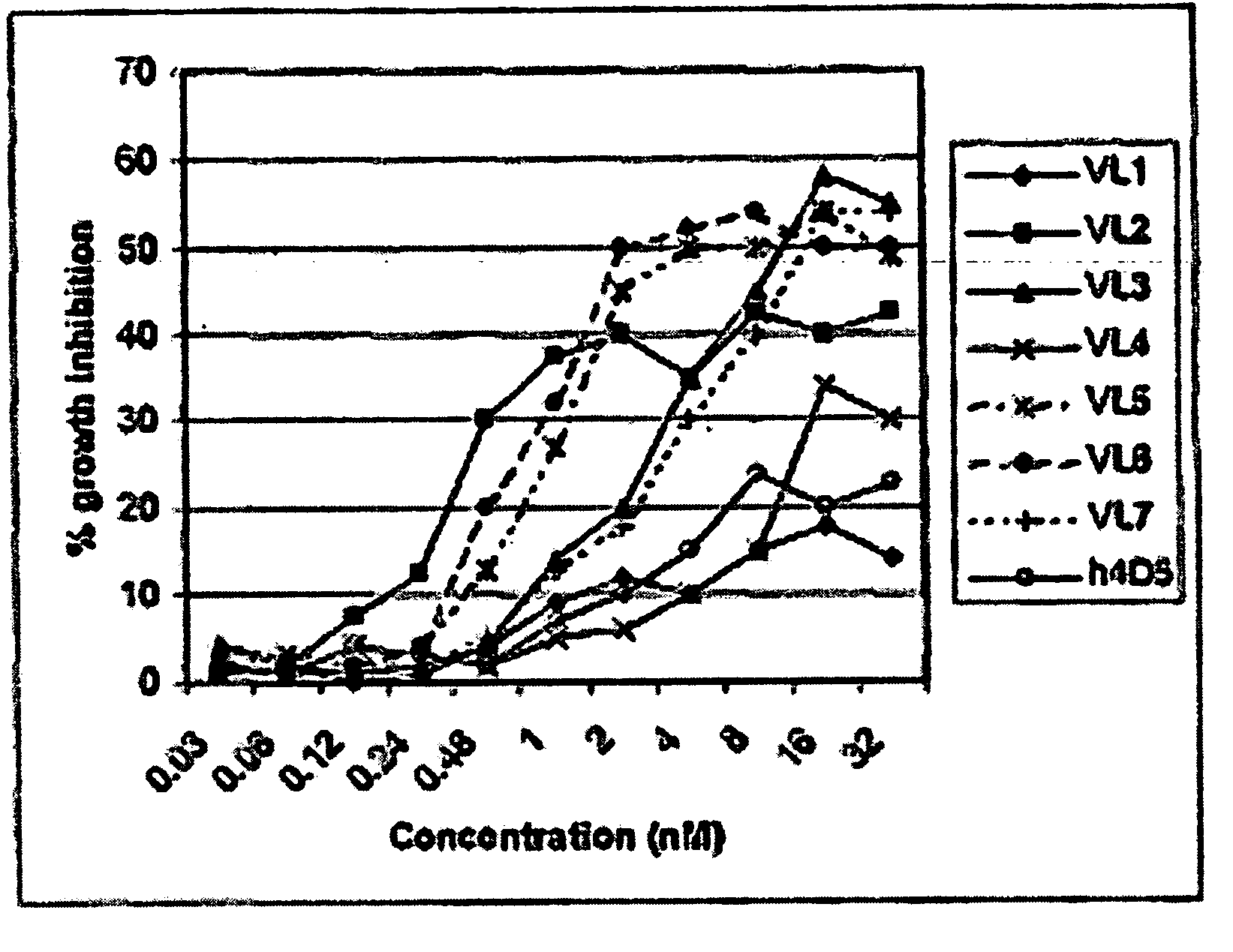
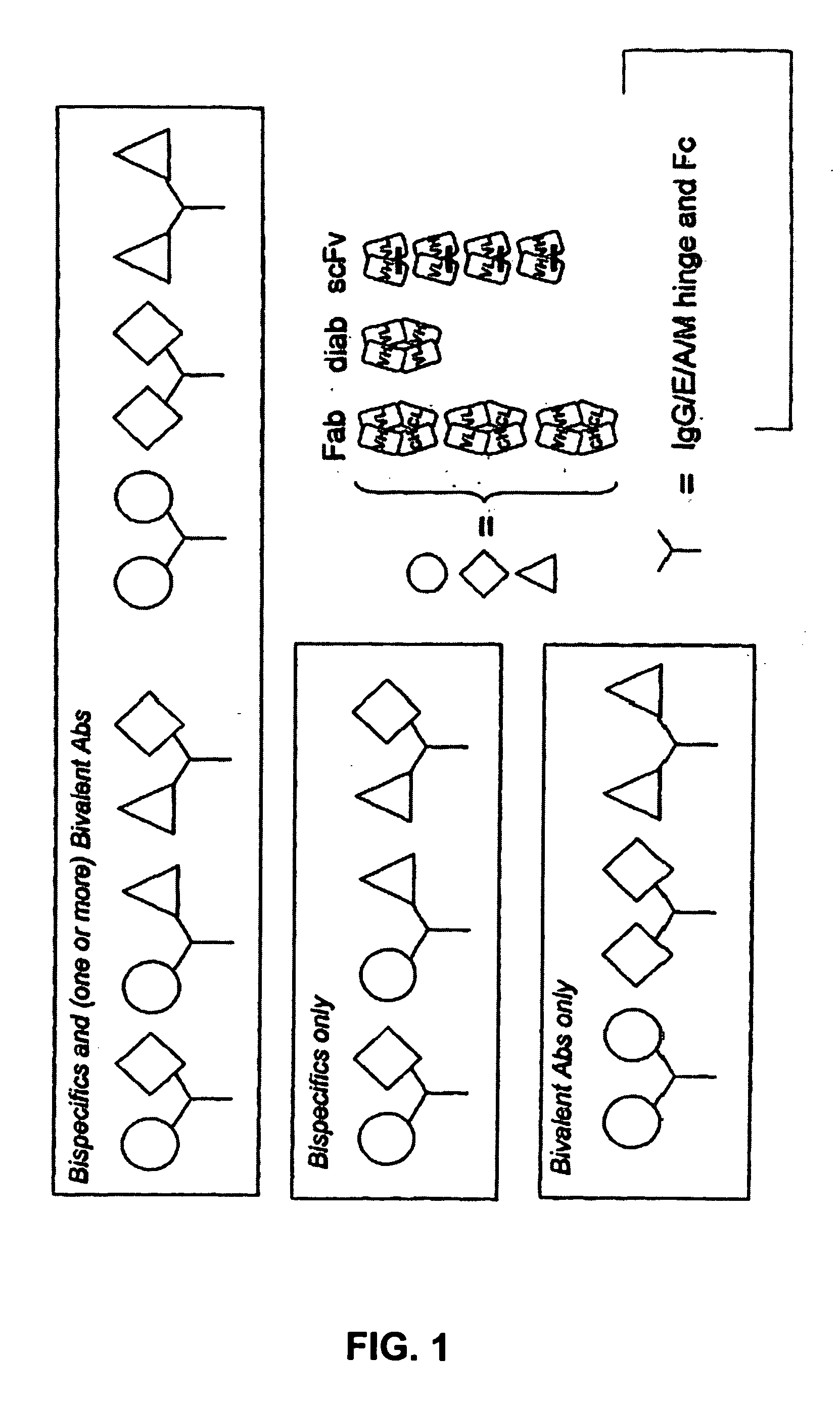
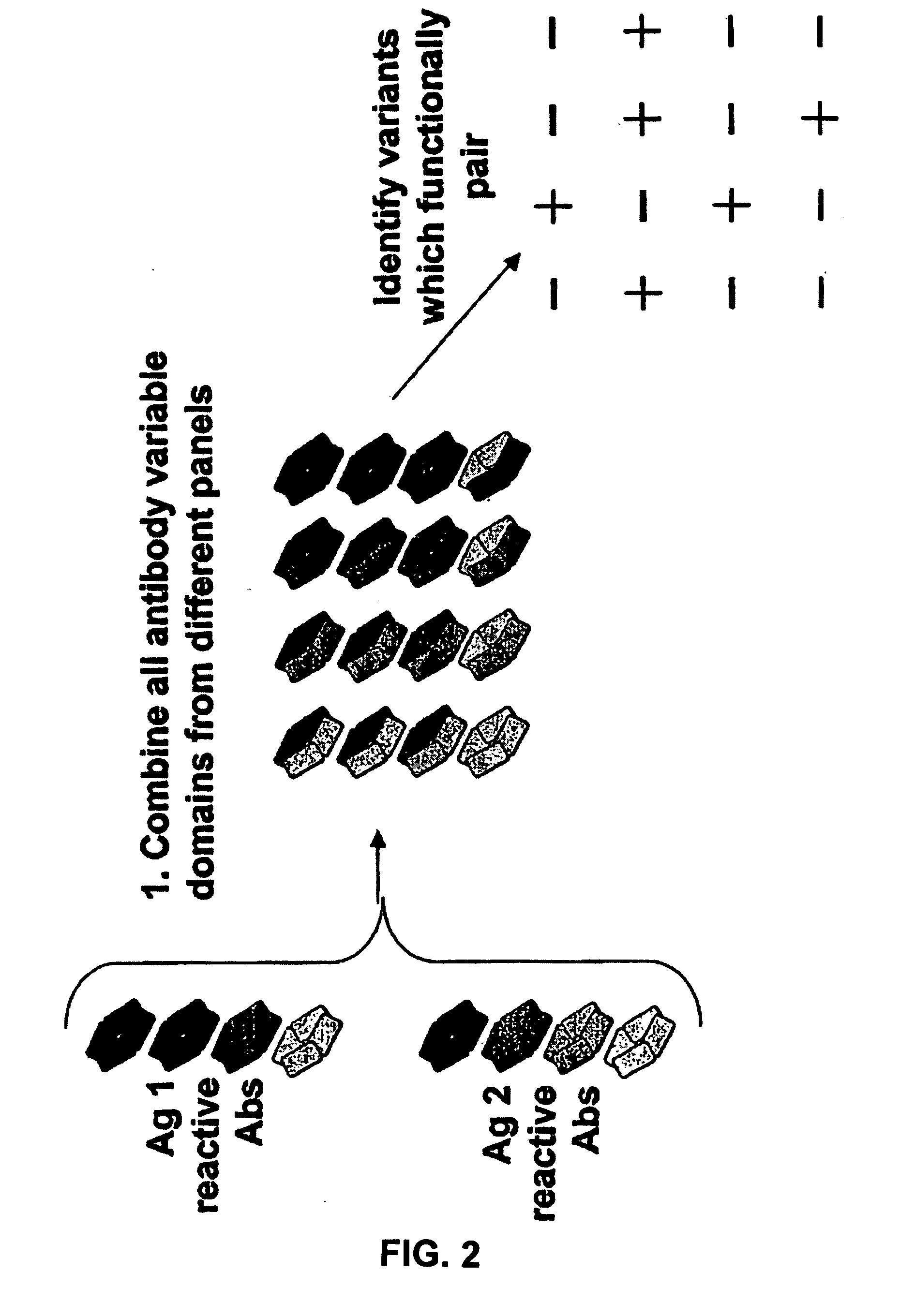
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Anti-Rabies virus IgG" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fab library for the preparation of anti VEGF and anti rabies virus fabs
InactiveUS20060160184A1Maintain good propertiesOptimization mechanismAnimal cellsAntibody mimetics/scaffoldsProtein moleculesImmunoglobulin IgE
The present invention provides combinations of specific binding proteins, such as immunoglobulins, that are designed to be true combinations, essentially all components of the combination being functional and compatible with each other. The invention further provides a method for producing a composition comprising at least two different proteinaceous molecules comprising paired variable regions, the at least two proteinaceous molecules having different binding specificities, comprising paired variable regions, at least two proteinaceous molecules having different binding specificities, comprising contacting at least three different variable regions under conditions allowing for pairing of variable regions and harvesting essentially all proteinaceous molecules having binding specificities resulting from the pairing.
