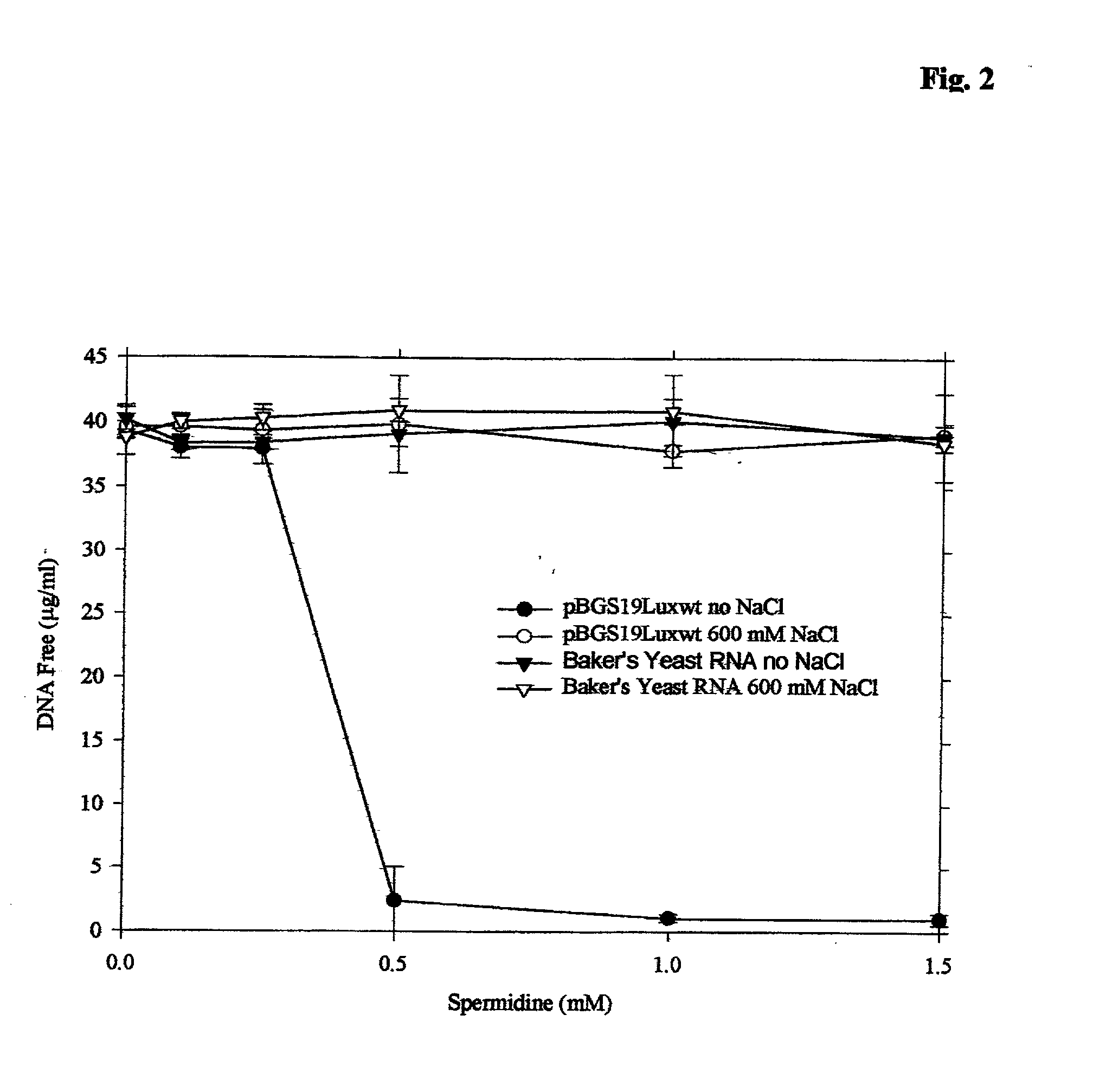
Apparatus, methods and compositions for biotechnical separations
InactiveUS20020010145A1Low costImprove performanceGenetic material ingredientsTyresFractional PrecipitationPlasmid dna
embodiments of the invention include purification of DNA, preferably plasmid DNA, by use of selective precipitation, preferably by addition of compaction agents Also included is a scaleable method for the liquid-phase separation of DNA from RNA. RNA may also be recovered by fractional precipitation according to the invention. RNA, commonly the major contaminant in DNA preparations, can be left in solution while valuable purified plasmid DNA is directly precipitated. Endotoxin can also be kept to very low levels. The invention includes mini-preps, preferably of plasmid and chromosomal DNA to obtain sequenceable and restriction digestible DNA in high yields in multiple simultaneous procedures. As a method of assay, a labeled probe is precipitated by hybridizing it to a target, (erg. chromosomal DNA, oligonucleotides, Ribosomal RNA, tRNA), and thereafter precipitating the probe / target complex with compaction agents and leaving in solution any unhybridized probe.
Owner:WILLSON RICHARD C III +1
Gene repair involving in vivo excision of targeting DNA
Methods of modifying, repairing, attenuating and inactivating a gene or other chromosomal DNA in a cell are disclosed. Also disclosed are methods of treating or prophylaxis of a genetic disease in an individual in need thereof.
Owner:CHILDRENS MEDICAL CENT CORP +1
Recombination systems and methods for eliminating nucleic acid sequences from the genome of eukaryotic organisms
The invention relates to recombination systems and methods for eliminating nucleic acid sequences from the chromosomal DNA of eukaryotic organisms, and to transgenic organisms—preferably plants—which comprise these systems or were generated using these methods.
Owner:SUNGENE +1
Methods of staining target chromosomal DNA employing high complexity nucleic acid probes
ActiveUS7115709B1Rapid and highly sensitive detectionEasy to analyzePeptide/protein ingredientsMicrobiological testing/measurementMetaphase chromosomeNucleic Acid Probes
Methods and compositions for staining based upon nucleic acid sequence that employ nucleic acid probes are provided. Said methods produce staining patterns that can be tailored for specific cytogenetic analyses. Said probes are appropriate for in situ hybridization and stain both interphase and metaphase chromosomal material with reliable signals. The nucleic acid probes are typically of a complexity greater than 50 kb, the complexity depending upon the cytogenetic application. Methods and reagents are provided for the detection of genetic rearrangements. Probes and test kits are provided for use in detecting genetic rearrangements, particularly for use in tumor cytogenetics, in the detection of disease related loci, specifically cancer, such as chronic myelogenous leukemia (CML), retinoblastoma, ovarian and uterine cancers, and for biological dosimetry. Methods and reagents are described for cytogenetic research, for the differentiation of cytogenetically similar but genetically different diseases, and for many prognostic and diagnostic applications.
