Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Rift Valley fever virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rift Valley fever (RVF) is a mosquito-borne zoonotic disease caused by Rift Valley fever virus (RVFV) that affects humans and ruminants. This virus belongs to the family Bunyaviridae and genus Phlebovirus.
Host targeted inhibitors of dengue virus and other viruses
ActiveUS20150166532A1Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeBiocideOrganic chemistryHerpes simplex diseaseDisease injury
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Host targeted inhibitors of dengue virus and other viruses
ActiveUS9879003B2Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeOrganic chemistryHerpes simplex diseaseJunin virus
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Reverse genetic system for Rift Valley fever virus and uses thereof
ActiveUS20070122431A1SsRNA viruses negative-senseViral antigen ingredientsAntiviral drugRna expression
The present invention describes a reverse genetic system for Phlebovirus such as Rift Valley fever virus. This system comprised of RNA expression plasmids and protein expression plasmids. Additionally, the present invention also discloses the modification of this system to generate a recombinant virus that expresses a non-viral foreign gene. Furthermore, the present invention discloses the use of this system in the development of anti-Rift Valley fever virus vaccines, screening of antivirals testing for anti RVF immune response and developing marker vaccines for Rift Valley fever virus. We also claim the utility of this approach to other phleboviruses.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system
ActiveCN102191338AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceYellow fever
The invention discloses a fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system consisting of primers, probes, a Premix Ex Taq reaction solution and a sterilized Tris buffer. With good singularity and high sensitivity, three pairs of primers and probes are very suitable for simultaneously detecting viruses of yellow fever, dengue fever and epidemicencephalitis B. And there is no cross reaction between the primers and probes and several other entomophily hemorrhagic fever viruses, such as Marburg virus and Rift Valley fever virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Multiplex fluorescent polymerase chain reaction (PCR) kit and primers for detecting Ebola viruses, Marburg viruses, Lassa viruses and Rift Valley fever viruses
InactiveCN102719557AAccurate detectionControl incomingMicrobiological testing/measurementFluorescence/phosphorescenceMarburg virusRift Valley fever virus
The invention provides a multiplex fluorescent polymerase chain reaction (PCR) kit and primers for detecting Ebola viruses, Marburg viruses, Lassa viruses and Rift Valley fever viruses. The multiplex fluorescent PCR kit comprises conventional reagents of an RT-PCR buffer and an RT-PCR enzyme mixed liquor and also comprises primers and probes for detecting the four viruses, wherein the primers are shown in sequences of SEG ID NO: 1-13 and the probes are shown in sequences of SEQ ID NO: 14-18. The multiplex fluorescent PCR kit, the primers and the probes realize rapid and accurate detection of pathogens of Ebola hemorrhagic fever, Marburg hemorrhagic fever, Lassa fever and Rift Valley fever, prevent the four infectious diseases from spreading into or out of the frontier port, are accurate and effective, have strong operability, and can be used for detection of the infectious diseases. Through the multiplex fluorescent PCR kit, the primers and the probes, suspect cases can be found timely and a capability of preventing the infectious diseases from spreading into our country is improved.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Bunyavirales vaccine
ActiveUS20210030864A1Efficient expressionSsRNA viruses negative-senseViral antigen ingredientsDiseaseBunyavirales
The present invention is directed to an artificial nucleic acid, particularly to an artificial RNA, and to polypeptides suitable for use in treatment or prophylaxis of an infection with a virus of the order Bunyavirales, particularly Severe fever with thrombocytopenia syndrome virus (SFTSV), Rift Valley fever virus (RVFV), or Crimean-Congo hemorrhagic fever virus (CCHFV), or a disorder related to such an infection. The present invention further concerns a Bunyavirales vaccine, particularly a SFTSV, RVFV, or CCHFV vaccine. The present invention is directed to an artificial nucleic acid, polypeptides, compositions and vaccines comprising the artificial nucleic acid or the polypeptides. The invention further concerns a method of treating or preventing a disorder or a disease, first and second medical uses of the artificial nucleic acid, polypeptides, compositions and vaccines. Further, the invention is directed to a kit, particularly to a kit of parts, comprising the artificial nucleic acid, polypeptides, compositions and vaccines.
Owner:CUREVAC SE
Reverse genetic system for rift valley fever virus and uses thereof
The present invention describes a reverse genetic system for Phlebovirus such as Rift Valley fever virus. This system comprised of RNA expression plasmids and protein expression plasmids. Additionally, the present invention also discloses the modification of this system to generate a recombinant virus that expresses a non-viral foreign gene. Furthermore, the present invention discloses the use of this system in the development of anti-Rift Valley fever virus vaccines, screening of antivirals testing for anti RVF immune response and developing marker vaccines for Rift Valley fever virus. We also claim the utility of this approach to other phleboviruses.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Preparation and application of hemorrhagic fever associated pathogen identifying gene chip
ActiveCN105087824APracticalShort detection cycleNucleotide librariesMicrobiological testing/measurementOligonucleotide chipOligonucleotide
The invention relates to a hemorrhagic fever associated pathogen identifying gene chip; the preparation method comprises preparation of a specific primer, preparation of a pathogen specific oligonucleotide probe, preparation of an oligonucleotide chip, establishment of an RT-PCR (reverse transcription-polymerase chain reaction) system and establishment of a hybrid system and a signal detection method. The gene chip prepared by the invention can be used for simultaneously identifying 16 hemorrhagic fever associated pathogen microorganisms, including Zaire Ebola virus, Sudan Ebola virus, marburg virus, lassa virus, junin virus, Machupo virus, rift valley fever virus, Crimea-Congo hemorrhagic fever virus, plasmodium, hantaan virus, SFTS (severe fever with thrombocytopenia syndrome) virus, dengue virus, yellow fever virus, Chikungunya virus, influenza A virus and influenza B virus. The gene chip has the characteristics of being rapid and accurate, high in throughput and high in sensitivity; and a new technological means is offered for the diagnosis of hemorrhagic fever pathogen, health supervision and the control and prevention of infectious diseases.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
Primer probe group and reagent box for detecting rift valley fever virus with RAA (Recombinase Aided Amplification) fluorescence method
InactiveCN108624720ARapid identificationAccurate identificationMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceFluorescence
The invention relates to a primer probe group and a reagent box for detecting a rift valley fever virus with an RAA (Recombinase Aided Amplification) fluorescence method, and belongs to the technicalfield of biological detection. The primer probe group for detecting the rift valley fever virus with the RAA fluorescence method comprises an upstream primer, a downstream primer and a probe, whereinthe nucleotide sequence of the upstream primer is shown in SEQ ID NO. 1; the nucleotide sequence of the downstream primer is shown in SEQ ID NO. 2; the probe is a substance with the nucleotide sequence shown in SEQ ID NO. 3, wherein the 28th basic group is a modified fluorescence reporter group, the 29th basic group is replaced with a tetrahydrofuran residue, and the 30th basic group is a modifiedquenching group. The primer probe group provided by the invention is quick, accurate and sensitive in detection and is convenient in operation, specificity is 100%, and the detection of the rift valley fever virus can be finished within 15 min.
