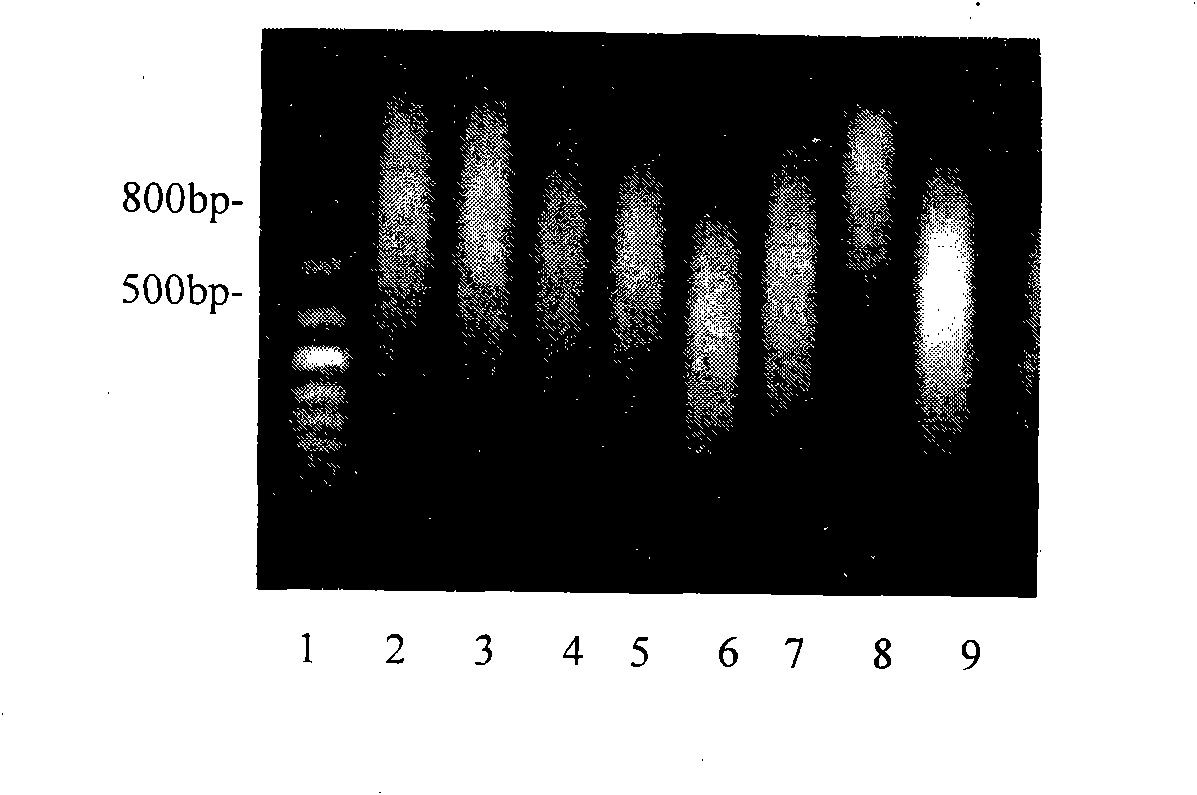
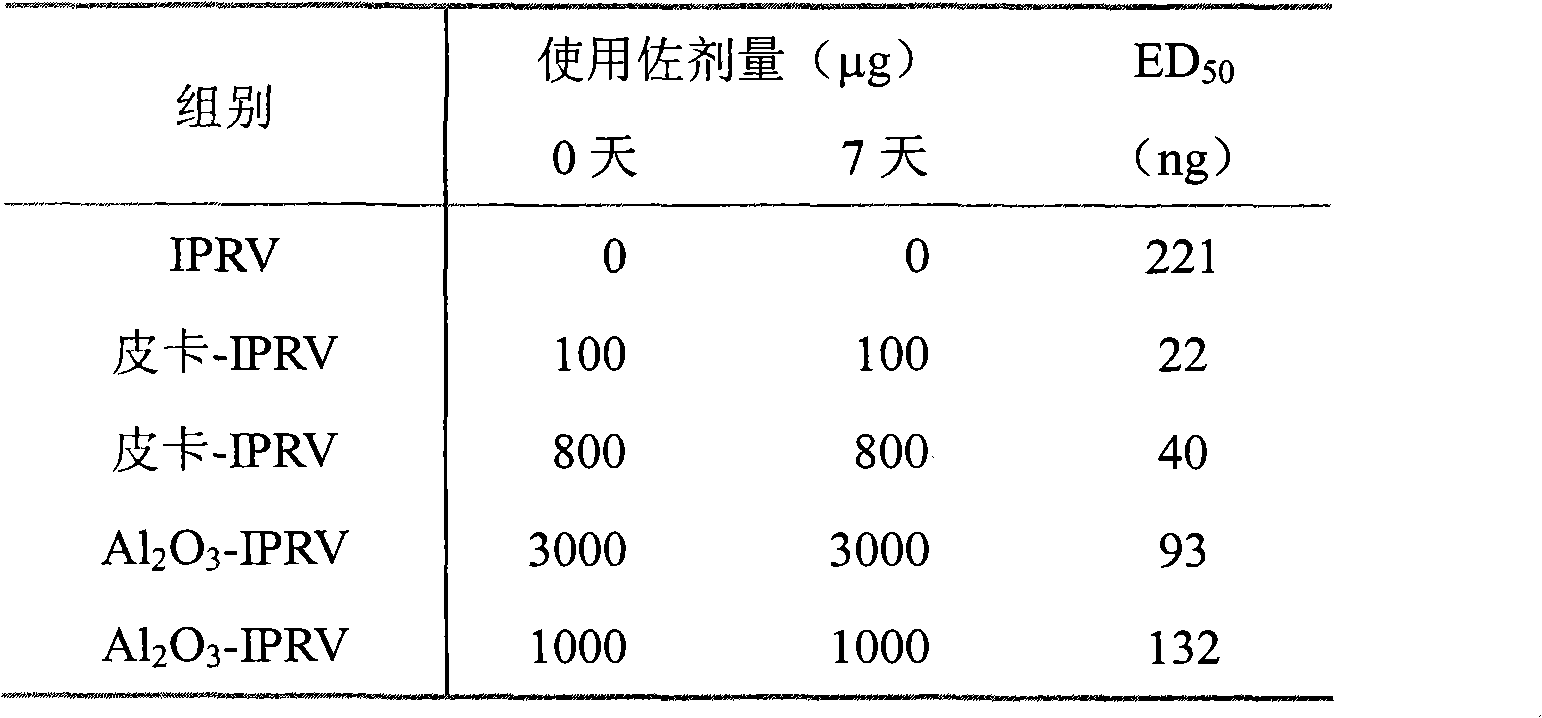
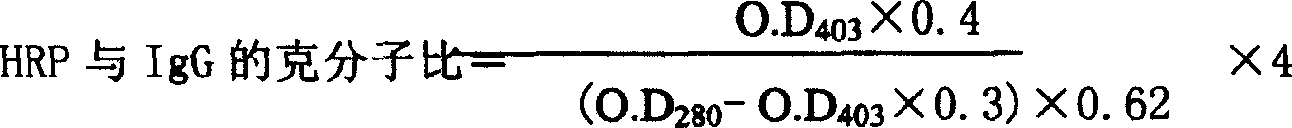Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Rabies Virus Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
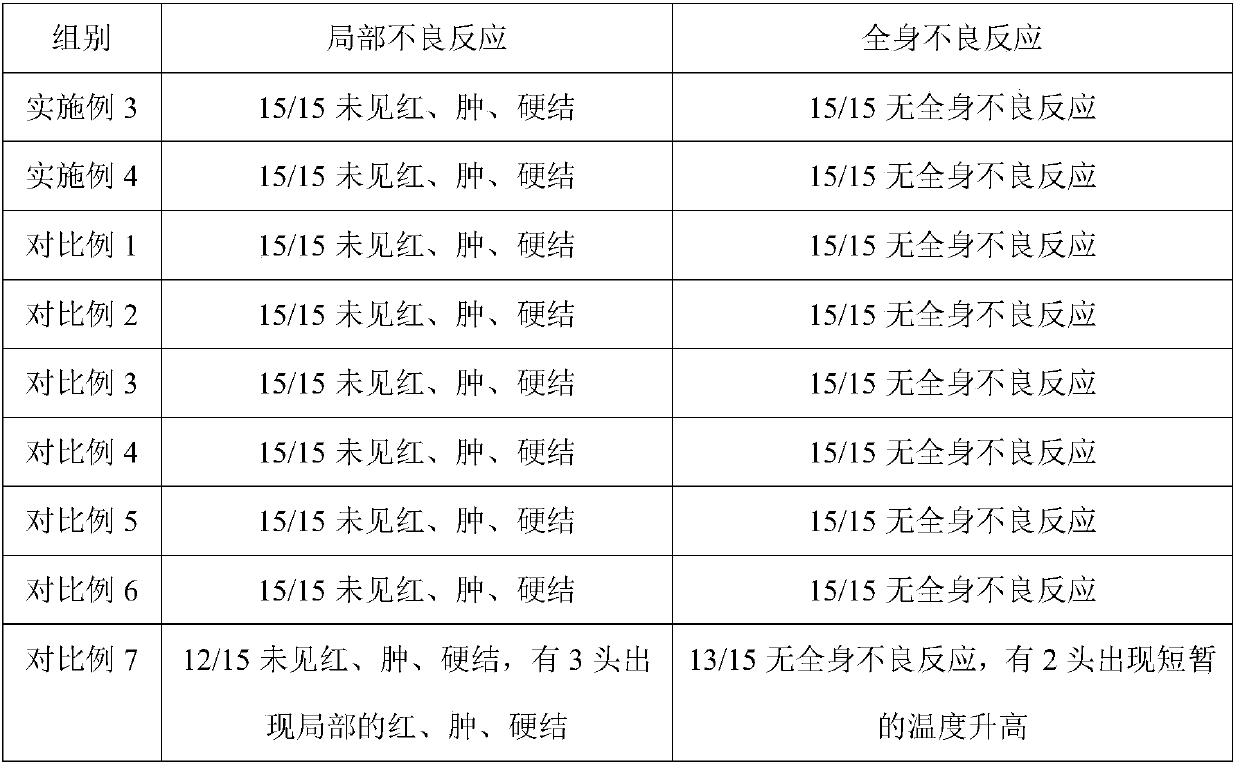
Detection of Rabies Virus Antigen in Dog Saliva Using a Latex Agglutination Test S. Kasempimolporn , * W. Saengseesom , B. Lumlertdacha , and V. Sitprija Queen Saovabha Memorial Institute (World Health Organization Collaborating Center for Research on Rabies Pathogenesis and Prevention), Thai Red Cross Society, Bangkok, Thailand
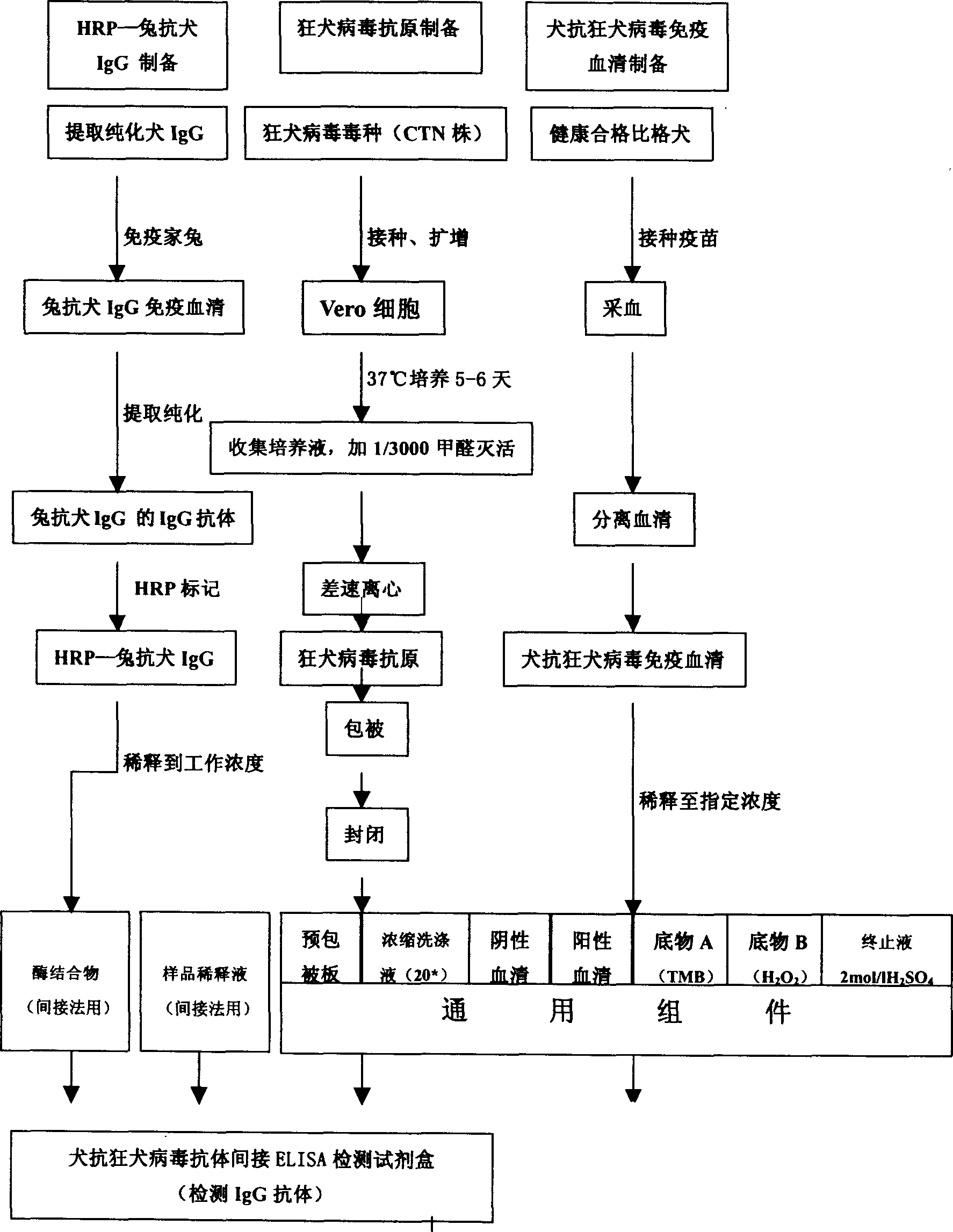
IgG kit for detecting streetvirus of dogs using indirect enzyme immunosorbent assay and preparation method thereof
The invention refers to a kind of detecting reagent box and the manufacturing method, concretely refers to the reagent which is indirect enzyme immune sorption experiment for detecting rabies virus IgG and the manufacturing method. The reagent box compositions are: beforehand enclosed rabies virus antigen enzyme label board, sample diluting solution, HRP-rabies resisting IgG enzyme compound, condensed washer solvent, substrate and stopping liquid. The specificity of the reagent can reach 100%; the sensitivity is 1:640; the accuracy (the variation coefficient) is 6.98%. The reagent uses indirect ELISA to detect the rabies virus IgG antibody.
Owner:湖北省预防医学科学院
Rabies antibody gold immunochromatography assay testing indicator paper and preparation technique
This invention provides one dog rabies virus antigen glue gold immune chromatography test paper and its process, which comprises the following steps: according to antigen antibody immune combination basic principle to label the sheep IgG by glue gold label covering on the glass fiber film as combination pad; covering the purification rabies virus antigen and gene engineer expressed antigen and rabit IgG covering on the NC film as test line and quality control line; when testing the antibody, forming antibody combination to expose red band.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Hydrophobia vaccine freezing drying preparations for stable human beings and the preparations thereof
ActiveCN101095950AImprove stabilityNo pollution in the processPowder deliveryAntiviralsFreeze-dryingMedicine
The invention relates to a kind of hydrophobia vaccine for human, which in detail relates to a freeze-drying hydrophobia vaccine for human and its preparation method. It is contained in physiological soluble buffer solution with pH being 7-8, the sodium chloride weight proportion is 0.4-1.0%, one or several from mycose, sucrose, gelatin, maltose and dextran is used as stabilizing agent, the concentration is 0.5-5%; the weight proportion of shaping agent for freeze-drying agent is 1-5%, and the purified hydrophobia viral antigen concentration is 4-20 IU per ml.
Owner:LIAONING CHENGDA BIOTECH
Method for preparing rabies virus antigen
ActiveCN101307317AReduce consumptionImprove securityAntiviralsDepsipeptidesAntigenBaculovirus expression
