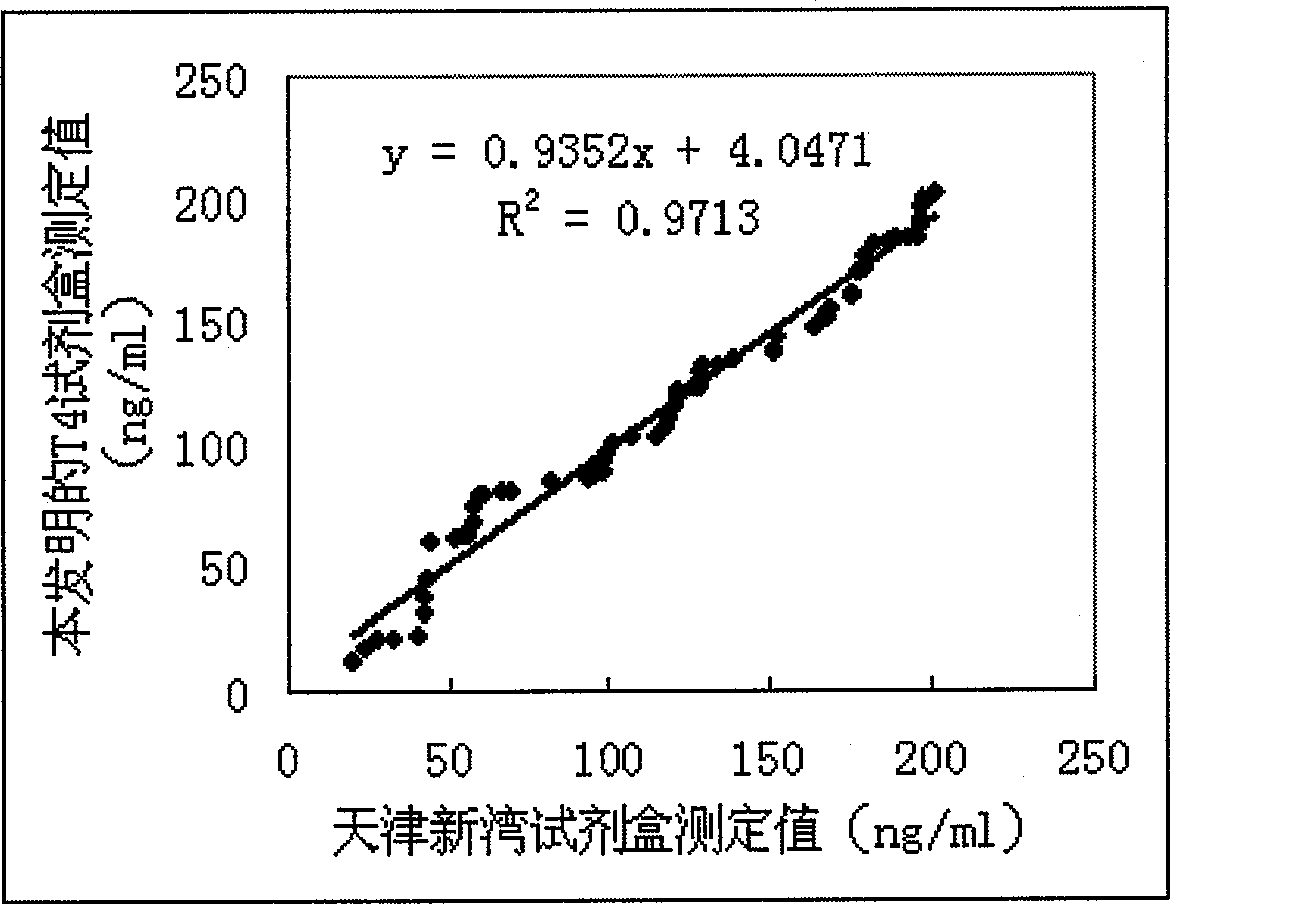
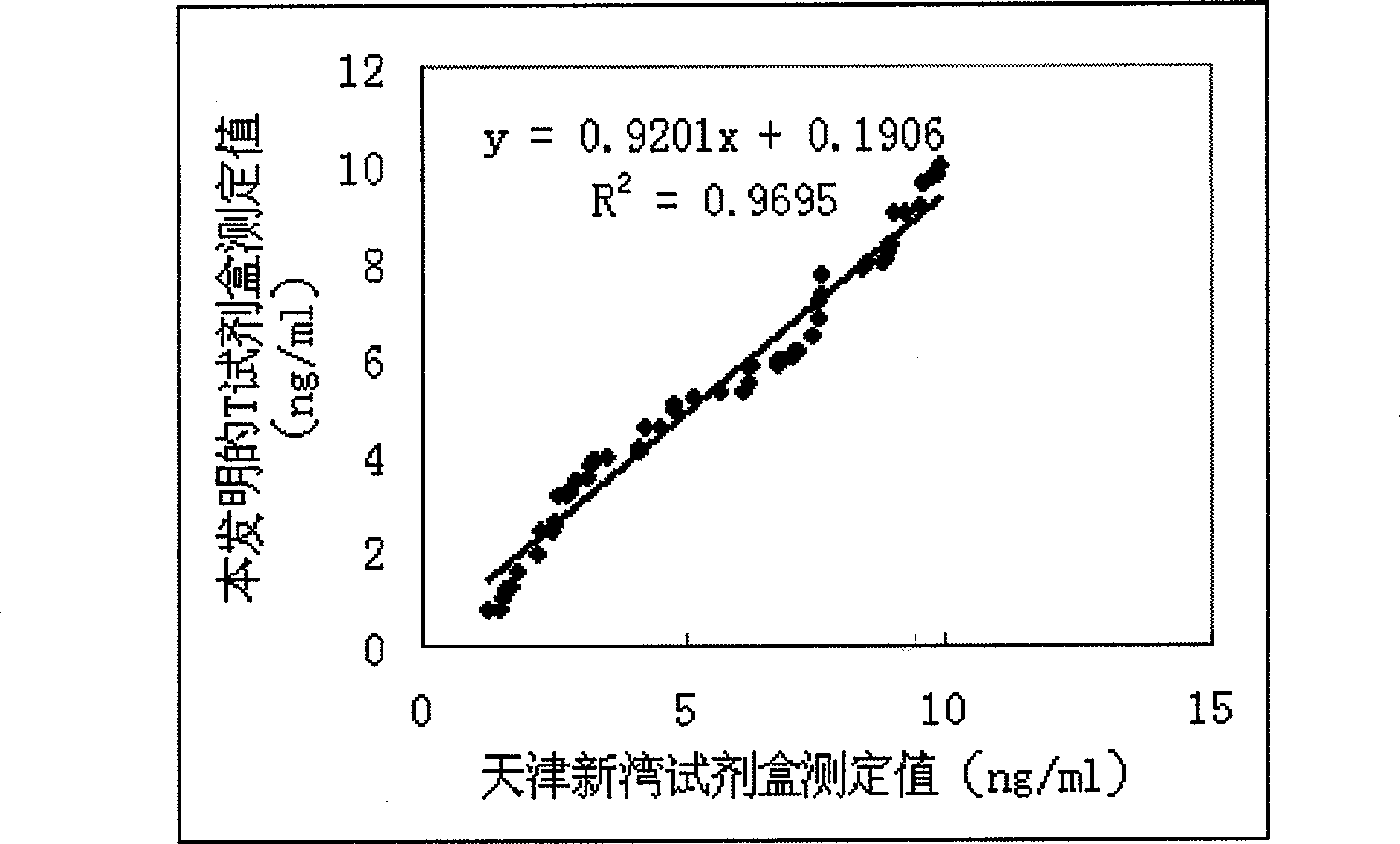
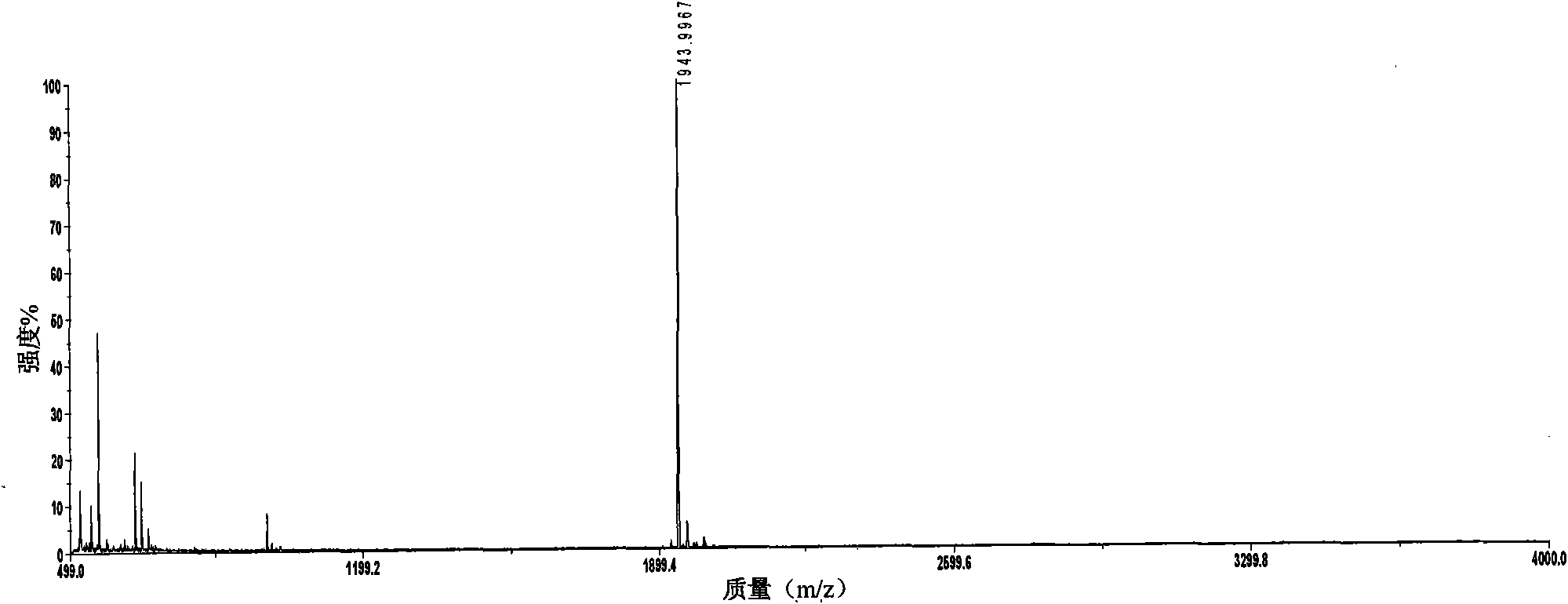
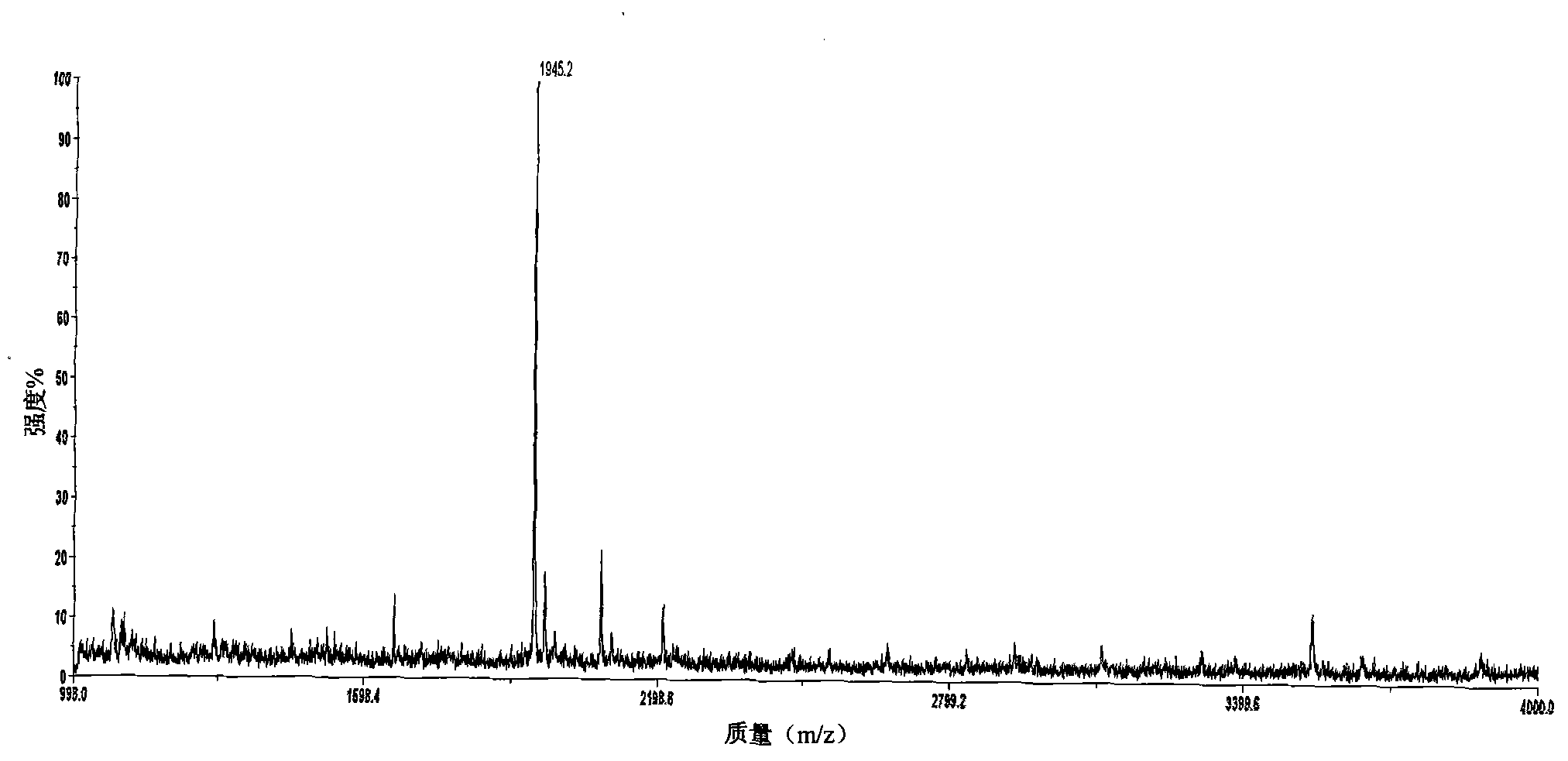
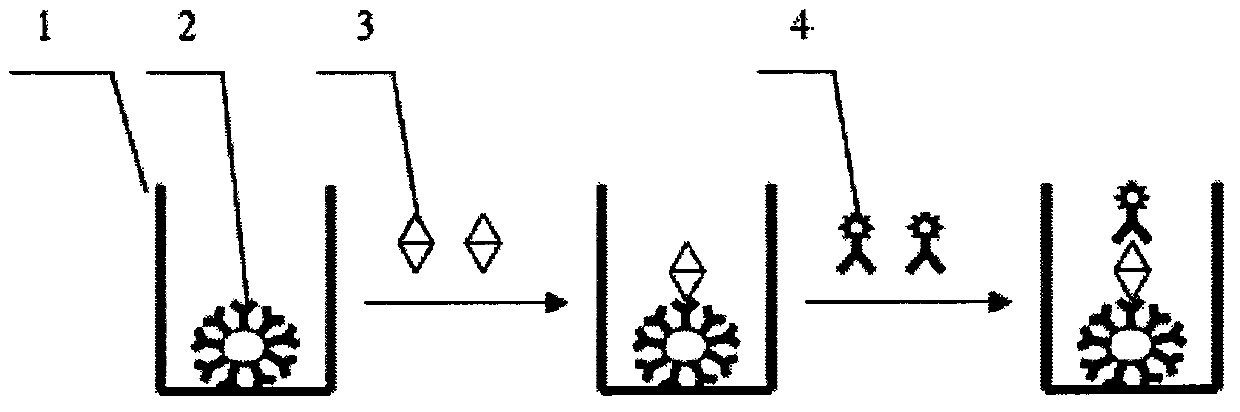
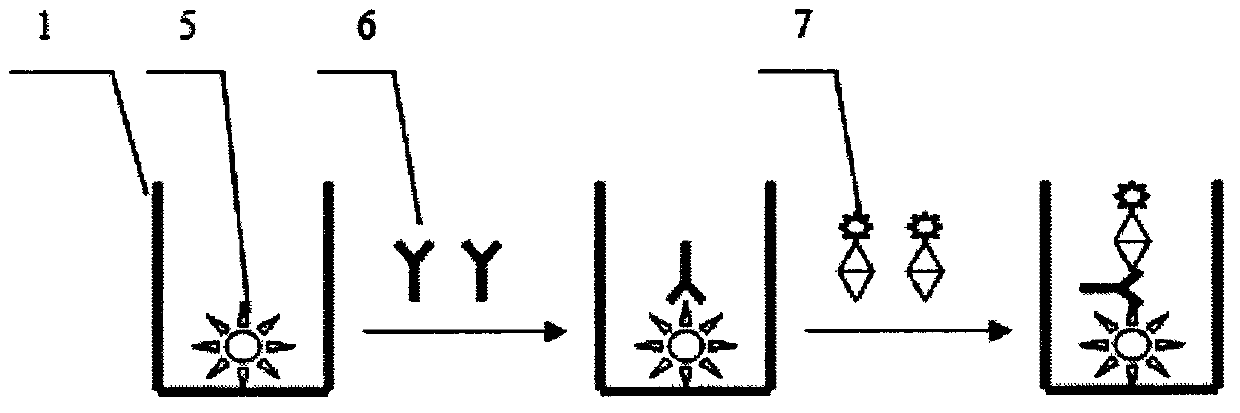
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Marker Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Magnetic granule competing method chemiluminescence immune analysis determination reagent kit for detecting hormone and preparing method thereof
InactiveCN101377491AGuaranteed SensitivityEasy to operateChemiluminescene/bioluminescenceBiological testingAntigenBiotin-streptavidin complex
The invention relates to the immunoassay medical field, particularly provides a magnetic particle competition method chemiluminescence immunoassay assay kit and a preparation method thereof used for detecting hormones. The kit according to the invention comprises: 1) a calibrator; 2) magnetic particles which are coated with streptavidin; 3) hormone antigens of enzyme markers; and 4) a chemiluminescence substrate. Further, the method for preparing the kit according to the invention has the following steps: 1) pure raw materials are used to prepare the calibrator; 2) the antigens are used to coat the magnetic particles; 3) the antigens of the enzyme markers are prepared; 4) the calibrator, the chemiluminescence substrate and the antigens of the enzyme markers are packaged in a separated way; and 5) a finished product is packaged. The kit has the advantages of convenience, rapidness, sensitivity, stability, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Method for analyzing acridine ester labeled antigen or acridine ester labeled antibody