Owner:浙江国际旅行卫生保健中心 +1
Fulminating-infectious-disease pathogen detecting primer pair and kit
ActiveCN105483293AReduced Pollution ChancesShorten detection timeMicrobiological testing/measurementAgainst vector-borne diseasesColor changesBiology
The invention discloses a fulminating-infectious-disease pathogen detecting primer pair and a kit. The primer pair comprises at least a pair of RT-LAMP primers of Ebola viruses, Lassa fever viruses, Marburg viruses, rift valley fever viruses, yellow fever viruses and Chikungunya fever viruses. By means of the primer system, the amplification reaction background is reduced, and sensitivity and specificity are quite good. The kit formed by the primer pair further comprises detecting liquid and a micro-fluidic chip; as an independent RT-PCR secondary amplification step of a detecting liquid system is omitted, detecting time is shortened; as the denaturation process and the renaturation process of nucleic acid do not exist, the polluted chance of RNA enzymes and the polluted chance of amplification nucleic acid are reduced, and the sensibility and the safety of detection are improved. By means of the constant-temperature sealed environment provided by a micro-fluidic chip system, rapid and constant-temperature amplification and automation result distinguishing of a nucleic acid extracting template are finally achieved, the requirement for test hardware is reduced, the use level of a reaction reagent is reduced, detection cost is reduced, and the result can be directly determined through color changes.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
New fluorescence quantitative polymerase chain reaction (PCR) detection method for rift valley fever virus and rift valley fever virus detection PCR system