Owner:MERUS NV
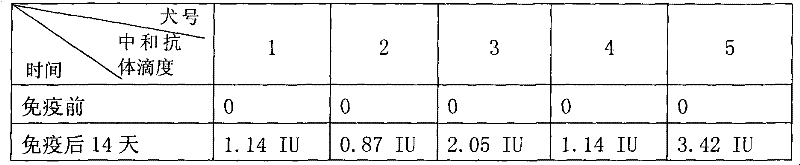
Anti-rabies virus monoclonal antibody and preparation method and application
InactiveCN101560255AExperimental costs are highEasy to operateImmunoglobulins against virusesTissue cultureImmunoblot AnalysisHybridoma cell
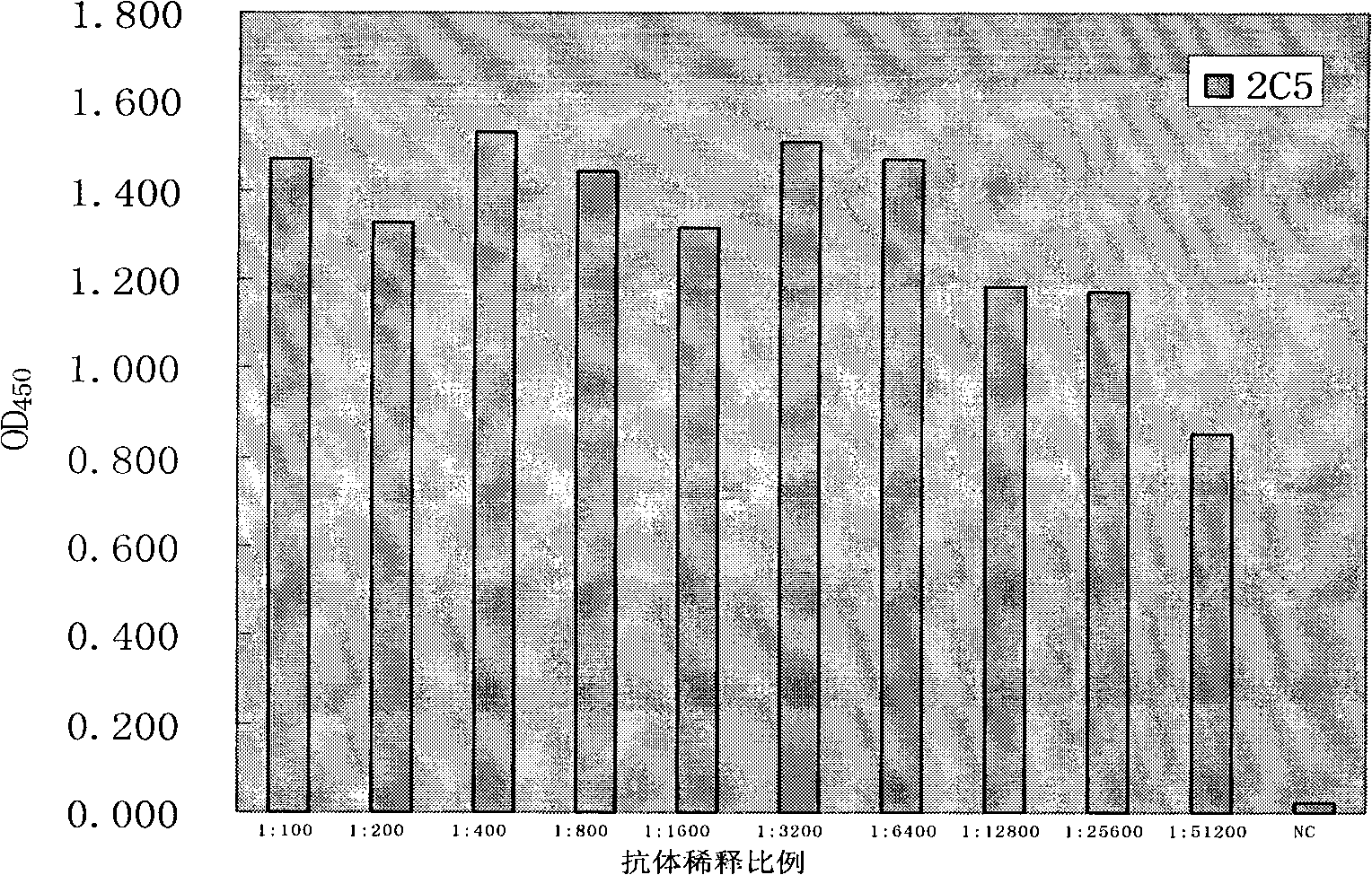
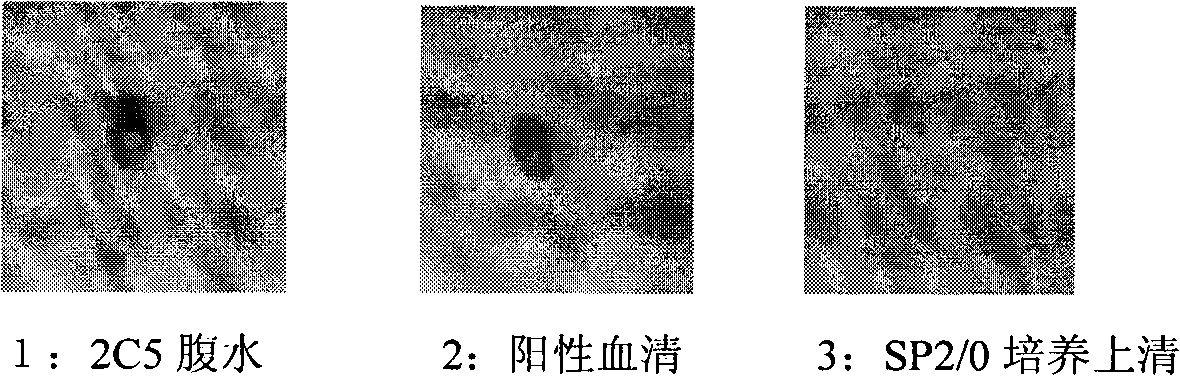
The invention discloses an anti-rabies virus monoclonal antibody and a preparation method and an application, belonging to the field of biomedicine and particularly relating to the preparation of a monoclonal antibody capable of identifying rabies virus and the application. The monoclonal antibody of the invention is screened by indirect Enzyme-linked immunosorbent assay (ELISA), and specificity and affinity thereof combined with antigen are identified by methods such as polyacrylamide gel electrophoresis analysis, speckle ELISA, immunoblot analysis and the like. The anti-rabies virus monoclonal antibody of the invention can be applied in multiple testing methods of antigen of rabies virus and can be also applied in the preparation of rabies virus detecting kit. The anti-rabies virus monoclonal antibody is secreted by anti-rabies virus monoclonal antibody hybridoma cell strain 2C5 with the preservation number being CGMCC No.3014.
Owner:NANJING MEDICAL UNIV +1
Rabies cure
InactiveUS20110020279A1Inhibit rabies virus multiplicationAvoid spreadingSsRNA viruses negative-senseOrganic active ingredientsSubarachnoid spacePresent method
This invention is for a method of treatment of rabies once the patient develops signs and symptoms of rabies with the intent to save the patients from death and disability using insulin combined with various anti rabies viral therapeutic, pharmaceutical, biochemical, and biological agents or compounds with added supportive therapies administered through OM, SAS, IVB, IV, and IA routes. An embodiment provides devices for intranasal delivery of therapeutic agents to olfactory mucosal area. Another embodiment uses the technology to deliver the therapeutic, pharmaceutical, biochemical, and biological agents or compounds to the subarachnoid space and ventricular system by using continuous catheters and Ommaya reservoir at the same time. The present method incorporates breaking the blood brain barrier to allow the entry of the anti rabies therapeutic agents into the neuropile. Additionally, an embodiment incorporates cooling of the brain and inducing hibernation to preserve the brain from damage due to rabies.
Owner:SHANTHA TOTADA R
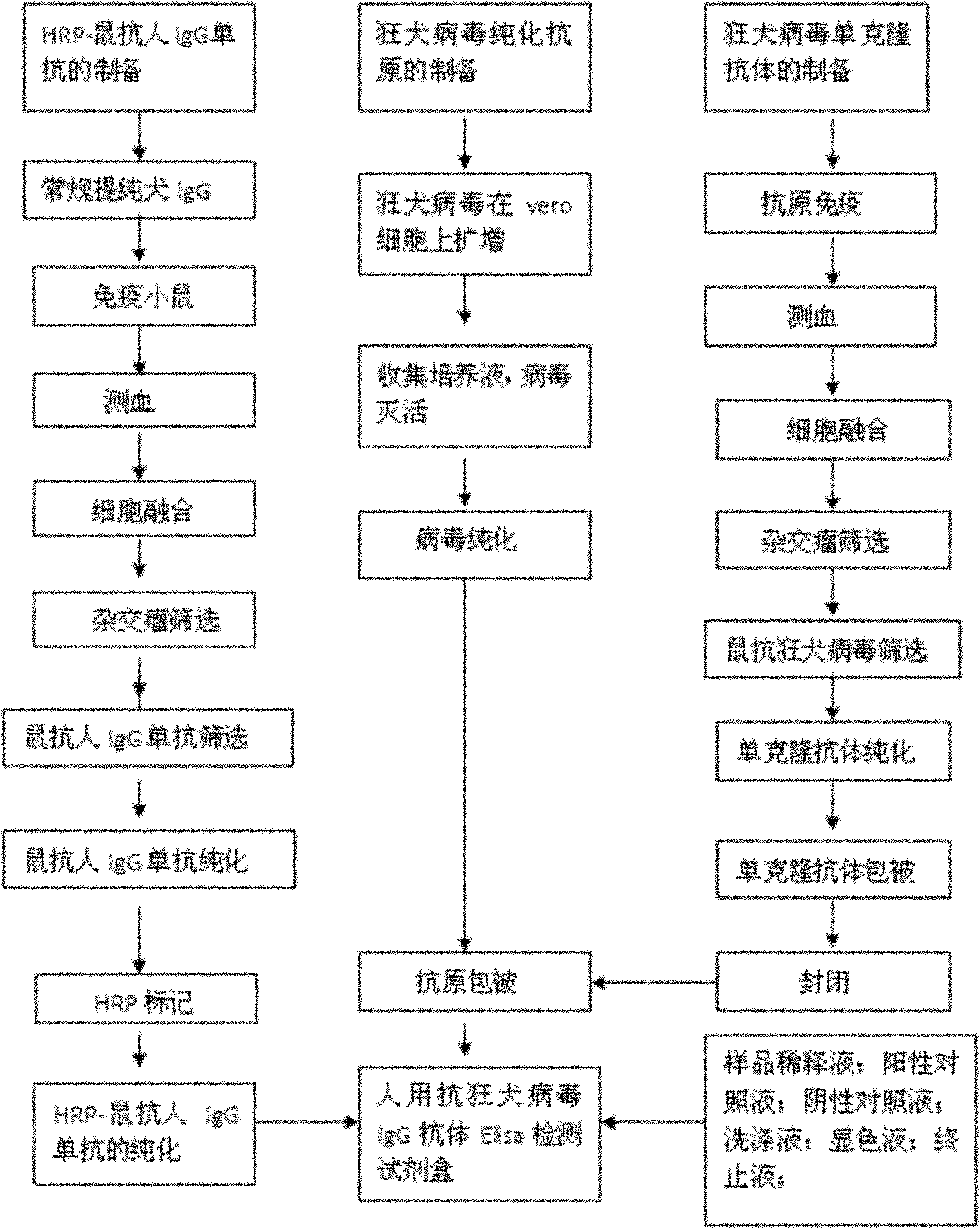
Human anti-rabies virus IgG antibody ELISA test kit
ActiveCN101936997AMake up for the shortcomings of low sensitivityHigh sensitivityDepsipeptidesMaterial analysisAntigenPositive control
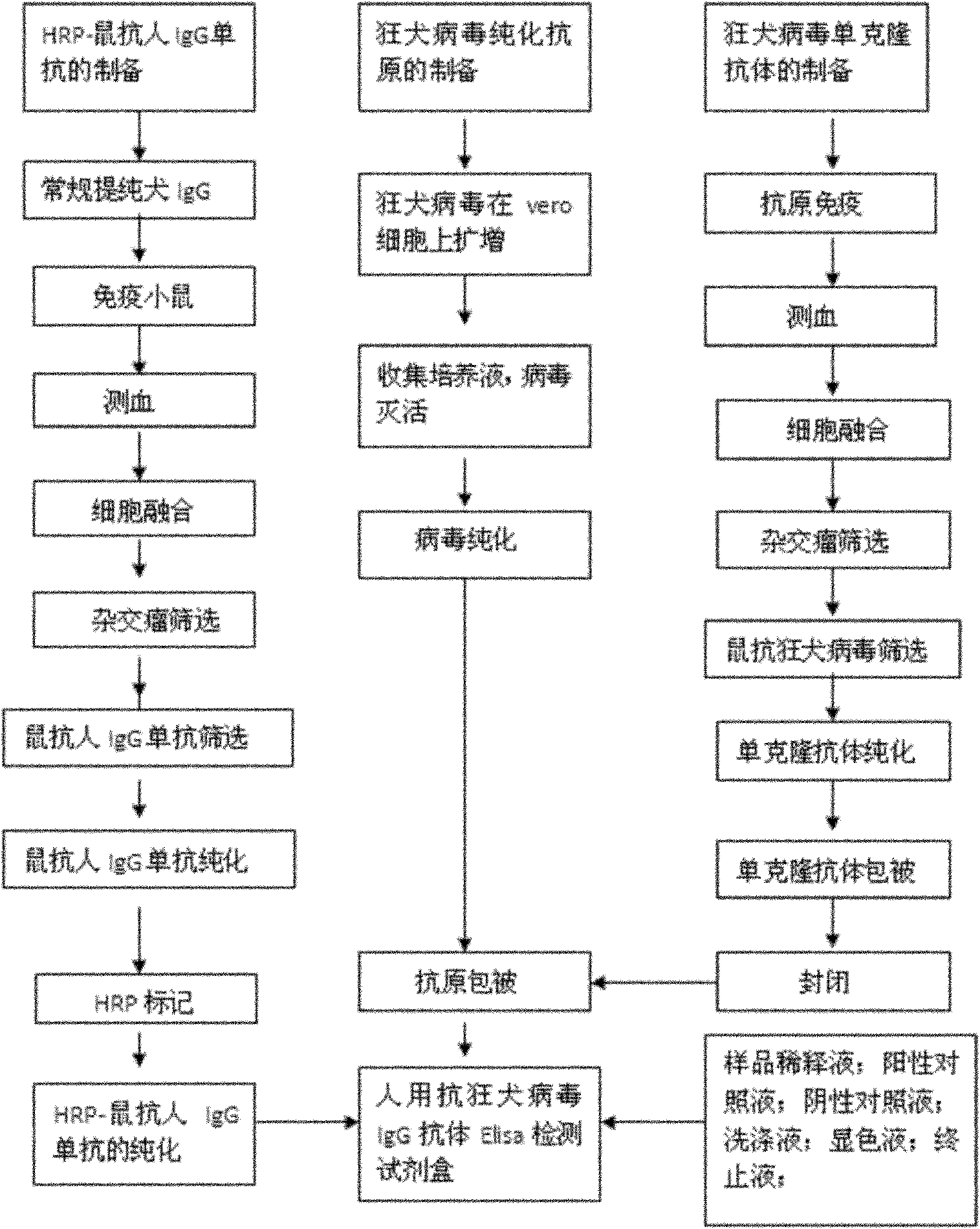
The invention relates to a human anti-rabies virus IgG antibody ELISA test kit. An ELISA plate is firstly coated with an anti-rabies virus monoclonal antibody, wherein the coating buffer solution is a 0.05M carbonate buffer solution of which the pH value is 9.6, and the coating amount is 0.1-1ug per hole; a blocking solution is a BSA or skimmed milk of which the mass concentration is 1-10%; the ELISA plate is coated with a rabies virus purified antigen after being blocked, wherein the coating amount is 0.1-1ug per hole; a sample diluent is a 0.01mol / L phosphate buffer