Owner:RGT UNIV OF CALIFORNIA
Process and equipment for plasmid purification
InactiveUS7026468B2Effective and controllable and economical operationConsistent low levelCation exchanger materialsIon-exchanger regenerationEscherichia coliLysis
A scalable alkaline lysis process, including procedures and devices for the isolation of large quantities (grams and kilograms) of plasmid DNA from recombinant E. coli cell. Effective, contgrollable, and economical operation, and consistent low level of host chromosomal DNA in the final plasmid product. Involves a series of new unit operations and devices for cell resuspension, cell lysis, and nuetralization.
Owner:URIGEN PHARMA INC
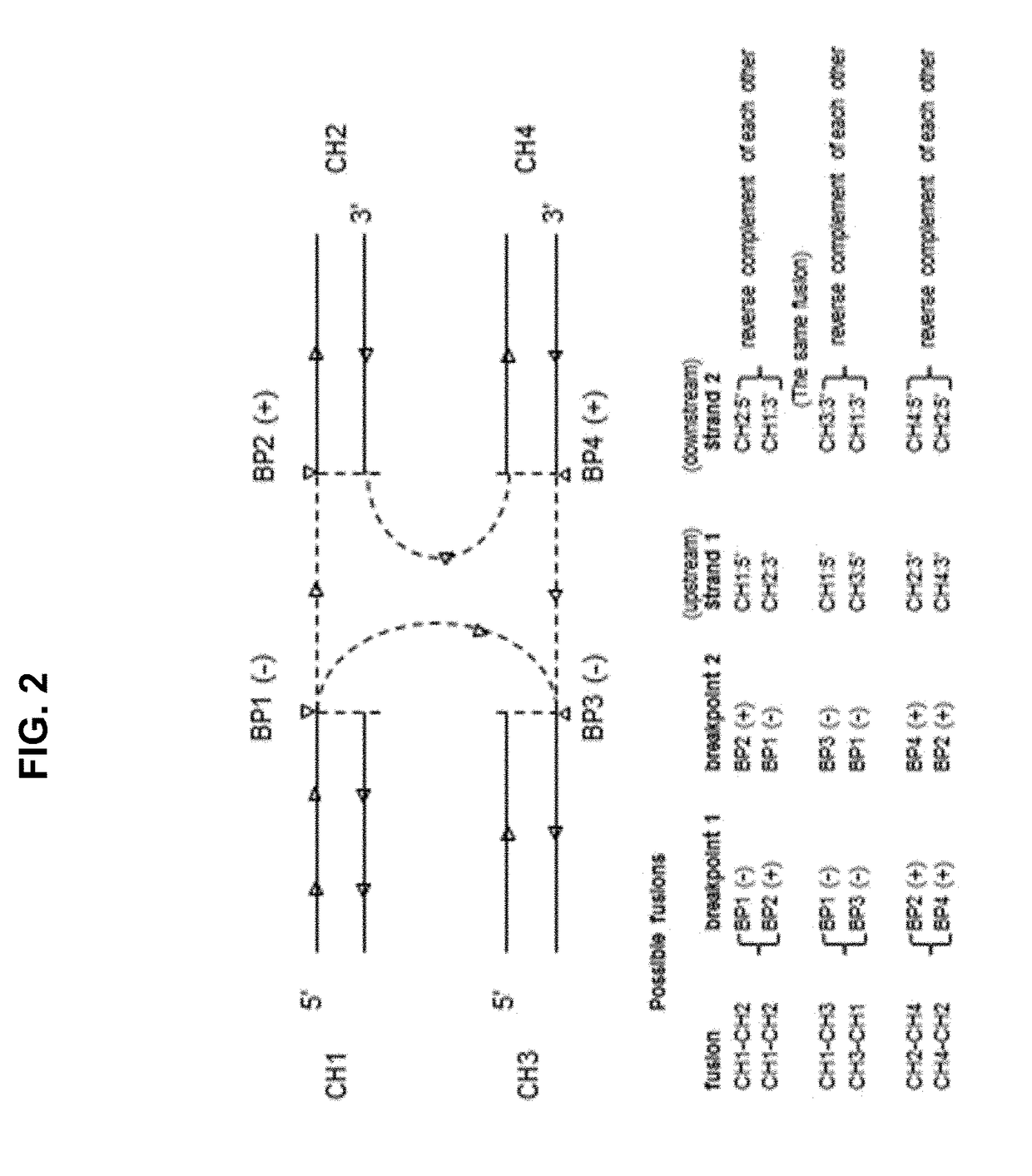
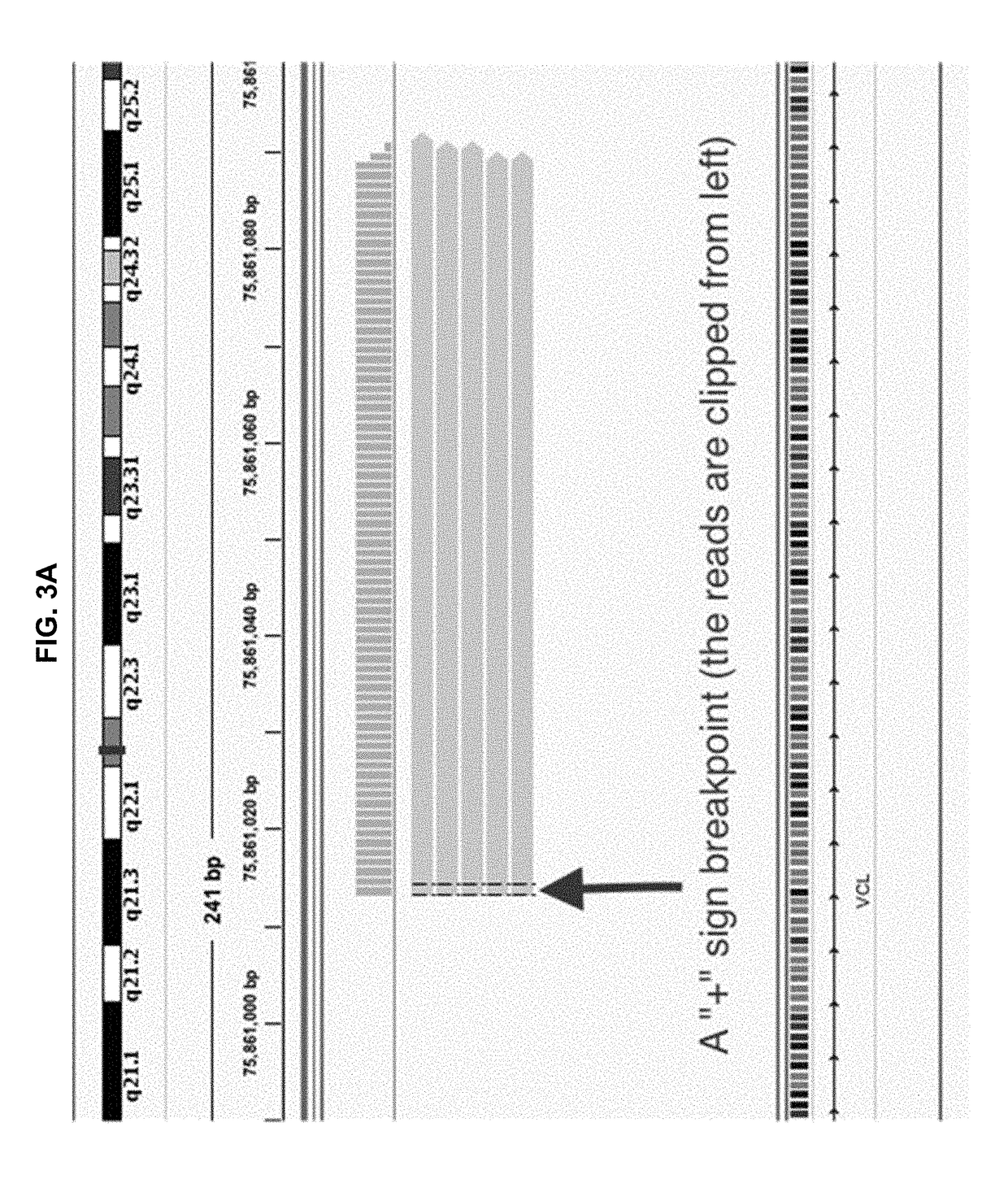
Methods and applications of gene fusion detection in cell-free DNA analysis
Systems and methods are disclosed for determining gene fusion by determining a fused read containing sequencing data of a portion of a fused chromosome DNA molecule; determining a predetermined point on the genome with least one mapped portion of the fused read clipped at the predetermined point (a breakpoint); identifying two mapped read portions from two breakpoints (breakpoint pair) as a potential fusion candidate; creating one or more fusion sets based on breakpoint pairs and clustering the fusion sets into one or more fusion clusters; and identifying each fusion cluster meeting a predetermined criterion as a gene fusion.
Owner:GUARDANT HEALTH
Alkaline pectinase genetic engineering bacteria and construction method thereof
InactiveCN101531988ABroaden research ideasBroaden the fieldBacteriaMicroorganism based processesPectinaseEnzyme Gene
The invention relates to an alkaline pectinase genetic engineering bacteria and a construction method thereof. The construction method comprises the steps: separating chromosomal DNA from a strain for producing alkaline pectinase, designing a primer, obtaining a target gene by PCR, constructing a recombinant plasmid, expressing in Bacillus subtilis, measuring gene sequence and carrying out comparative analysis. The gene obtained and expressed in the method is different from the enzyme gene sequences which have been reported and has the differences on the composition of individual amino acids;the culture medium adopted for fermentation of the engineering bacteria is applicable to the industrial production, an expression host is protease deficient which is conducive to the separation, the purification and the stable preservation of enzyme in the late stage, and the enzyme activity of fermentation liquid of the engineering bacteria is improved by 22 times compared with the starting strain, which can achieve 330U / ml.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Diagnosis, prognosis and treatment of pulmonary diseases
InactiveUS20060078558A1Reducing airway resistance responseImprove responsivenessOrganic active ingredientsPeptide/protein ingredientsDiseaseObstructive Pulmonary Diseases