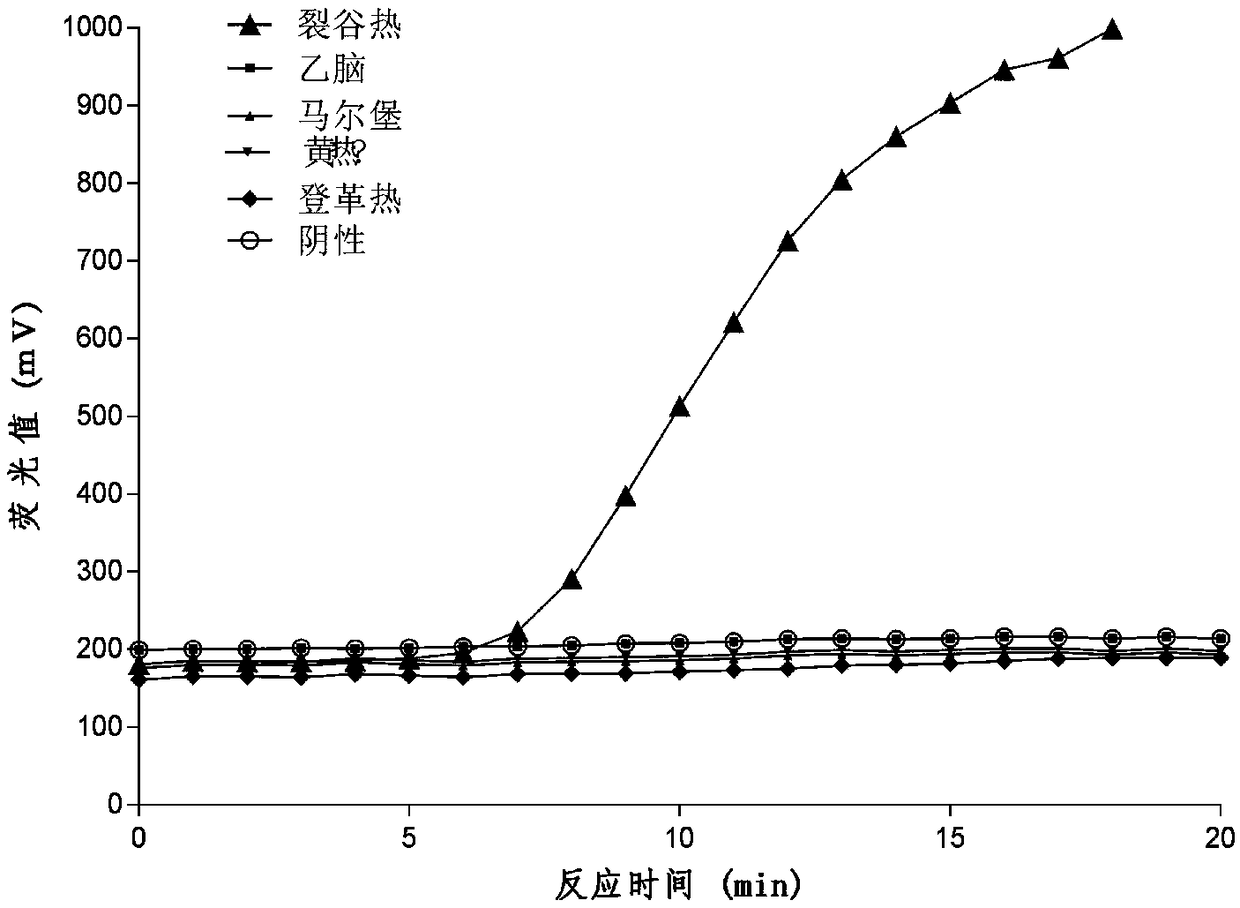
ActiveCN102140528AQuick QualificationQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceRift Valley fever virus
The invention discloses a new fluorescence quantitative polymerase chain reaction (PCR) detection method for rift valley fever virus and a rift valley fever virus detection PCR system. The system consists of primers and a probe, Premix Ex Taq reaction solution, and sterile Tris water, wherein the primers and the probe have good specificity and high sensitivity, and are suitable for no cross reaction of the rift valley fever and Dengue virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Colloidal gold immunochromatographic test strip for detecting anti-RVF virus antibodies in human and animal serum
The invention discloses a colloidal gold immunochromatographic test strip for detecting anti-RVF virus antibodies in human and animal serum, belonging to the technical field of biological detection. The test strip contains a colloidal gold-labeled specific RVF virus recombinant antigen glass fiber membrane, and a nitrocellulose membrane coated with a specific RVF virus recombinant antigen detection line and coated with a murine anti-RVF virus recombinant antigen monoclonal antibody quality control line. The colloidal gold immunochromatographic test strip disclosed by the invention not only has the advantages of easiness, convenience and quickness, high specificity, high sensitivity, accuracy and reliability, low cost and the like, but also can be used for reading results with naked eyes directly without special instruments, thus having very important medical value for the diagnosis, prevention and treatment of Rift Valley fever virus.