The invention provides a method for making rabies virus antigen. The method comprises the following steps that: the antigen gene of rabies virus or the combined expression combination of the antigen gene is respectively cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the transfer expression carrier and baculovirus undergo cotransfection so as to carry out homologous recombination or transposition, thereby obtaining recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding rabies antigen; and the expressed antigen is ingathered and purified so as to obtain rabies virus antigen. The method adopts a baculovirus expression system to make safe and efficient rabies virus antigen in a domestic silkworm bioreactor; moreover, due to having extremely high safety, the made antigen can be directly used to make injection vaccine and oral vaccine used for animal immunization. The method can substantially reduce the production cost of rabies virus antigen, and has the advantages of safety, high efficiency, less energy consumption and low cost, etc.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Vaccine composition and preparation method and application thereof
The invention provides a method for preparing a vaccine composition. The method comprises the following steps: (1) preparing a liquid containing a porcine pseudorabies virus; (2) adding a nonionic surfactant or a solution containing the nonionic surfactant into the liquid in the step (1) and solubilizing envelope proteins in the liquid containing the porcine pseudorabies virus; (3) removing the nonionic surfactant in the step (2) to obtain a purified porcine pseudorabies virus antigen; and (4) preparing the vaccine composition by using the antigen prepared in the step (3). The invention further provides the vaccine composition containing porcine pseudorabies antigen prepared by the preparation method. Immunosuppression components are removed from the vaccine composition and meanwhile, immunity active ingredients are retained to the maximum extent. The vaccine composition has a relatively good protective effect on porcine lung diseases; when the vaccine composition and a live virus antigen of porcine reproductive and respiratory syndrome are jointly acted, the vaccine composition has no inhibiting effect to the live virus antigen, showing that the vaccine composition can be jointly used with various other antigen components.
Owner:PU LIKE BIO ENG
Kit for detecting rabies virus antibody
ActiveCN108872572AImprove featuresIncreased sensitivityBiological material analysisAntigenMagnetic bead
The invention discloses a kit for detecting a rabies virus antibody, which belongs to the technical field of biological diagnosis. The kit comprises a magnetic bead coupled with a rabies virus antigen, a second antibody combined with the rabies virus antibody and provided with a marker, a rabies virus antibody standard product, a rabies virus antibody quality control product, a sample dilute solution, and a washing solution, wherein the DNA sequence of the rabies virus antigen is as shown by Seq No. 1, and an amino acid sequence of the rabies virus antigen is as shown by Seq No. 2. By adoptingthe kit, a to-be-detected substance can be separated by adopting a tubular magnetic ball, then the detection is performed by adopting a chemical luminescent method, the interference of impurities canbe avoided, the possibility of false positive can be reduced, and the detection sensitivity can be improved.