solution (PBS) which contains bovine serum albumin (BSA) with a mass concentration of 0.1-10% and NaN3 with a mass concentration of 0.01-0.05 and has a pH value of 7.2-7.4; an enzyme conjugate is a horse radish peroxidase-mouse anti-human IgG enzyme conjugate; a concentrated cleaning solution is a 0.01mol / L PBS which contains tween-20 with a volume concentration of 0.05% and has a pH value of 7.2-7.4; a zymolyte A solution is a 3,3'-5,5'-tetramethyl benzidine solution, and a zymolyte B solution is an oxydol solution; and a stop solution is a 1mol / L H2SO4 solution, and a positive control and a negative control are arranged in the kit. The specificity of the kit is up to 100%, and the sensitivity is 1:640. The kit is used for evaluating the immunity effect of humans inoculated with rabies vaccines.
Owner:WUHAN CHOPPER BIOLOGY
Rabies virus resistant specific humanized antibody and application thereof
ActiveCN104193823AStrong neutralizing activityImmunoglobulins against virusesAntiviralsPhage antibodiesBacteriophage
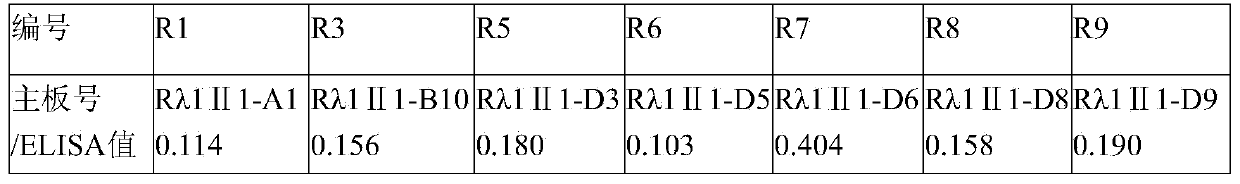
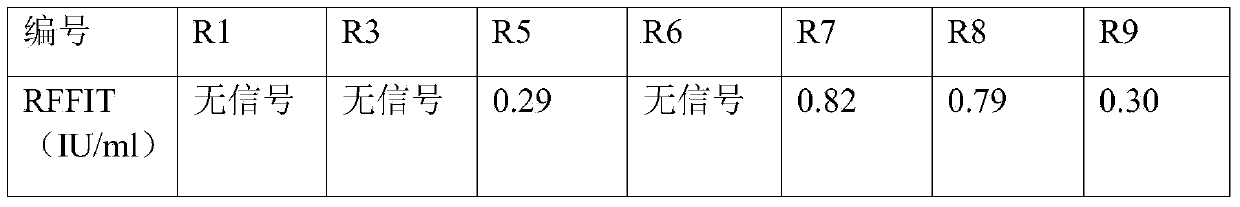
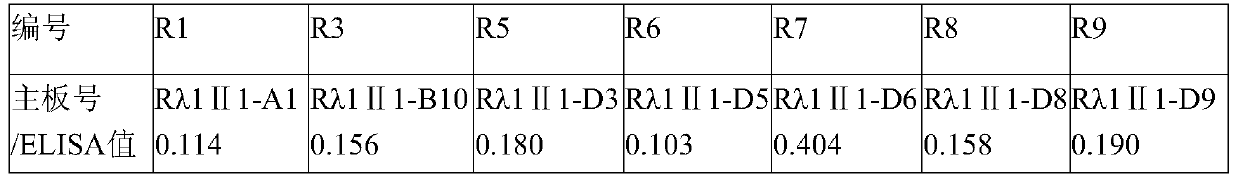
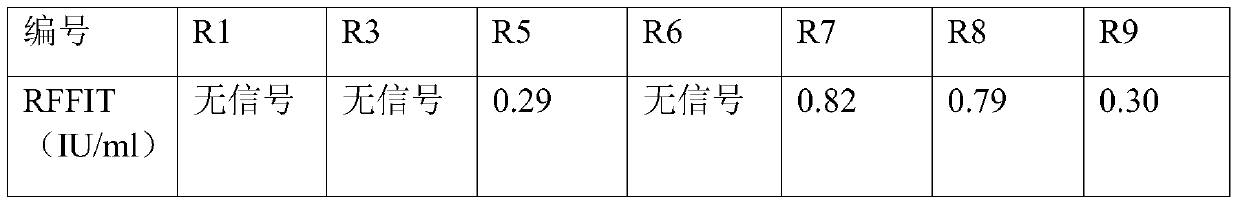
The invention aims at providing a rabies virus resistant neutralizing antibody, and particularly provides a humanized or completely humanized monoclonal antibody to meet the requirement for clinically diagnosing and / or treating rabies. A phage antibody library is prepared by adopting a phage antibody library technology and taking 32 parts of high-potency healthy human peripheral blood inoculated with rabies vaccines as a raw material; 7 ELISA positive antibodies are obtained through three rounds of screening from the phage antibody library; and furthermore, the neutralizing activity of the 7 ELISA positive antibodies is measured through an RFFIT method, wherein four ELISA positive antibodies, namely R5, R7, R8 and R9, have higher neutralizing activity in all. The rabies virus resistant neutralizing antibody with high affinity, which is provided by the invention, can be used for substituting ERIG and HRIG to carry out active and / or passive immune therapy on rabies virus seriously-exposed persons.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Anti-rabies virus IgG antibody colloidal gold immunochromatographic assay reagent plate and preparation method
The invention relates to an anti-rabies virus IgG antibody colloidal gold immunochromatographic assay reagent plate and a preparation method. Glass fiber paper and cellulose nitrate films are laid on a polyethylene plate and a polyvinyl chloride substrate film; the cellulose nitrate film is coated with an assay line and a contrast line; and a gold-labeled probe polyester film is adhered on the assay line side; water-absorbing filter paper is adhered on the contrast line side, wherein the assay line is coated with rabies virus purified antigen, and the contrast line is coated with anti-SPA purified antigen. The reagent plate of the invention has the advantages of rapid detection, high detection accuracy, high specificity, convenient carrying, simple and convenient operation and can be usedfor detecting rabies virus antibodies of various animals and human. The assay reagent plate can be stored at the normal temperature for 1 year without special equipment or apparatus and has high assay repeatability.