Owner:GUIZHOU PROVINCIAL PEOPLES HOSPITAL
Primers and probe for detecting fragment S of rift valley fever virus
InactiveCN102433392AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesForward primerSwine Fever Virus
The invention provides primers and a probe for detecting the fragment S of a rift valley fever virus, wherein, a forward primer is 5'-GGATTACTTTCCTGTGATATCTGTTG-3'; a reverse primer is 5'-GTATCCTGGGAGGRCCATCWC-3', R refers to A or G and w refers to T or A); and a probe is 5'-F1-ACTCCACTGACACAACACGACGACCACT-Q1-3', F1 is a fluorescent reporter and Q1 is a fluorescent quencher. The invention also provides a real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) detection method for the fragment S of the rift valley fever virus. In the method, pseudovirus grains of the rift valley fever virus are taken as a template, and the primers and the probe are used to carry out real-time fluorescent RT-PCR, thus judging whether the detected virus is the rift valley fever virus according to the detected result. The probe provided by the invention has high sensitivity and good specificity, and has no cross reaction with all of Africa swine fever virus, irides virus and goatpox virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
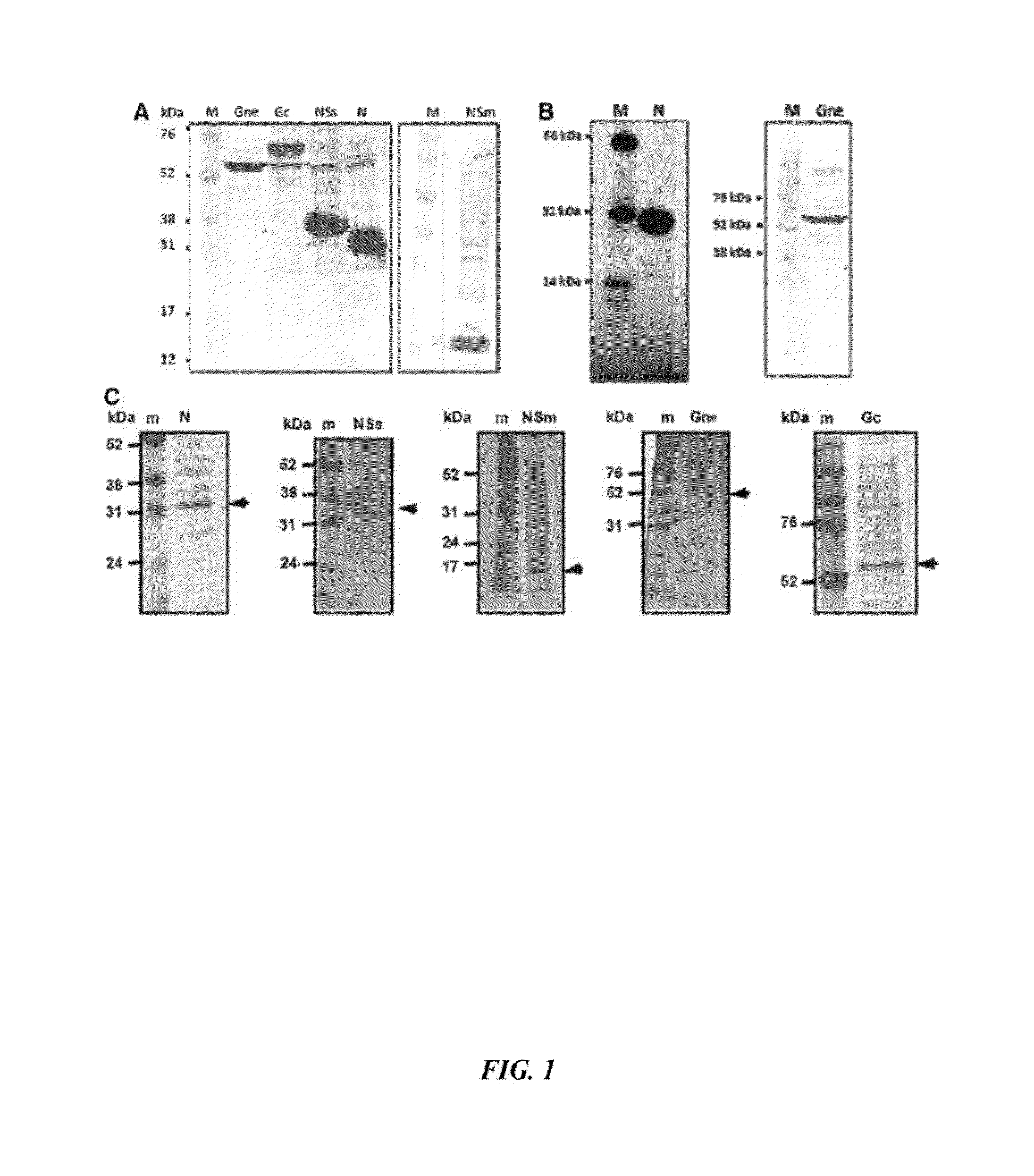
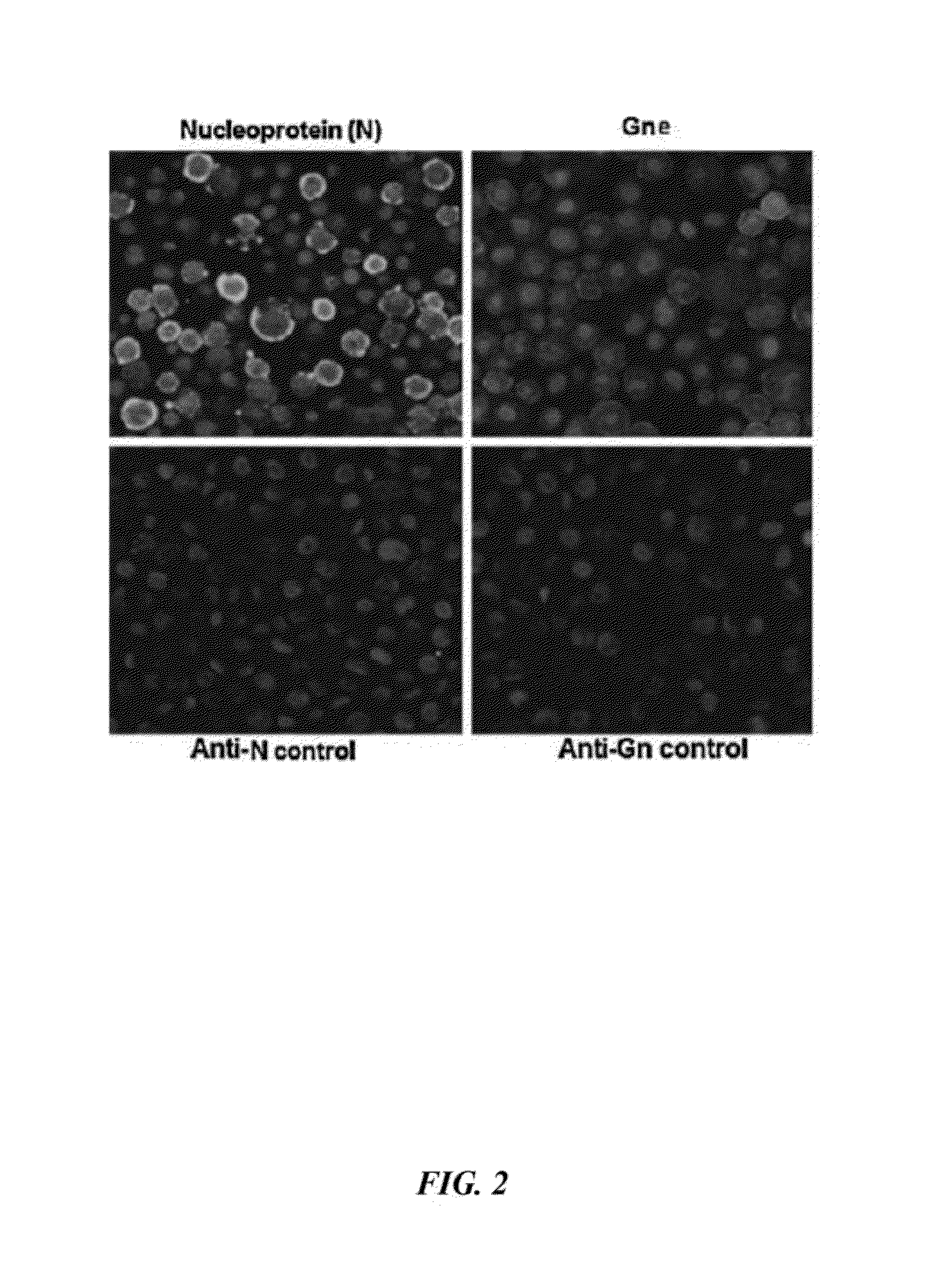
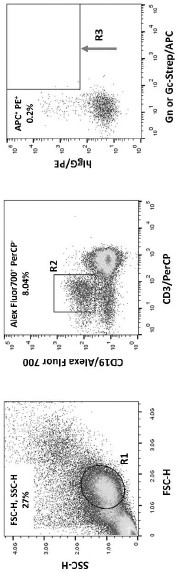
Rift Valley Fever Virus Glycoproteins, GN and GC, and their Use
The present invention describes subunit vaccines containing Gn and Gc glycoproteins of the Rift Valley Fever Virus, including nucleic acids encoding such glycoproteins, host cells, vectors, and immunoreagents generated with the glycoproteins, methods of vaccination, methods of diagnosis, and kits.