Owner:GUANGZHOU YOUDI BIOTECH CO LTD
Method for detecting canine rabies virus antibody and detection kit
The invention provides a method for detecting canine rabies virus antibody (IgG) and a detection kit. The kit is composed of a coated plate and a reagent reaction system, and comprises a rabies virus antigen-coated reaction plate, a standard substance, positive contrast serum, a washing lotion (20X), a sample weak solution, an enzyme-marked combination substance, a developer A, a developer B and a stopping solution. The method is characterized in that the method uses an ELISA method to determine that the content of the canine rabies virus antibody (IgG) reaches a absorbance corresponding to a protective level by detecting the standard substance of the kit, and by compared the absorbance of the sample to be detected with the absorbance of the standard substance, the content of the canine rabies virus antibody (IgG) contained in the sample to be detected is judged whether to reach an immunization protective level. The method and the kit can simultaneously detect a lot of samples, and a detection result is remarkably with a result by a neutralization experiment, has high accuracy degree, is suitable for monitoring an immunization inoculation effect and determining an individual immunization state, and can be used for investigating animal eqpidemic diseases.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD
Rabies virus antibody (IgG) enzyme-linked immunoassay kit and detection method thereof
The invention provides a rabies virus antibody (IgG) enzyme-linked immunoassay kit and a detection method thereof. The detection kit is composed of a purified rabies virus antigen coated microporous plate, enzyme labeled SPA and other reagents. The detection method adopts an indirect method principle to detect the rabies virus IgG antibody in human or animal serum or blood plasma, and is suitable for rabies vaccine immunized serology effect evaluation and epidemiology investigation. An enzyme labeled antibody applied in the invention is a Staphylococal protein A (SPA), and the SPA can be combined with an Fc fragment in IgG molecules in human or mammal serum, so the kit provided by the invention has all the characteristics of an ELISA kit, can be used for human rabies virus antibody detection, and can also be used for detecting the immune effect of various species of animals.
Owner:成大生物(本溪)有限公司
Dog anti rabies virus antibody colloidal gold immunochromatography assay detection reagent plate and preparation method thereof
This invention relates to one anti-rabies virus antigen gold immune analysis test agent board and its process method, which comprises the following steps: paving glass fiber paper and aqua fortis fiber film on the underlay film of polyethylene board and polychloroethylene underlay film; covering the film onto the test line and comparison line; the test line is added with gold detector ester polymer film; the comparison line side is added with water absorptive pad; the test line is to cover the virus protein. This invention process method is to cover virus protein analysis film by glue gold label rabbit anti-virus IgG multi-clone antigen to process gold detection combination pad.
Owner:WUHAN J H BIO TECH
Vaccine product with respectively packaged freeze-dried vaccine and dilute solution thereof and method for preparing dilute solution
The invention provides a vaccine product with respectively packaged freeze-dried vaccine and dilute solution thereof and method for preparing the dilute solution, which aims to solve the problem that the patent No. 93105862.7 records a vaccine in liquid state made by the combination of polyinosinic cytidilic acid containing kanamycin and calcium ions with lyssa virus antigen, both of which are in liquid states, has a guarantee period that is strictly limited to result in the limitation of the application of the product; and the method for preparing the adjuvant of polyinosinic cytidilic acid containing kanamycin and calcium ions is not disclosed in the patent No. 93105862.7. The invention has the following essentials: (1) freeze-dried vaccine and dilute solution thereof are respectively packaged, and the dilute solution is the polyinosinic cytidilic acid containing kanamycin and calcium ions; (2) the method for preparing the dilute solution. The invention overcomes the defect that the freeze-dried vaccine can not use PIKA adjuvant, and widens the practical application range of the PIKA adjuvant.
Owner:LIAONING YISHENG BIOLOGY PHARMACY
Porcine circovirus II type-porcine pseudorabies double-combination vaccine, and preparation methods and application thereof
InactiveCN103920146ALow costReduce the number of vaccinationsViral antigen ingredientsAntiviralsImmune effectsCircovirus
The invention relates to a porcine circovirus II type-porcine pseudorabies double-combination vaccine which is characterized by including at least one porcine circovirus II type antigen and at least one porcine pseudorabies virus antigen. The invention also relates to two preparation methods of the double-combination vaccine. One method comprises the steps: inactivating the porcine circovirus II type to obtain a porcine circovirus II type inactivated vaccine; and mixing the porcine pseudorabies virus antigen with a freeze-drying protective agent to obtain a porcine pseudorabies freeze-drying living vaccine, and then dissolving the porcine pseudorabies freeze-drying living vaccine with the inactivated vaccine as a diluent to obtain the porcine circovirus II type-porcine pseudorabies double-combination vaccine composition. The other method comprises the steps: mixing the porcine circovirus II type antigen with the porcine pseudorabies virus antigen in proportion, and then mixing with the freeze-drying protective agent to obtain the double-combination freeze-drying vaccine. With use of the double-combination vaccine, the cold chain operating cost can be greatly reduced; and the combination vaccine has a synergistic role in the immune effect on the porcine pseudorabies virus antigen, and does not affect the immune effect of the porcine circovirus antigen.
Owner:PU LIKE BIO ENG
Test paper for colloidal gold immunochromatograohic assay of IgG (immunoglobulin G) antibody of dog anti-rabies virus and preparation method of test paper
ActiveCN103399154AQuick checkMeet the needs of on-site testingMaterial analysisMonoclonal antibodyColloid
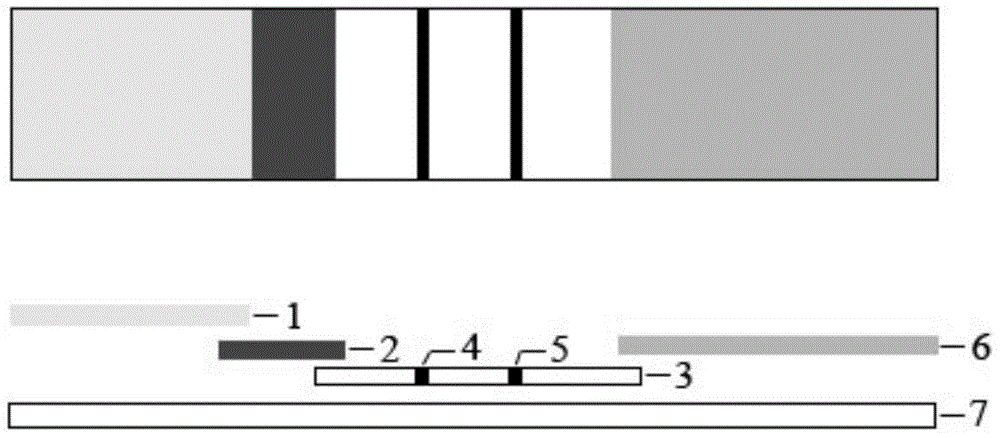
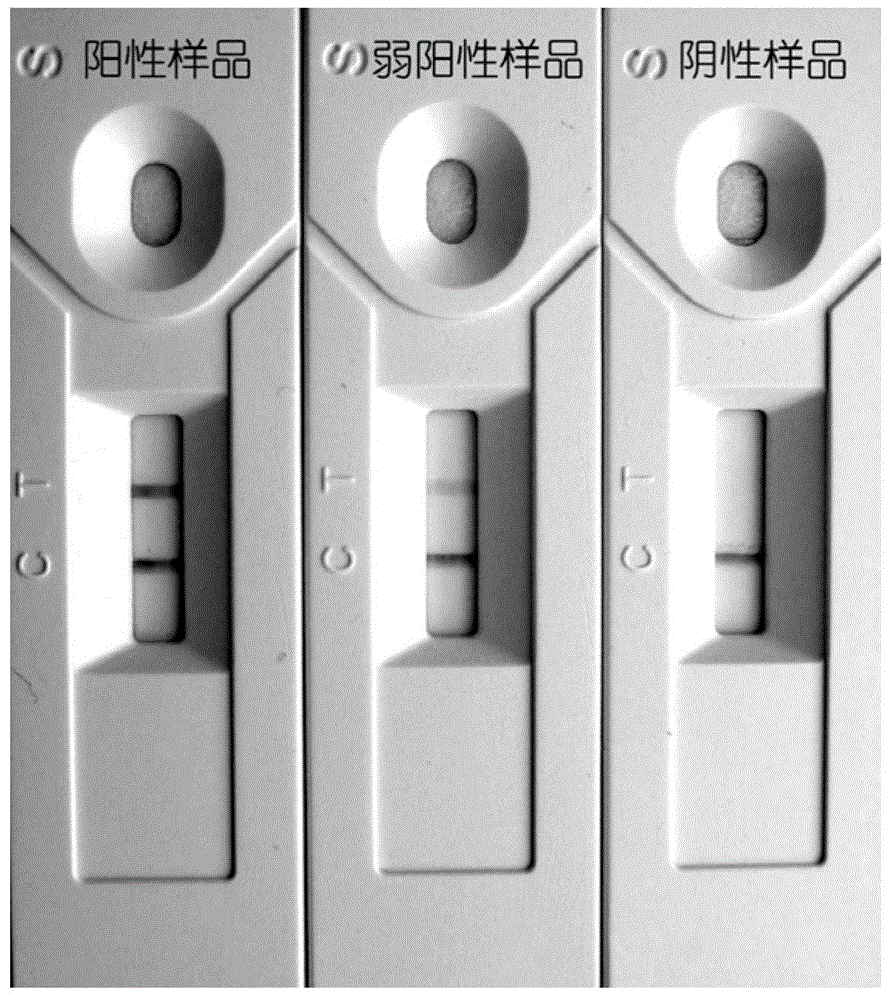
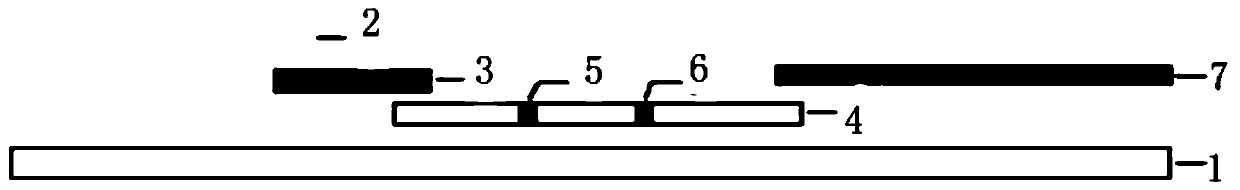
The invention discloses a piece of test paper for colloidal gold immunochromatograohic assay of an IgG (immunoglobulin G) antibody of dog anti-rabies virus and a preparation method of the test paper. The test paper comprises a sample absorbing region, a gold-labeled probe region, a solid-phase antibody region, a water absorbing region and a supporting plate, wherein the sample absorbing region, the gold-labeled probe region, the solid-phase antibody region and the water absorbing region are laid on the supporting plate and are partially overlapped in sequence; the sample absorbing region is coated with rabies virus antigen; the gold-labeled probe region is coated with a gold-labeled probe 1 and a gold-labeled probe 2 which are a monoclonal antibody of the colloidal gold-labeled rabies virus and rabbit IgG respectively; the solid-phase antibody region has a control line C1 (coated with a goat anti-rabbit IgG antibody), a testing line T (coated with a goat anti-dog IgG antibody), a control line C2 (coated with a goat anti-rabbit IgG antibody) and a control line C3 (coated with a goat anti-mouse IgG antibody). The test paper disclosed by the invention has the advantages that the test is fast, the accuracy rate is high, the specificity is strong, the test paper is simple and convenient to carry and operate, and the level of the rabies virus antibody is judged according to the color depths of the control lines.
