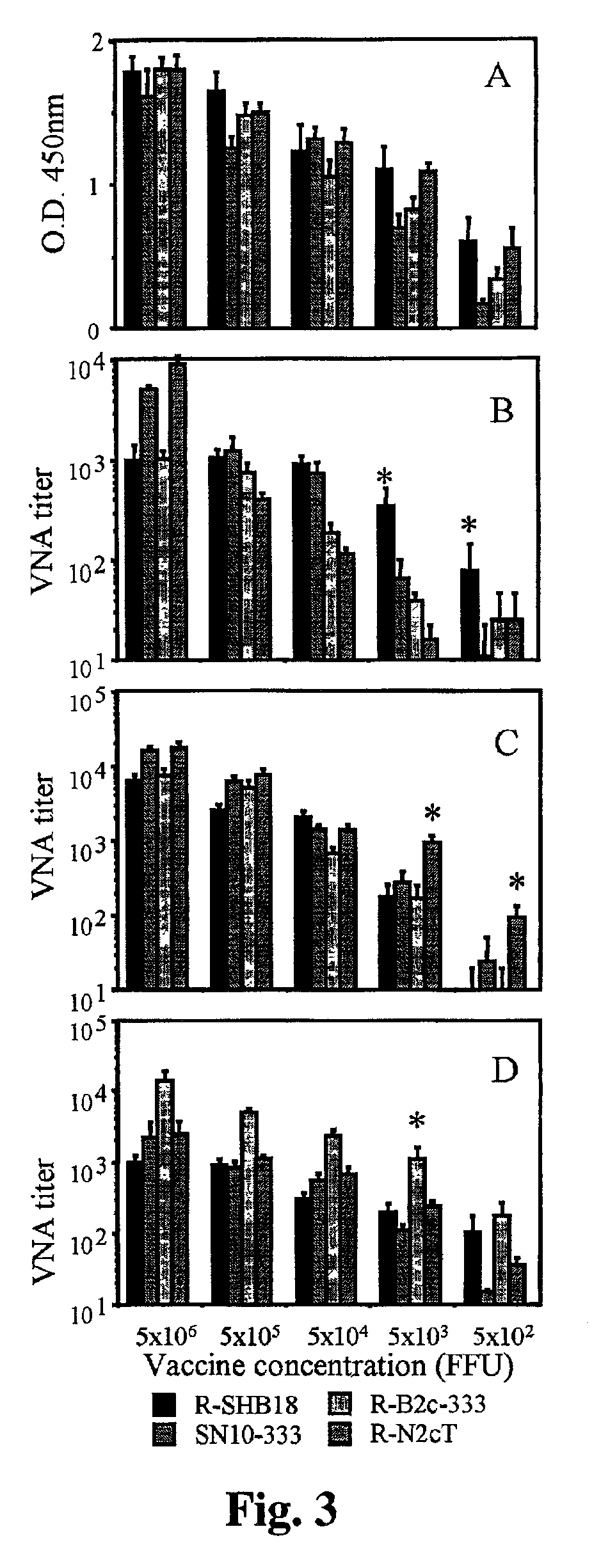
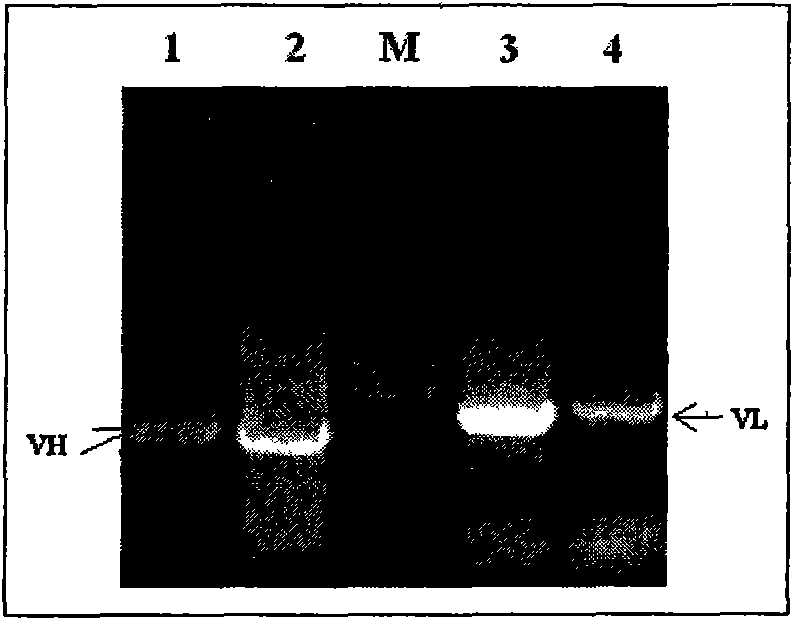
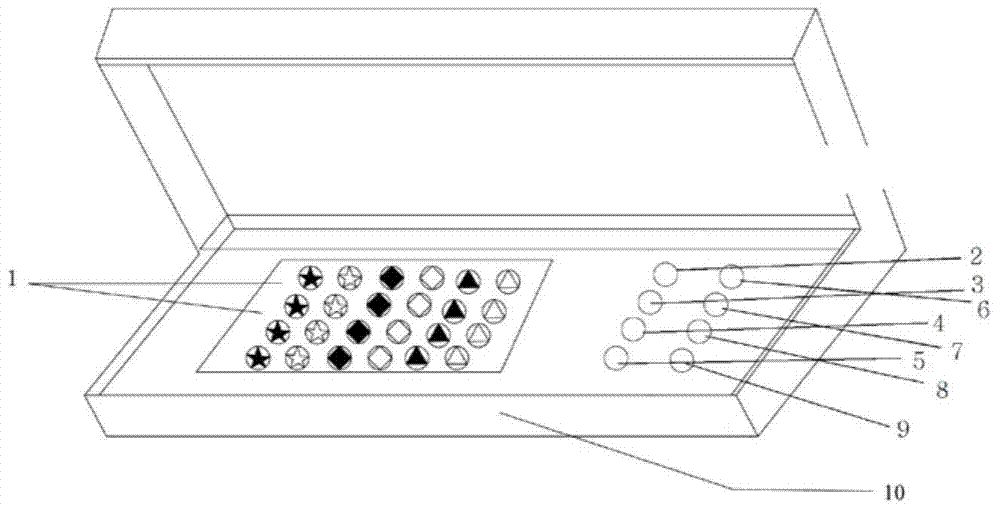
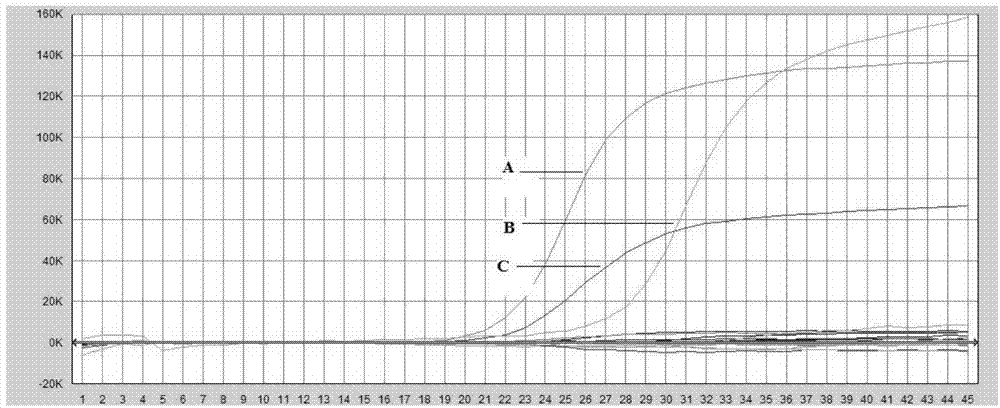
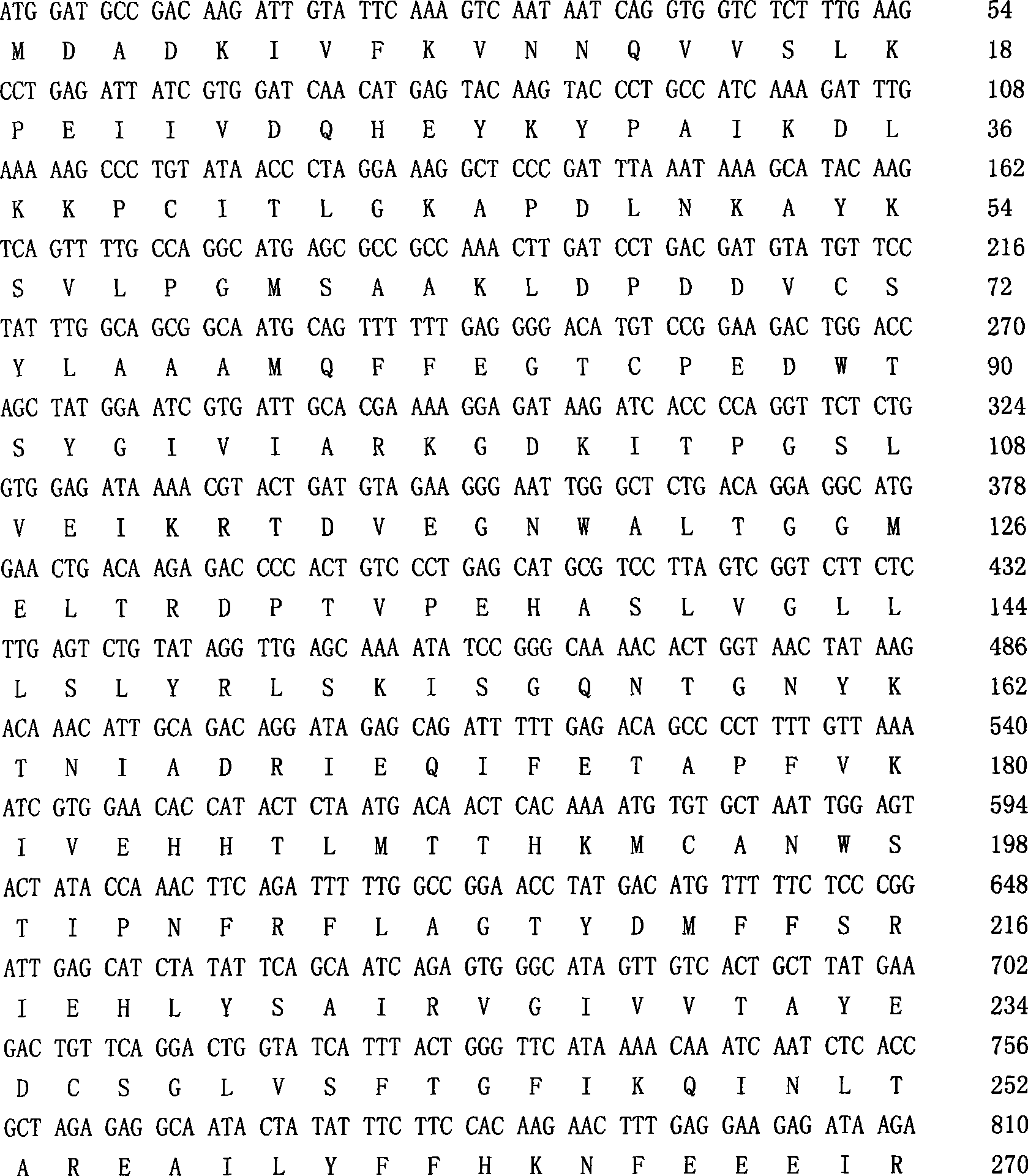
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
339 results about "Rabies virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rabies lyssavirus, formerly Rabies virus, is a neurotropic virus that causes rabies in humans and animals. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva. Rabies lyssavirus, like many rhabdoviruses, has an extremely wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles and insects.
Fab library for the preparation of anti VEGF and anti rabies virus fabs
InactiveUS20060160184A1Maintain good propertiesOptimization mechanismAnimal cellsAntibody mimetics/scaffoldsProtein moleculesImmunoglobulin IgE