Owner:WUHAN CHOPPER BIOLOGY
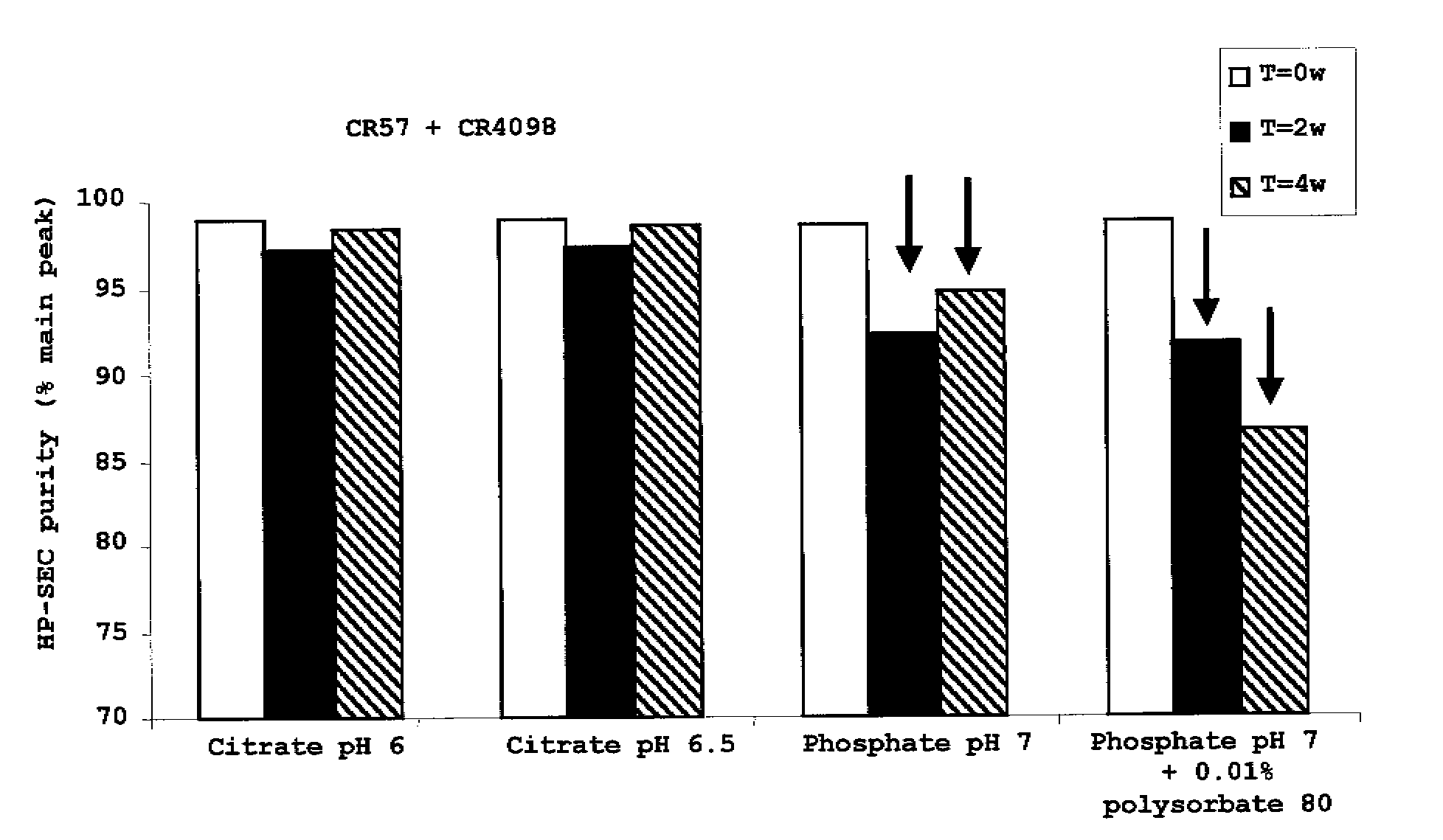
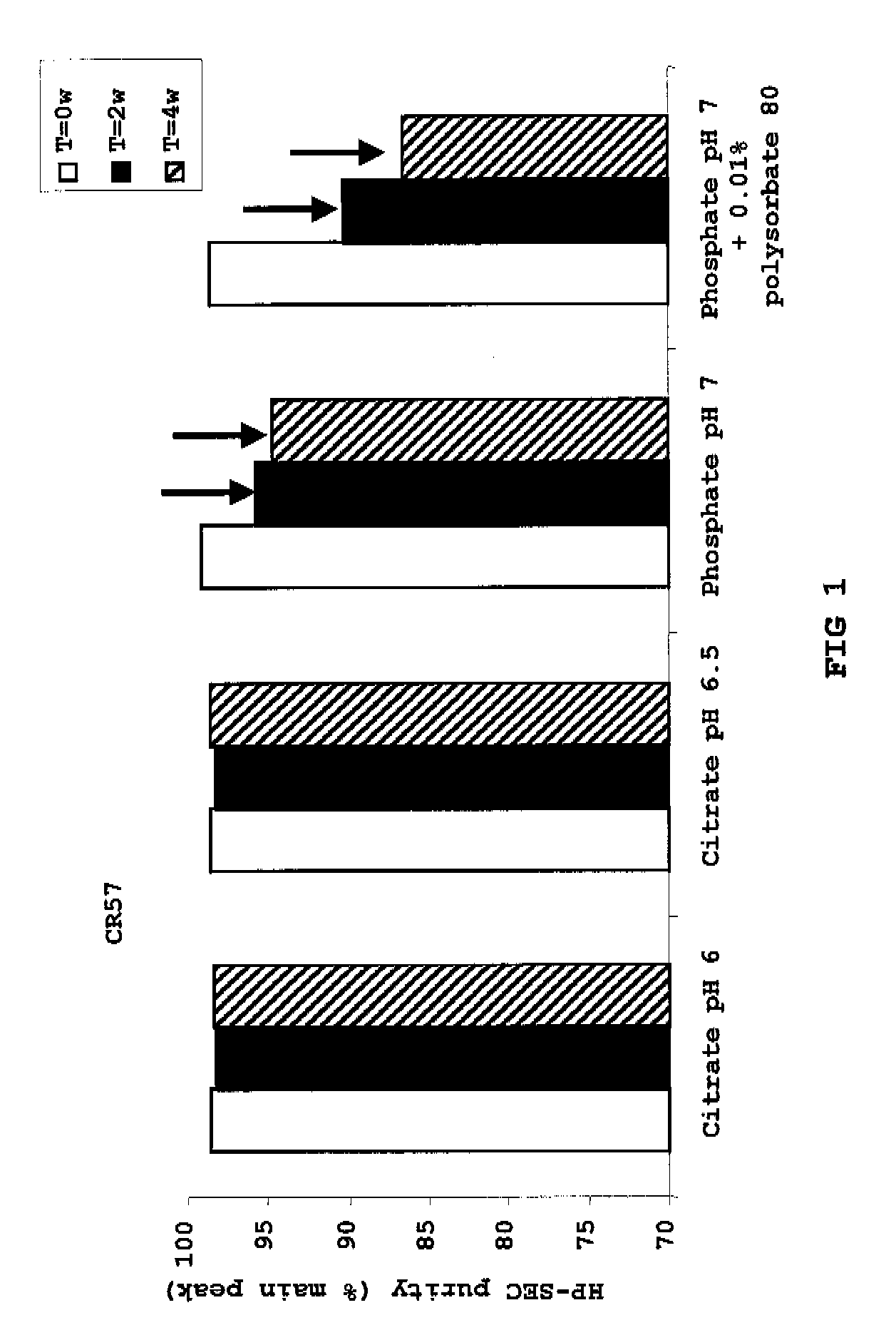
Liquid Anti-rabies antibody formulations
InactiveUS20100034829A1Improve instabilityNervous disorderPharmaceutical delivery mechanismPharmaceutical formulationAntibody
The present invention provides pharmaceutical antibody formulations, in particular liquid pharmaceutical formulations comprising anti-rabies virus antibodies. The formulations can be used in the post exposure prophylaxis of rabies.
Owner:JANSSEN VACCINES & PREVENTION BV
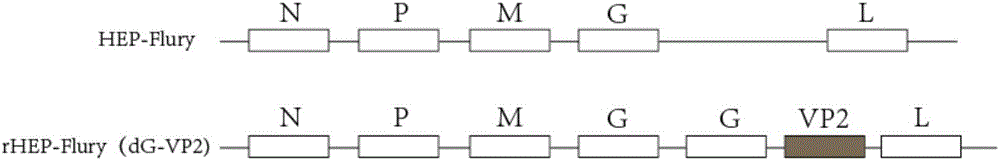
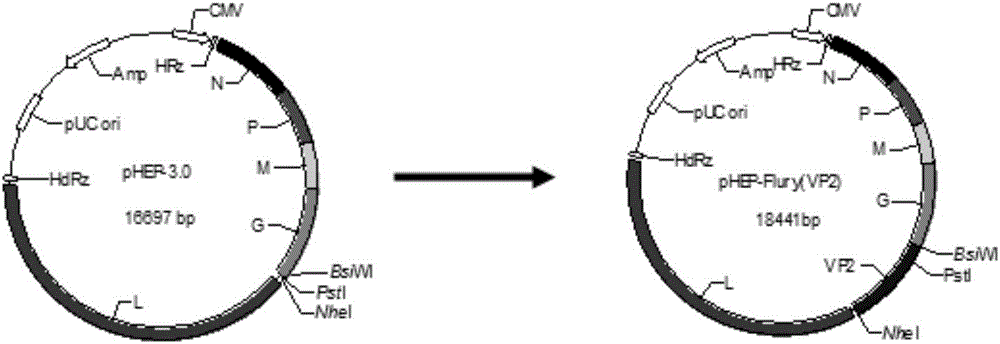
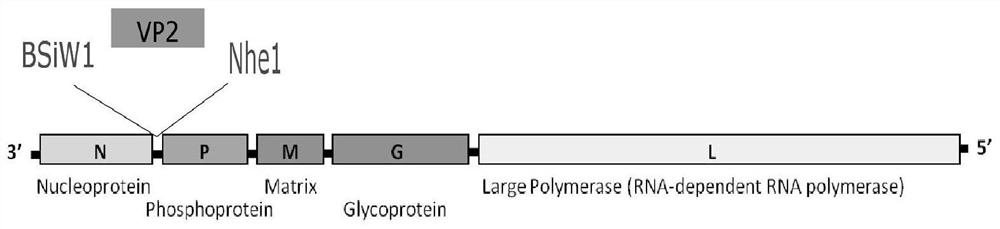
Recombinant rabies virus carrying two genes G and gene VP2 and application of recombinant rabies virus
InactiveCN106244602AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsVp2 geneCanine parvovirus
The invention discloses a recombinant rabies virus rHEP-Flury(dG-VP2) carrying two genes G and a gene VP2. According to the recombinant virus, a rabies virus HEP-Flury strain serves as a framework, the VP2 gene shown as SEQ ID NO.1 is inserted into HEP-Flury, an additional gene G of the rabies virus is inserted, and the recombinant plasmid pHEP-Flury(dG-VP2) carrying the two genes G and the gene VP2 is obtained; finally, rescuing screening is carried out, and the recombinant rabies virus rHEP-Flury(dG-VP2) is obtained. The recombinant rabies virus rHEP-Flury(dG-VP2) carries the two genes G and expresses VP2 protein, a high rabies virus resisting antibody and canine parvovirus VP2 resisting antibody level can be generated through immune induction, the cost of vaccines for dogs can be reduced, and very good application and popularization prospects are achieved.
Owner:SOUTH CHINA AGRI UNIV
Liquid anti-rabies antibody formulations
InactiveUS7959922B2Nervous disorderImmunoglobulins against virusesPharmaceutical formulationAntibody
The present invention provides pharmaceutical antibody formulations, in particular liquid pharmaceutical formulations comprising anti-rabies virus antibodies. The formulations can be used in the post exposure prophylaxis of rabies.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for constructing virus live vector recombinant vaccine by utilizing transposon
InactiveCN101850116AGenetic material ingredientsViruses/bacteriophagesSwine Fever VirusRecombinant vaccines
The invention discloses a method for constructing virus live vector recombinant vaccine by utilizing transposon. Green fluorescent protein is taken as a report gene, expression boxes respectively expressing rabies virus glycoprotein and swine fever E2 protein genes are constructed and are cloned to the shuttle vector of the transposon, under the action of mediation of transposase, recombination with purified canine adenovirus type II virus and herpes virus type I entire genome are respectively carried out, then transfection agent (liposome and the like) is utilized to respectively transfect the recombination product with MDCK and Vero cells, thus obtaining four strains of recombinant viruses taking green fluorescent protein as report gene, namely recombinant canine adenovirus type II virus expressing glycoprotein, recombinant canine adenovirus type II virus expressing E2 protein, recombinant herpes virus type I expressing glycoprotein and recombinant herpes virus type I expressing E2 protein. Immunity test shows that the canine adenovirus type II virus expressing E2 gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to swine fever virus infection in swine and canine adenovirus type II virus expressing glycoprotein gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to rabies virus infection in dog.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Chimeric G protein based rabies vaccine
A novel chimeric protein of rabies virus designed to express a chimeric G protein at a high level in transgenic plants. A gene was also designed and chemically synthesised to encode the chimeric G protein and expressed at high level in plant tissue. The gene was expressed in transgenic tobacco plants to examine its therapeutic efficacy against infection by rabies virus. The chimeric G protein was enriched in plant membranes. The BalbC mice were immunised with the plant leaf expressed G-protein. Plant derived chimeric G protein elicited higher immune response as compared to the commercial vaccine. The mice displayed protective immunity when they were challenged with live virus. Chimeric G protein expressed at high level in plant leaves was demonstrated to function as a commercially valuable subunit vaccine against rabies virus infection.