Owner:WUHAN CHOPPER BIOLOGY
Porcine parvovirus-porcine pseudorabies combined inactivated vaccine, and preparation method and application thereof
InactiveCN105521488AImprove securityEnough protectionViral antigen ingredientsAntiviralsAntigenAdjuvant
The invention discloses a porcine parvovirus-porcine pseudorabies combined inactivated vaccine, and a preparation method and an application thereof. The preparation method comprises the following steps: respectively inactivating a porcine parvovirus liquid and a porcine pseudorabies liquid with pyrrole to obtain a porcine parvovirus antigen and a porcine pseudorabies antigen, and mixing the porcine parvovirus antigen and the porcine pseudorabies antigen with an oil phase adjuvant to prepare the combined inactivated vaccine. No immunosuppression exists between the two antigen components in the combined inactivated vaccine, the combined inactivated vaccine prepared from the porcine parvovirus antigen and the porcine pseudorabies antigen according to a volume ratio of 2:3 has better safety and immunoprotection effects than single vaccines, and the porcine parvovirus and the porcine pseudorabies can be substantially immunoprotected through one-shot syringe injection. The porcine parvovirus-porcine pseudorabies combined inactivated vaccine has the advantages of good safety, avoiding of untoward effects of multi-time immunoprophylaxis, reduction of the immunization cost, and use convenience, and can be applied in effective prevention of the porcine parvovirus and the porcine pseudorabies.
Owner:哈药集团生物疫苗有限公司
Test paper bar for testing colloidal gold of protective antibody of rabies virus
InactiveCN1963509AThe result is clear and easy to distinguishEasy to operateMaterial analysisViral glycoproteinColloid
This invention provides one glue gold test bar to test rabies virus protective antibody, which covers rabies virus protein and rabies multiple clone antibodies on the NC film and combines glue gold rabies virus antigen and applies film analysis double antigen clamper method and tests specimen rabies virus protective antibody.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
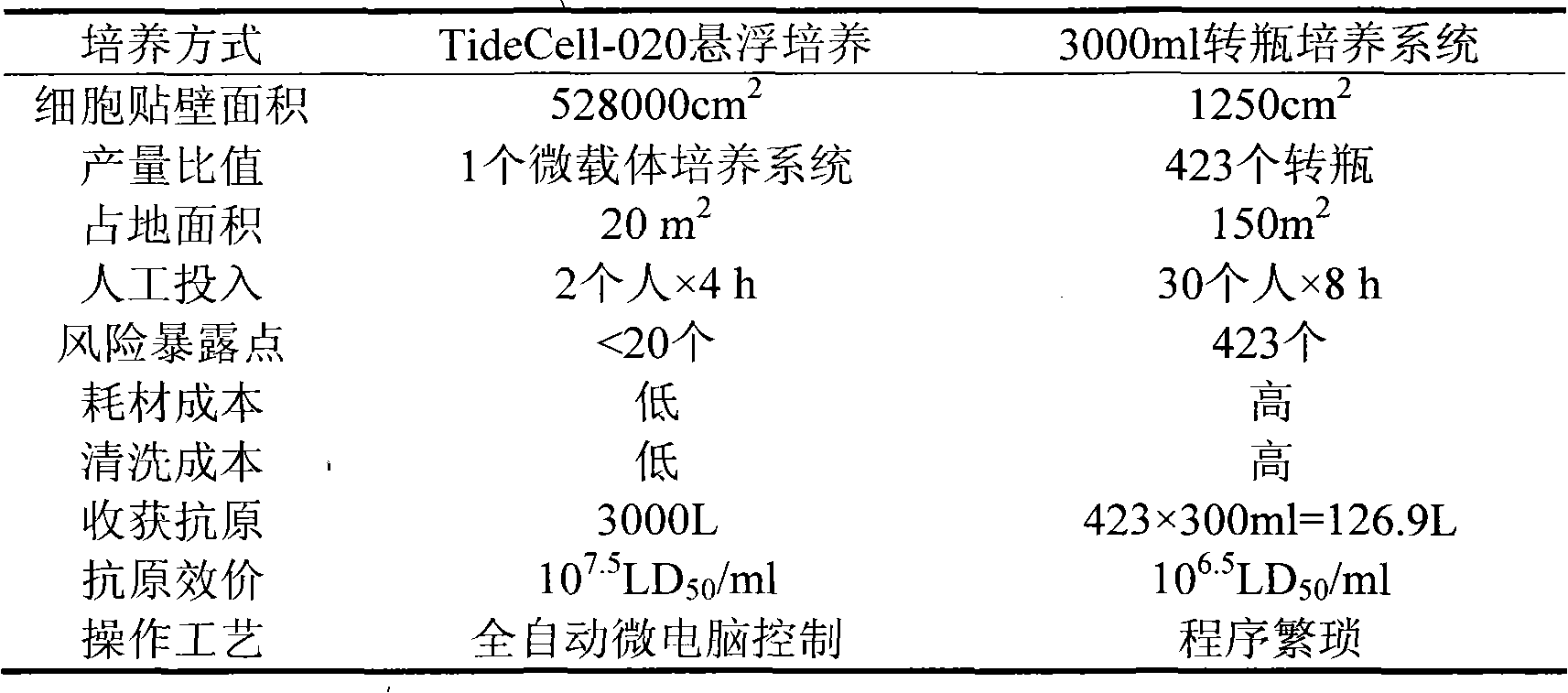
Method for producing rabies virus antigens for animals at a large scale
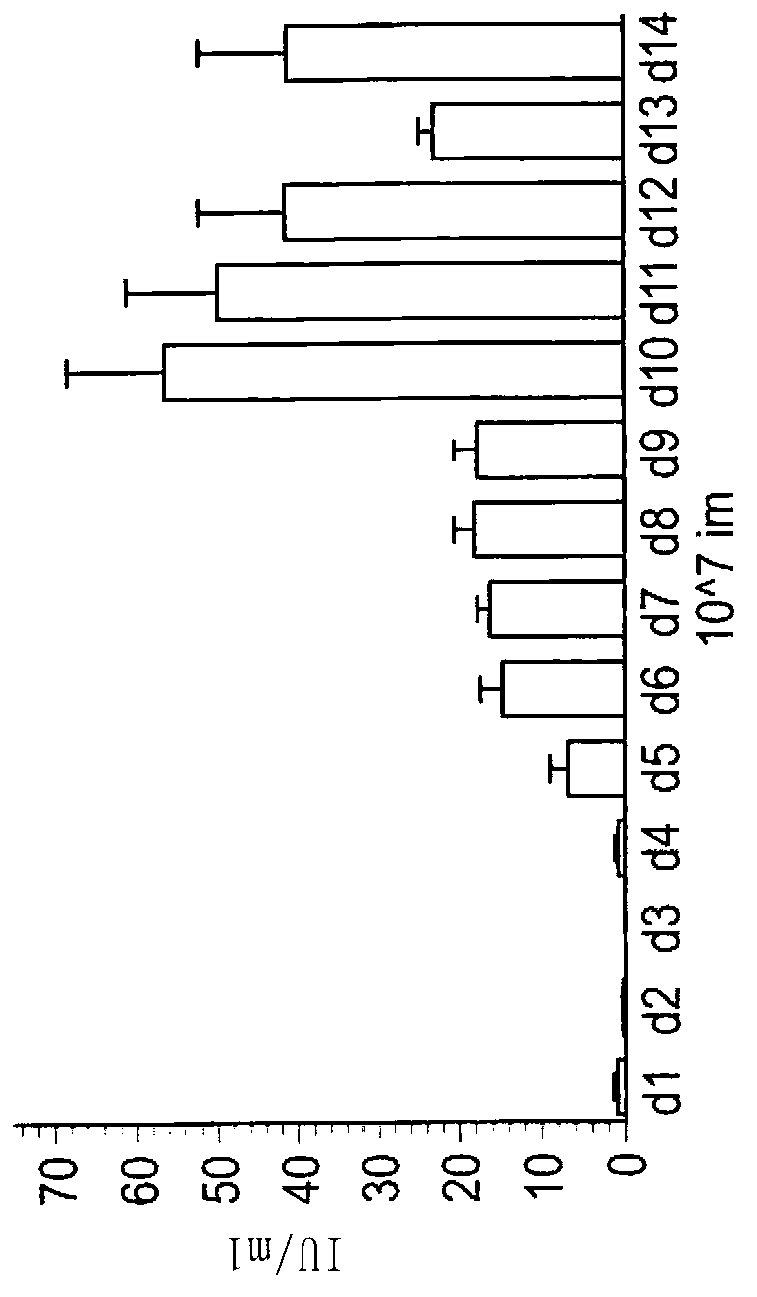
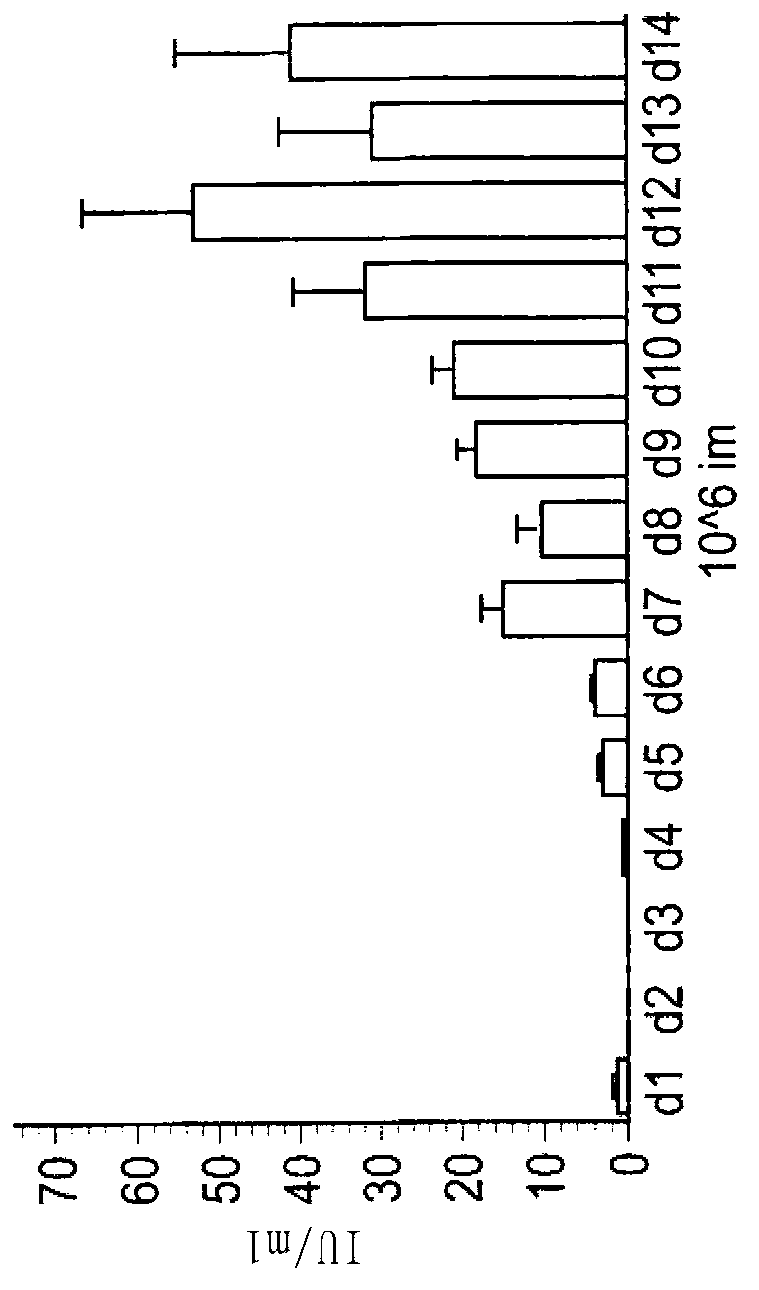
The invention discloses a method for producing rabies virus antigens for animals at a large scale, which produces the rabies virus antigens at a large scale by utilizing a bioreactor by a cell microcarrier suspension culture system. The method comprises the following steps: inoculating cells for preparing the antigens into a carrier tank containing a culture solution and microcarriers to enable the cells to be attached to the microcarriers; in a proper culture environment, enabling the cells to grow on the microcarriers until the quantity of the cells is 5-40 times more than inoculum density; making rabies viruses into a virus suspension, and enabling the virus suspension to be adsorbed on the cells; culturing the viruses in the proper culture environment by using a cell maintenance culture solution; continuously culturing for 3-5 days and then harvesting a virus solution for the first time, wherein a semicontinuous process is adopted and the ratio of a changed solution is 50 percent; continue culturing for 9-11 days, and harvesting the changed solution once every 24 hours; mixing the harvested virus solution with the virus solution of the bioreactor; and carrying out freeze thawing at the temperature of -20 DEG C and inactivation purification to obtain the rabies virus antigens. The method has large production scale, high single-scale yield and relatively low production cost.
Owner:PU LIKE BIO ENG
Pseudorabies/porcine parvovirus infection combined inactivate vaccine and suspension culture preparation method
InactiveCN108421037AHigh antigen contentLarge batches of antigensViral antigen ingredientsInactivation/attenuationAdjuvantPorcine parvovirus antigen