The present invention provides methods to protect a subject from a respiratory disorder involving an airway obstructive disease such as asthma or chronic obstructive pulmonary disease. Provided are methods to protect a subject from an airway obstructive disease using gene therapy. Methods are provided for supplying FoxA2 function to cells of the lung and airway, such as smooth muscle and epithelial cells, by FoxA2 gene therapy. The FoxA2 gene, a modified FoxA2 gene, or a part of the gene may be introduced into the cell in a vector such that the gene remains extrachromosomal or may be integrated into the subjects chromosomal DNA for expression. These methods provide for administering to a subject in need of such treatment a therapeutically effective amount of a FoxA2 gene, or pharmaceutically acceptable composition thereof, for overexpressing the FoxA2 gene. Such methods of expressing the administered FoxA2 gene in the lungs and airway provide for: (1) preventing or alleving bronchial hyperresponsiveness; (2) preventing or alleving of an airway obstructive disease, e.g., bronchial hyperreactivity, airway hyperresponsiveness, asthma or chronic obstructive pulmonary disorder (“COPD”); (3) reducing the airway resistance response to inhaled natural or synthetic bronchoconstrictors or allergens or to exercise; and (4) enhancing responsiveness (relaxation) of airway tissues to β-agonists.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Method for relative quantitation of chromosomal DNA copy number in single or few cells
The present invention is directed to methods for determining the presence or absence of a genetic defect in an IVF embryo prior to transfer comprising performing real-time PCR and 2−ΔΔCT analyses to determine normalized copy number of at least one invariant locus on at least one chromosome collected from at least one cell of the embryo and selecting a candidate IVF embryo determined to be without genetic defect for transfer.
Owner:SCOTT JR RICHARD T +1
Vector for transformation of Labyrinthulomycota
An object of the present invention is to provide a transformation system for Labyrinthulomycota that allows the elucidation of biosynthetic mechanisms of lipids such as PUFA and carotenoids as well as for the construction of a high production system and the design and development of novel functional lipid molecules by the control of the mechanisms. The present invention provides a vector for the transformation of Labyrinthulomycota with a transgene, which comprises at least (1) a nucleotide sequence which is homologous to a part of chromosomal DNA of Labyrinthulomycota and is capable of homologous recombination with the chromosomal DNA, (2) a selection marker gene having a promoter sequence located upstream and a terminator sequence located downstream, and (3) a cloning site for transgene insertion having a promoter sequence located upstream and a terminator sequence located downstream.