InactiveCN101609090AReduce consumptionProduct quality and safetyChemiluminescene/bioluminescenceBiological testingChemical structureAntigen
The invention relates to a chemiluminescent immunoassay method, in particular to a method for analyzing an acridine ester labeled antigen or an acridine ester labeled antibody and an immunoassay kit prepared by same. An acridine ester label is provided with a special luminescent group in a chemical structure, and the special group directly participates in luminescent reaction in the analyzing process of luminescent immunization; the substance does not have background luminescence usually, can be used for detecting a sample with low concentration or trace concentration in the reaction and is a luminescent agent with high luminescent efficiency; and molecules of acridine ester I and acridine ester II and acridine amide III can be combined with an antibody (antigen) to generate the labeled antibody with high chemiluminescent activity and immunological reaction specificity.
Owner:BEIJING ELCOTEQ BIO TECH
Polypeptide immunoassay kit and detection method thereof
InactiveCN102062780AStrong specificityStrong elution abilityImmunoglobulins against animals/humansMaterial analysis by electric/magnetic meansAntigenSolid phases
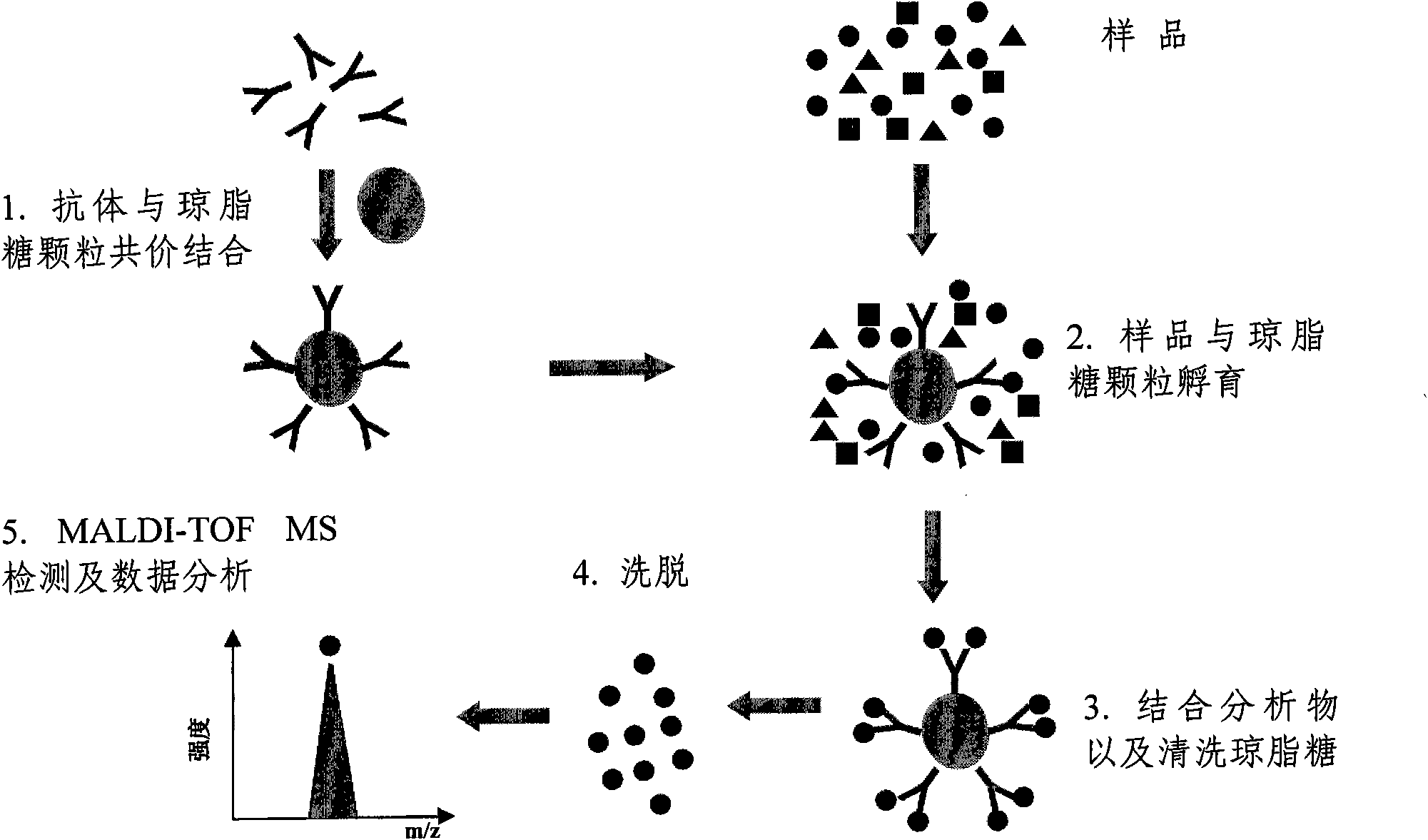
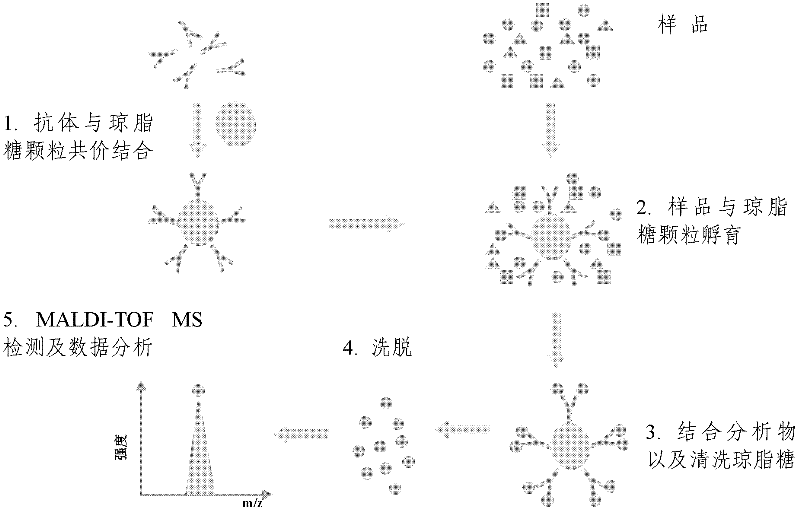