Owner:KANSAS STATE UNIV RES FOUND +1
Rift valley fever virus RT-LAMP (reverse transcription-loop-mediated isothermal amplification) detection method
InactiveCN103952494AEasy to operateImprove securityMicrobiological testing/measurementMicroorganism based processesWater bathsBetaine
The invention relates to a rift valley fever virus RT-LAMP (reverse transcription-loop-mediated isothermal amplification) detection method which comprises the following detection process: step a, establishing an amplification reaction system which comprises 5 mul of a RNA sample, 2.5 mul of a Bst (bacillus stearothermophilus) DNA polymerase buffer (10x), 1 mul of a Bst DNA polymerase (8U / mul), 1 mul of AMV reverse transcriptase (10U / mul), 1 muld of NTP (25mmol / mul), 5 mul of Betaine (5 mumol / mul), 1 mul of MgSO4 (100nmol / mul), 1 mul of F3 and B3 (5pmol / mul ), 1 mul of BIP and FIP (70pmol / mul), and the balance of H2O for supplementing to 25 mul; wherein the F3,B3,BIP and FIP are four primers corresponding to L gene; step b, evenly mixing the components, reacting for 1.5-2H in water bath at 65 DEG C; and step c, using 1.5% agarose gel for electrophoresis, and then observing the results. The new rift valley fever virus RT-LAMP technology has the advantages of being fast, sensitive, simple in operation, high in safety, low in price, in no need of special equipment, and the like, particularly applicable to popularization and application in basic inspection and quarantine institutions, and provides a quick and practical diagnostic method for early diagnosis of rift valley fever virus.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Rift valley fever virus humanized monoclonal antibodies and application thereof
ActiveCN109734800AHigh activityEffective treatmentImmunoglobulins against virusesAntiviralsAntigenBaculovirus expression
The invention discloses rift valley fever virus humanized monoclonal antibodies and application thereof and belongs to the technical field of medicine. According to the antibodies, RVFV glycoproteinsGn and Gc expressed by baculovirus are adopted as antigens, memory B cells which can be combined with the RVFV glycoproteins Gn and Gc are screened from PBMCs of a patient suffering from rift valley fever, and through experiments such as single B cell sequencing, in-vitro neutralization and in-vivo protection, eight Gn humanized monoclonal antibodies capable of efficiently neutralizing RVFV infection and one Gc humanized monoclonal antibody are identified. The humanized monoclonal antibodies have very high in-vitro neutralization activity (IC50 can be as low as 1.93 + / - 0.6 pM and reaches a pMlevel), can effectively treat mice infected with RVFV, prevents the mice from being infected with RVFV, and has an extremely high application value on clinical treatment and prevention of the infection of RVFV.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Rift Valley fever virus nucleic acid molecule characteristic standard sample and preparation method thereof
InactiveCN104388584AImprove uniformityImprove stabilityMicrobiological testing/measurementMicroorganism based processesFreeze-dryingQuality control
The invention discloses a preparation method of a Rift Valley fever virus nucleic acid molecule characteristic standard sample, which comprises the following steps: synthesizing the sequence, cloning the vector, connecting the target segment and vector, converting the plasmid, extracting the recombinant plasmid, freeze-drying, preserving and the like to obtain the Rift Valley fever virus nucleic acid molecule characteristic standard sample. The standard sample prepared by the invention comprises virus characteristic sequence information and is suitable for the virus and content level in the analytical sample. The standard sample disclosed by the invention has the advantages of favorable uniformity, high stability and high purity, and can be stored for a long time. The preparation method is simple in process, can provide a standard sample for Rift Valley fever virus detection research, medical research and the like to implement comparison among different laboratory results, thereby ensuring the quality control on the laboratory. The preparation method provides a standard sample for quick accurate detection on Rift Valley fever virus by quarantine inspection mechanisms, import and export trade and other enterprises.