Owner:FUJIFILM CORP +1
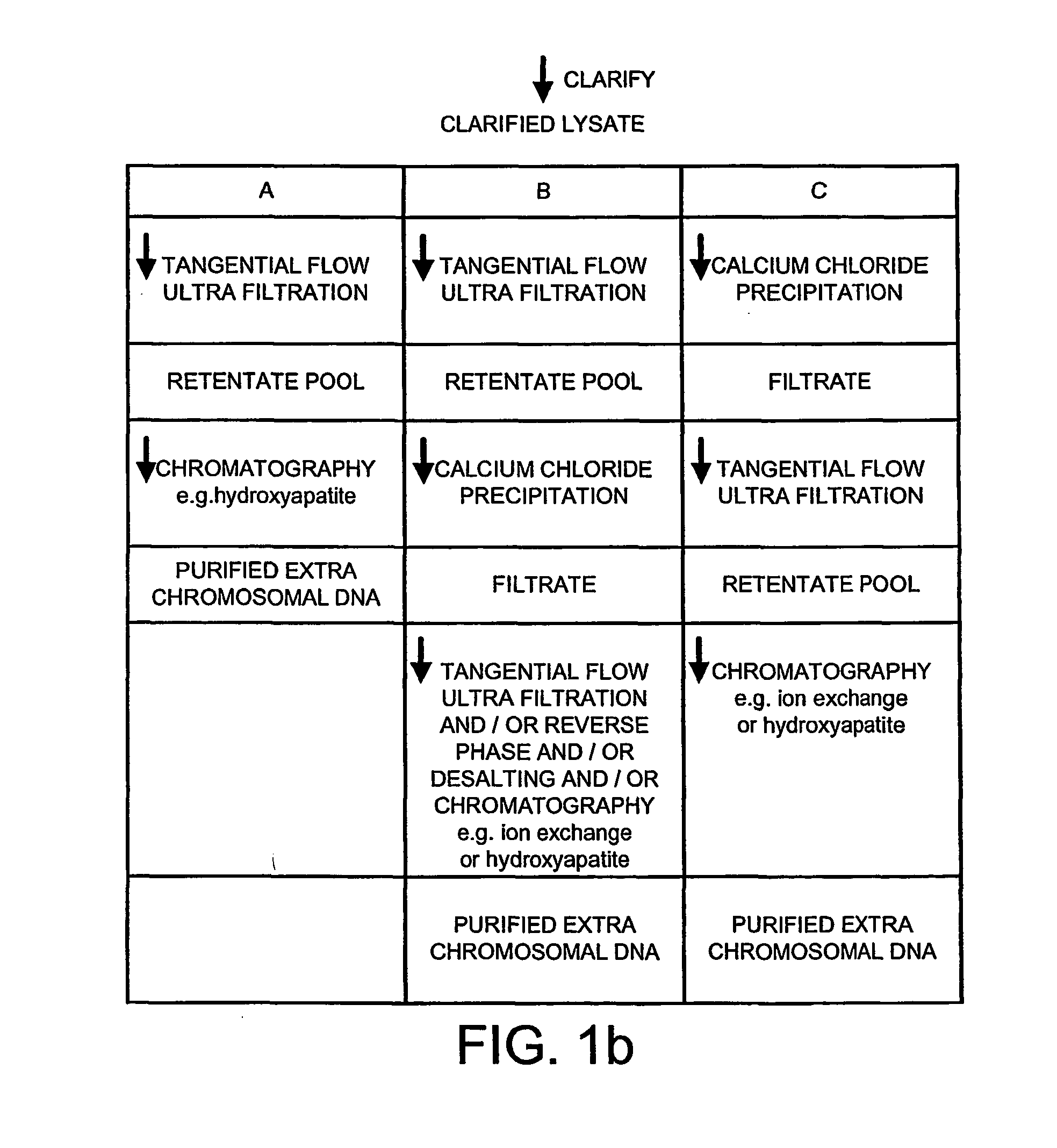
Method of separating extra-chromosonal dna from other cellular components
The invention relates to a method of separating extra-chromosomal DNA from RNA. It also relates to DNA produced by the method and pharmaceuticals derived from such DNA, for example, DNA vaccines. The method comprises separating extra-chromosomal DNA from RNA without first digesting the RNA, and comprises: i) lysing cells comprising extra-chromosomal DNA to form a lysate; ii) clarifying the lysate; and iii) subjecting the clarified lysate to tangential flow ultra filtration under conditions, which allow a substantial amount of the RNA to pass through a membrane whilst the extra-chromosomal DNA is retained. The preferred conditions include the use of a diafiltration buffer of low conductivity and diafiltration volumes of over 10 volume equivalents.
Owner:GLAXO GRP LIMITED OF GLAXO WELLCOME HOUSE
Chromosomal DNA integration method
Owner:BIO ARCHITECTURE LAB
Method for introducing a gene into labyrinthulomycota
An object of the present invention is to provide a transformation system for Labyrinthulomycota that allows the elucidation of biosynthetic mechanisms of lipids such as PUFA and carotenoids as well as for the construction of a high production system and the design and development of novel functional lipid molecules by the control of the mechanisms. The present invention provides a method for introducing a transgene into a cell of Labyrinthulomycota, which comprises introducing into a cell of Labyrinthulomycota a recombinant vector comprising a transgene and a nucleotide sequence which is homologous to a part of chromosomal DNA of Labyrinthulomycota and is capable of homologous recombination with the chromosomal DNA, and then inducing homologous recombination in this homologous nucleotide sequence.