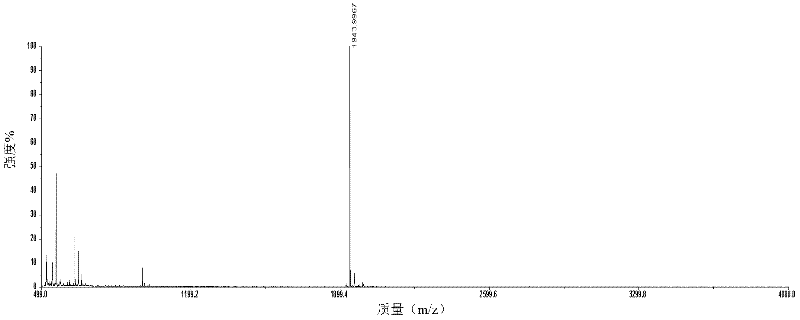
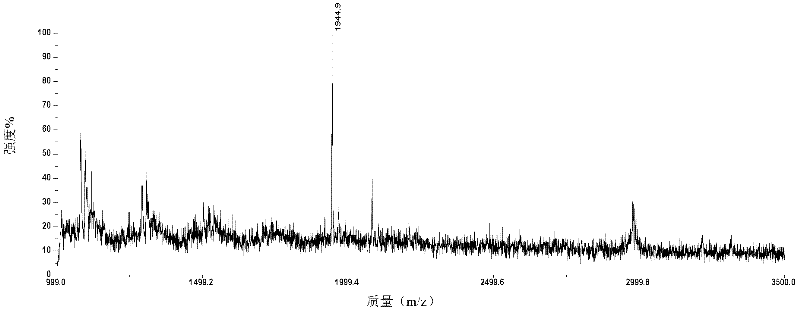
The invention relates to a polypeptide immunoassay kit, which comprises a protein G or protein A agarose serving as a solid phase carrier, a purified serum polypeptide antibody and PBS buffer solution. The invention also relates to a polypeptide immunomic spectrometry analysis method, which comprises the following steps: coupling a purified serum antibody with a proper solid carrier; allowing the coupled product to bond with a polypeptide marker antigen specifically, and separating the antigen from the carrier by using eluent; and finally, detecting polypeptide antigen in the eluent by high-pass matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOFMS). Thus, the complete immunomic spectrometry analysis method is established.
Owner:BEIJING C & N INT SCI TECH +1
Apparatus and methods for steroid hormone testing
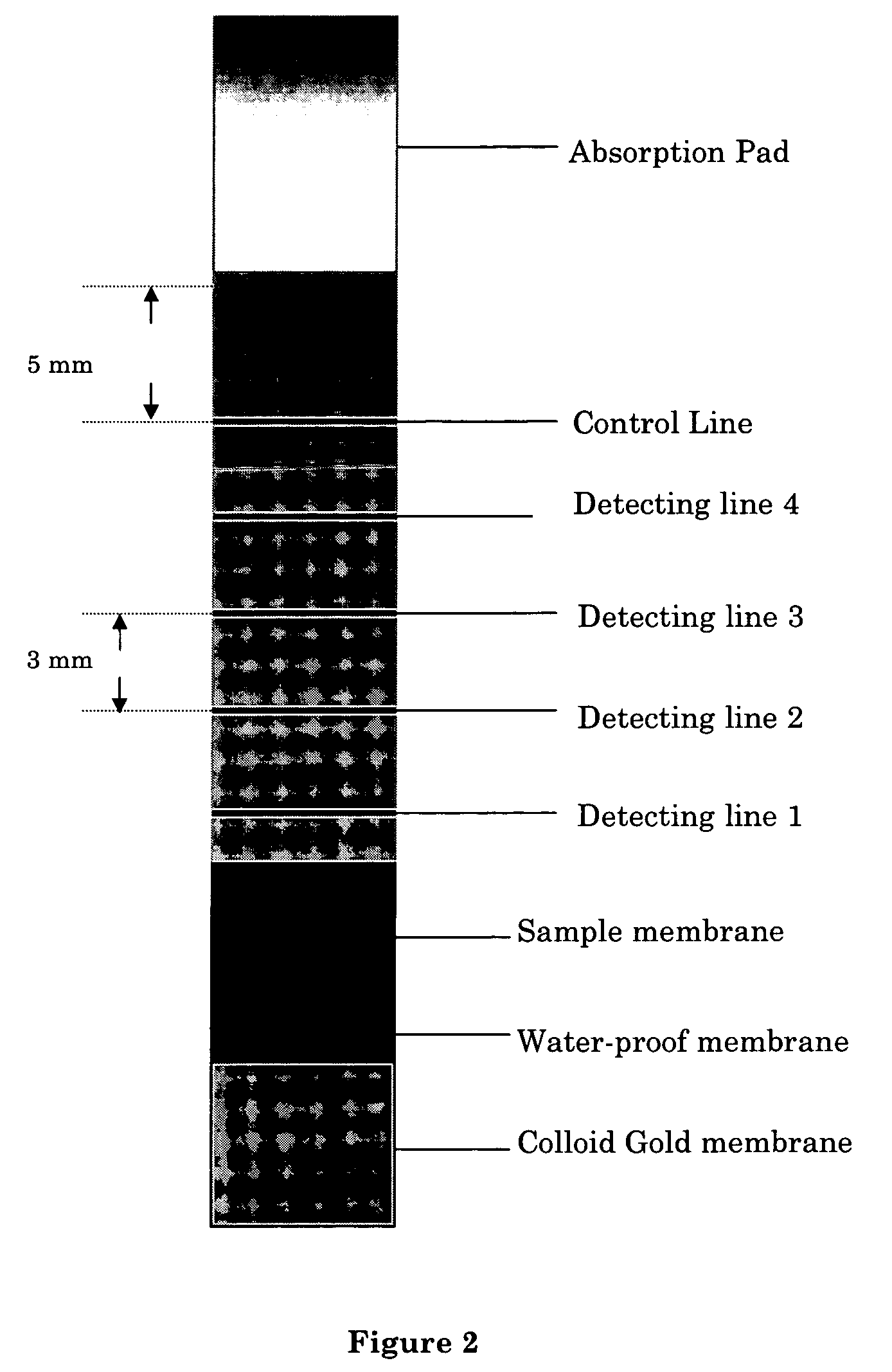
An immuno-chromatographic detection device for detecting an analyte in sample, such as estrogen in a urine or saliva sample, the device comprising (a) a binding membrane having immobilized thereon (i) an test antibody against said analyte in at least one detection region, and (ii) a control antibody against a control antigen known to be present in the sample in a control region, (b) a sample membrane located at a first end of the binding membrane for receiving the sample, wherein the sample membrane is in chromatographic connection with the binding membrane, and (c) a label membrane containing (iii) a labeled antigen that is capable of binding to the test antibody and upon binding with the test antibody exhibits an observable change at the at least one detection region, and (iv) a labeled control antigen that is capable of binding to the control antibody and upon binding with the control antibody exhibits an observable change at the control region, wherein the sample membrane is separated from the label membrane by a waterproof membrane which is removable to allow the sample membrane and label membrane to be connected chromatographically. Also provided are kits comprising the device, method for detecting the analyte, and methods for manufacturing the device and kit.
Owner:NJ INT
Composite nano colloidal gold chitosan immune carrier and preparation method thereof
The invention provides a composite nano colloidal gold chitosan immune carrier, wherein, colloidal gold is uniformly dispersed in chitosan. The invention also provides a preparation method of the composite nano colloidal gold chitosan immune carrier and a labeled antigen-antibody. According to the invention, the characteristic that chitosan has good affinity for the antigen and antibody is applied in the colloidal gold, thus the adsorption capability of the colloidal gold for the antigen-antibody is increased, and the amount of the colloidal gold is reduced. The composite nano colloidal gold chitosan immune carrier can be widely used for immunodetection or immunodiagnosis in the fields of biomedicine or agriculture and the like.