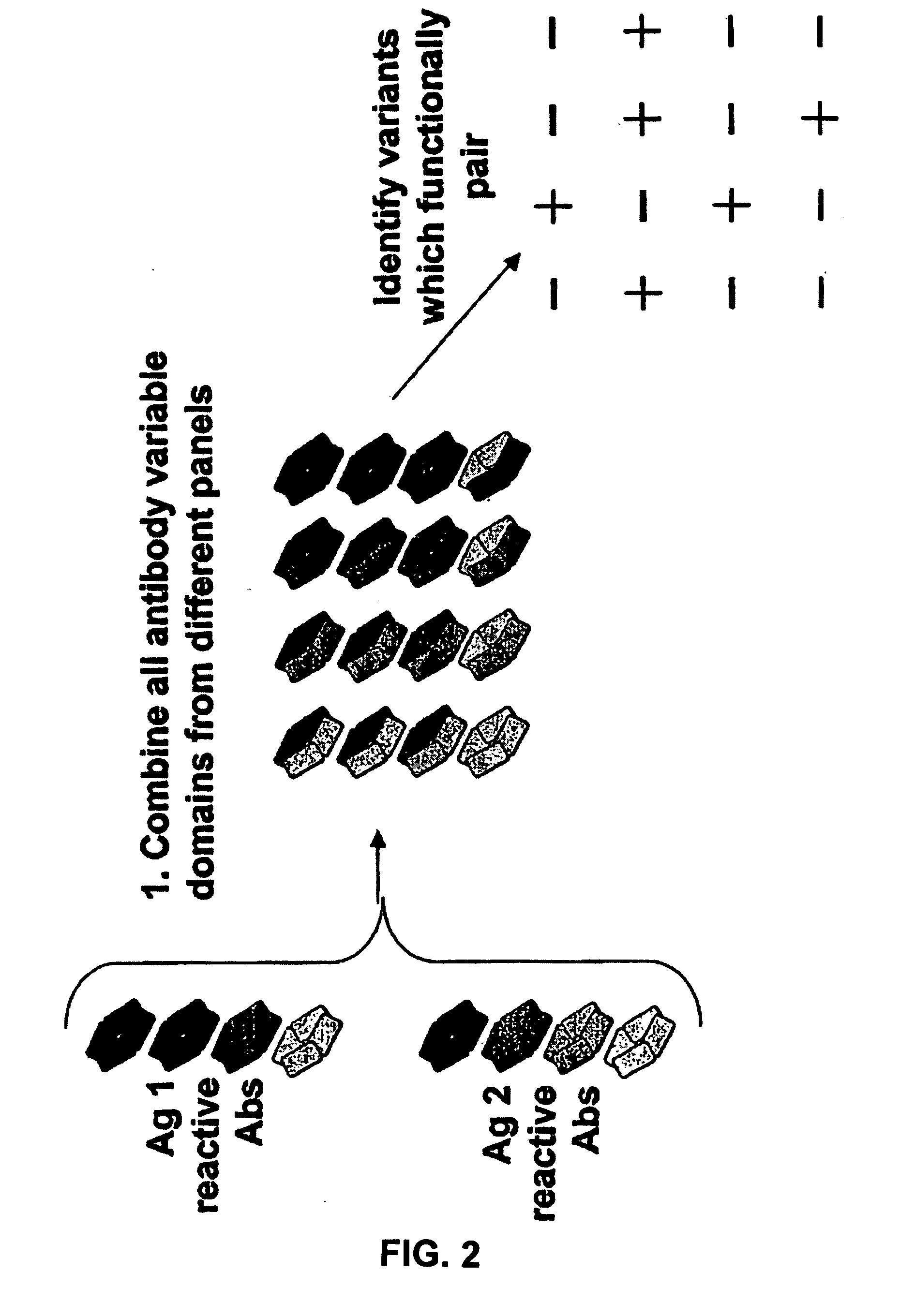
The present invention provides combinations of specific binding proteins, such as immunoglobulins, that are designed to be true combinations, essentially all components of the combination being functional and compatible with each other. The invention further provides a method for producing a composition comprising at least two different proteinaceous molecules comprising paired variable regions, the at least two proteinaceous molecules having different binding specificities, comprising paired variable regions, at least two proteinaceous molecules having different binding specificities, comprising contacting at least three different variable regions under conditions allowing for pairing of variable regions and harvesting essentially all proteinaceous molecules having binding specificities resulting from the pairing.