Owner:HIROSHIMA UNIVERSITY +1
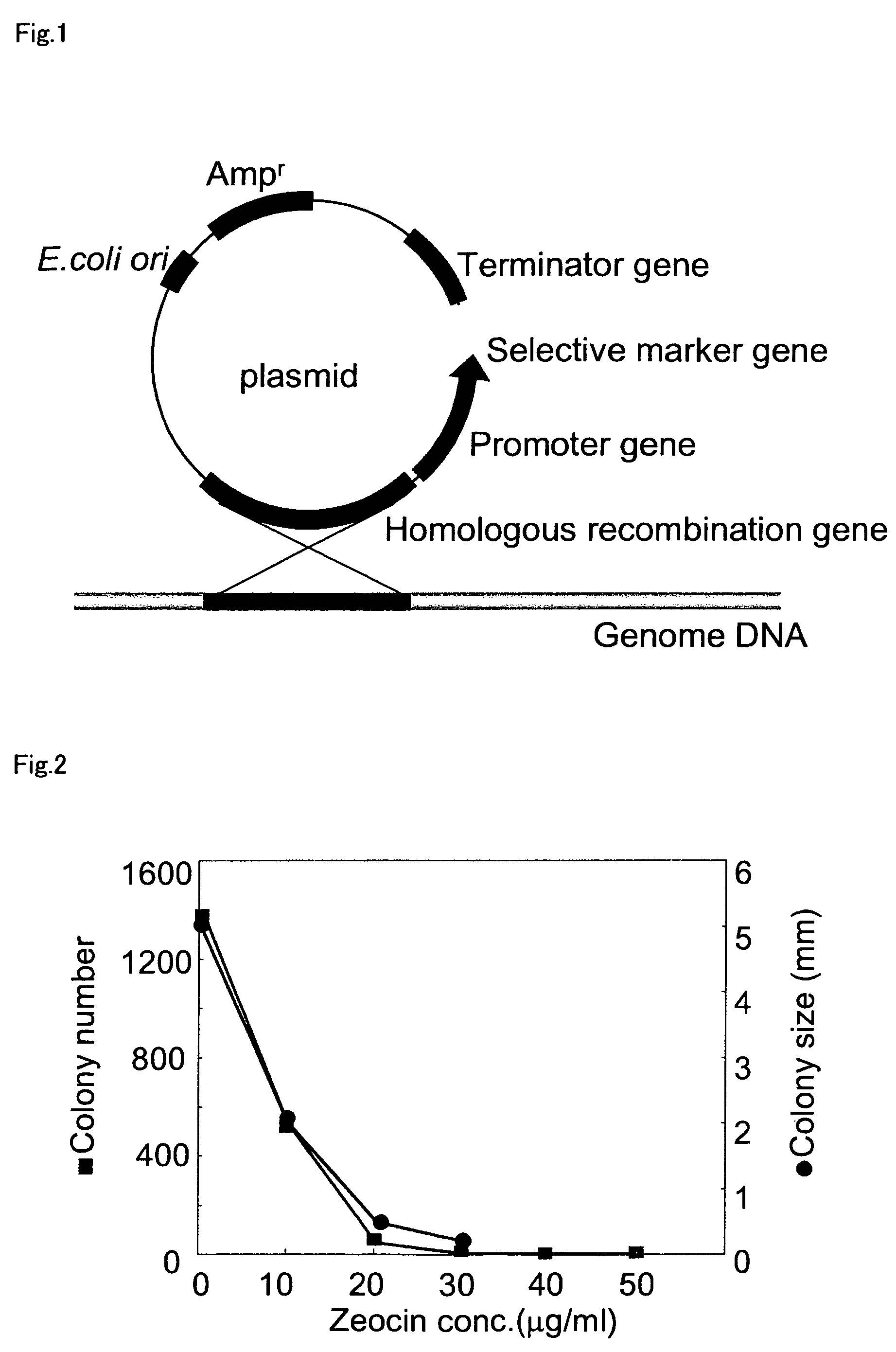
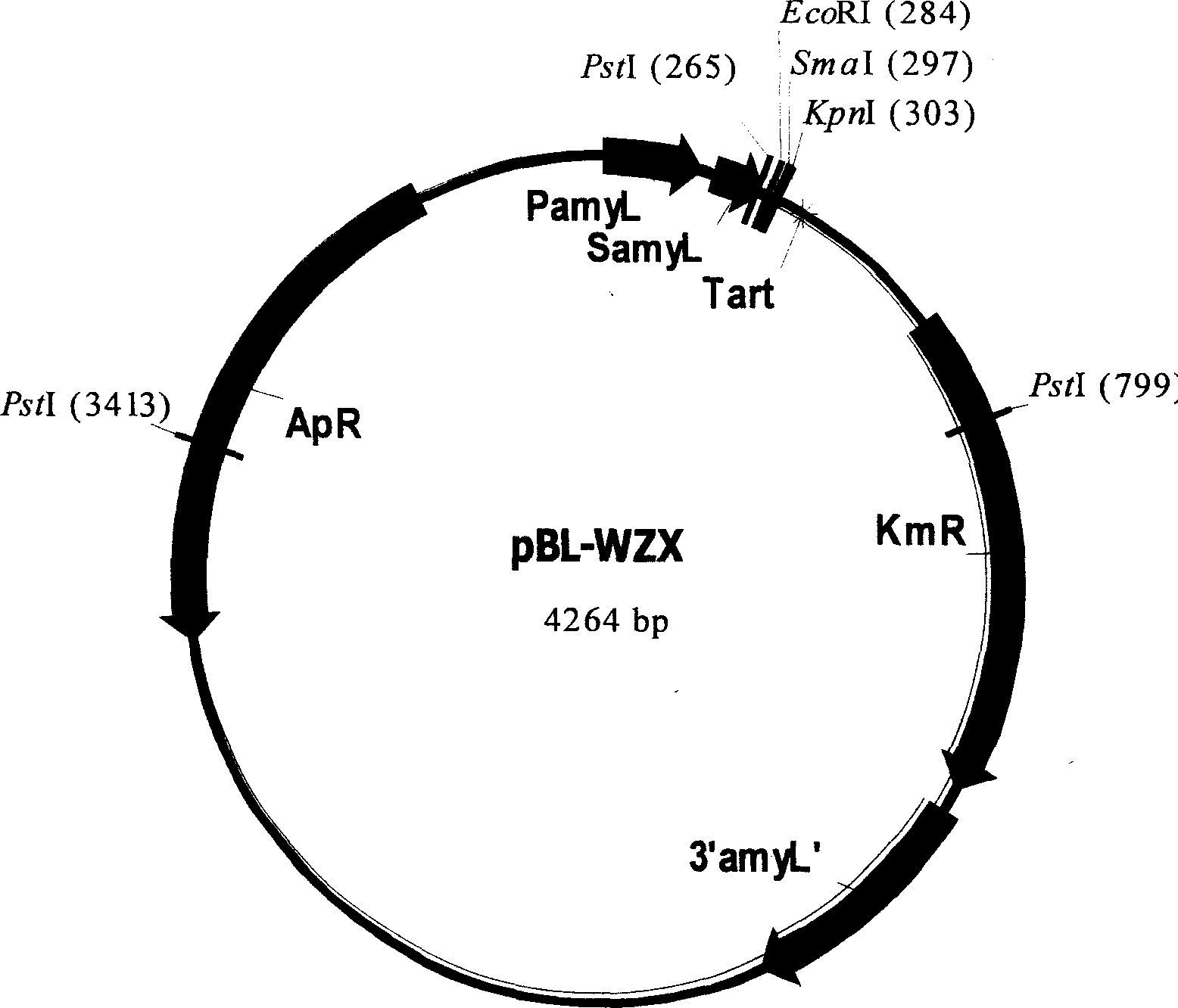
Expression vector for secreting expression of exogenous gene in Escherichia coli or bacillus and its construction
The present invention is expression vector to secretion express foreign gene in colibacillus or bacillus and its construction, and belongs to the field of gene engineering technology. The expression vector pBL-WZX contains one promoter sequence with function in both colibacillus and bacillus, one signal peptide sequence with function in both colibacillus and bacillus, one polyclonal site for foreign gene to be cloned, one artificially transcription termination sequence, one genetic marker for cloning selection between colibacillus and bacillus, and one DNA sequence for foreign gene to complete integration and gene copy number proliferation in the chromosome DNA of bacillus. The expression vector pBL-WZX can induce the transcription and translation of foreign gene and secrete the gene product to outside cell, and has foreign gene secretion expression interventing level up to 1 mg / mL in colibacillus and 3.1 mg / mL in bacillus separately.
