Anti-rabies virus monoclonal antibody and preparation method and application
A technology of rabies virus and monoclonal antibody, applied in the field of biomedicine, can solve the problems of cumbersome operation of detection methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Immunogen preparation and immunization
[0043] The immunogen was prepared by using inactivated rabies virus CTN strain to purify the virus (a purity of more than 99%, donated by Zhang Aihua, Wuhan Institute of Virology).
[0044] Animal immunization: Purify the above-mentioned inactivated rabies virus CTN strain virus, measure the protein concentration to be 0.67 mg / mL, add Freund's complete adjuvant to mix at 1:1, oscillate and emulsify for half an hour, and subcutaneously immunize BALB / c mice with 100 μg; Two weeks later, the antigen was emulsified with Freund's incomplete adjuvant, and the same method was used to immunize again; two weeks after the second immunization, 200 μg of purified antigen was injected intraperitoneally for booster immunization, and fusion was performed three days later.
Embodiment 2
[0045] The fusion of embodiment 2 hybridoma cells
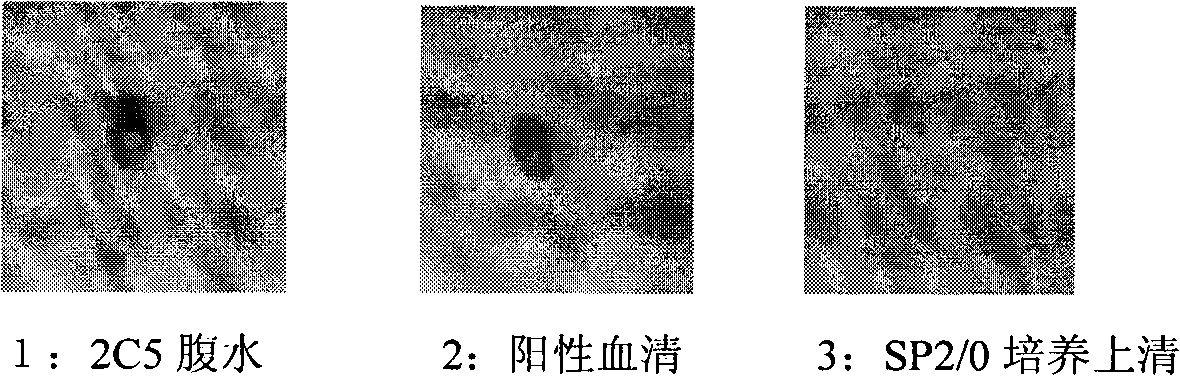
[0046] Fusion of hybridoma cells is performed according to conventional methods. Positive blood was obtained by removing the eyeball before fusion. Spleen cells were fused with SP2 / 0 cells in logarithmic growth phase with PEG-2000. At the same time, blood was collected from BALB / c immunized mice