Owner:MERUS NV
Polynucleotide delivering complex for target cell
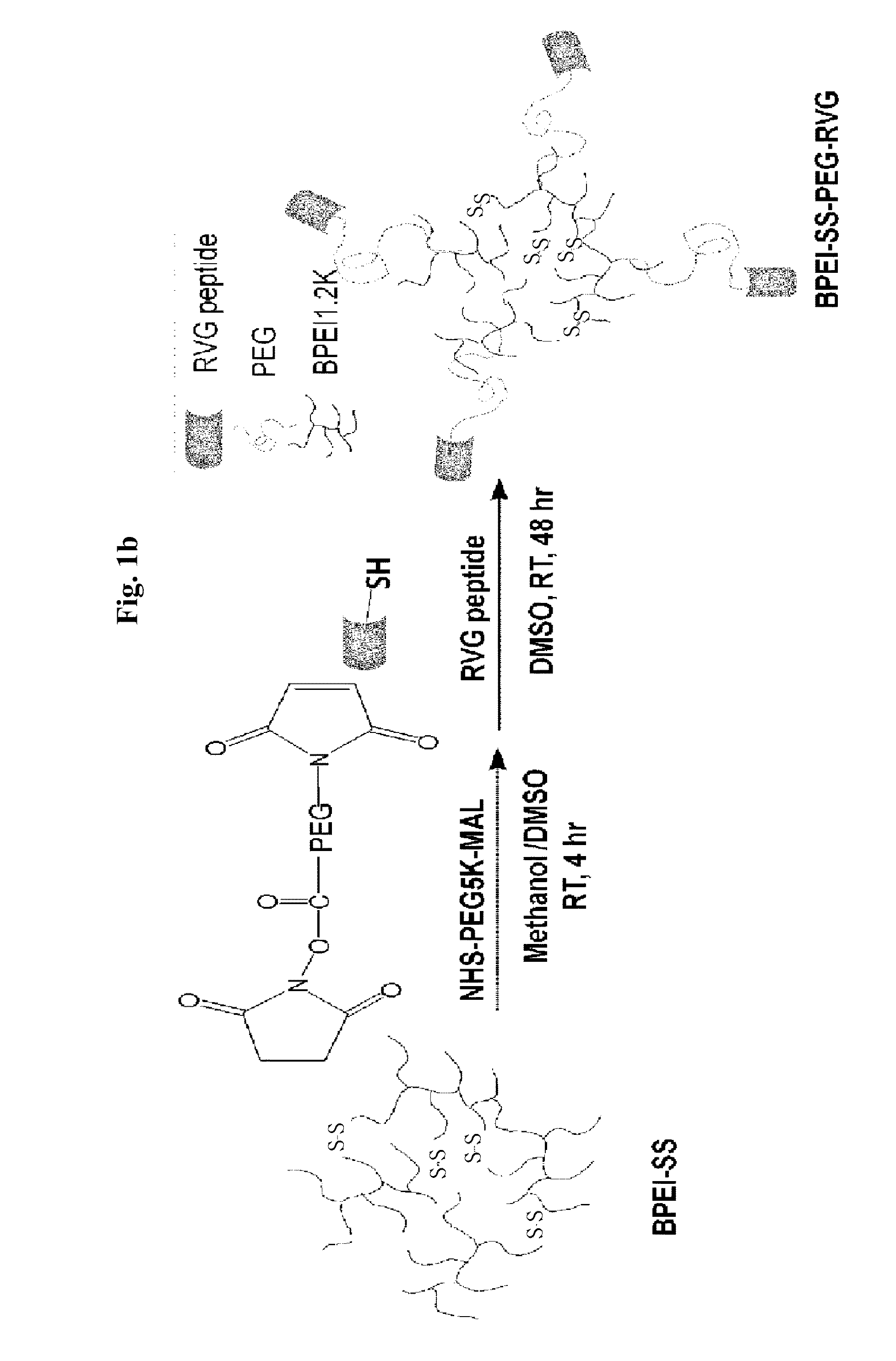
InactiveUS8759103B2Good biocompatibilityStrong physiological functionSsRNA viruses negative-sensePeptide/protein ingredientsNucleotidePolynucleotide
The present invention provides a polynucleotide delivery system including a cationic polymer to which a rabies virus glycoprotein (RVG) peptide is bound, wherein the cationic polymer includes a biodegradable bond, and a method of delivering polynucleotides to a target cell by using the delivery system.
Owner:POSTECH ACAD IND FOUND
Binding molecules capable of neutralizing rabies virus and uses thereof
InactiveUS20080070799A1SsRNA viruses negative-senseMicrobiological testing/measurementBiologyRabies virus
Provided are binding molecules that specifically bind rabies virus and are able to neutralize the virus. Further provided are nucleic acid molecules encoding the binding molecules, compositions comprising the binding molecules and methods of identifying or producing the binding molecules. The binding molecules can be used in the diagnosis, prophylaxis and / or treatment of a condition resulting from rabies virus. In certain embodiments, they can be used in the post-exposure prophylaxis of rabies.
