Hepatitis E virus fluorescent quantitative PCR detection method
A hepatitis E virus, fluorescence quantitative technology, applied in the field of bioengineering, can solve the problems of large difference in detection rate, different antigens, reduce missed detection rate and other problems, achieve high accuracy, low false positive rate, avoid The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
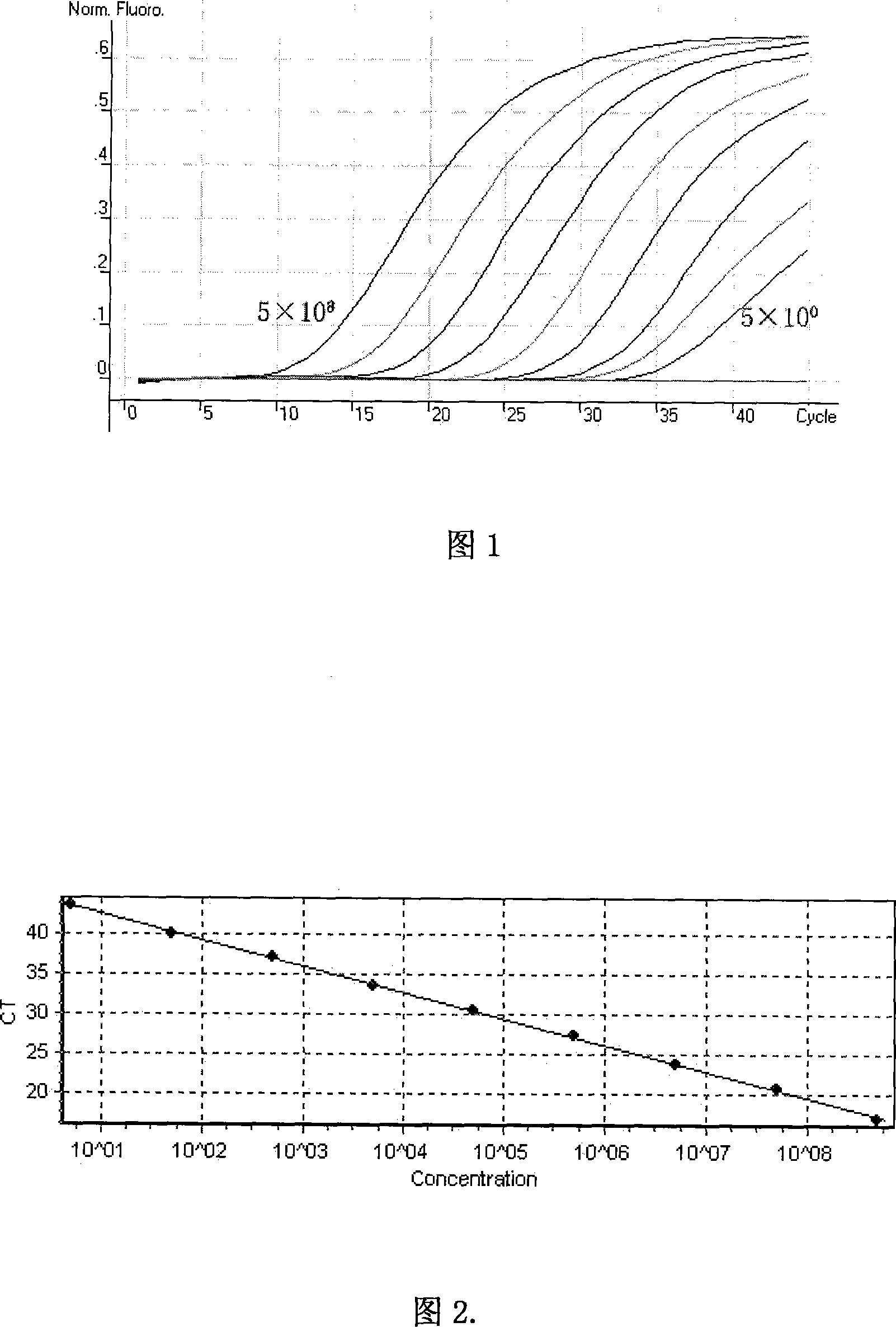
Image
Examples
Embodiment 1
[0043] Design primers and Taqman probes
[0044] Experimental steps:
[0045] The complete sequence of HEV was collected in Genbank and analyzed by bioinformatics, and the conserved segment of the sequence was selected as a candidate segment for designing primers and probes. D11092, L08816, L25547 and M94177), determined the highly conserved region located in ORF2 as the target gene sequence for PCR amplification. A pair of PCR primers and Taqman probes were designed using primer express software and primer premier5.0 software. The fluorescent reporter group at the 5' end of the probe was FAM, and the fluorescent quencher group labeled at the 3' end was TAMRA. Primers and probes are located in the ORF2 region, and the target amplified fragment is 151bp. The hepatitis E virus-specific quantitative PCR primer refers to an oligonucleotide chain with a length of 21 bp.
[0046] The primer and probe sequences are:
[0047] Upstream primer: HEV-1(+): GAATGCTCAGCAGGATAAGGGT;
[...
Embodiment 2
[0051] Construction and preparation of quantitative standards
[0052] Experimental procedure
[0053] 1. Construction of quantitative standards
[0054] The HEV gene fragment containing the target sequence was cloned by RT-PCR, recombined into the vector pET-28a, and the DNA sequence was determined. The constructed recombinant plasmid was used as a quantitative standard and named pET28a-ORF2.
[0055] 2. Preparation of Quantitative Standards
[0056] Plasmid pET28a-ORF2 was extracted and purified by alkaline cleavage method, and the OD measured by UV spectrophotometer 260 、OD 280 and OD 230 The value was used to calculate the plasmid concentration, detect the purity of the plasmid, and convert to copy number according to Avogadro constant.
[0057] Calculated as follows:
[0058] Plasmid concentration (μg / μl) = OD 260 × Dilution factor × 50 / 1000
[0059] Plasmid copy number = A*N / 1000M
[0060] A: represents the plasmid concentration (μg / μl);
[0061] M: represents...
Embodiment 3
[0064] Isolation and concentration of viruses in virus samples
[0065] Experimental procedure
[0066] 1. Virus Extraction and Concentration
[0067] (1). PBS with a pH of 7.4 was used to fully shake and mix with the feces samples of the acute phase of the hepatitis E patients to make a 10% feces suspension. If the virus content of the sample is high, continue to step 2 directly; if the virus content is low, continue to the following steps:
[0068] (2). After centrifuging at 4°C and 8000rpm for 10 minutes, take the supernatant;
[0069] (3). Continue to centrifuge at 12000rpm for 15min at 4°C, and take the supernatant;
[0070] (4). Add appropriate amount of PEG 6000 and sodium chloride to the supernatant, and let stand overnight at 4°C;
[0071] (5). Centrifuge at 12,000 rpm for 15 minutes at 4°C to obtain a precipitate, and use Trizol to extract RNA from the precipitate.
[0072] 2. Viral Nucleic Acid Extraction
[0073] (1). Add 1ml Trizol to every 150μl of 10% fece...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com