Owner:福建福大百特生物科技有限公司
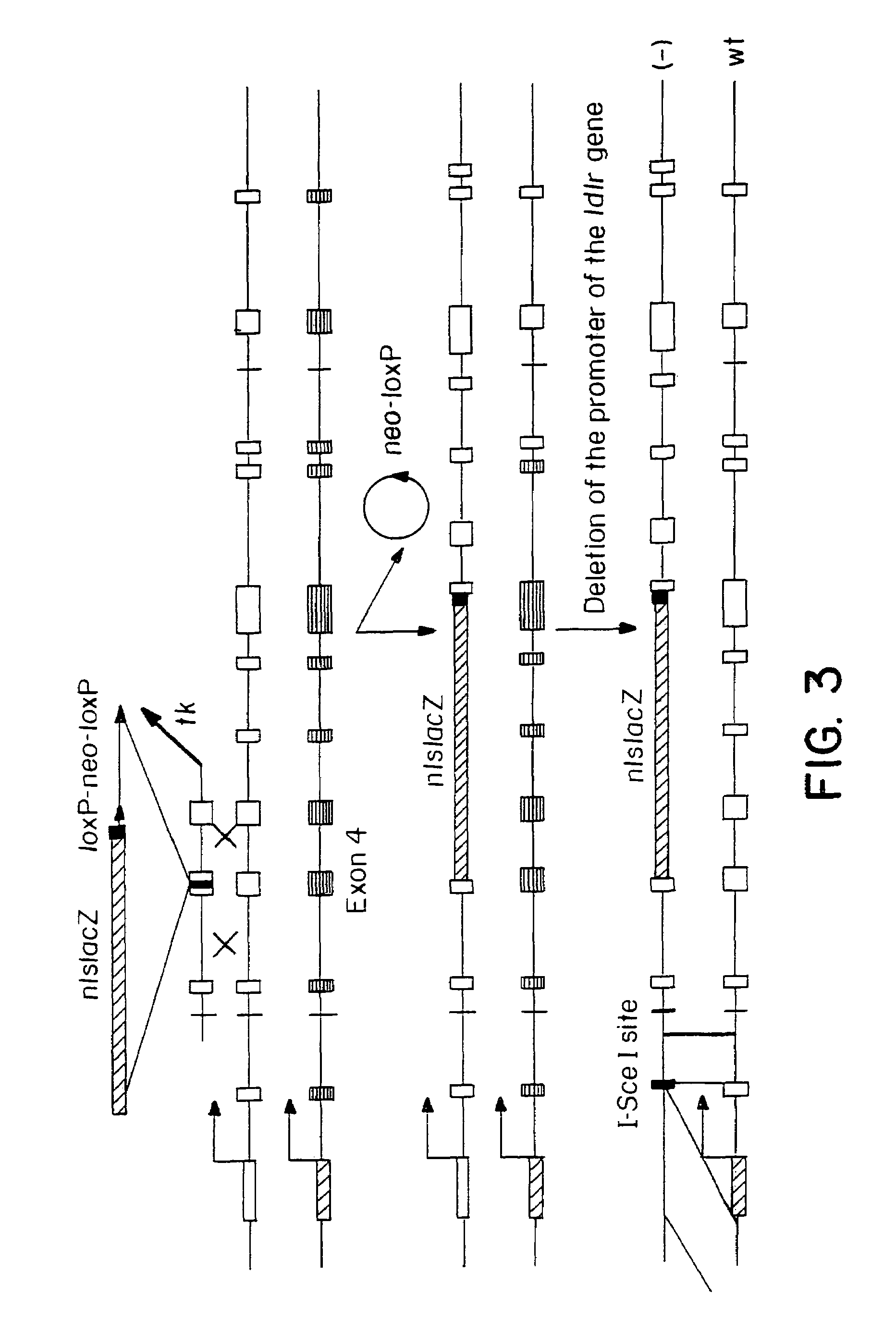
Chromosomal modification involving the induction of double-stranded DNA cleavage and homologous recombination at the cleavage site
InactiveUS7629326B2Efficiently introducedHighly efficient recombinational eventsBiocideHydroxy compound active ingredientsDiseaseBond cleavage
Methods of modifying, repairing, attenuating and inactivating a gene or other chromosomal DNA in a cell are disclosed. Also disclosed are methods of treating or prophylaxis of a genetic disease in an individual in need thereof. Further disclosed are chimeric restriction endonucleases.