Owner:JANSSEN VACCINES & PREVENTION BV
Anti-rabies virus monoclonal antibody and preparation method and application
InactiveCN101560255AExperimental costs are highEasy to operateImmunoglobulins against virusesTissue cultureImmunoblot AnalysisHybridoma cell
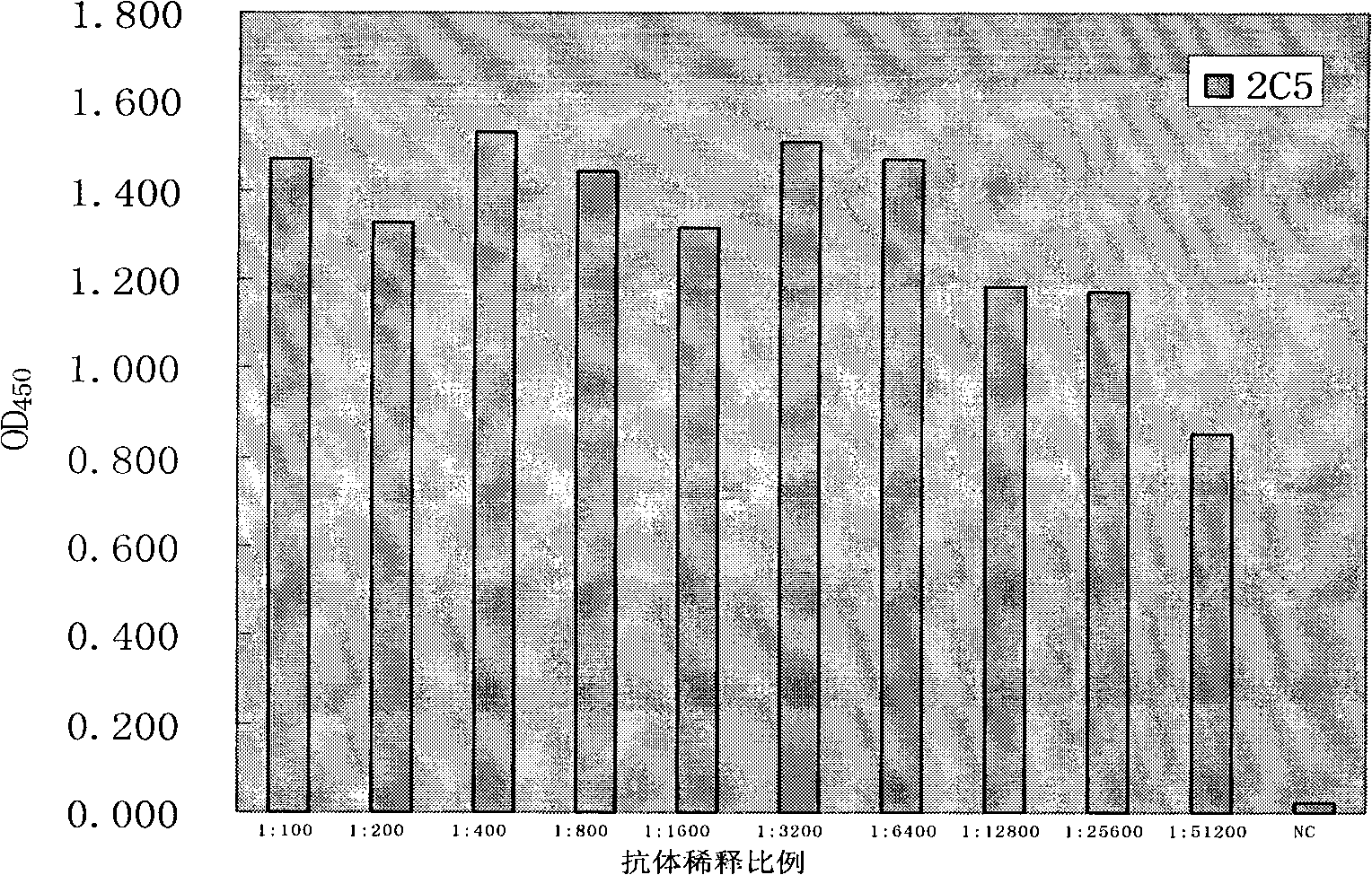
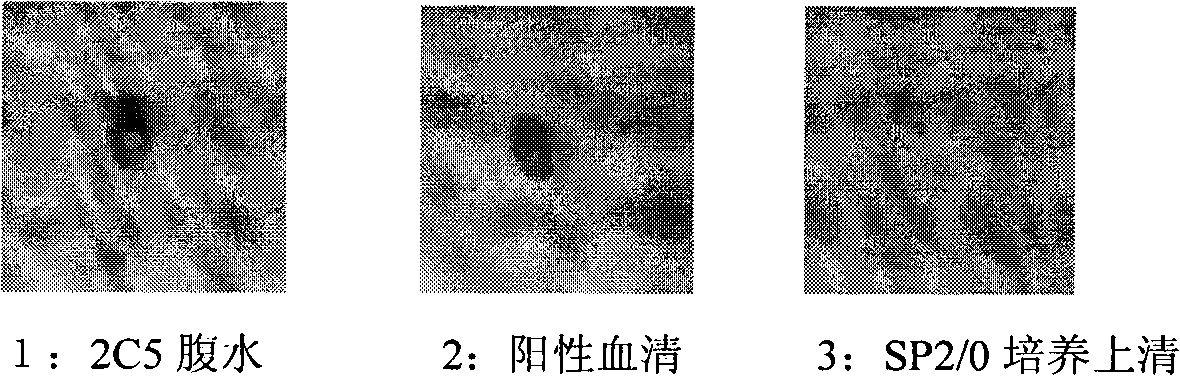
The invention discloses an anti-rabies virus monoclonal antibody and a preparation method and an application, belonging to the field of biomedicine and particularly relating to the preparation of a monoclonal antibody capable of identifying rabies virus and the application. The monoclonal antibody of the invention is screened by indirect Enzyme-linked immunosorbent assay (ELISA), and specificity and affinity thereof combined with antigen are identified by methods such as polyacrylamide gel electrophoresis analysis, speckle ELISA, immunoblot analysis and the like. The anti-rabies virus monoclonal antibody of the invention can be applied in multiple testing methods of antigen of rabies virus and can be also applied in the preparation of rabies virus detecting kit. The anti-rabies virus monoclonal antibody is secreted by anti-rabies virus monoclonal antibody hybridoma cell strain 2C5 with the preservation number being CGMCC No.3014.
Owner:NANJING MEDICAL UNIV +1
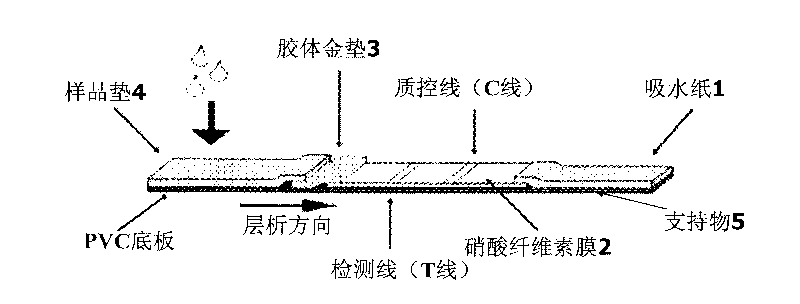
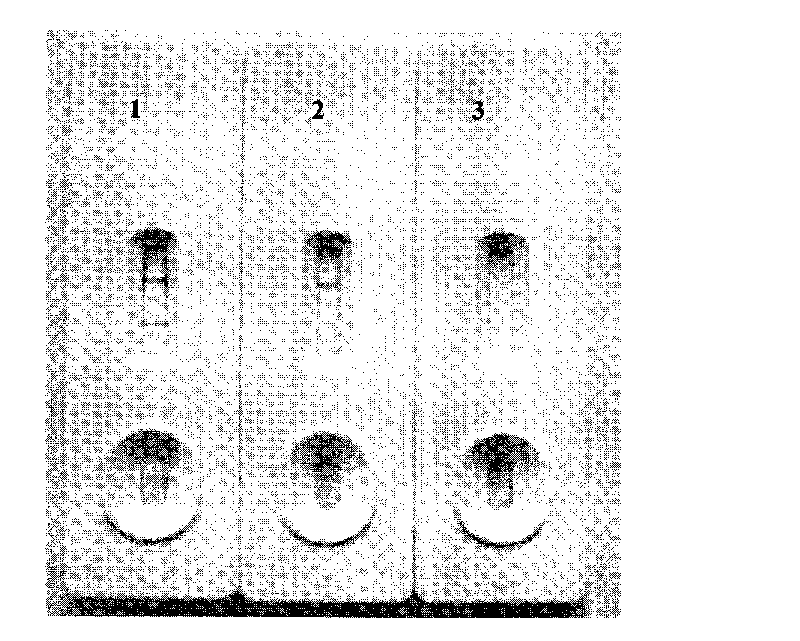
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Binding molecules capable of neutralizing rabies virus and uses thereof
The invention provides binding molecules that specifically bind to rabies virus and are capable of neutralizing the virus. The invention further provides nucleic acid molecules encoding the binding molecules, compositions comprising the binding molecules and methods of identifying or producing the binding molecules. The binding molecules can be used in the diagnosis, prophylaxis and / or treatment of a condition resulting from rabies virus. Preferably, they can be used in the post-exposure prophylaxis of rabies.