and ICR mice provided with feeder cells as positive and negative sera for control when screening monoclonal antibodies. The fused cells were suspended in HAT medium, distributed into 96-well plates, and placed at 37°C, 5% CO 2 Cultivate in the incubator, add fresh HAT for 5-7 days, observe frequently, change the medium and detect in due course.
Embodiment 3
[0047] The detection of embodiment 3 hybridoma cells
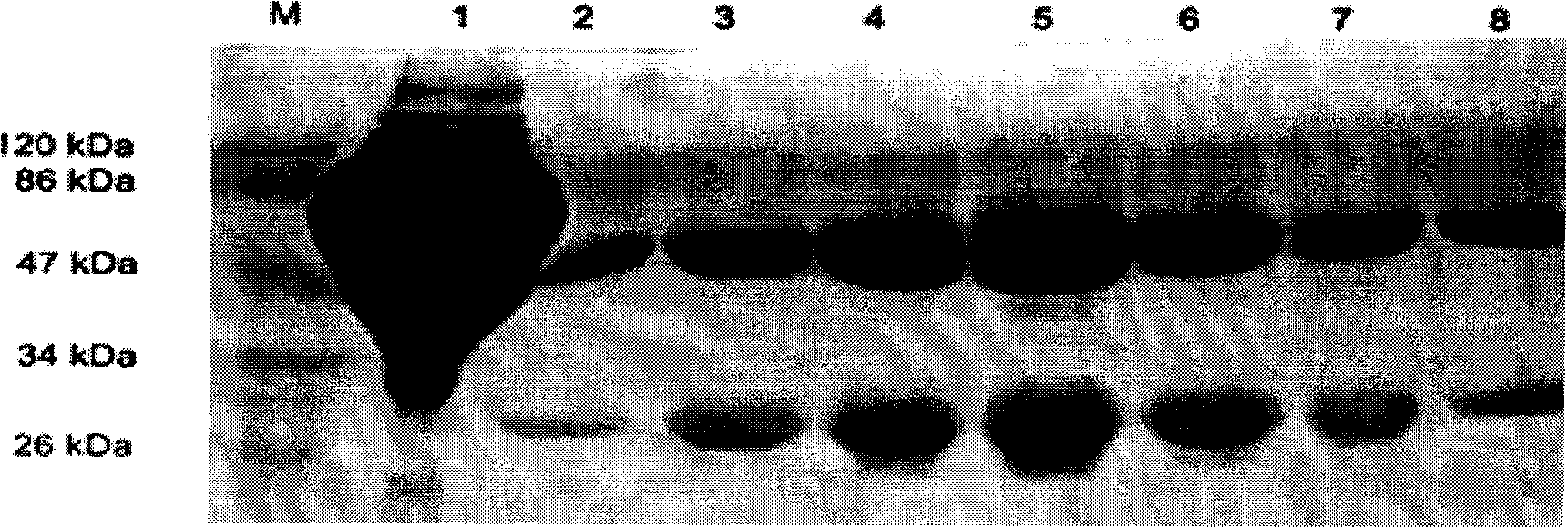
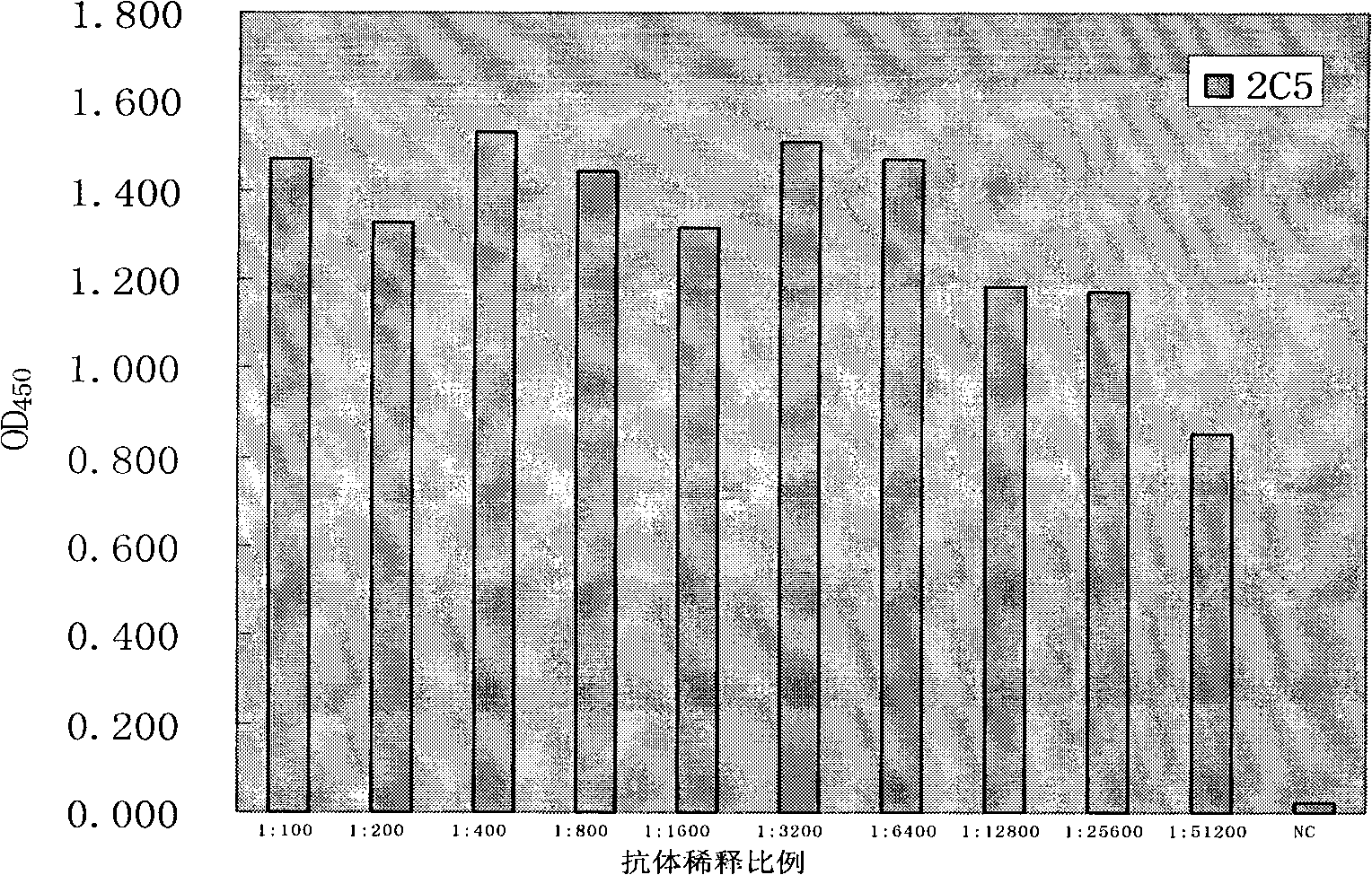
[0048] Enzyme plate coated on the plate, do square array test, that is: use the purified virus of inactivated rabies virus CTN strain as antigen, coat ELISA plate with PBS doubling dilution horizontally, use positive serum as primary antibody, Dilute longitudinally by 100 times, 200 times, 400 times, 800 times, and select the optimal coating concentration (5 μg / mL) according to the reaction results. The coating of the microtiter plate is briefly described as follows: Take the purified antigen protein and mix it with carbonate buffer solution, that is, 0.2mol / L Na 2 CO 3 8mL, 0.2mol / L NaHCO 3 17mL, add deionized water to 100mL, pH9.6, dilute to the coating concentration, and set a negative control at the same time; add 100μL / well to the ELISA plate, 37℃, 3 hours; PBST, 0.01mol / L, pH7.3 , PBS+0.5% Tween-20, wash 3 times, 5 minutes / time; add PBS / FCS, namely 0.01mol / L, pH7.3, PBS+10%FCS or 1%BSA, 200μL / well, 37℃ to block ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com