Humanized neutralizing antibody (RVFab8) against rabies virus glycoprotein
A rabies virus, glycoprotein technology, applied in the direction of antiviral immunoglobulin, antiviral agent, antibody, etc., can solve the problems of infection, limited application, and limitation of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
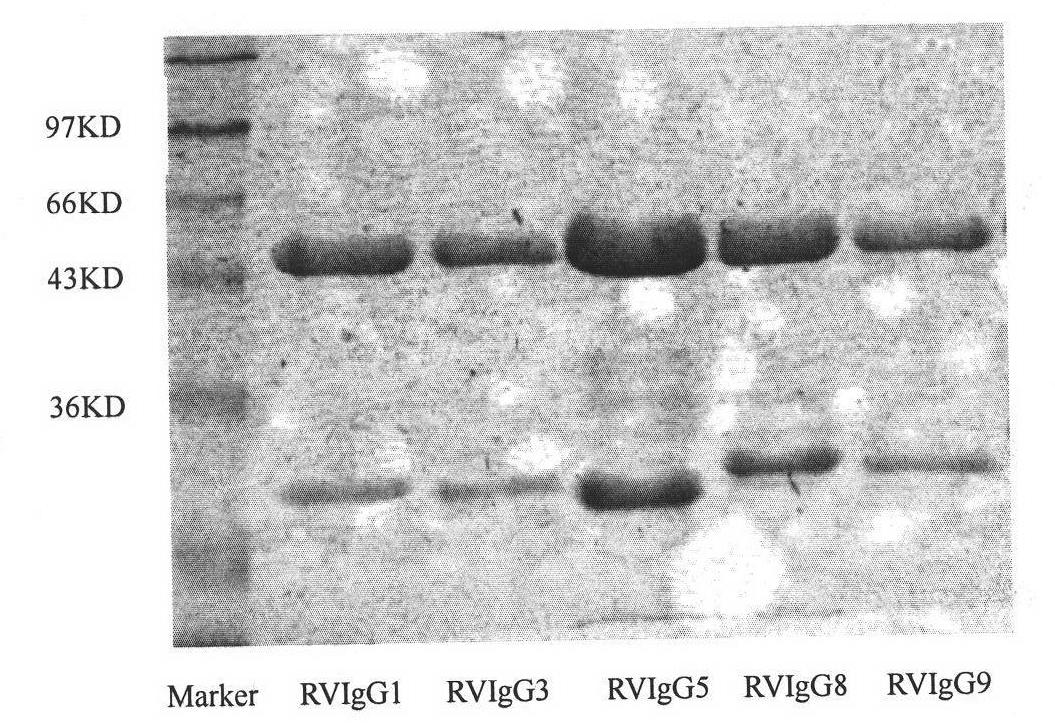
Image
Examples
Embodiment Construction
[0026] The specific implementation manners of the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention.
[0027] Unless otherwise specified, the technical means used in the embodiments are conventional means well known to those skilled in the art.
[0028]Materials and methods
[0029] 1. Viruses, cells, and vectors: rabies virus aG strain, CTN strain, CVS strain and ERA strain were provided by the Institute of Viral Disease Prevention and Control, Chinese Center for Disease Control and Prevention. The PM strain used for human immunization is an imported human vaccine strain, and the cells used for the neutralization experiment of rabies virus in vitro are BHK-21 (ATCC). The strain is XLI-Blue (Stratagene, USA), and the vector is pComb3 (40kb), provided by Scripps Research I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com