Owner:JANSSEN VACCINES & PREVENTION BV
Preparing method for recombinant human anti-rabies monoclonal antibodies
Owner:NCPC NEW DRUG RES & DEV
Recombinant Poxviral Vectors Expressing both Rabies and OX40 Proteins, and Vaccines Made Therefrom
The present invention provides vectors that contain and co-express in vivo or in vitro immunogenic polypeptides or antigens together with an OX40L polypeptide, which functions as a genetic adjuvant. Together, the immunogenic polypeptide and the OX40L polypeptide elicit an immune response in animal or human, which is greater than the immune response elicited by the immunogenic polypeptide alone. In a particular example, the invention provides vectors encoding a Rabies G immunogenic polypeptide and a canine OX40L genetic adjuvant, which vectors elicit strong immune responses in canine against rabies virus
Owner:MERIAL INC
Binding molecules capable of neutralizing rabies virus and uses thereof
InactiveUS20080226652A1SsRNA viruses negative-senseVirus peptidesPost-exposure prophylaxisRabies virus
Provided are binding molecules that specifically bind to rabies virus and are capable of neutralizing the virus. Further provided are nucleic acid molecules encoding the binding molecules, compositions comprising the binding molecules and methods of identifying or producing the binding molecules. The binding molecules can be used in the diagnosis, prophylaxis and / or treatment of a condition resulting from rabies virus. In certain embodiments, they can be used in the post-exposure prophylaxis of rabies.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for extracting rabies virus
InactiveCN101270350AImprove removal efficiencyGood removal effectSsRNA viruses negative-senseMicroorganism based processesHollow fibreFiber
The invention provides a method for extracting rabies virus, and is to solve the defects that great discrepancy of quality indices of different batches occurs; removal of remaining DNA becomes difficult; the protein content of the remaining host is too high; and great side effects appear clinically when the single method of molecular sieve gel chromatography is adopted for extracting rabies virus vaccine. The essential of the invention is that a hollow fiber ultrafiltration column or ultrafiltration membrane with the molecular weight cut-off of 750KD or 500KD is used to condense and partially purify harvested liquid of virus; anion exchange chromatography or molecular sieve gel chromatography is adopted to separate and purify samples; molecular sieve gel chromatography or anion exchange chromatography is adopted to separate and purify samples got in step (2). The method for extracting rabies virus has the characteristics of great productive capacity, high product quality, excellent batch stability, being remarkably effective in removal of remaining DNA and HCP, and reducing the potential safety hazard of vaccine.
Owner:LIAONING YISHENG BIOLOGY PHARMACY
Multiplex real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2
InactiveCN102071259AEasy to identifyEasy diagnosisMicrobiological testing/measurementMicroorganism based processesAgricultural scienceFluorescence
The invention discloses a multiplex SYBR Green I real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2. The primer is obtained through synthesis according to design. The multiplex SYBR Green I real-time fluorescence PCR detection method for detecting porcine rabies virus, porcine parvovirus and porcine circovirus type 2 by utilizing the primer comprises the following steps: extracting the DNA of a sample, and then, detecting the sample by utilizing a SYBR Green I real-time fluorescence PCR reaction system and a SYBR Green I real-time fluorescence PCR amplification program. The invention has the beneficial effects that three types of viruses, namely the porcine rabies virus, the porcine parvovirus and the porcine circovirus type 2, can be simultaneously and effectively diagnosed and detected; non-specific swine fever virus, porcine reproductive and respiratory syndrome virus and swine influenza virus can not be detected; and the invention is beneficial to identification and diagnosis of the breeding disorder virus of a pregnant swine, and has better sensitivity, repeatability and stability.
Owner:HENAN AGRICULTURAL UNIVERSITY
Genetically engineered rabies recombinant vaccine for immunization of stray dogs and wildlife
InactiveUS7074413B2Reduce absorptionEnhance immune responseSsRNA viruses negative-senseVectorsWildlifeRecombinant vaccines
Live, attenuated recombinant rabies virus vaccines are generated using reverse genetics to combine the antigenic determinants that render the rabies virus non-pathogenic with the determinants that are responsible for the elicitation of an effective anti-rabies immune response. These vaccines do not affect the antigenic, and therefore the immunogenic, properties of the virus. The present invention further relates to recombinant rabies virus vaccines that express a pro-apoptotic protein, such as cytochrome c, to increase the capacity to induce apoptosis, thereby enhancing the protective immunity against rabies. This new generation of live rabies virus vaccines represents a safe and effective approach to the eradication of rabies in wildlife, and subsequently humans and livestock.
Owner:UNITED STATES OF AMERICA +1
Anthropogenic antivirulin glycosidoprotein neutralizing genetic engineering antibody RD9 and preparation and application thereof
InactiveCN101550189AOvercoming the disadvantages of instabilityHigh affinityMicrobiological testing/measurementGenetic material ingredientsSingle strandGenetic engineering
The invention relates to an anthropogenic antivirulin glycosidoprotein neutralizing genetic engineering antibody RD9 and a preparation and an application thereof. The antibody is VH-connecting peptide-VK of a three-structural single-stranded antibody comprising a variable region of heavy chain and a variable region of light chain by using 12 sequences of amino acid of the 1 (CH1) 5' end of a constant region of a heavy chain as the connecting peptide AKTTAPSVYPL, and the antibody realizes efficient expression in a prokaryotic system. The biological characteristic studies show that the RD9 is an anthropogenic genetic engineering antibody which has high appetency and better stability and can specially centralize rabies virus as well as genes and gene products of the antibody can be used for preparing clinical drugs for preventing and treating the rabies; the preparation method of the antibody is easy for massive industrial production. The invention solves the problem in the other technology of the application of genes of the variable region of the heavy chain and the variable region of a light chain of the antibody and polypeptide coded by the genes in the drugs for preventing and treating the rabies.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Kit for fluorescence quantitative PCR (polymerase chain reaction) detection of main porcine viruses
ActiveCN104263853AImprove the level of detection technologyHigh sensitivityMicrobiological testing/measurementClassical swine feverPseudorabies
The invention relates to a kit which utilizes a probe fluorescence quantitative PCR technology, is divided into two systems and can fast obtain and detect a variety of viruses causing porcine reproductive disorders and diarrheal diseases from clinical samples, wherein the A system is used for detecting porcine circovirus type 2 (PCV2), porcine parvovirus (PPV) and pseudorabies virus (PRV); the B system is used for detecting porcine epidemic diarrhea virus (PEDV), porcine reproductive and respiratory syndrome virus (PRRSV) and classical swine fever virus (CSFV). The detection kit is reasonable in design, simple in a using method, fast, accurate and high in sensitivity, is suitable for port inspection and quarantine management, livestock farming, animal protection and other departments, and simultaneously has extensive scientific research values and commercial prospects.