The invention belongs to the technical field of veterinary biological products and particularly relates to a pseudorabies / porcine parvovirus infection combined inactivate vaccine and a suspension culture preparation method. The preparation method comprises the following steps: preparing a pseudorabies virus suspension culture antigen and a porcine parvovirus infection virus suspension culture antigen; proportionally mixing the inactivated pseudorabies virus and porcine parvovirus infection virus antigen solutions, and then adding an adjuvant to fully emulsify to obtain the pseudorabies / porcineparvovirus infection combined inactivate vaccine. The suspension culture processes of the pseudorabies virus antigen solution and the porcine parvovirus infection virus antigen solution are established, the virus antigen solutions prepared through the suspension culture process have the advantages of high antigen content, large batch of the antigen and stable batch, the preparation method greatlyreduces the use of manpower, the occupied area and space of a culture system is reduced, and the production cost of an enterprise is lowered; meanwhile, the pseudorabies / porcine parvovirus infectioncombined inactivate vaccine is used, two viruses are prevented through one syringe, the immunity times of an animal are reduced, the stress times of the animal are reduced, and the production cost ofthe vaccine and the culture cost of a farmer are greatly reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Method for preparing rabies virus antigen by chloroplast
InactiveCN101812468AImprove immunityAssembly precisionUnicellular algaeMicroorganism based processesAntigenTreatment effect
The invention provides a method for preparing a rabies virus antigen by chloroplast, comprising the following steps: (1) constructing a chlamydomonas chloroplast expression vector of rabies virus antigen gene; (2) screening the constructed expression vector transgenosis chlamydomonas to obtain transgenosis chlamydomonas; and (3) culturing transgenosis chlamydomonas to express the rabies virus antigen gene in chlamydomonas to obtain the rabies virus antigen. In the method of the invention, a chlamydomonas chloroplast expression system is utilized to produce rabies virus antigens safely and efficiently in a chlamydomonas bioreactor, the expressed protein has high specificity and good immunity effect, and can not produce non-purposive immunoreaction. The cost of the method of the invention is significantly lower than that of a traditional method for preparing the rabies virus antigen, the method has no need of investing and building factories and no generation of three wastes (waste gas, waste water and industrial residue), and the consumption of electric power, water resource and other resources is extremely low. The antigen prepared by the method of the invention has very high security and no need of purification, and can be directly used as animal supplementary feed, thus having treatment effect.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Rabies virus IgG antibody immune gold-labeled test paper and preparation method thereof
InactiveCN104360061AEasy to operateStrong specificityBiological material analysisAntigenNitrocellulose
The invention relates to rabies virus IgG antibody immune gold-labeled test paper. The test paper consists of a serum treatment pad, a gold-labeled release pad, a nitrocellulose membrane, an absorbent pad and a back plate, wherein the serum treatment pad, the gold-labeled release pad, the nitrocellulose membrane and the absorbent pad are sequentially superposed and adhered to the back plate, and the superposition distance between every two parts is 2-3mm; a purified rabies virus antigen-colloidal gold compound is coated on the gold-labeled release pad; a detection line and a quality control line are coated on the nitrocellulose membrane; the detection line is fixedly provided with SPA; and the quality control line is fixedly provided with anti-dog rabies virus positive IgG. The test paper disclosed by the invention is easy to operate, high in specificity and high in sensitivity, special instruments and equipment and professionals are not needed, whether vaccine immunity animals generate antibodies on a protection level can be rapidly screened, and a reference basis is provided for vaccine immunity and immune procedure formulation of rabies.