Owner:COUNCIL OF SCI & IND RES +2
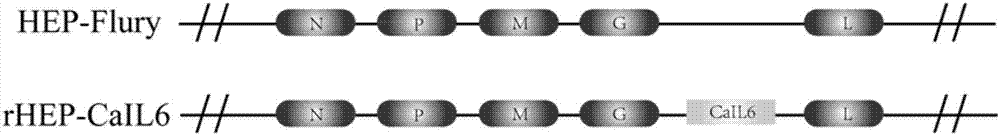
Recombinant rabies virus carrying interleukin 6 gene and application thereof
InactiveCN106967691AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsRabies virus strainEukaryotic plasmids
The invention discloses a recombinant rabies virus rHEP-CaIL6 carrying an immune enhancement factor interleukin (IL) 6 gene and application thereof. The recombinant virus takes rabies virus HEP-Flury strain as the skeleton, the IL6 gene of canine is inserted to a position between the G and L gene of HEP-Flury to obtain a recombinant plasmid pHEP-CaIL6, and finally saving screening is carried out to obtain the recombinant rabies virus strain rHEP-CaIL6. The recombinant virus carries the immune enhancement factor, can enhance the immune response and induce the production of higher rabies virus neutralizing antibody, thus better protecting the body from resisting the attack of lethal rabies virus, also can produce a neutralizing antibody with protective ability at a low dose, and lowers the cost of canine vaccines. Moreover, the IL6 gene is recombined into the rabies virus, thus achieving stable expression of IL6 protein, also avoiding the overexpression thereof, and overcoming the defect that excessive IL6 can cause pathological injury.
Owner:SOUTH CHINA AGRI UNIV
Chimeric G protein based rabies vaccine
Owner:COUNCIL OF SCI & IND RES +2
Liquid anti-rabies antibody formulations
InactiveCN101557799ANervous disorderPharmaceutical delivery mechanismPharmaceutical formulationPost-exposure prophylaxis
The present invention provides pharmaceutical antibody formulations, in particular liquid pharmaceutical formulations comprising anti-rabies virus antibodies. The formulations can be used in the post exposure prophylaxis of rabies.
Owner:JANSSEN VACCINES & PREVENTION BV
Rabies virus IgG antibody immune gold-labeled test paper and preparation method thereof
InactiveCN104360061AEasy to operateStrong specificityBiological material analysisAntigenNitrocellulose
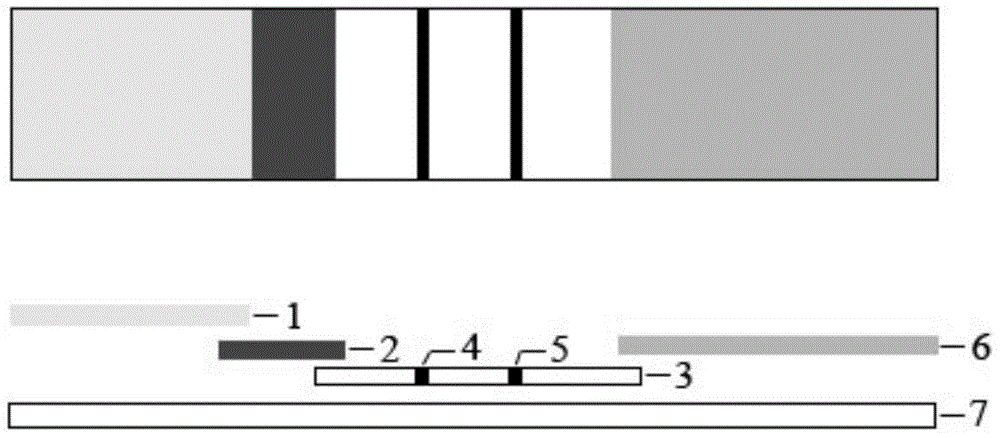
The invention relates to rabies virus IgG antibody immune gold-labeled test paper. The test paper consists of a serum treatment pad, a gold-labeled release pad, a nitrocellulose membrane, an absorbent pad and a back plate, wherein the serum treatment pad, the gold-labeled release pad, the nitrocellulose membrane and the absorbent pad are sequentially superposed and adhered to the back plate, and the superposition distance between every two parts is 2-3mm; a purified rabies virus antigen-colloidal gold compound is coated on the gold-labeled release pad; a detection line and a quality control line are coated on the nitrocellulose membrane; the detection line is fixedly provided with SPA; and the quality control line is fixedly provided with anti-dog rabies virus positive IgG. The test paper disclosed by the invention is easy to operate, high in specificity and high in sensitivity, special instruments and equipment and professionals are not needed, whether vaccine immunity animals generate antibodies on a protection level can be rapidly screened, and a reference basis is provided for vaccine immunity and immune procedure formulation of rabies.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Fully-humanized rabies virus resisting neutralizing antibody
ActiveCN106432485AStrong specificityGood neutralization effectImmunoglobulins against virusesAntiviralsNeutralizing antibodyDNA
The invention discloses a fully-humanized rabies virus resisting neutralizing antibody. DNA sequences for encoding the antibody and binding fragments thereof, host cells containing DNA, and an expression vector are included. The invention further discloses a preparation method of the fully-humanized rabies virus resisting neutralizing antibody with high affinity and application of the antibody in medicine for treating and / or preventing rabies.
Owner:CHANGCHUN BCHT BIOTECH
Recombinant rabies viruses in which canine distemper virus main immune genes are embedded and application of recombinant rabies viruses
InactiveCN109943576ALow costMicroorganism based processesAntiviralsRabies virus strainCanine distemper virus CDV
The invention discloses recombinant rabies viruses BNSP-CDV-F and BNSP-CDV-H in which canine distemper virus main immune genes are embedded. The recombinant viruses take a rabies virus strain SAD-B19as a framework, CDV-F and CDV-H genes shown in SEQ ID NO.1 and SEQ ID NO.2 are separately inserted between an N gene and P gene of a recombinant plasmid SAD-19 full-length cDNA, and finally recombinant rabies virus strains BNSP-CDV-F and BNSP-CDV-H are saved through a reverse genetic operation technology. The recombinant viruses BNSP-CDV-F and BNSP-CDV-H can express fusion protein and hemagglutinin protein of canine distemper viruses; after a vaccine prepared by mixing the recombinant viruses is used for immunizing animals, a great number of anti-rabies virus antibodies and main immune gene antibodies resistant to the canine distemper viruses can be induced and generated. The cost of the vaccine can also be reduced, and the recombinant viruses BNSP-CDV-F and BNSP-CDV-H have a good application and popularization prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Novel rabies virus fake virus system as well as preparation and application thereof
InactiveCN103173495AEliminate the effects ofHigh titerInactivation/attenuationFluorescence/phosphorescenceEpitopeHigh-Throughput Screening Methods
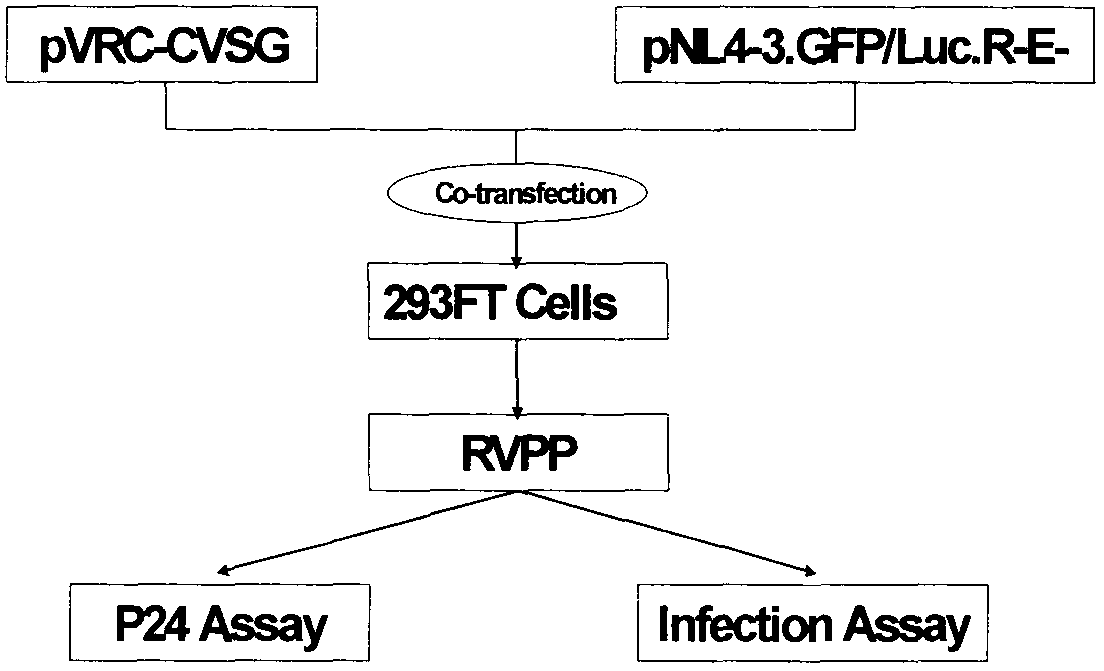
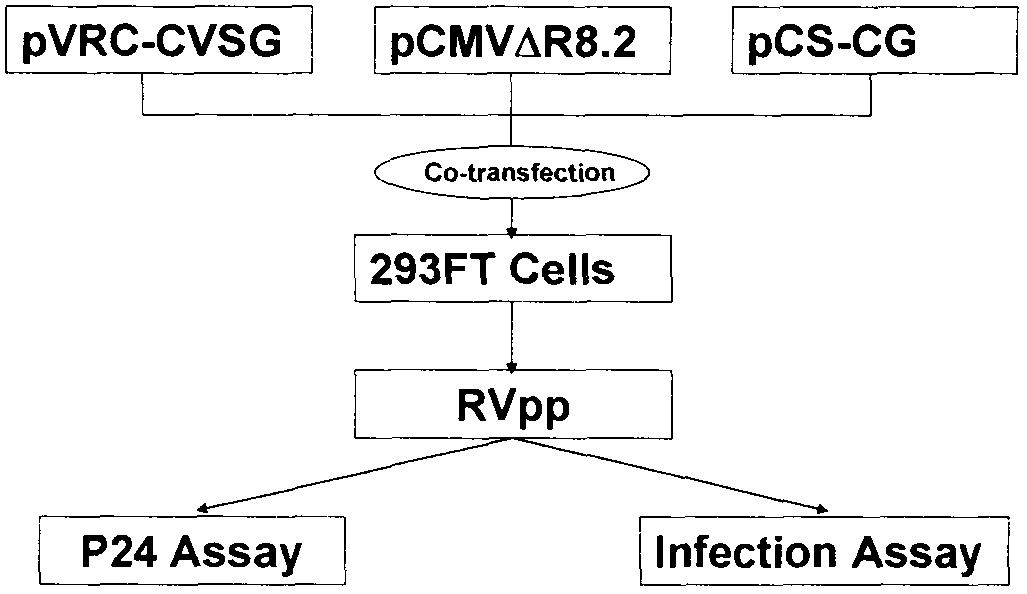
The invention relates to a novel rabies virus fake virus system as well as preparation and application thereof, belonging to the field of biotechnology. The novel rabies virus fake virus system comprises a carrier system and host cells, wherein the carrier system consists of pVRC-RVG series shuttle plasmids and auxiliary plasmids of a rabies virus glucoprotein gene; and when the shuttle plasmids and auxiliary plasmids in the carrier system are transfected to the host cells together, rabies virus fake viruses can be obtained from culture supernatant. The novel rabies virus fake virus system can be used for evaluating neutralizing antibodies of rabies viruses, screening neutral monoclonal antibody epitopes of the rabies viruses, carrying out high throughput screening on rabies virus medicaments, and preparing a novel rabies vaccine, so that the novel rabies virus fake virus system is a brand-new technical system for construction, preparation and application of rabies virus fake viruses.