Owner:薛芳
Antiviral rift valley fever virus peptides and methods of use
ActiveUS9556237B2Easy to produceUseful prophylacticSsRNA viruses negative-sensePeptide/protein ingredientsEbola virusAndes virus
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE ARMY ON BEHALF OF THE U S ARMY MEDICAL RES INST OF INFECTIOUS DISEASES
Dual-fluorescence quantitative RT-PCR primers and probes for identifying and detecting rift valley fever virus
InactiveCN110295254AEasy to operateHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSpecific detectionTrue positive rate
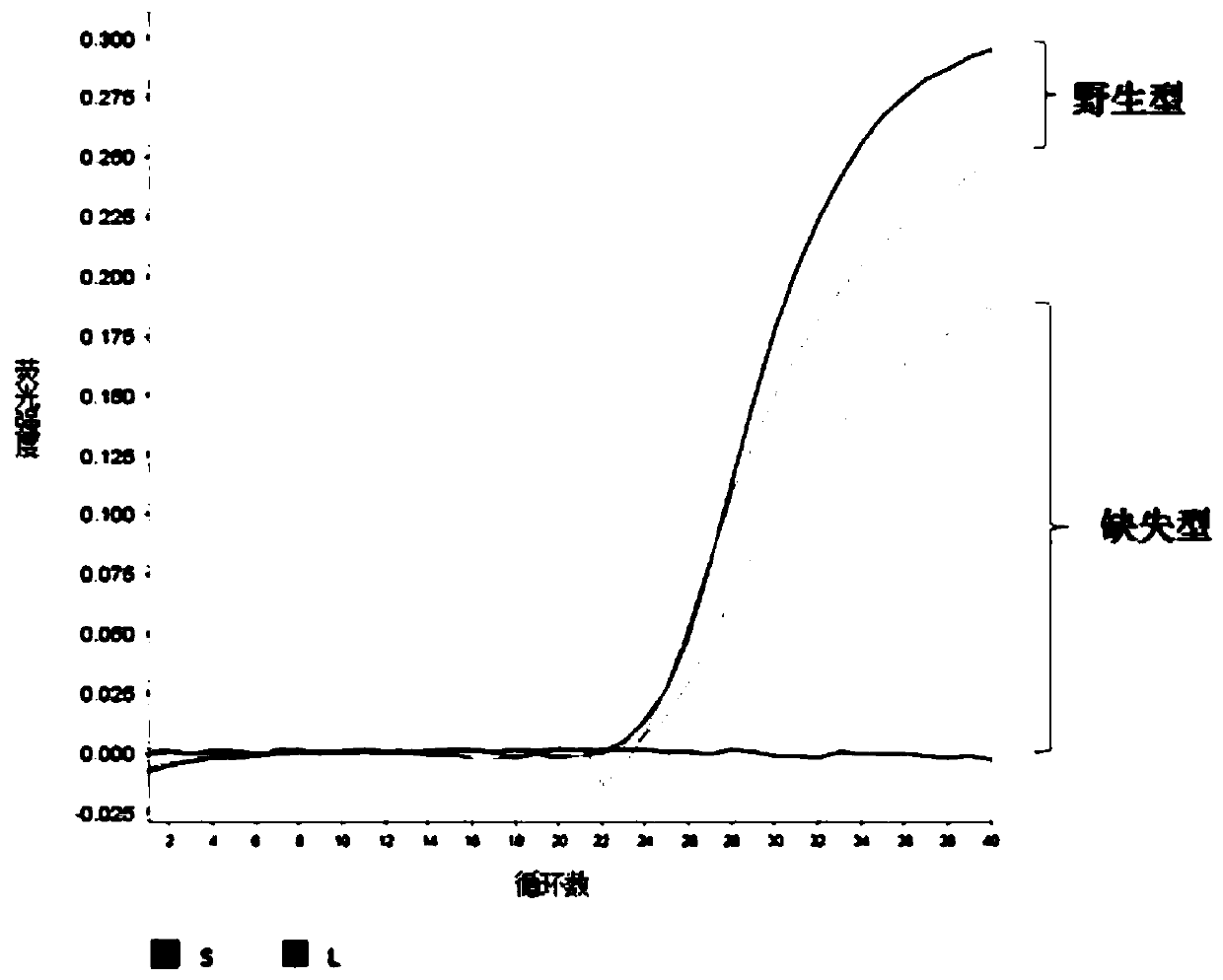
The invention provides dual-fluorescence quantitative RT-PCR primers and probes for identifying and detecting rift valley fever virus and belongs to the field of biotechnology. The pair of specific detection primers L-F and L-R and the fluorescent probe L-P for an L gene of rift valley fever virus and the pair of specific detection primers S-F and S-R and the fluorescent probe S-P for an S fragment of rift valley fever virus are designed, and the concentration and reaction conditions of the specific primers and fluorescent probes in a method are optimized. The provided dual-fluorescence quantitative RT-PCR method can be used for detecting the infection of rift valley fever virus and meanwhile identifying rift valley fever virus wild strains and NSs deletion strains including Clone 13 and other deletion strains, so that infected animals are distinguished from immunized animals. The primers and the probes have the advantages of simple operation, high sensitivity and specificity, good repeatability and the like and can not only reduce the cost but also gain valuable time for epidemic detection and control.
Owner:JIANGSU ACAD OF AGRI SCI
Primer and probe for detecting rift valley fever virus
InactiveCN105002302AEfficient detectionObvious amplification curveMicrobiological testing/measurementMicroorganism based processesForward primerPositive control
The invention aims at providing a primer and probe for detecting rift valley fever virus by applying an RPA technology. The sequence of a forward primer body is showed in SEQ ID NO:1, the sequence of a reverse primer body is showed in SEQ ID NO:2, and the sequence of a probe is showed in SEQ ID NO:3. According to the primer and probe for detecting the rift valley fever virus by applying the RPA technology, a great number of RPA primers and probes are designed according to a rift valley fever virus genomic sequence, and a primer and probe combination which can rapidly and effectively detect rift valley fever virus nucleic acid can be screened out from the great number of the RPA primers and probes. Rapid detection is conducted by utilizing the set of the primer and probe, an obvious amplification curve can be obtained by taking rift valley fever virus nucleic acid RNA in vitro transcription as a positive control, and other pathogenic nucleic acids such as foot and mouth disease virus, contagious pustular dermatitis virus, mycoplasma capricolum and bluetongue virus which serve as a template to conduct the reaction do not have the amplification curve.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Gene chip for detecting 6 viruses of zoonosis-borne diseases and application thereof
InactiveCN102031311AStable and specific universal detectionStable and specific realization of universal detectionNucleotide librariesMicrobiological testing/measurementHepacivirusNipah virus
The invention discloses a gene chip for viruses of zoonosis-borne diseases and application thereof. The gene chip for the viruses of the zoonosis-borne diseases is a gene chip for detecting at least one virus in six viruses of hepatitis E virus, Nipah virus, rabies virus, Rift Valley fever virus, foot and mouth disease virus and Vesicular stomatitis virus, and is fixed with a hepatitis E virus detection probe, a Nipah virus detection probe, a rabies virus detection probe, a Rift Valley fever virus detection probe, a foot and mouth disease virus detection probe and a Vesicular stomatitis virus detection probe. The invention also discloses a method for detecting the Rift Valley fever virus, the Nipah virus, the foot and mouth disease virus, the Vesicular stomatitis virus, the hepatitis E virus and / or the rabies virus. The invention can stably and specifically implement generic detection of the six viruses of the zoonosis-borne diseases, has the advantages of simpleness, convenience, quickness, good specificity and high sensitivity, and can be used for detecting clinical samples.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Triple detection method of arbovirus liquid chips