Owner:CHANGCHUN SR BIOLOGICAL TECH
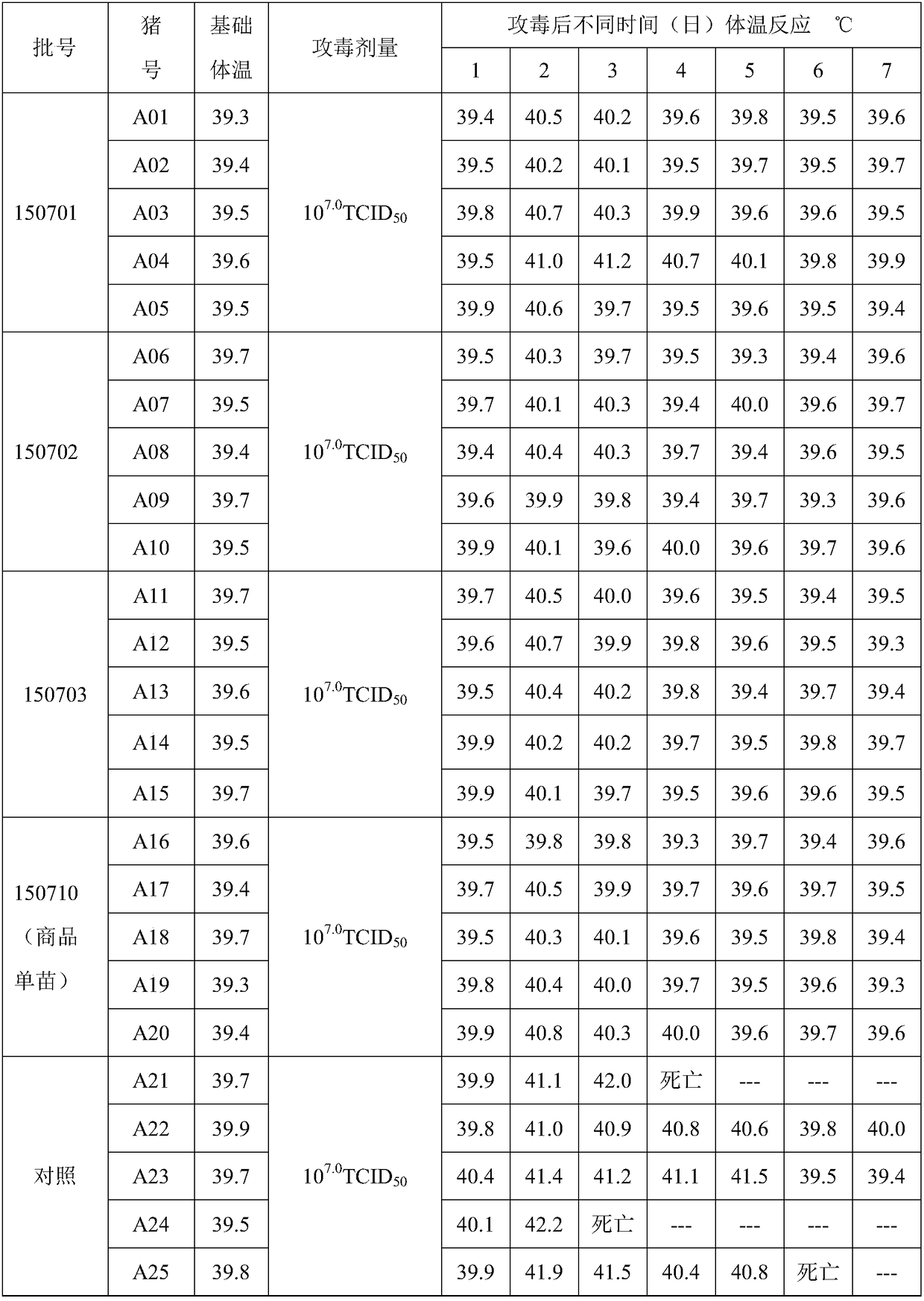
Rabies vaccine glycoprotein content detection method
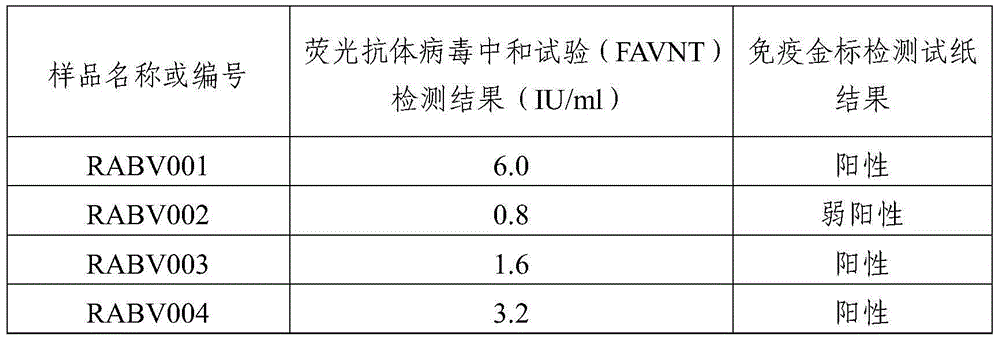
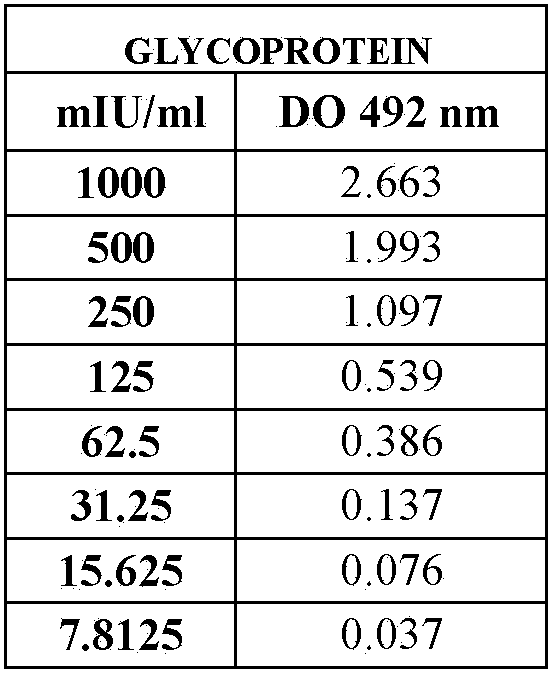
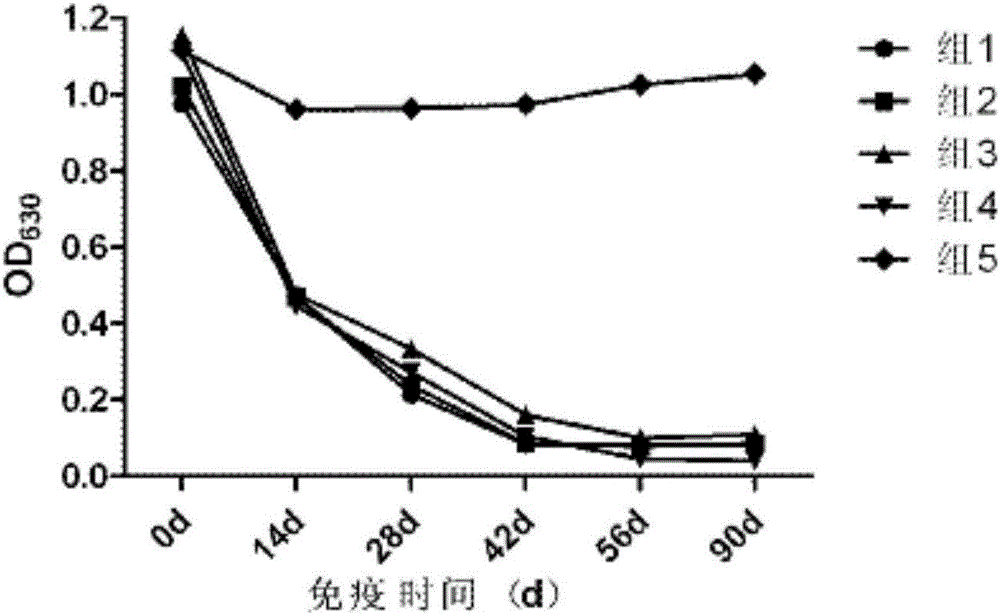
ActiveCN103777021AAntigen content predictionReliable Quantitative Numerical BasisBiological testingSorbentRabies Virus Antigen
The invention relates to a rabies vaccine glycoprotein content detection method which is characterized by using a sandwich ELISA (enzyme-linked immuno sorbent assay) method for rabies virus antigen content detection, and the rabies vaccine glycoprotein content detection method is as follows: firstly, coating a 96-hole elisa plate with rabies virus polyclonal antibody, adding a to-be-tested sample for incubation at 37 DEG C for 1.5 hours; cleaning the elisa plate for 5 times; then adding the mouse anti-rabies virus monoclonal antibody for incubation at 37 DEG C for 1.5 hours; cleaning the elisa plate for 5 times, adding an anti-mouse enzyme conjugate for incubation at 37 DEG C for 1 hour; cleaning the elisa plate for 6 times, adding a substrate for coloration; using a microplate reader for detecting, reading an OD (optical density) value; and calculating the rabies virus glycoprotein content. The rabies vaccine glycoprotein content detection method can be used for quality control of rabies vaccine industry, and can quickly identify the authenticity of rabies vaccine.
Owner:LIAONING CHENGDA BIOTECH
Parapoxvirus vectors containing rabies virus antigen
The present invention relates to recombinant parapoxviruses which carry in their genomes comprising heterologous DNA derived from a rabies virus, to the preparation of such constructs, and to their use in immunogenic compositions and vaccines. It further relates to the use of recombinant parapoxviruses for diagnostics.