Owner:中国疾病预防控制中心病毒病预防控制所
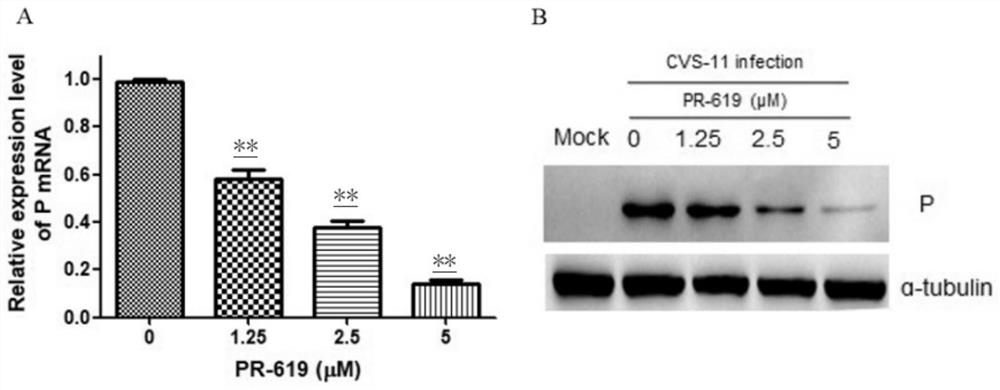
Application of deubiquitinating enzyme inhibitor in preparation of medicine for resisting rabies virus
ActiveCN112933090AAntiviralsHeterocyclic compound active ingredientsDeubiquitinating enzymeBioinformatics
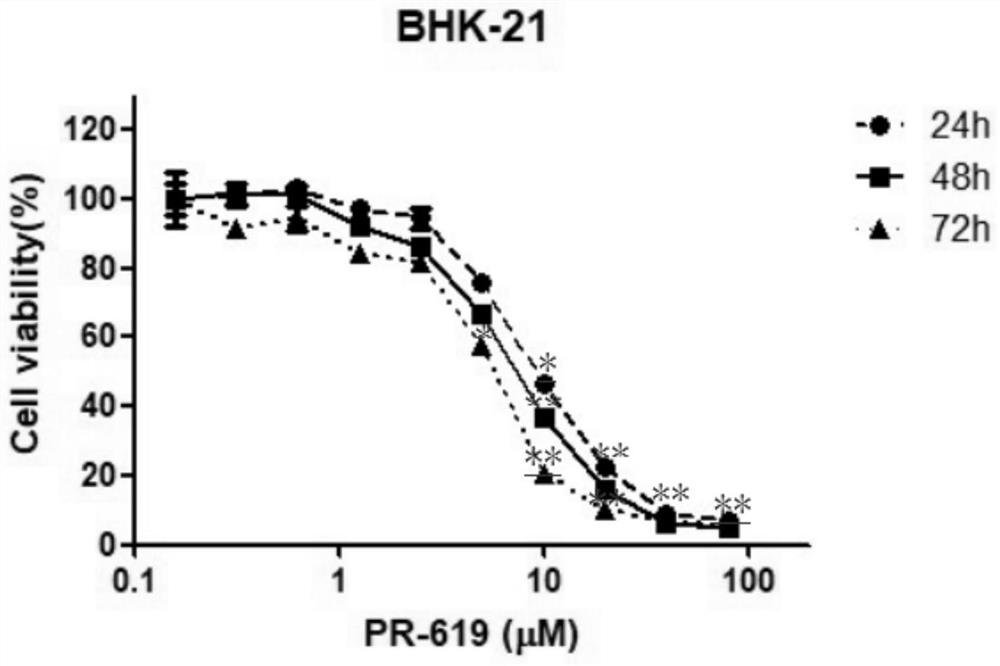
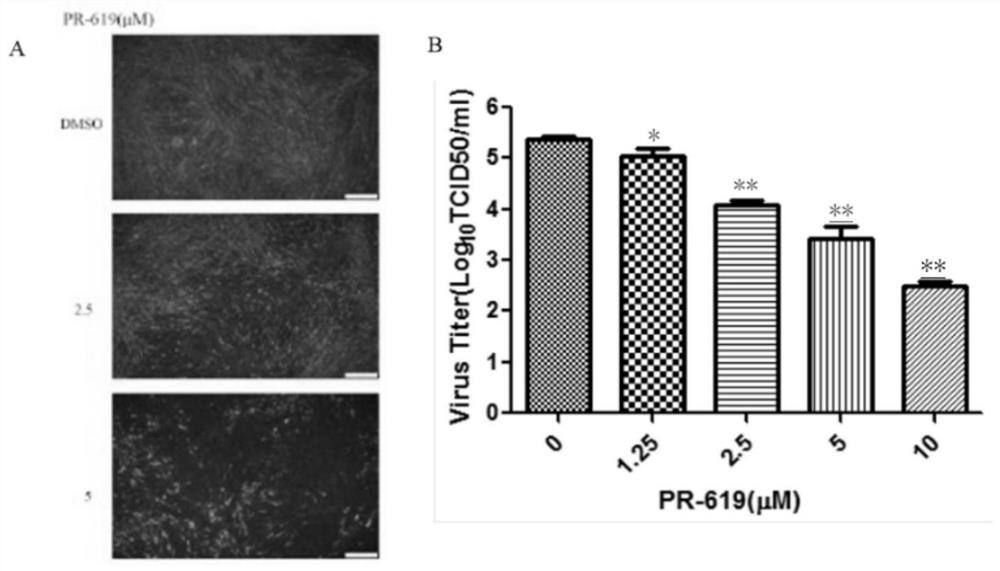
The invention provides application of a deubiquitinating enzyme inhibitor in preparation of a medicine for resisting rabies virus, and relates to the technical field of biological medicine. Experiments show that PR-619 has anti-rabies virus activity for the first time, and it is determined that PR-619 has prevention and treatment effects on rabies virus infection.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Recombinant poxviral vectors expressing both rabies and OX40 proteins, and vaccines made therefrom
The present invention provides vectors that contain and co-express in vivo or in vitro immunogenic polypeptides or antigens together with an OX40L polypeptide, which functions as a genetic adjuvant. Together, the immunogenic polypeptide and the OX40L polypeptide elicit an immune response in animal or human, which is greater than the immune response elicited by the immunogenic polypeptide alone. In a particular example, the invention provides vectors encoding a Rabies G immunogenic polypeptide and a canine OX40L genetic adjuvant, which vectors elicit strong immune responses in canine against rabies virus.
Owner:MERIAL INC
Recombinant rabies virus of chimeric canine parvovirus VP2 gene and application of recombinant rabies virus
PendingCN112852759ASsRNA viruses negative-senseViral antigen ingredientsRabies virus strainChimera Protein
The invention provides a recombinant rabies virus of a chimeric canine parvovirus VP2 gene, and belongs to the technical field of immunology. The recombinant rabies virus takes a rabies vaccine candidate strain SAD-B19 as a skeleton, and the VP2 gene of a CPV-2a strain currently popular in China is inserted into an SAD-B19 genome; the recombinant rabies virus strain of the chimeric VP2 protein is obtained through rescue, after immunization, high-level anti-rabies virus antibodies and anti-canine parvovirus VP2 antibodies can be induced to be generated, and the vaccine is low in cost, safe and effective.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Rabies virus antibody test paper, preparation method thereof and detection method thereof
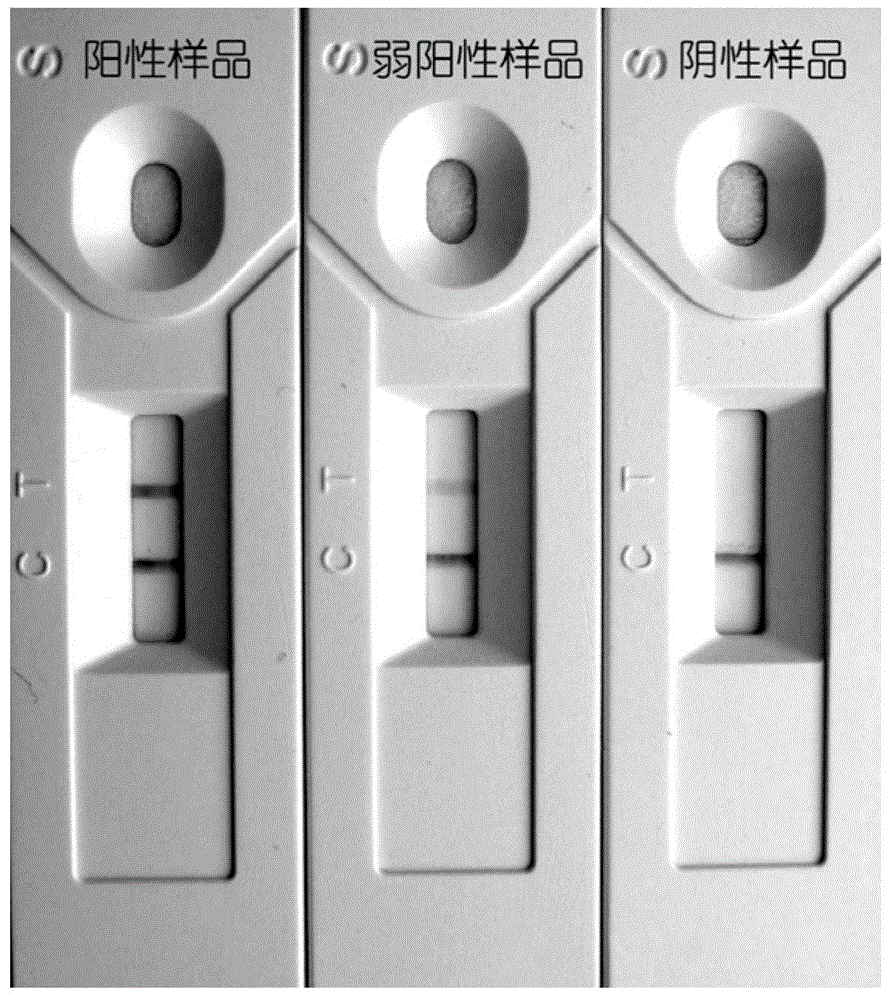
ActiveCN109959789AEliminate distractionsAvoid false positivesBiological testingAgainst vector-borne diseasesNeutralizing antibodyRabies Virus Antigen
The invention discloses rabies virus antibody test paper and a preparation method thereof and a detection method thereof, which belong to the technical field of antibody test paper. The problems of false positive risk and safety hazard in the prior art are solved. The test paper comprises, from one end to the other end, a sample pad which coats a rabies virus antigen, a gold standard pad which coats a monoclonal antibody against the rabies virus antigen, a nitrocellulose membrane which coats a detection line and a quality control line, and an absorbent pad, wherein the sample pad, the gold standard pad, the nitrocellulose membrane and the absorbent pad overlap each other and are attached to the upper surface of a backboard. The rabies virus antigen coated by the sample pad is rabies virus-like particles. According to the invention, the used labeled antigen is the rabies virus-like particles expressed by insect cells, which avoids false positives; the monoclonal antibody against the rabies virus G protein competes with a neutralizing antibody in the blood to improve the detection accuracy and sensitivity; the virus-like particles are free of nucleic acid components; and biosafety risks produced by the use of whole viruses as marker antigens are eliminated.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Anti-rabies virus G protein scFv20 antibody and application thereof
PendingCN112430264AImprove featuresHigh affinityImmunoglobulins against virusesAntiviralsPhage antibodiesAntiendomysial antibodies
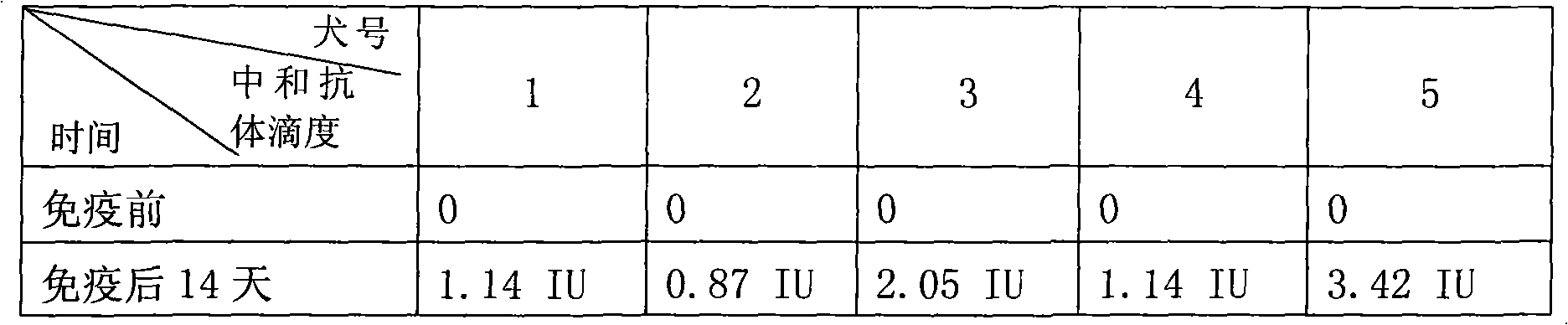
The invention provides an anti-rabies virus G protein scFv20 antibody and application thereof. A light chain amino acid sequence is shown as SEQ ID NO.1, and a heavy chain amino acid sequence is shownas SEQ ID NO.2. According to the invention, a phage antibody library technology is applied, 130 cases of peripheral blood lymphocytes of healthy volunteers immunized by rabies vaccines are used to construct a fully humanized anti-rabies virus scFv phage antibody library, and purified RVG protein is used as an antigen to enrich and screen the phage antibody library to obtain a strain of fully humanized anti-RVG protein scFv with neutralizing activity, named scFv20; wherein the neutralization titer is determined to be 0.5 IU / mg.