InactiveCN104120191AEasy to operateLow costMicrobiological testing/measurementMicroorganism based processesMultiplexMicrosphere
The invention discloses a triple detection method of arbovirus liquid chips. The method comprises the following steps: respectively designing specific primers and probes aiming at three types of arboviruses comprising Chikungunya viruses, Crimean-Congo hemorrhagic fever and Rift Valley fever viruses, determining the specificity of the primers and the probes via a single PCR and clone sequencing, and then carrying out multiplex-PCR amplification under the condition that the concentration ratio of the forward and reverse primers in an amplification reaction system is 1:1-1:8; then coupling the probes with fluorescent coding microspheres so as to prepare a microsphere mixing liquid and carrying out coupling quality control by coupling the probes; finally, carrying out a hybridization reaction on multiplex-PCR products and the microsphere mixing liquid, simultaneously adding streptomycin-phycoerythrin diluted by a nucleic acid detection buffer solution, and analyzing reaction products by utilizing a liquid chip detecting instrument. The method is good in detection specificity and can be used for simultaneously detecting the three types of arboviruses, and has high detection sensitivity due to the adoption of an asymmetric PCR amplification method in the process of the multiplex-PCR amplification.
Owner:李云峰
Composition for preparation of low-toxicity rift valley fever virus, preparation method of composition and RVFV attenuated vaccine
InactiveCN110484517AGuaranteed synthesisImprove toleranceSsRNA viruses negative-senseViral antigen ingredientsAttenuated vaccineRift Valley fever virus
The invention discloses a composition for preparation of a low-toxicity rift valley fever virus, a preparation method of the composition and an RVFV attenuated vaccine, and relates to the field of biotechnology. According to a reverse genetic system, introduction of excess basic groups is avoided, the primitiveness of a viral genome is kept to the greatest degree, and the reverse genetic system can stably express a low-toxicity rift valley fever virus strain in the later period, the defect of toxicity recovery is avoided, and the limitation is broken that by means of a traditional method, an attenuated strain can be obtained through continuous passage many times.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Rift valley fever virus humanized monoclonal antibody and application thereof
ActiveCN111978400AHigh activityEffective treatmentImmunoglobulins against virusesAntiviralsAntigenBaculovirus expression
The invention discloses a rift valley fever virus humanized monoclonal antibody and an application thereof, and belongs to the technical field of medicines. According to the invention, RVFV glycoprotein Gn and Gc expressed by rod-shaped viruses are used as antigens; memory B cells capable of being combined with the RVFV glycoprotein Gn and Gc are screened from PBMCs of a rift valley fever rehabilitation patient; and through experiments of single B cell sequencing, in-vitro neutralization, in-vivo protection and the like, eight strains of Gn humanized monoclonal antibodies capable of efficiently neutralizing RVFV infection and a strain of a Gc humanized monoclonal antibody are identified. The humanized monoclonal antibody disclosed by the invention has extremely high in-vitro neutralizationactivity (IC50 can be as low as 1.93 + / - 0.6 pM, reaching a pM level), can effectively treat mice infected by RVFV and prevent infection of RVFV on the mice, and has extremely high application valuein clinical treatment and prevention of RVFV infection.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Primers and probe for detecting fragment S of rift valley fever virus
InactiveCN102433392BStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesForward primerAfrican swine fever