Owner:ZOETIS SERVICE LLC
Porcine pseudorabies virus and porcine circovirus type II bivalent vaccine, and applications thereof
ActiveCN106511993AImprove securityImprove protection efficiencyViral antigen ingredientsAntiviralsAntigenDisease
The invention belongs to the technical field of vaccine, and more specifically relates to a porcine pseudorabies virus and porcine circovirus type II bivalent vaccine, and applications thereof. The porcine pseudorabies virus and porcine circovirus type II bivalent vaccine comprises inactivated porcine pseudorabies virus XF-1 strain, and porcine circovirus type II-WH strain. It is confirmed by the porcine pseudorabies virus and porcine circovirus type II bivalent inactivated vaccine that XF-1 strain can be prepared into combined vaccines used for preventing two or more than two diseases with other well-known antigens in the field of vaccine, a cognition error that concurrent infection of porcine circovirus type II and porcine pseudorabies virus is caused is eliminated, combination application of porcine circovirus type II and porcine pseudorabies virus at an appropriate rate is capable of achieving excellent effect, and vaccine development prospect is promising.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Rabies virus antibody quantitative detection kit as well as preparation method and detection method thereof
ActiveCN110007080AHigh sensitivityImprove accuracyBiological material analysisBiological testingNitrocelluloseQuality control
The invention discloses a rabies virus antibody quantitative detection kit as well as a preparation method and a detection method thereof, belongs to the technical field of antibody detection kits, and solves the problems that the antibody detection accuracy is not high enough and potential safety hazards exist in the prior art. The kit comprises an immunochromatographic detection test strip and asample diluent, the immunochromatographic detection test strip sequentially comprises a sample pad coated with a rabies virus antigen, a marker pad coated with a monoclonal antibody of the rabies virus antigen, a nitrocellulose membrane coated with a detection line and a quality control line and a water absorption pad from one end to the other end, and the sample pad, the marker pad, the nitrocellulose membrane and the water absorption pad are mutually stacked and attached to the upper surface of a back plate. According to the invention, the rabies virus antibody in blood is detected by adopting a competition method, and real antibody numerical values in a blood sample can be directly displayed according to a standard curve in combination with strip reading equipment, so that the detection accuracy and sensitivity are improved.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Rabies virus antibody colloidal gold detection test strip for human and livestock joint detection and preparation method thereof
The invention discloses a rabies virus antibody colloidal gold detection test strip for human and livestock joint detection and a preparation method of the rabies virus antibody colloidal gold detection test strip. The rabies virus antibody colloidal gold detection test strip comprises a colloidal gold labeled binding area and a detection area, wherein a detection line and a quality control line are arranged on the detection area, rabies virus antigens labeled by colloidal gold and albumen protein gold particles labeled by colloidal gold are wrapped by the colloidal gold labeled binding area, staphylococcus albumins are wrapped by the detection line, and ovalbumin antibodies are wrapped by the quality control line. According to the rabies virus antibody colloidal gold detection test strip, the detection cost can be reduced, the detection sensitivity can be improved, false positive can be avoided, and the human and livestock joint detection of rabies virus antibodies is achieved. Furthermore, the instability problem appearing when monoclonal antibodies or polyclonal antibodies are used as the quality control line can be effectively solved through the quality control line, and the rabies virus antibody colloidal gold detection test strip has a great significance in large-batch sample detection, field application and immunized human self-detection and has very large market development potentials.
Owner:HUAZHONG AGRI UNIV
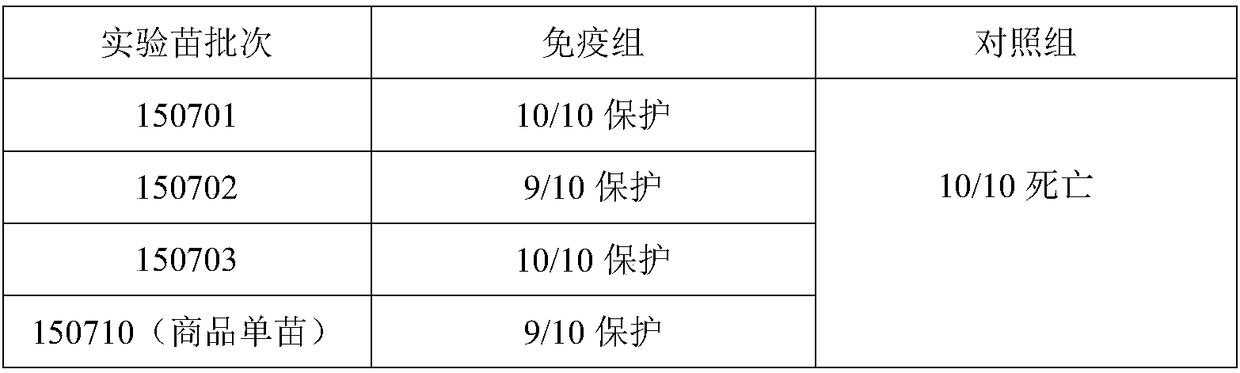
Preparation method for living-vector vaccine against rabies, product thereof and application thereof
InactiveCN106853247AComprehensive immune responseGood immune effectSsRNA viruses negative-senseViral antigen ingredientsAntigenRabies
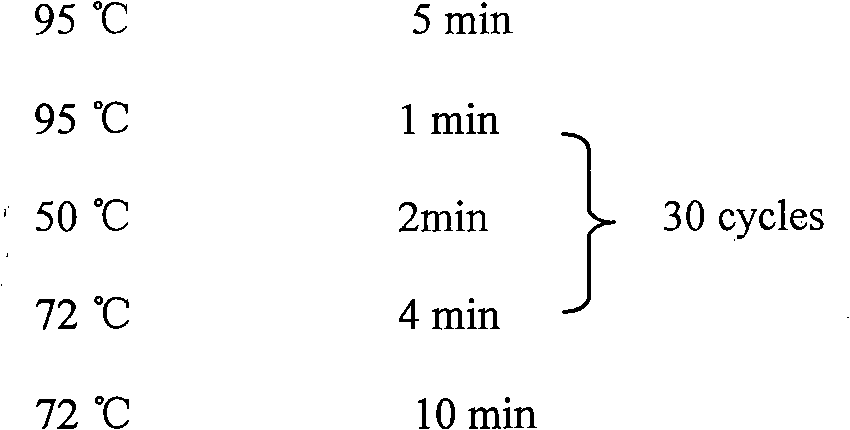
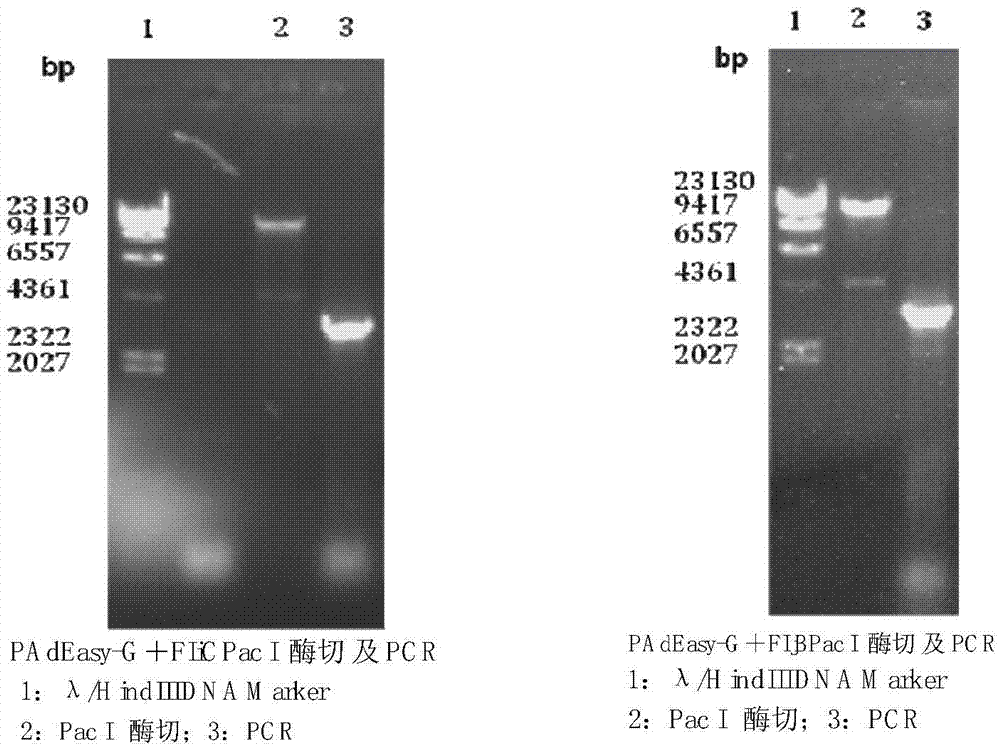
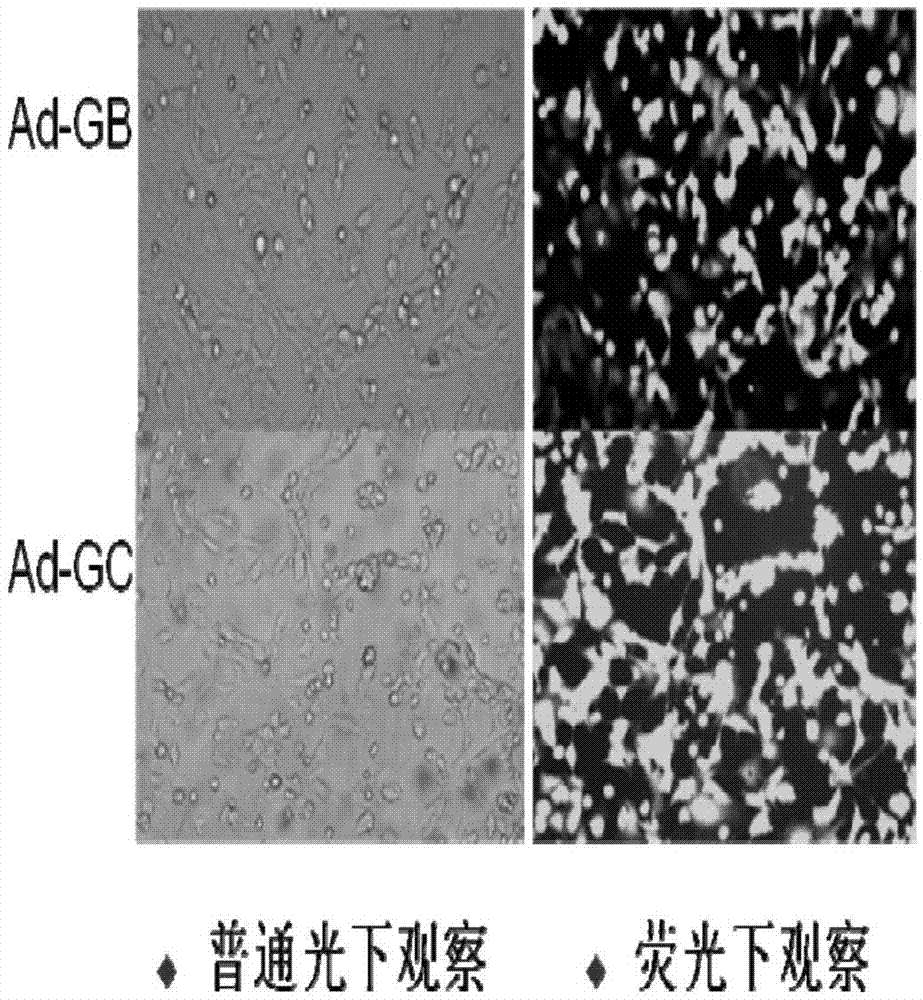
The invention discloses a preparation method for a living-vector vaccine against rabies, a product thereof and application thereof. The method comprises the following steps: 1, designing primers and amplifying a rabies virus antigen protein gene G and a bacterial flagellin gene FIjB or FIiC; 2, inserting the antigen protein gene G and the gene FIjB or FIiC between a specific promoter and a specific terminator of a replication-competent defective adenovirus shuttle vector so as to construct an adenovirus shuttle vector containing a gene G-FIjB or a gene G-FIiC; 3, subjecting the obtained recombinant replication-competent defective adenovirus shuttle vector and an adenovirus skeleton vector to homologous recombination so as to obtain recombinant adenovirus plasmids; and 4, producing recombinant adenoviruses with a uniform genomic structure from the recombinant adenovirus plasmids. Moreover, the invention also provides the living-vector vaccine against rabies prepared by using the method. Compared with the prior art, the living-vector vaccine against rabies prepared in the invention is safer and more effective, is short in a preparation period and has good application prospects in the field of prevention or treatment of rabies.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Rabies virus antibody test paper, preparation method thereof and detection method thereof