Owner:广州中科基因检测服务有限公司
Fluorescent quantitative DCR kit for detecting swine pseudo rabies virus and its use
ActiveCN1710101AAccurate determination of starting copy numberMeet the requirements for rapid differential diagnosis of pseudorabies virusMicrobiological testing/measurementFluorescenceNucleotide
This invention discloses a kind of measuring PRVHS fluorescence quantitative PCR reagent box and application. The reagent box has a) DNA cracking liquid, b)Taq DNA polymerization enzyme, c) pG-HB-contained nucleoside series of standard positive template, d)the fluorescence quantitative reaction liquid containing positive and negative primer and fluorescence probe series. Advantages: simple and easy for operation, fast and accurate quantitation and so on.
Owner:常州同泰生物药业科技股份有限公司
Method and application for fast retrograde transsynaptic labeling of nerve cells
The invention belongs to the construction field of auxiliary viruses, and discloses a method and an application of fast retrograde transsynaptic labeling of nerve cells. The invention uses double-stranded adeno-associated virus (SCAAV) as a helper virus vector to load TVA receptor and rabies virus outer membrane glycoprotein (RVG) respectively, and packaged into virus for specific recognition andretrograde transsynaptic marker of ENVA outer membrane wrapped defective recombinant rabies virus. As shown by that live test result, the recombinant defective rabies virus RV-[delta]G-X-ENVA and helper virus SCAAV combined system, can realize fast retrograde transsynaptic labeling, which saves 1-2 week experiment time, saves material resources and manpower, provides a better research tool for theapplication of defective rabies virus in neural network reverse transsynaptic marker, and lays a good technical support for the structure and function analysis of neural network.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Process for preparing veterinary rabies inactivated and freeze-dried vaccine through suspension culture cell
ActiveCN102228686AQuality improvementSmall difference between batchesInactivation/attenuationAntiviralsAntigenHamster
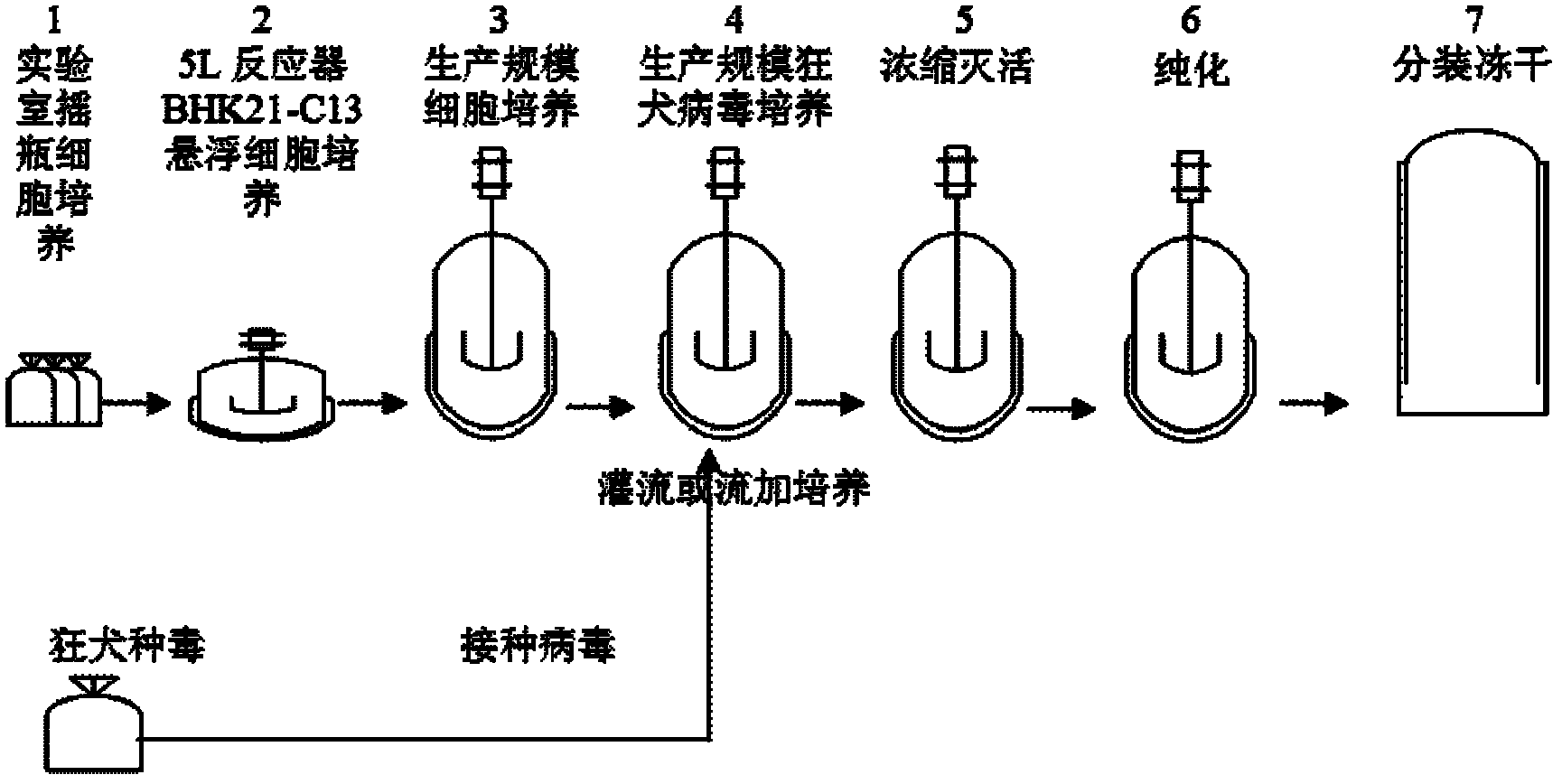
The invention relates to a process for preparing a veterinary rabies inactivated vaccine, in particular to a process for preparing a veterinary rabies inactivated and freeze-dried vaccine through a suspension culture cell. The method has the key point that: a bioreactor is used for large-scale suspension culture of baby hamster kidney BHK21-C13 cells and rabies virus is inoculated, and the rabiesvirus is subjected to mass propagation through a fed-batch and perfusion technology, so that a high-concentration and high-titer rabies antigen is obtained and concentrated, inactivated and purified to prepare the veterinary rabies inactivated vaccine; and thus, technical problems such as complexity, low antigen content, low effectiveness, large dosage, poor batch-to-batch variation and the like existing in the conventional process are solved.