Owner:JIANGSU YITONG BIOTECHNOLOGY CO LTD
ELISA (Enzyme-Linked Immunosorbent Assay) kit for detecting polypeptide marker antigen
ActiveCN102539767AMicrobiological testing/measurementImmunoglobulins against animals/humansAntigenHigh flux
The invention discloses an ELISA (Enzyme-Linked Immunosorbent Assay) kit for detecting a polypeptide marker antigen. The kit contains a monoclonal antibody of the polypeptide marker antigen; and the monoclonal antibody is secreted by a hybridoma cell strain CGMCC No.5269 of a polypeptide marker monoclonal antibody capable of resisting primary liver cancer. The invention also provides a method for detecting the polypeptide marker antigen. The monoclonal antibody provided by the invention has the advantage of strong specificity aiming at the polypeptide marker antigen; and the method for detecting the polypeptide marker antigen is simple, convenient and rapid in operation step and beneficial to clinical detection, and can be used for carrying out high-flux and low-cost detection of an enzyme linked detector.
Owner:BEIJING C & N INT SCI TECH +1
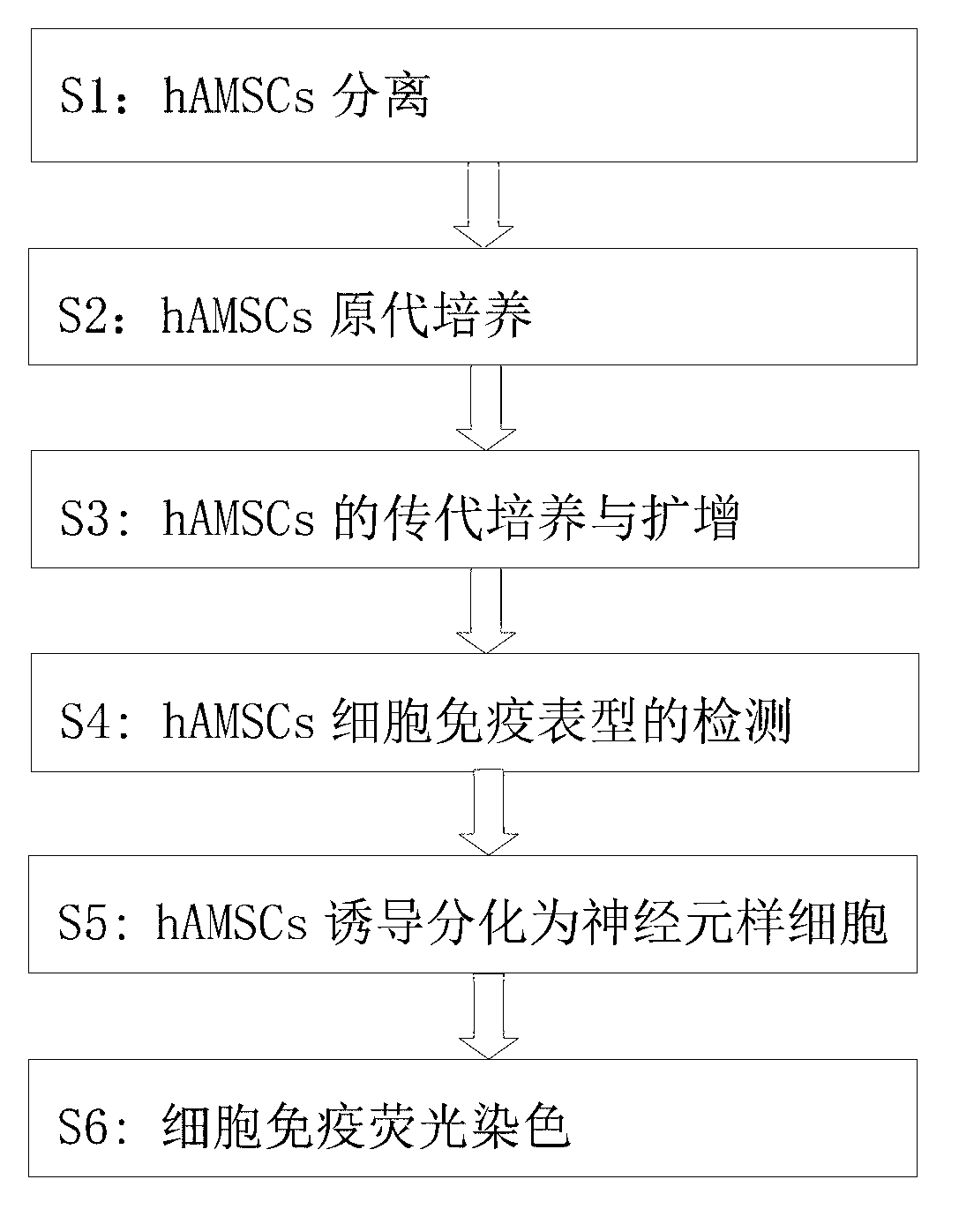
Method for inducing human amniotic mesenchymal stem cells to differentiate into neuron-like cells
InactiveCN103013917ANervous system cellsSkeletal/connective tissue cellsGerm layerGlial fibrillary acidic protein
The invention provides a method for inducing differentiating human amniotic mesenchymal stem cells (hAMSCs) to differentiate into neuron-like cells by adopting all-trans retinoic acids, a basic fibroblast growth factor (bFGF) and an epidermal growth factor (EGF). The method comprises the following steps of: separating the hAMSCs, carrying out primary culture of the hAMSCs, subculturing and amplifying the hAMSCs, detecting hAMSCs immunophenotyping, inducing the hAMSCs to differentiate into the neuron-like cells and carrying out cellular immunity fluorescence staining. According to the inducing method provided by the invention, umbilical cord mesenchymal stem cells are induced to differentiate into neutral stem cells by using the all-trans retinoic acids in combination of the bFGF and the EGF; the neural stem cells not only have the typical morphology of nerve cells, but also express neuron marker antigen neuron-specific emolase and astrocyte marker antigen glial fibrillary acidic proteins; and the capability of a mesenchymal cell trans-germinal layer differentiating into non mesenchymal cells is realized so that the mesenchymal cells are likely to turn into more ideal seed cells for clinical application in further.