Owner:INST PASTEUR +1
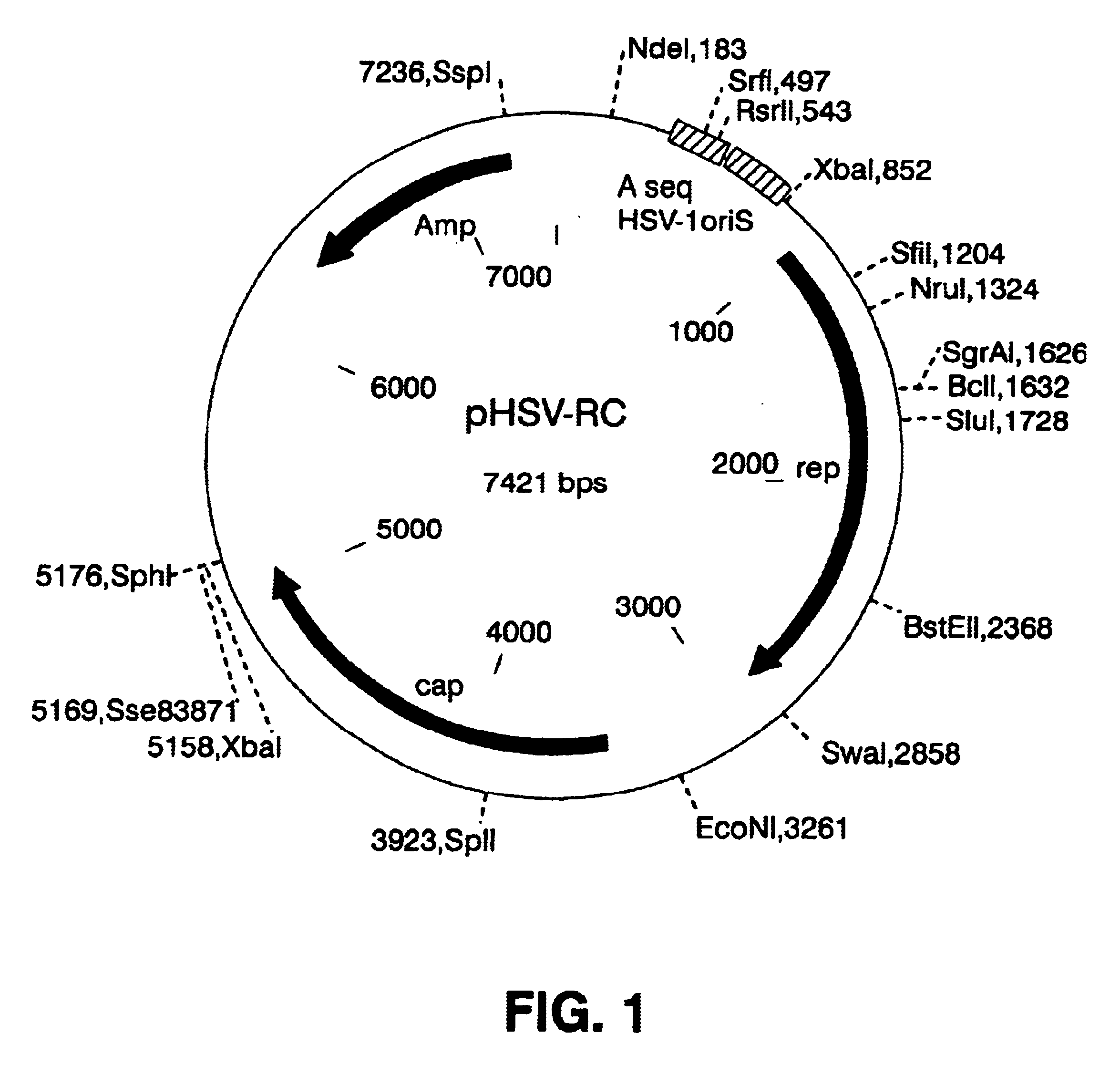
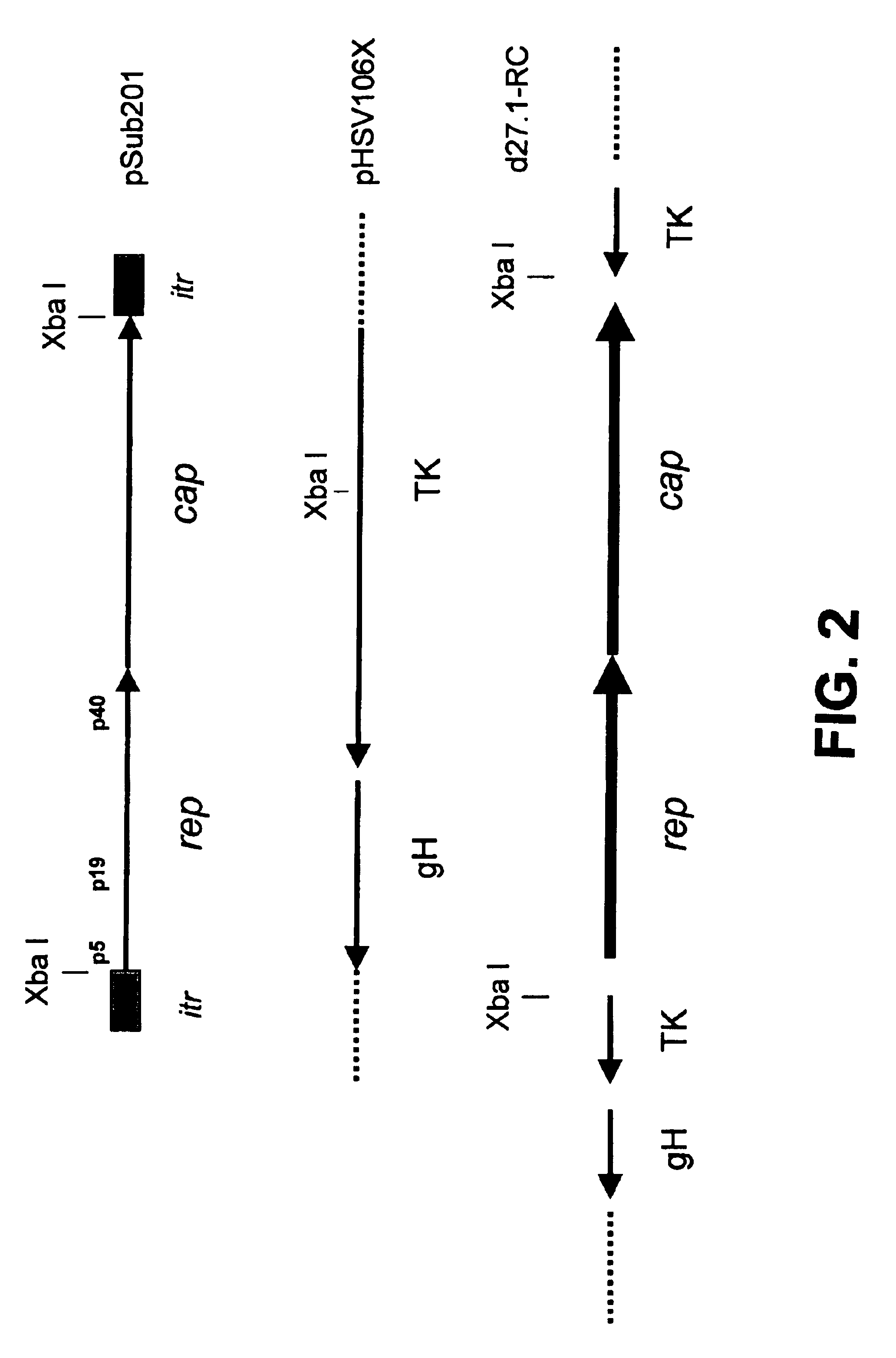
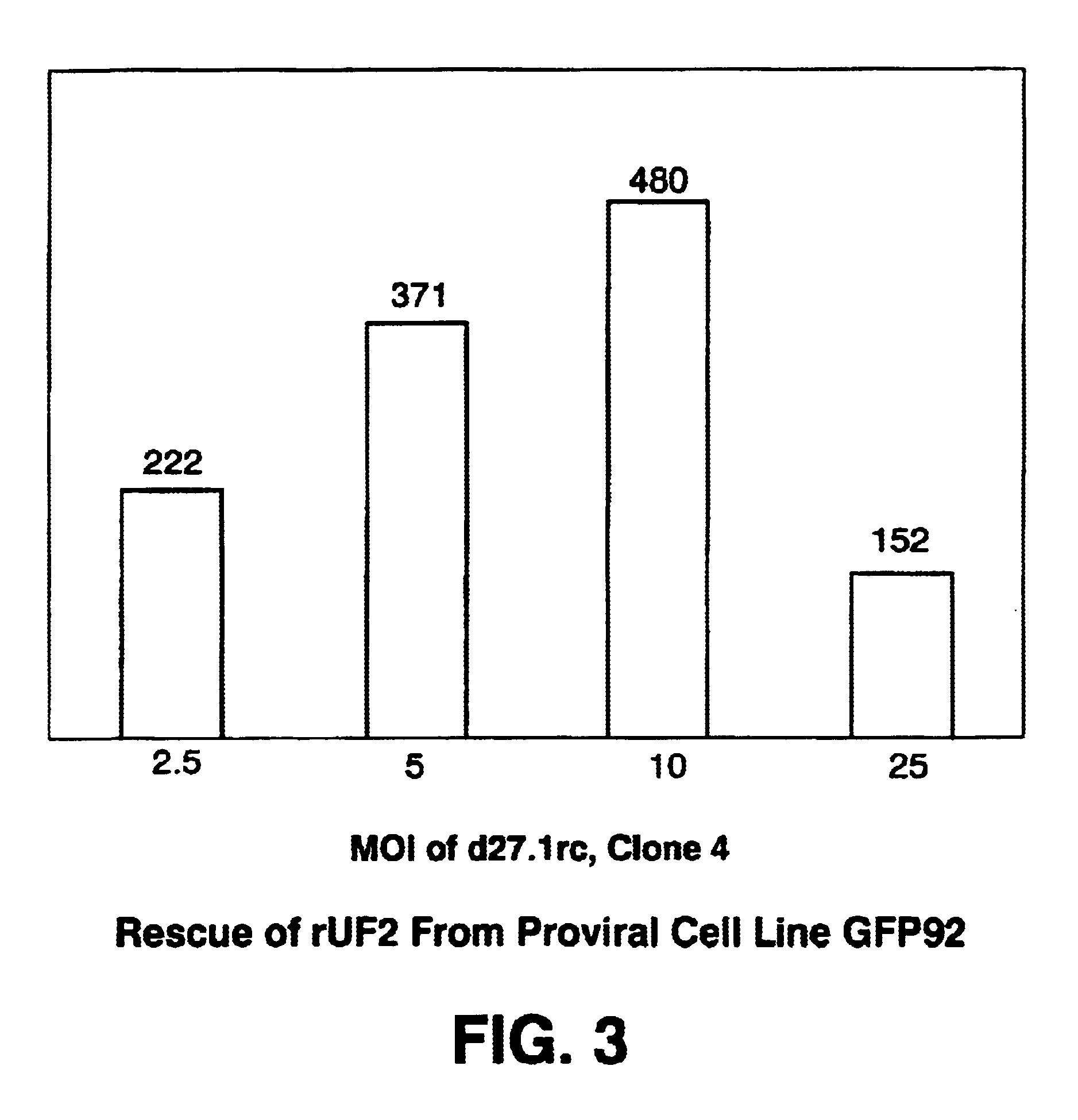
Methods for large-scale production of recombinant AAV vectors
InactiveUS6783972B1Large scaleCost-effective and reliableSugar derivativesGenetic material ingredientsNucleotideCell staining
Disclosed are HSV-1 amplicons that supply all necessary helper functions required for rAAV packaging and methods for their use. These HSV-1 amplicons have been shown to be capable of rescuing and replicating all forms of rAAV genomes including rAAV genomes introduced into cells by infection of rAAV virions, rAAV genomes transfected into cells on plasmids or proviral rAAV genomes integrated into cellular chromosomal DNA. Also provided are methods for preparing high-titer rAAV vector compositions suitable for gene therapy and the delivery of exogenous polynucleotides to selected host cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Acetate-free purification of plasmid DNA on hydroxyapatite
InactiveUS6406892B1Ion-exchange process apparatusOther chemical processesChromatographic separationApatite
Plasmid DNA is purified from a cell lysate by acidifying the lysate with a combination of a mineral acid and an inorganic salt in place of the commonly used acetate buffer, allowing chromosomal DNA and other impurities to precipitate out and recovering the supernatant, and applying the supernatant directly to a hydroxyapatite chromatographic separation medium. The use of the mineral acid and organic salt avoids the degradation of the hydroxyapatite that frequently accompanies repeated use with acetate and related compounds.
Owner:BIO RAD LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com