Owner:JINYUBAOLING BIO PHARMA CO LTD
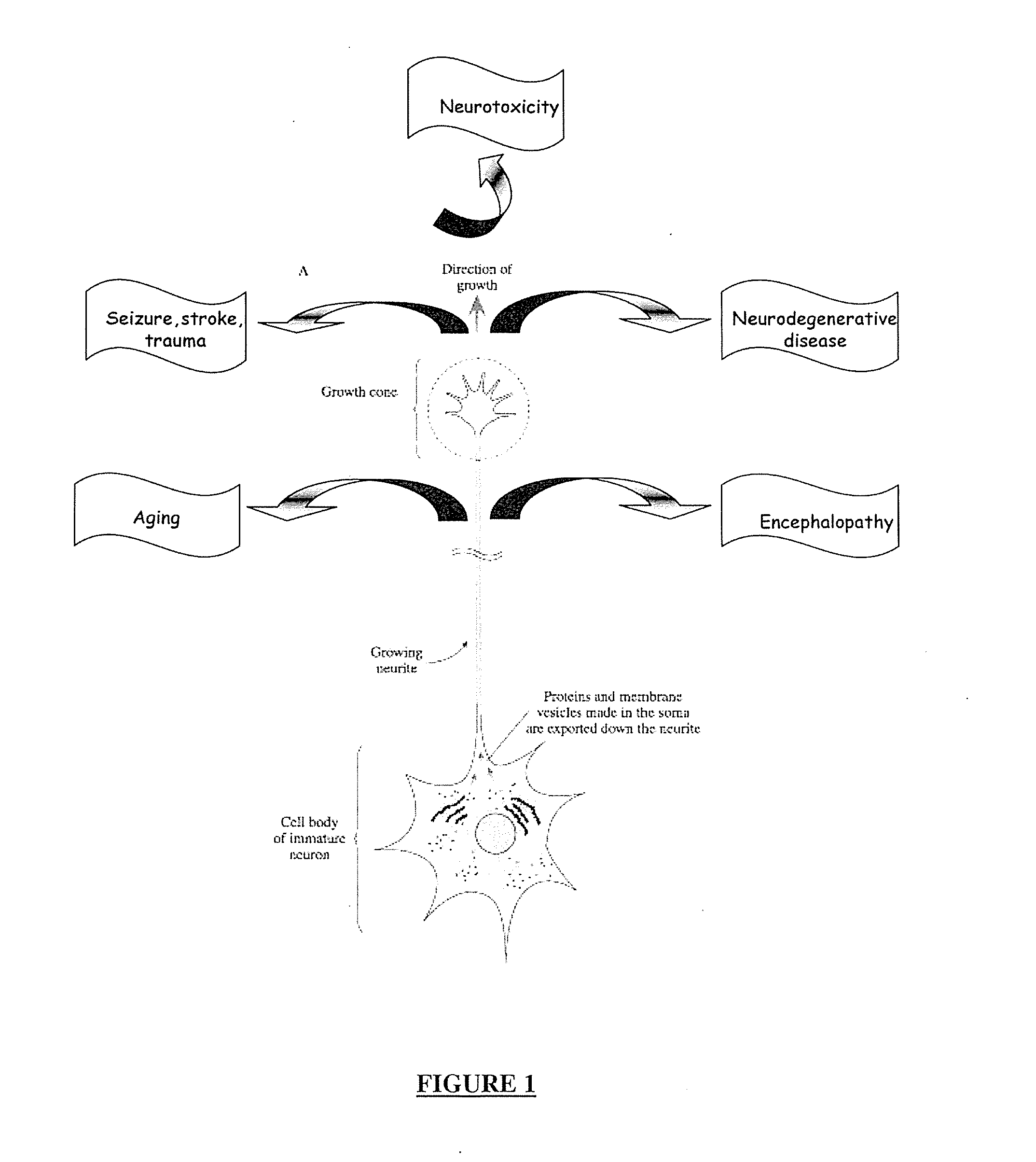
Neuron generation, regeneration and protection
ActiveUS20120100116A1Positive neurite outgrowth effectGood effectOrganic active ingredientsVirusesNervous systemNeoplasm
The invention demonstrates that, contrary to apoptotic rabies virus G proteins, certain non-apoptotic rabies virus G proteins, such as the G protein of the CVS-NIV strain, have a neurite outgrowth promoting effect. The invention further demonstrates that this neurite outgrowth promoting effect is due to the cytoplasmic tail of said non-apoptotic rabies virus G proteins, more particularly to their PDZ-BS, which shows a single-point mutation compared to the one of apoptotic rabies virus G proteins. The invention provides means for inducing and / or stimulating neurite outgrowth, which are useful in inducing neuron differentiation, for example for the treatment of a neoplasm of the nervous system, as well as in regenerating impaired neurons, for example for the treatment of a neurodegenerative disease, disorder or condition or in the treatment of a microbial infection, or in protecting neurons from neurotoxic agents or oxidative stress.
Owner:INST PASTEUR +1
Time resolved immunofluorescence detection kit for rabies virus nucleoprotein, and preparation method thereof
ActiveCN103323599AEasy to manufactureExtended storage timeFluorescence/phosphorescenceProtein.monoclonalImmunofluorescence
The invention discloses a time resolved immunofluorescence detection kit for rabies virus nucleoprotein, and a preparation method thereof. The content of rabies virus nucleoprotein in a sample is quantificationally determined by using principles of a double-antibody sandwich method and time resolved immunofluorescence. The detection kit comprises a standard, a coating reaction plate, a lanthanide ion marked anti-rabies virus nucleoprotein monoclonal antibody, an analysis buffer, a washing liquid and a fluorescence enhancing liquid, wherein a first anti-rabies virus nucleoprotein monoclonal antibody is adsorbed on the coating reaction plate, and a second anti-rabies virus nucleoprotein monoclonal antibody is marked with the lanthanide ion. A preparation method comprises: adding a to-be measured sample in the coating reaction plate, incubating, washing, adding the lanthanide ion marked second anti-rabies virus nucleoprotein monoclonal antibody, incubating, washing, adding the fluorescence enhancing liquid, and determining in a time resolved immunofluorescence detector. The technical scheme of the invention has advantages of high sensitivity, good singularity, simple operation, wide detection scope and the like.
Owner:GUANGZHOU PROMISE BIOLOGICAL PROD
PRV (pesudorabies virus) gB monoclonal antibody and application thereof
The invention belongs to the technical field of immunobiology and particularly relates to application for a patent of a PRV (pesudorabies virus) gB monoclonal antibody and application thereof. The antibody is obtained by screening PRV variant strains HeNZK-2014 and PRV gB proteins with hybridoma cells. The hybridoma cells capable of stably secreting the PRV gB protein monoclonal antibody is obtained through screening, so that the gB protein monoclonal antibody with high potency is obtained, a finished kit is prepared from the antibody, and a matched one-step competitive ELISA detection method is established. Through a series of verification for specificity, sensitivity and repeatability, the result indicates that according to the PRV gB antibody provided in the application and the established one-step competitive ELISA detection method, a comparatively accurate and comparatively stable detection result is achieved, the result can be better used for detection and evaluation of the PRV gB antibody, and the foundation is laid for preventing and controlling a PRV.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Recombinant pseudo-rabies virus expressing swine parvovirus VP2 gene and vacine and its preparation method
The present invention relates to main structure gene VP2 of artificial constructed pseudorabies virus, PrV and porcine parvovirus, PPV. In the pseudorabies virus genome in which the main toxicity gene (TK) and virus generation nonessential gene (gG) are deleted the VP2 gene of porcine parvovirus can be site-specifically inserted to make it be positioned in strong late promoter downstream of pseudorabies virus, and the inserted exogenous gene coded protein has good immunogenicity, and can stimulate swine to produce protective immune reaction for resisting two virulent challenges of porcine parvovirus and pseudorabies virus. Said invention also includes recombinant pseudorabies virus, Hzau AVL-PRppvV-VP2, vaccine prepared by using it and its preparation method.