Owner:陆华
High-sensitivity enzyme-linked immunoassay method
InactiveCN102495207AHigh sensitivityOvercoming Insensitivity InsufficiencyChemical analysis using catalysisImmune profilingDisease factors
Owner:EAST CHINA JIAOTONG UNIVERSITY
Enzyme-linked immunosorbent kit for inspecting porcine immunoglobulin G and application thereof
InactiveCN101571540AImprove accuracySimple structureColor/spectral properties measurementsElisa kitGlobulin G
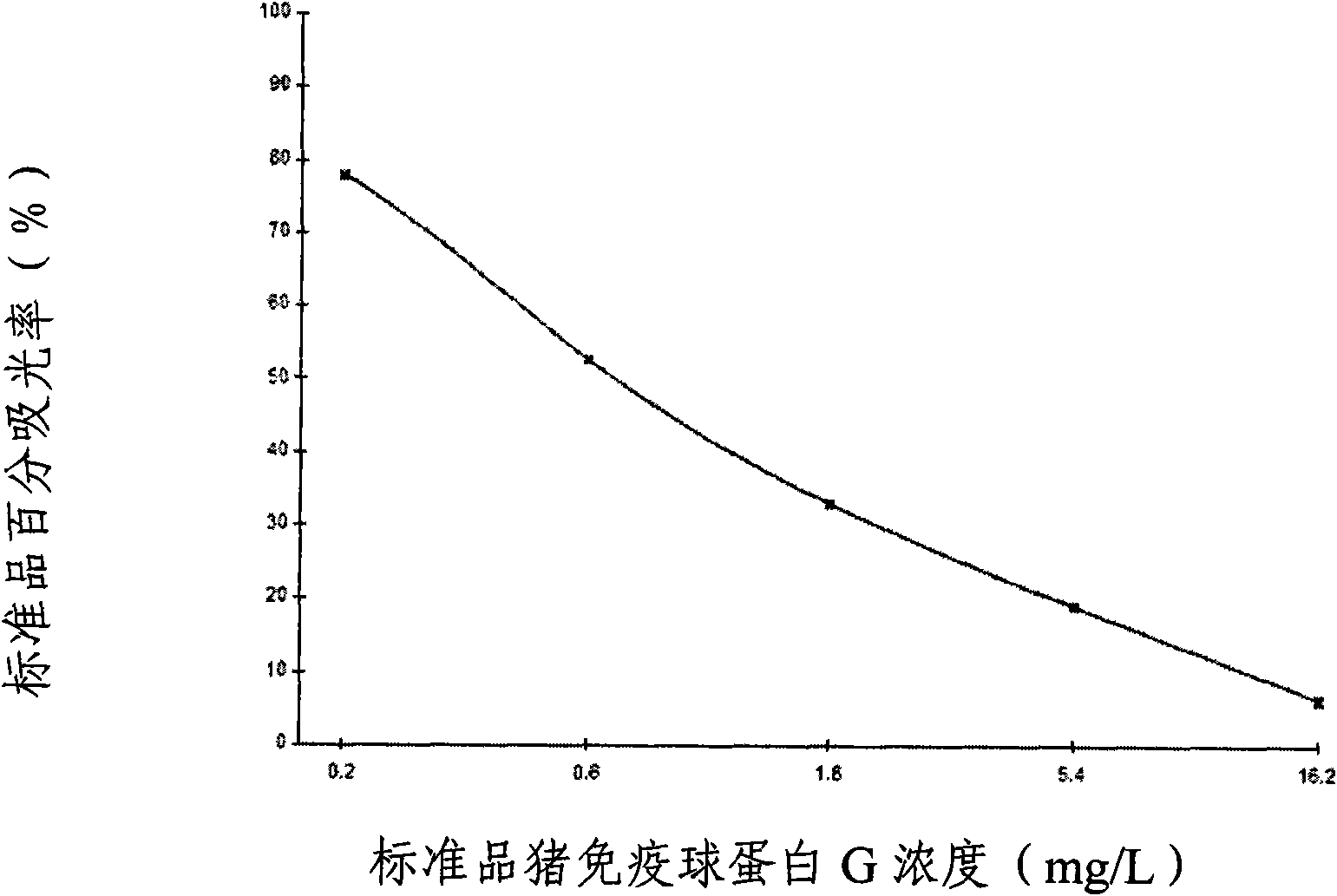
The invention provides an enzyme-linked immunosorbent kit for inspecting porcine immunoglobulin G, comprising an ELISA plate which is coated with coating antigen, an enzyme label, porcine immunoglobulin G specific antibody working liquid (being contained when the antigen is coated on the ELISA plate and the enzyme label is enzyme labeling antibody or antibody is coated on the ELISA plate and the enzyme label is enzyme labeling antigen), porcine immunoglobulin G standard product solution, substrate color development solution, stop solution, concentrated washing liquid and concentrated complex solution. The invention further discloses a method which applies the enzyme-linked immunosorbent kit for inspecting the porcine immunoglobulin G, and the method comprises the steps of firstly carrying out the pre-treatment on a sample, then using the kit for inspecting and finally analyzing the inspection result. The provided enzyme-linked immunosorbent kit can be used for inspecting the content ofthe porcine immunoglobulin G in porcine plasma protein powder and other samples, the operation is simple, the cost is low, and the enzyme-linked immunosorbent kit can be monitored on-site and is appl icable in screening mass samples.
Owner:贵州谱尼测试技术有限公司
Method for preparing porcine hepatitis E virus (HEV) total antibody enzyme-linked immuno sorbent assay (ELISA) detection kit
ActiveCN103837680AEfficient detectionHigh sensitivityVirus peptidesFermentationEpidemiologyAntigen binding
The invention discloses a method for preparing a porcine hepatitis E virus (HEV) total antibody enzyme-linked immuno sorbent assay (ELISA) detection kit. The method for preparing the porcine HEV total antibody ELISA detection kit comprises the following steps: preparing an HEV coating antigen; preparing an HEV labeled antigen; preparing an HEV labeled antigen-HRP marker; and diluting an S antigen according to a certain ratio by adopting a carbonate buffer solution, adding the diluent into an ELISA plate with the volume of 100mu l for each pore, packaging the ELISA plate in an aluminum coating bag filled with a drying agent, and finishing the coating. An ELISA detection method for rapidly and efficiently detecting porcine HEV total antibodies is established, the coating antigen and antibodies in serum are specifically bonded according to a dual antigen sandwich method principle, an antigen-antibody complex and the labeled antigen are bonded to carry out antigen-antibody reaction, the two antigens are bonded with different antigen-binding sites, the method is high in sensitivity, high in specificity and high in repeatability, a corresponding technical guarantee is provided for HEV epidemiology large-scale surveys, and a theoretical basis is provided for well preventing and controlling porcine HEV epidemic diseases.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence
InactiveCN104914244AAvoid missing detectionGuaranteed specificityBiological material analysisEnzyme immunoassaysImmunoresponse
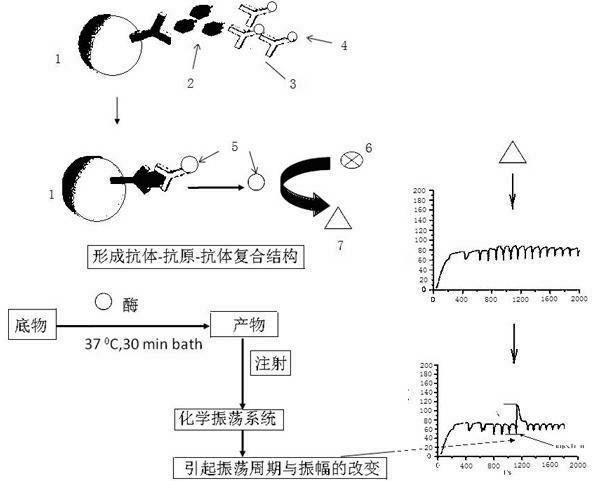
The invention provides a kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence. According to the invention, the principles of chemiluminescence are utilized; anti-FITC-coated magnetic particles are bonded with an FITC-coated reagent and then undergo immunoreaction with a detected object; after immunoreaction of an AP-labeled substance, an immunoreaction chain (as shown in a figure 1 which is described in the specification) is constructed; luminous sensitivity of a substrate catalyzed by AP is far higher than enzyme immunoassay development, so reaction sensitivity is enhanced; meanwhile, FITC is used to simultaneously marking antigen and antibody, so hepatitis C virus antibody and core antigen are detected at the same time. The kit provided by the invention can more accurately detect hepatitis C, is free of leak detection, achieves the effect of early discovery and has a high application value in prevention of hepatitis C.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Method of selecting an antibody, a hybridoma, a monoclonal antibody and use thereof
InactiveUS20030064389A1Avoid it happening againAnimal cellsMicrobiological testing/measurementTest sampleMonoclonal antibody
The present invention provides a method of selecting an antibody against a target substance to be measured which comprises selecting the antibody against the target substance by antigen-antibody reaction in the presence of a substance interfering with the antigen-antibody reaction. That is, an antigen and a labeled antigen are reacted with the antibody in the presence of an interfering substance such as an environment pollutant, and on the basis of the degree of reaction thereof, the antibody against the target substance highly resistant to the interfering substance is selected. Thereby, even if a test sample is contaminated with a substance interfering with antigen-antibody reaction, the antibody highly resistant to a substance interfering with antigen-antibody reaction is not influenced by the interfering substance and gives a correct value in the quantification.
Owner:JAPAN ENVIROCHEM
Detection method and detection kit for SARS-CoV-2 neutralizing antibody
InactiveCN112394180AEnhanced detection signalWide linear rangeBiological testingImmunoassaysAntigenReceptor
The invention discloses a detection method and a detection kit for an SARS-CoV-2 neutralizing antibody and belongs to the technical field of biomedical detection. The invention discloses a detection method of an SARS-CoV-2 neutralizing antibody. The detection method is a latex enhanced immunoturbidimetry detection method based on hACE2-RBD amplification. The detection kit comprises a first latex microsphere marked by an S protein receptor binding domain RBD of SARS-CoV-2 and a second latex microsphere marked by human hACE2. By labeling the antigen on the latex microsphere, the detection signalis amplified, detection sensitivity is improved, and the detection range is widened. Whether the detected person is an infected person or not can be determined, and the infection risk of the detectedperson can be obtained. The method is advantaged in that detection of the neutralizing antibody can also be used for evaluating the clinical effect after inoculation of the SARS-CoV-2 vaccine, and has great significance for research, development and inoculation of the SARS-CoV-2 vaccine.