Owner:NANJING MEDICAL UNIV
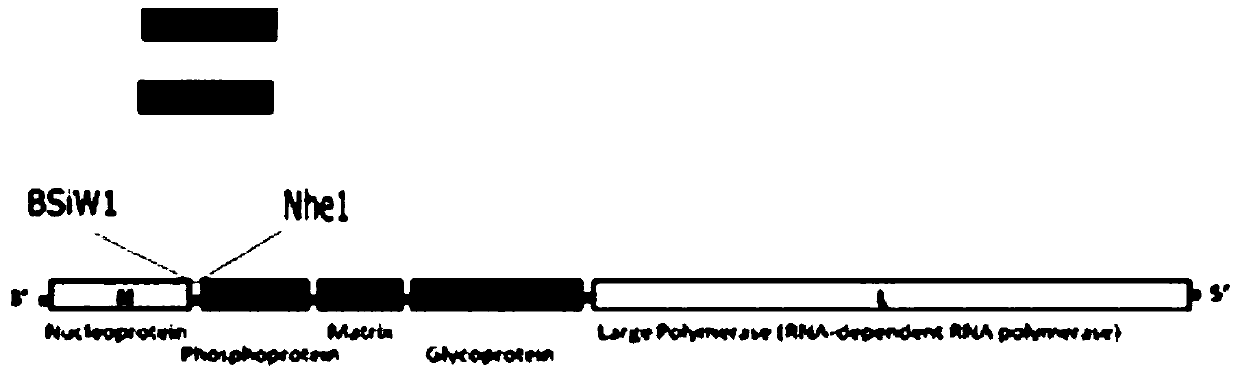
Recombinant virus of standard attack strain CVS-11 and preparation method thereof
InactiveCN102864126AStrong specificityImprove economyMicroorganism based processesViruses/bacteriophagesAntigenStaining
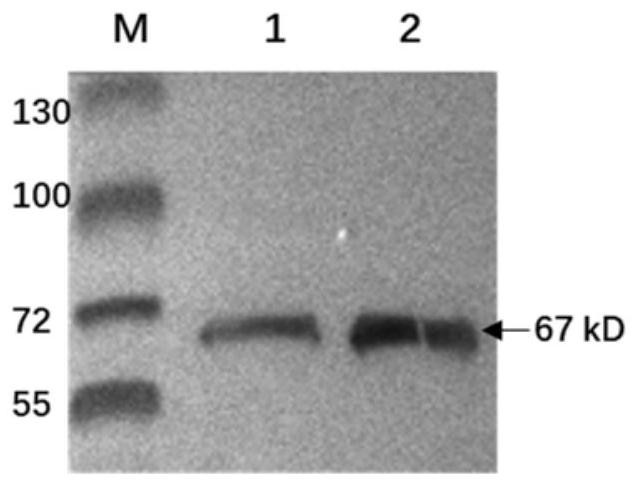
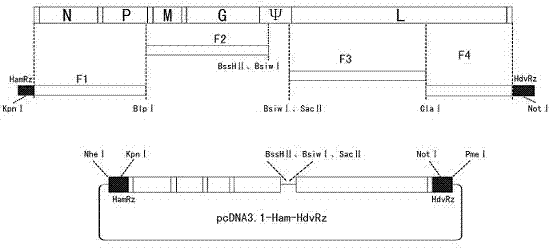
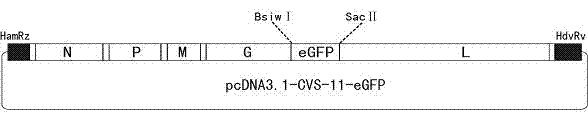
The invention provides a recombinant virus of standard attack strain CVS-11 and a preparation method thereof. The invention establishes a reverse genetic manipulation system for rabies virus international standard attack strain CVS-11, provides an effective platform for further modifying or reconstructing the CVS-11 genome structure, and lays foundation for researching pathopoiesis mechanism, antigen site, virulence determined site and the like of rabies virus on the molecular level. On the basis of the CVS-11 reverse genetic manipulation system, the invention establishes a recombinant virus rCVS-11-eGFP for saving international standard attack strain CVS-11. The rCVS-11-eGFP expresses eGFP in the proliferation process, and can be used for directly observing the proliferation state and diffusion of the virus. By utilizing the characteristics of no toxicity, capability of self-luminescence without dependence on any accessory factor or substrate, and the like, the rCVS-11-eGFP is used as a detection antigen for determining the anti-rabies virus neutralizing antibody titer, and no operations, such as stain fixation and the like, are needed in the result determination process, so that the invention is economic and convenient.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Human anti-rabies virus IgG antibody ELISA test kit
ActiveCN101936997BMake up for the shortcomings of low sensitivityHigh sensitivityDepsipeptidesMaterial analysisAntigenPositive control
The invention relates to a human anti-rabies virus IgG antibody ELISA test kit. An ELISA plate is firstly coated with an anti-rabies virus monoclonal antibody, wherein the coating buffer solution is a 0.05M carbonate buffer solution of which the pH value is 9.6, and the coating amount is 0.1-1ug per hole; a blocking solution is a BSA or skimmed milk of which the mass concentration is 1-10%; the ELISA plate is coated with a rabies virus purified antigen after being blocked, wherein the coating amount is 0.1-1ug per hole; a sample diluent is a 0.01mol / L phosphate buffer solution (PBS) which contains bovine serum albumin (BSA) with a mass concentration of 0.1-10% and NaN3 with a mass concentration of 0.01-0.05 and has a pH value of 7.2-7.4; an enzyme conjugate is a horse radish peroxidase-mouse anti-human IgG enzyme conjugate; a concentrated cleaning solution is a 0.01mol / L PBS which contains tween-20 with a volume concentration of 0.05% and has a pH value of 7.2-7.4; a zymolyte A solution is a 3,3'-5,5'-tetramethyl benzidine solution, and a zymolyte B solution is an oxydol solution; and a stop solution is a 1mol / L H2SO4 solution, and a positive control and a negative control are arranged in the kit. The specificity of the kit is up to 100%, and the sensitivity is 1:640. The kit is used for evaluating the immunity effect of humans inoculated with rabies vaccines.
Owner:WUHAN CHOPPER BIOLOGY
Anti-rabies virus monoclonal antibody and application
InactiveCN101560255BExperimental costs are highEasy to operateImmunoglobulins against virusesTissue cultureAntigen bindingBlot
Owner:NANJING MEDICAL UNIV +1
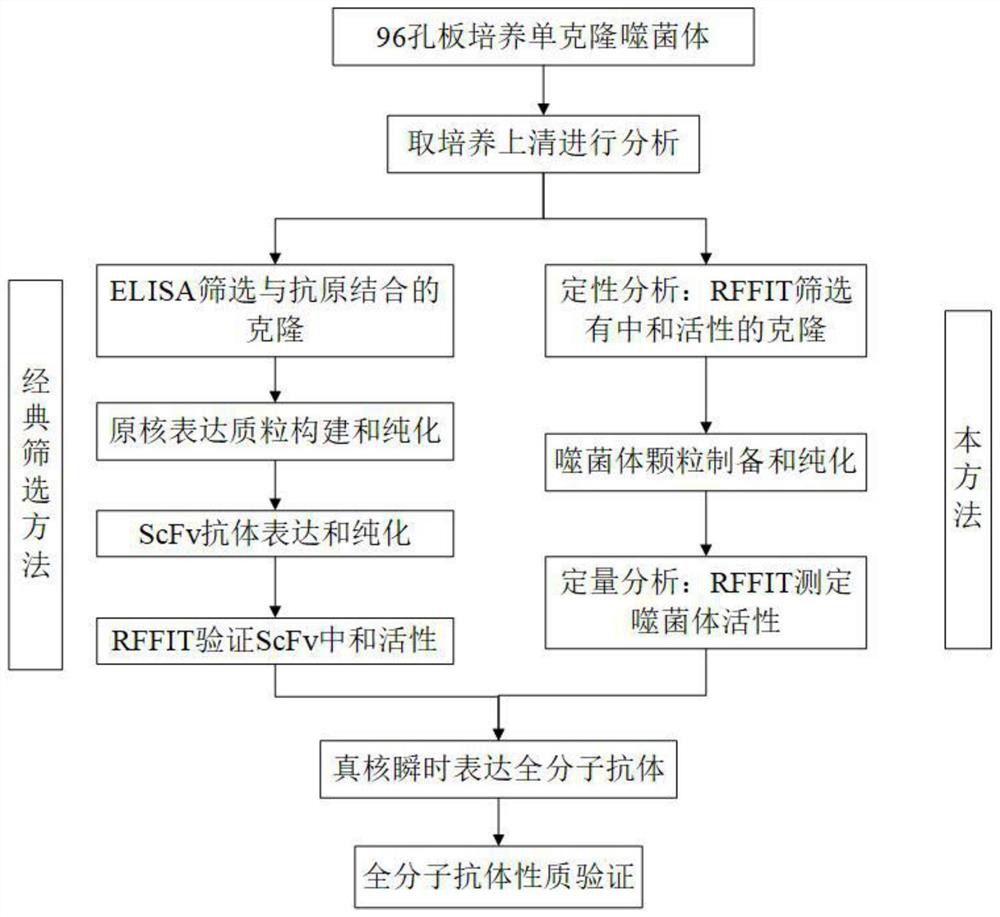
Method for screening and verifying anti rabies virus neutralizing antibodies from phage antibody library and screening kit