Owner:HUAZHONG AGRI UNIV
Rabies virus NP-ELISA antibody detection reagent kit
InactiveCN101413948AImprove securityStrong specificityMaterial analysisEscherichia coliPositive control
The invention relates to a dog rabies virus NP-ELISA antibody detection kit, wherein, an antibody detection plate is a detachable 96-hole enzyme labeled plate which is coated by rabies virus nucleoprotein NP, enzyme conjugate working liquid is a horseradish peroxidase labeled anti-dog IgG monoclonal antibody, a positive control is dog rabies standard positive serum, a negative control is a dog standard negative serum, the rabies virus nucleoprotein NP is a fragment of rabies virus ERA vaccine strain NP gene 1350bp which is amplified by RT-PCR technology, the rabies virus nucleoprotein NP is inserted into the downstream of a prokaryotic expression vector pET-28a to construct a pET-NP recombinant expression plasmid, Escherichia coli BL21 DE3 is converted by the calcium chloride method, 0.01mM IPTG induced expression is carried out, ultrasonic waves are used for the lysis of an expression strain, and the purification by a Ni-NTA affinity chromatography column is carried out for the preparation. The using concentration of the rabies virus nuclear NP recombinant protein for coating the NP antibody detection plate is 2 mug / ml, and the using amount is 100 mu 1 / hole. The dog rabies virus NP-ELISA antibody detection kit has the advantages of strong specificity, high sensitivity and good safety.
Owner:ZHEJIANG UNIV
Humanized neutralizing antibody (RVFab8) against rabies virus glycoprotein
The invention discloses a humanized neutralizing antibody (RVFab8) against rabies virus glycoprotein, which is obtained through screening by utilizing phage display technology. The antibody specifically identifies the granule antigen of the rabies virus, is against the rabies virus glycoprotein G, has obvious immunofluorescence reaction and enzyme linked immunosorbent assay with the rabies virus and has the neutralizing activity function against rabies virus infection. The antibody can be prepared into the specific antibody drugs for preventing and treating rabies, thereby being clinically used for preventing and treating rabies caused by the rabies virus.
Owner:NCPC NEW DRUG RES & DEV
Attenuated Live Triple G Protein Recombinant Rabies Virus Vaccine for Pre- and Post-Exposure Prophylaxis of Rabies
The invention provides a recombinant rabies viruses comprising three copies of a mutated G gene wherein each G gene encodes a rabies virus glycoprotein having the amino acid 194 mutated to a serine and the amino acid 333 is mutated to a glutamic acid. The recombinant rabies virus is nonpathogenic in immunodeficient mammals and can be used in a vaccine to induce an immune response protect mammals from infection by rabies virus as well as clear a pre-existing rabies virus infection from neural tissues.
Owner:THOMAS JEFFERSON UNIV
Completely humanized neutralizing antibody for anti-rabies viruses
ActiveCN103910796ALow priceQuickly builds immune protectionHybrid immunoglobulinsImmunoglobulins against virusesAntigenAntigen epitope
The invention discloses a completely humanized neutralizing antibody for anti-rabies viruses. The amino acid sequence of a heavy chain of the antibody is shown in sequence table SEQ ID NO1, the amino acid sequence of a light chain of the antibody is shown in sequence table SEQ ID NO5, the complementary determining region (CDR) of a variable region of the heavy chain of the antibody is shown as follows: CDR1: SEQIDNO2, CDR2:SEQIDNO3 and CDR3: SEQIDNO4, and the complementary determining region (CDR) of a variable region of the light chain of the antibody is shown as follows: CDR1:SEQIDNO6, CDR2:SEQIDNO7, and CDR3: SEQIDNO8. The beneficial effects of the completely humanized neutralizing antibody are that: the completely humanized neutralizing antibody disclosed by the invention ahs the advantages of being completely humanized, good in specificity, high in affinity, good in neutralizing effect, and low in price, can be used as a biological engineering antibody drug to quickly establish immune protection force against rabies virus, can be used for passive immunotherapy of acute infection, and also can be used for preparing detection reagents for detecting the rabies virus, finding effective neutralization antigen epitope and developing recombinant proteins and subunit vaccines for the rabies virus.
Owner:CHANGCHUN BCHT BIOTECH
Preparation method and application of Cre and Flp dependent retrograde tracing recombinant pseudo-rabies virus
ActiveCN107699589AMicrobiological testing/measurementPreparing sample for investigationRetrograde tracingViral Vaccine
The invention discloses a preparation method and application of a Cre and Flp dependent retrograde tracing recombinant pseudo-rabies virus. The preparation method comprises the following steps: (1) preparing a Cre and Flp system dependant retrograde tracing recombinant pseudo-rabies virus; and (2) using the virus in a labeled neural circuit, and successfully preparing a recombinant pseudo-rabies virus with independent and controllable Cre and Flp by the platform. The preparation method successfully obtains recombinant pseudo-rabies virus with independent Cre and Flp that can express fluorescent protein. The virus can be widely used in neural circuit labeling, establishment of drug screening platform, study on virus inhibiting mechanism of drugs, development of viral vaccine and diagnosticreagent, establishment of animal model, virus replication, analysis of pathogenic mechanism, and the like.
Owner:布林凯斯(武汉)生物技术有限公司
Hydrophobia split vaccine for human body
The invention discloses a hydrophobia cracking vaccine and preparing method, which comprises the following steps: fixing toxic plant seeded cell, culturing, obtaining virus liquid, cracking the hydrophobia virus particle through chemical method, extracting, condensing, purifying hydrophobia film segment with barb protrusion and base protein, allocating further to obtain the product.
Owner:安徽智飞龙科马生物制药有限责任公司 +2
Rabies virus Flury-LEP vaccine strain reverse genetic operating system and LEP green fluorescent protein recombination viral vector
InactiveCN101586120AMicrobiological testing/measurementMicroorganism based processesPhosphoric acidViral vector
The invention relates to a rabies virus Flury-LEP vaccine strain reverse genetic operating system comprising a recombination vector for coding LEP full length genome cDNA and an auxiliary plasmid system thereof, wherein, the auxiliary plasmid system respectively codes nucleoprotein of LEP, phosphoric acid protein P and polyase protein L. the invention also relates to LEP green fluorescent protein recombination viral vector built by the reverse genetic operating system, concretely, the rabies virus Flury-LEP vaccine strain reverse genetic operating system is pCI-LEP and auxiliary plasmid systems pCAGG-N, pCAGG-P and pCAGG-L, the LEP green fluorescent protein recombination viral vector is pCI_LEP_EGEP. The invention also relates to recombination viruses rescued by the rabies virus Flury-LEP vaccine strain reverse genetic operating system and the LEP green fluorescent protein recombination viral vector, and applications thereof.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant new castle disease LaSota attenuated vaccine strain expressing rabies virus glucoprotein (GP protein)
The invention relates to a Newcastle disease LaSota lentogen vaccine which expresses rabiesvirus glycosidoprotein (GP protein); more specifically, the Newcastle disease LaSota lentogen vaccine is rL-ERAGP. The invention also discloses a method for preparing the Newcastle disease LaSota lentogen vaccine and an application of the Newcastle disease LaSota lentogen vaccine in preparing bivalent vaccine used to prevent rabies and Newcastle diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com