The invention provides primers and a probe for detecting the fragment S of a rift valley fever virus, wherein, a forward primer is 5'-GGATTACTTTCCTGTGATATCTGTTG-3'; a reverse primer is 5'-GTATCCTGGGAGGRCCATCWC-3', R refers to A or G and w refers to T or A); and a probe is 5'-F1-ACTCCACTGACACAACACGACGACCACT-Q1-3', F1 is a fluorescent reporter and Q1 is a fluorescent quencher. The invention also provides a real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) detection method for the fragment S of the rift valley fever virus. In the method, pseudovirus grains of the rift valley fever virus are taken as a template, and the primers and the probe are used to carry out real-time fluorescent RT-PCR, thus judging whether the detected virus is the rift valley fever virus according to the detected result. The probe provided by the invention has high sensitivity and good specificity, and has no cross reaction with all of Africa swine fever virus, irides virus and goatpox virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
New fluorescence quantitative polymerase chain reaction (PCR) detection method for rift valley fever virus and rift valley fever virus detection PCR system
ActiveCN102140528BQuick QualificationQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceViral testRift Valley fever virus
The invention discloses a new fluorescence quantitative polymerase chain reaction (PCR) detection method for rift valley fever virus and a rift valley fever virus detection PCR system. The system consists of primers and a probe, Premix Ex Taq reaction solution, and sterile Tris water, wherein the primers and the probe have good specificity and high sensitivity, and are suitable for no cross reaction of the rift valley fever and Dengue virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Hybridoma cell strain capable of secreting anti-rift valley fever virus NSs protein monoclonal antibody and application thereof
ActiveCN111041000AHas a blocking effectImprove featuresBiological material analysisMicroorganism based processesElisa kitProtein.monoclonal
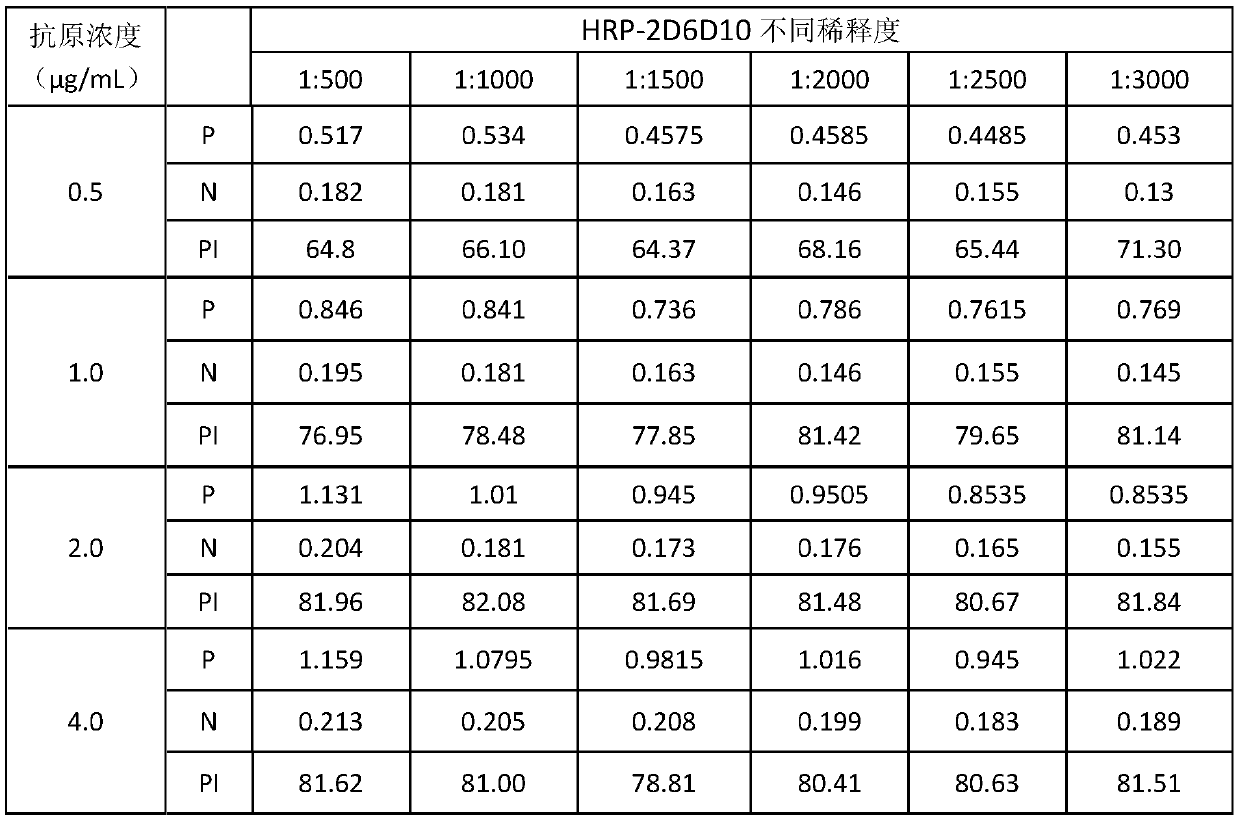
The invention provides a hybridoma cell strain capable of secreting an anti-rift valley fever virus NSs protein monoclonal antibody and an application thereof, and belongs to the technical field of biology. The preservation number of the hybridoma cell strain 2D6D10 capable of secreting an anti-rift valley fever virus NSs protein monoclonal antibody is CCTCC NO: C2019269. The invention further provides an anti-rift valley fever virus NSs protein monoclonal antibody secreted by the hybridoma cell strain and an application of the anti-rift valley fever virus NSs protein monoclonal antibody in preparation of a rift valley fever virus NSs protein antibody detection kit. The hybridoma cell strain 2D6D10 disclosed by the invention can generate a monoclonal antibody aiming at RVFV non-structuralprotein NSs protein, and the monoclonal antibody has a blocking effect, so that the blocking ELISA kit disclosed by the invention can be used for differential diagnosis of virus infection and inactivated vaccines or deletion vaccine immune antibodies.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Gene chip for detecting 6 viruses of zoonosis-borne diseases and application thereof
InactiveCN102031311BStable and specific universal detectionStable and specific realization of universal detectionNucleotide librariesMicrobiological testing/measurementFoot mouth disease virusRabies virus
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Rift Valley Fever Virus glycoproteins, Gn and Gc, and their use
The present invention describes subunit vaccines containing Gn and Gc glycoproteins of the Rift Valley Fever Virus, including nucleic acids encoding such glycoproteins, host cells, vectors, and immunoreagents generated with the glycoproteins, methods of vaccination, methods of diagnosis, and kits.
Owner:KANSAS STATE UNIV RES FOUND +1
A kind of monoclonal antibody a38 against Rift Valley fever virus and its application
ActiveCN114605528BHigh binding activityGood neutralizing effectImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesPharmaceutical drug
The invention discloses a monoclonal antibody against Rift Valley fever virus. The antibody is obtained by screening with flow sorting-single cell PCR technology and has a unique CDR partition. The invention also discloses that the antibody is used in the preparation of Rift Valley fever. Application of heat therapy drugs. The monoclonal antibody disclosed in the invention has high-efficiency and specific anti-RVF activity, and also has the characteristics of high expression, high degree of humanization and good stability, and is suitable for industrial production.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com