ActiveCN109959789AEliminate distractionsAvoid false positivesBiological testingAgainst vector-borne diseasesNeutralizing antibodyRabies Virus Antigen
The invention discloses rabies virus antibody test paper and a preparation method thereof and a detection method thereof, which belong to the technical field of antibody test paper. The problems of false positive risk and safety hazard in the prior art are solved. The test paper comprises, from one end to the other end, a sample pad which coats a rabies virus antigen, a gold standard pad which coats a monoclonal antibody against the rabies virus antigen, a nitrocellulose membrane which coats a detection line and a quality control line, and an absorbent pad, wherein the sample pad, the gold standard pad, the nitrocellulose membrane and the absorbent pad overlap each other and are attached to the upper surface of a backboard. The rabies virus antigen coated by the sample pad is rabies virus-like particles. According to the invention, the used labeled antigen is the rabies virus-like particles expressed by insect cells, which avoids false positives; the monoclonal antibody against the rabies virus G protein competes with a neutralizing antibody in the blood to improve the detection accuracy and sensitivity; the virus-like particles are free of nucleic acid components; and biosafety risks produced by the use of whole viruses as marker antigens are eliminated.
Owner:CHANGCHUN SR BIOLOGICAL TECH
A kind of rabies virus antibody (Igg) ELISA kit and its detection method
The invention provides a rabies virus antibody (IgG) enzyme-linked immunoassay kit and a detection method thereof. The detection kit is composed of a purified rabies virus antigen coated microporous plate, enzyme labeled SPA and other reagents. The detection method adopts an indirect method principle to detect the rabies virus IgG antibody in human or animal serum or blood plasma, and is suitable for rabies vaccine immunized serology effect evaluation and epidemiology investigation. An enzyme labeled antibody applied in the invention is a Staphylococal protein A (SPA), and the SPA can be combined with an Fc fragment in IgG molecules in human or mammal serum, so the kit provided by the invention has all the characteristics of an ELISA kit, can be used for human rabies virus antibody detection, and can also be used for detecting the immune effect of various species of animals.
Owner:成大生物(本溪)有限公司
Vaccine compound and preparation method and application thereof
InactiveCN109568573AImprove protectionAntiviralsAntibody medical ingredientsAntigenRabies Virus Antigen
Owner:PULIKE BIOLOGICAL ENG INC
A method for producing porcine pseudorabies virus antigen by whole suspension cell culture
ActiveCN107142249BImprove securityIncrease productivityViral antigen ingredientsAntiviralsFreeze thawingRabies Virus Antigen
Owner:广东渔跃生物技术有限公司 +1
Rabies vaccines and applications based on self-assembled ferritin nano-antigen particles and prepared therefrom
ActiveCN112442130BOperational securitySimple and fast operationSsRNA viruses negative-senseAntibody mimetics/scaffoldsMutantVertebrate Animals
The invention discloses a rabies vaccine and application based on self-assembled ferritin nanometer antigen particles and prepared therefrom. In the invention, the rabies virus G antigen protein is fused with the self-assembled ferritin nanoparticle subunit to obtain a fusion protein. In the present invention, the rabies virus G antigen protein is subjected to site mutation, and the soluble expression amount and expression efficiency of the obtained mutant are significantly improved. The present invention utilizes prokaryotic expression system, silkworm and AcMNPV-insect cell eukaryotic expression system to express recombinant protein, or performs gene presentation in vertebrate body by recombinant baculovirus to produce antigen and induce antibody production. The rabies vaccine provided by the present invention displays antigenic proteins on the surface of the ferritin cage structure of Helicobacter pylori, causing broadly neutralizing anti-rabies virus antibodies, improving immune efficacy and expanding the scope of immunity, and has the potential of a universal vaccine with cross-immunity efficacy . The vaccine preparation method of the present invention is safe and convenient, and is suitable for rapid large-scale production.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
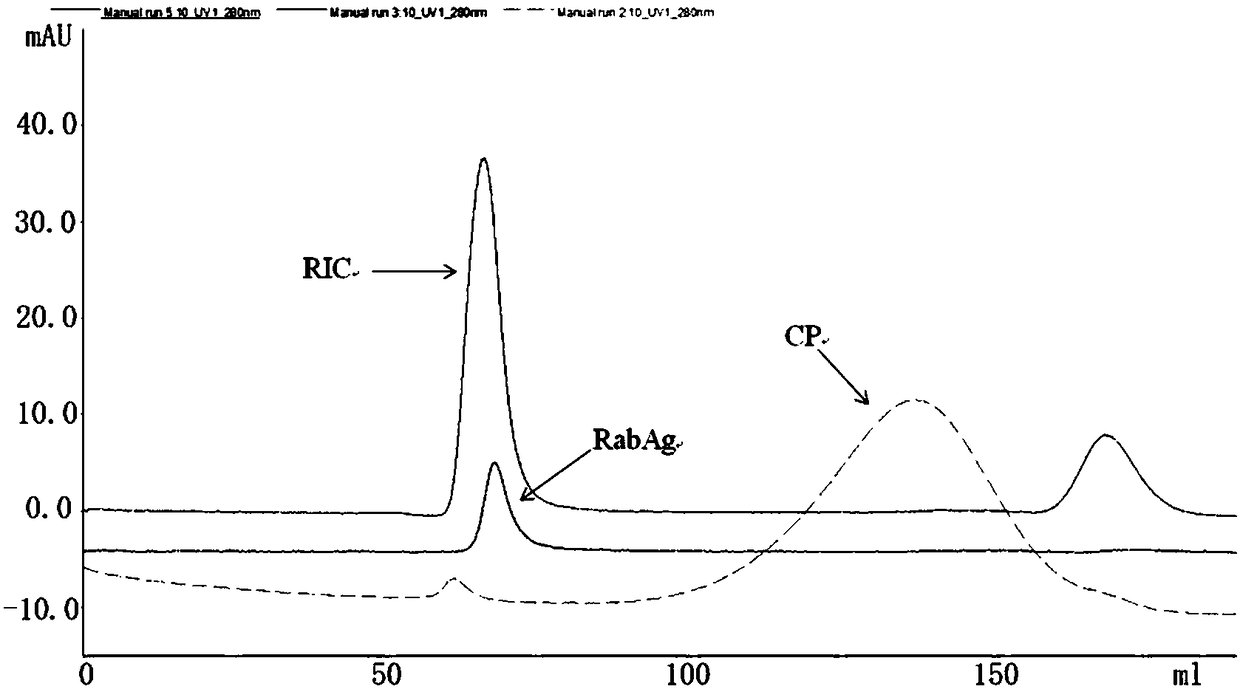
Rabies immunogenic conjugate
ActiveCN108175855ASsRNA viruses negative-senseViral antigen ingredientsConjugate vaccineChemical Linkage
The invention relates to a rabies immunogenic conjugate (RIC). The rabies immunogenic conjugate comprises a rabies virus antigen (RabAg) and carrier protein (CP), and is characterized in that the rabies virus antigen at least comprises the rabies virus antigen connected with the carrier protein through a chemical bond; the carrier protein at least comprises the carrier protein connected with the rabies virus antigen through the chemical bond; the chemical bond connects the rabies virus antigen and the carrier protein to form a rabies virus antigen-carrier protein immunogenic conjugate. The invention further relates to a novel rabies conjugate vaccine (RCV) prepared according to the rabies immunogenic conjugate and a preparation method thereof. The invention preliminarily proves that the rabies immunogenic conjugate overcomes the defects in the prior art and has a relatively good effect.
Owner:复星安特金(成都)生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com