ActiveCN110655571AModerately high in vitro anti-rabies virusHigh activityImmunoglobulins against virusesPhage antibodiesNeutralizing antibody
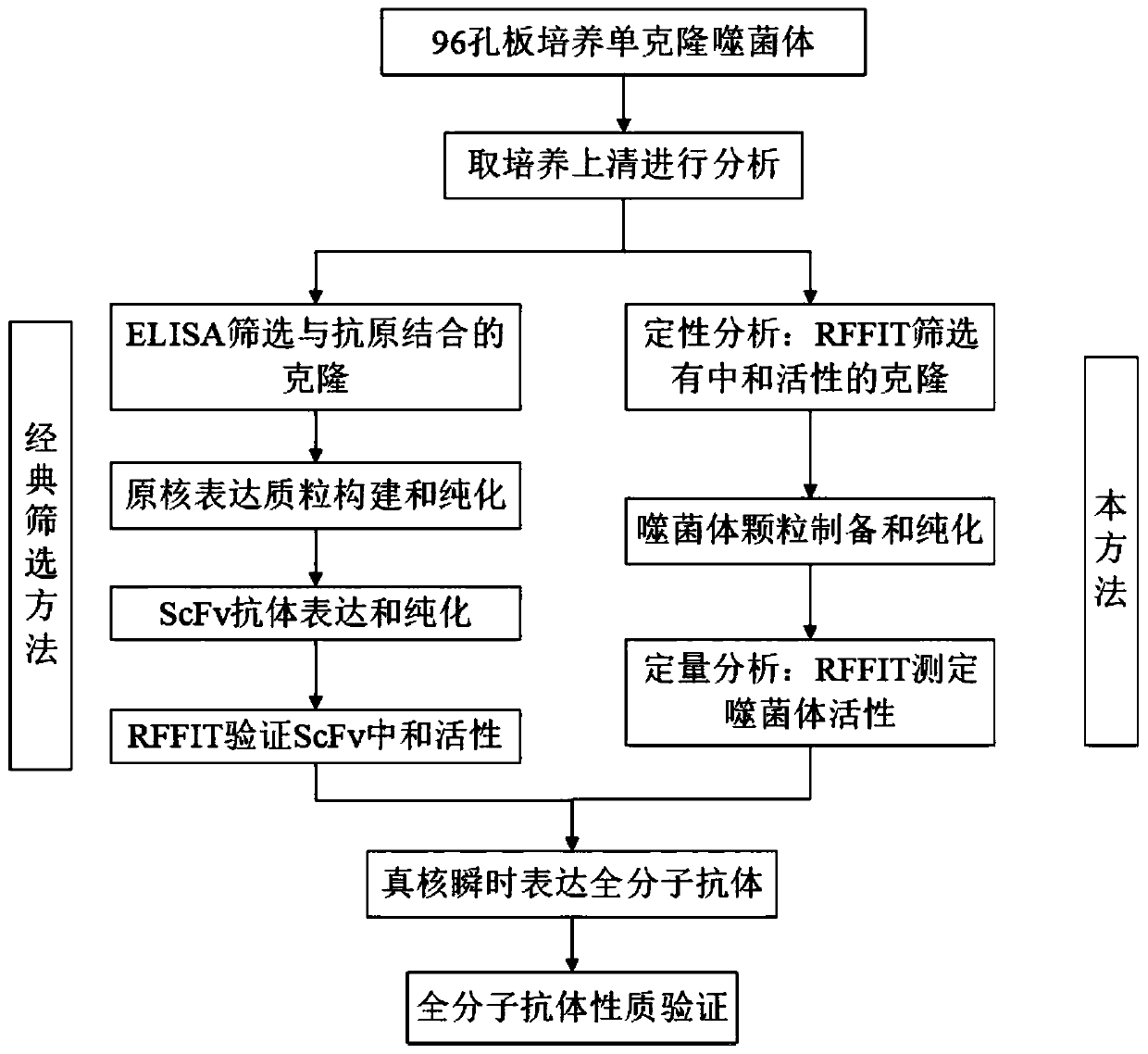
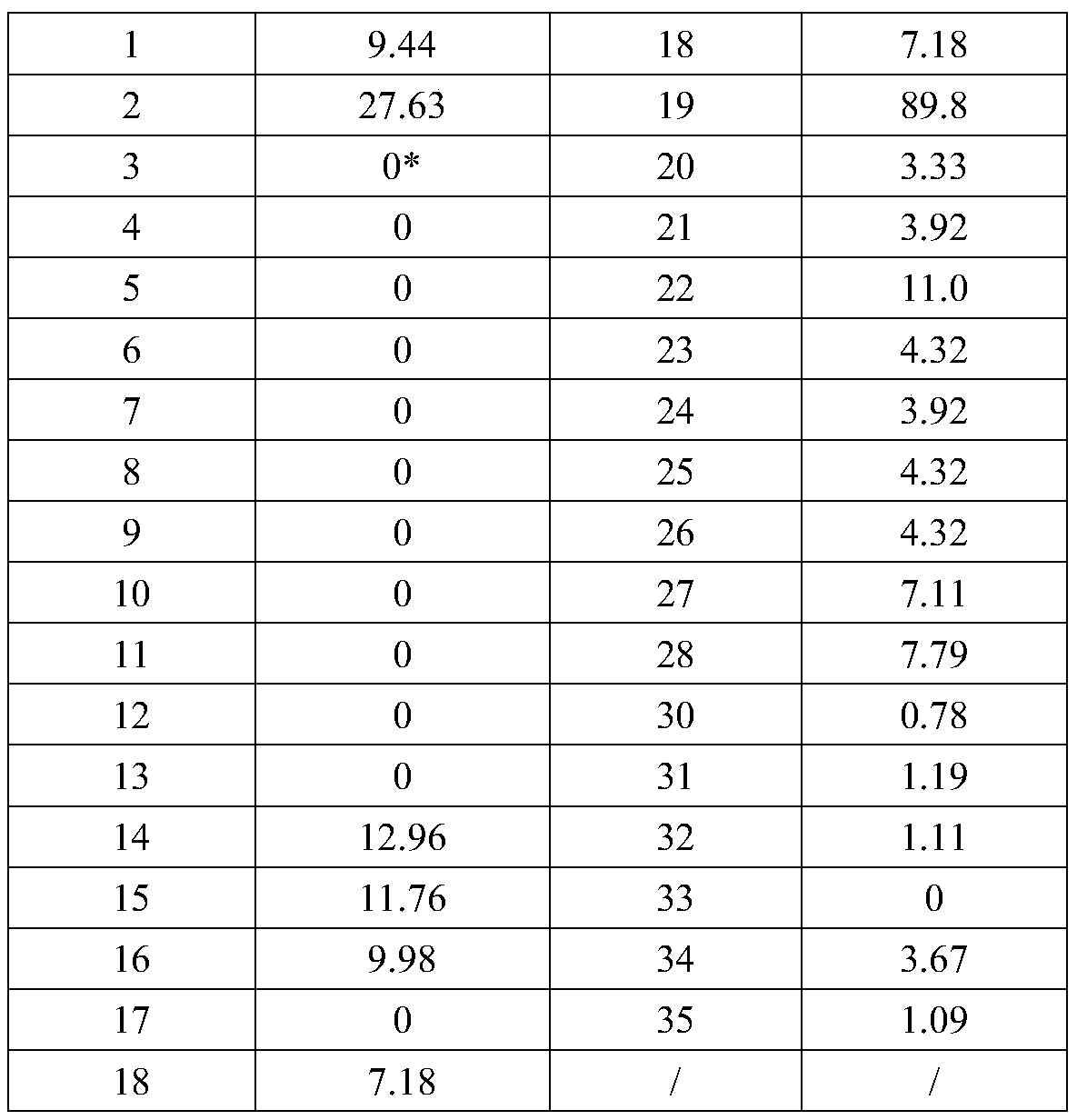
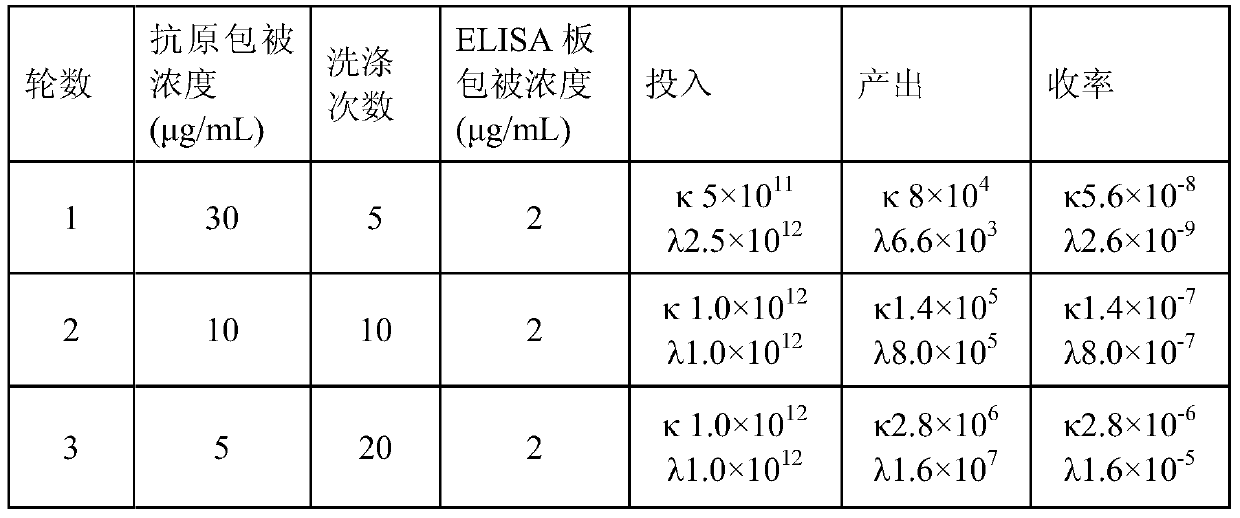
The invention relates to the technical field of biomedical engineering, and discloses a method for screening and verifying anti rabies virus neutralizing antibodies from a phage antibody library. Themethod includes the steps that A, a monoclonal phage antibody is cultured, and a culture supernatant is taken; B, qualitative analysis is performed, specifically, the culture supernatant in the step Ais placed in a 96-well plate, and co-incubated with a diluted FITC labeled anti rabies virus nucleoprotein antibody after neutralization with a DMEM medium and neutralizing viruses, BSR cell suspension culture and precooled acetone fixation sequentially, clone sequencing that can significantly inhibit virus infection is selected, repeated sequences are eliminated, and anti rabies virus antibody sequences with different neutralization activities are acquired preliminarily; C, quantitative analysis is performed, specifically, different sequences with neutralization activities are selected, phage antibody particles are prepared, purified and diluted for a certain number of times, and then the neutralization activity in vitro is determined by a RFFIT method; and D, the transient expression and activity analysis of a whole molecule antibody are further performed.
Owner:LANZHOU INST OF BIOLOGICAL PROD
A kind of anti-rabies virus specific human antibody and its application
ActiveCN104193823BStrong neutralizing activityImmunoglobulins against virusesAntiviralsImmune therapyNeutralizing antibody
Owner:LANZHOU INST OF BIOLOGICAL PROD
Method for constructing virus live vector recombinant vaccine by utilizing transposon
InactiveCN101850116BGenetic material ingredientsViruses/bacteriophagesRecombinant vaccinesSwine Fever Virus
The invention discloses a method for constructing virus live vector recombinant vaccine by utilizing transposon. Green fluorescent protein is taken as a report gene, expression boxes respectively expressing rabies virus glycoprotein and swine fever E2 protein genes are constructed and are cloned to the shuttle vector of the transposon, under the action of mediation of transposase, recombination with purified canine adenovirus type II virus and herpes virus type I entire genome are respectively carried out, then transfection agent (liposome and the like) is utilized to respectively transfect the recombination product with MDCK and Vero cells, thus obtaining four strains of recombinant viruses taking green fluorescent protein as report gene, namely recombinant canine adenovirus type II virus expressing glycoprotein, recombinant canine adenovirus type II virus expressing E2 protein, recombinant herpes virus type I expressing glycoprotein and recombinant herpes virus type I expressing E2 protein. Immunity test shows that the canine adenovirus type II virus expressing E2 gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to swine fever virus infection in swine and canine adenovirus type II virus expressing glycoprotein gene and herpes virus type I live vector recombinant vaccine all can induce immunoreaction resistant to rabies virus infection in dog.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Method and screening kit for screening and validating anti-rabies virus neutralizing antibody from phage antibody library
ActiveCN110655571BHigh activityMeet the requirements for antibody activityImmunoglobulins against virusesPhage antibodiesRabies virus
Owner:LANZHOU INST OF BIOLOGICAL PROD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com