Owner:NANJING LEADING BIOMEDICAL TECH CO LTD
Immunological marker detection method, reagent, and detection kit
InactiveCN106226523AHigh sensitivityImprove accuracyFluorescence/phosphorescenceAntigenSerum samples
Owner:FUJIAN COSUNTER PHARMA
Time resolution fluorescence detection kit based on phosphorescence luminous technology, and preparation method and application of detection kit
InactiveCN103293300ARealize quantitative detectionQuick QualificationMaterial analysisAntigenPlatinum
The invention provides a time resolution fluorescence detection kit based on a phosphorescence luminous technology, and a preparation method and application of the detection kit. The kit comprises magnetic particles covered by an antigen or an antibody, an antigen or an antibody marked with a phosphorescence luminous material, a standard product of an object to be detected, a washing solution and a micro-pore plate. The kit adopts the phosphorescence luminous material, namely platinum / palladium porphyrin, as a biological marker. The preparation method of the kit comprises the following steps of: preparing the magnetic particles covered by the antigen or the antibody, marking the antigen or the antibody by the phosphorescence luminous material, preparing the standard product of the object to be detected, and preparing the washing solution. The invention further discloses the application of the kit to quantitative determination of a biological sample. The kit is divided into a sandwich method mode, a competition method mode, an indirection method mode and a capturing method mode according to different immune reaction manners of objects to be detected; rapid and sensitive qualitative and quantitative detection analysis on the different objects to be detected in samples can be carried out by different detection modes according to different properties of the objects to be detected.
Owner:SHENZHEN AIRUI BIO TECH
Method for detecting small molecule compound based on prussian blue bionic marker
The invention relates to the field of electrochemical immunological techniques, and particularly relates to a method for detecting a small molecule compound based on a prussian blue bionic marker. The method comprises the following steps: using an analogue of a prussian blue micro-particle marker to-be detected object with catalytic activity like that of catalase as a marker antigen; immobilizing an antibody with a magnetic ferroferric oxide particle, dispersing the magnetic ferroferric oxide particle in a reaction liquid; and forming an oxidation current signal in a competitive immune reaction and electrochemical detection system and further realizing quantitative measurement on the to-be-detected object. According to the method, a small molecule antigen is molecularly marked by adopting prussian blue instead of a bio-enzyme and a competence immune reaction is performed by combining with the magnetic particle, thus the high-sensitivity quantitative detection on the small molecule compound can be realized through an electric signal generated by a catalytic oxidation reaction of the electrochemical detection marker antigen to hydrogen peroxide and paradioxybenzene because the size of the electric signal is in linear dependence with the concentration of a to-be-detected small molecule.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
Aftosa totivirus particle vaccine composition as well as preparation method and application thereof
ActiveCN104548085AGood effectKeep aliveAntiviralsPharmaceutical non-active ingredientsAlcoholVaccine antigen
The invention discloses an aftosa totivirus particle vaccine composition as well as a preparation method and an application thereof. The aftosa totivirus particle vaccine composition disclosed by the invention contains totivirus particles of aftosa virus and a stable cryoprotectant, wherein the stable cryoprotectant is composed of the following matters: polyhydric alcohol, a buffer solution and a nonionic surfactant. The stable cryoprotectant provided by the invention stabilizes the vaccine antigen, protects the titer and the effect of the marker vaccine, protects the antigen stability of the marker vaccine or the antigen stability of the marker antigen library in a production process and a cold chain process, and can maintain the titer or the activity of the marker vaccine for a longer time under liquid and refrigeration conditions.
Owner:吕宏亮 +2
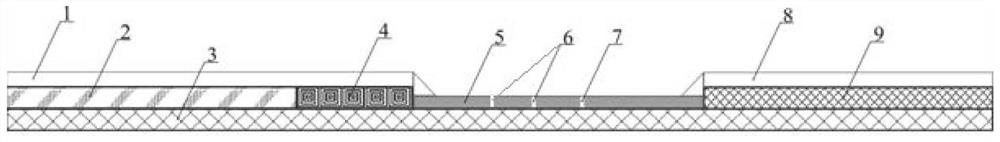
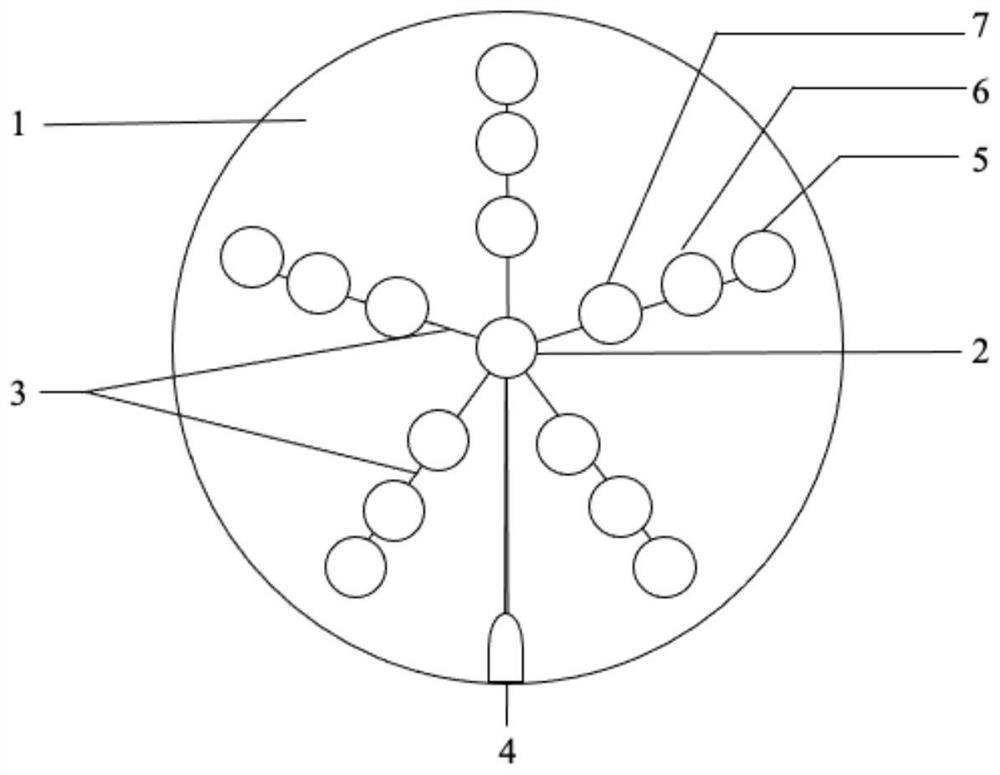
Miniature blood detector for rapidly detecting acute myocardial infarction marker, and detection method thereof
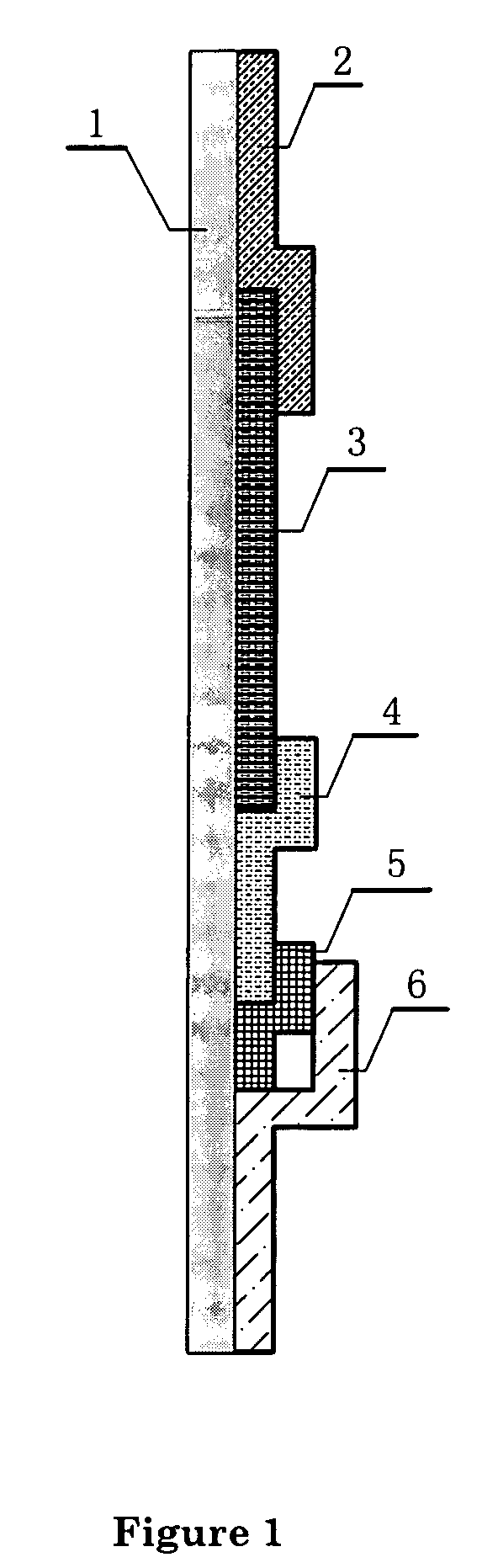
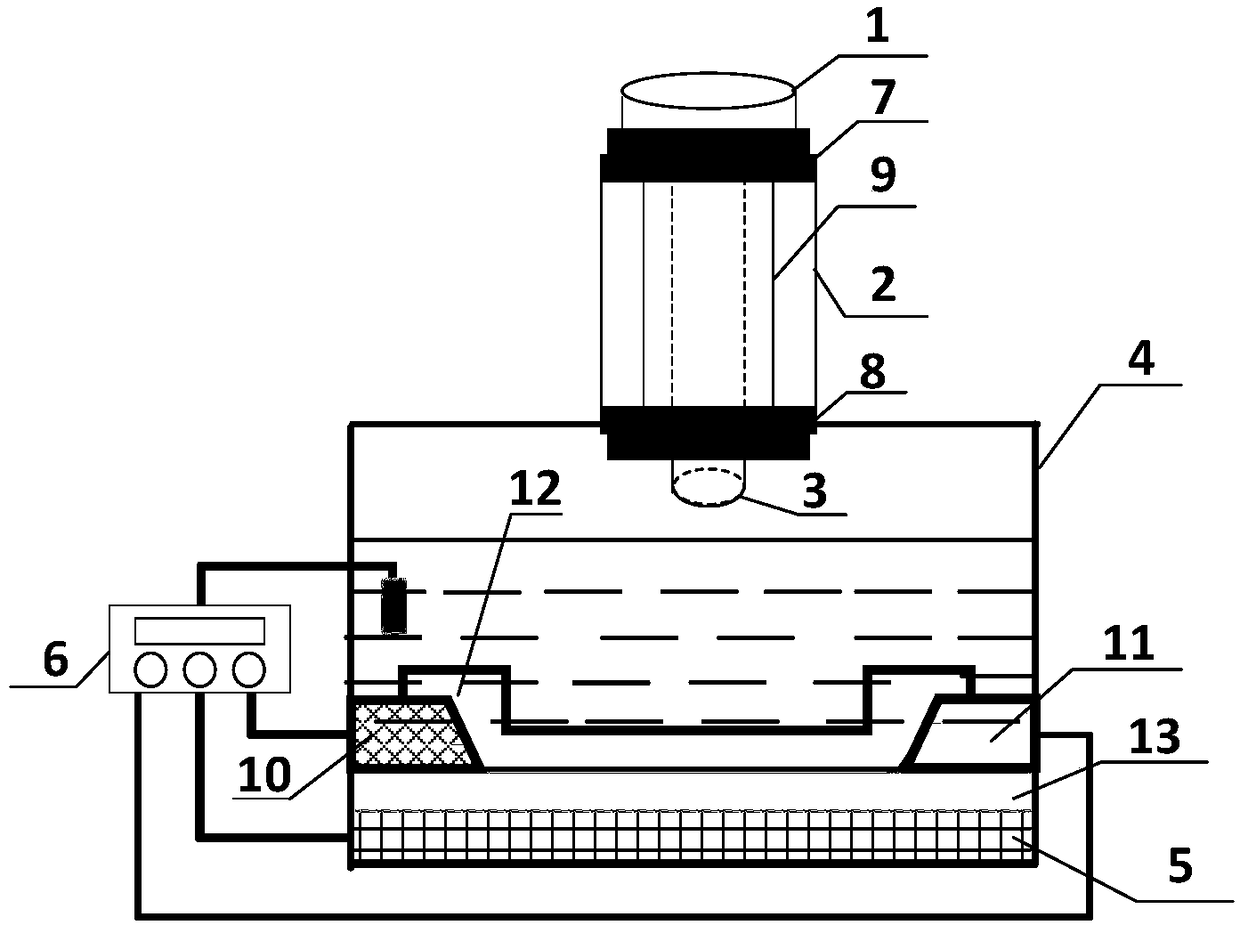
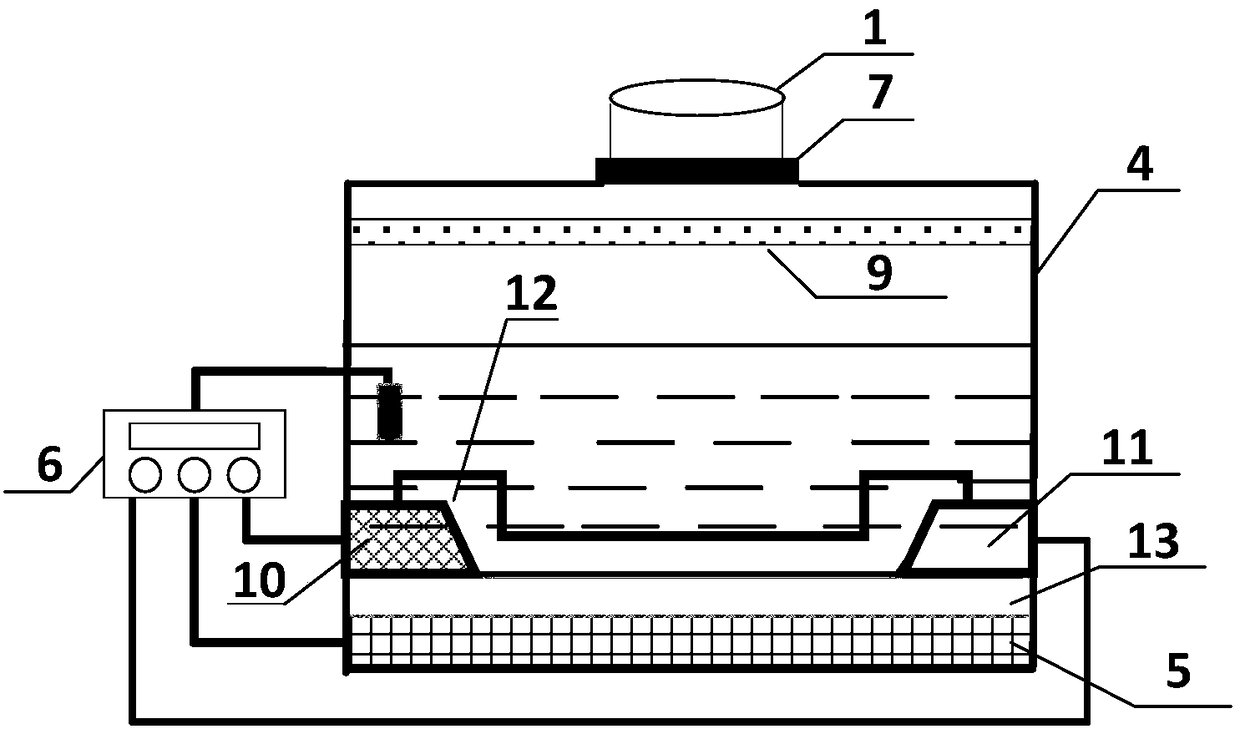
ActiveCN108918889ASimplify inspection stepsAchieve early diagnosisDisease diagnosisBiological testingCross-linkSeparation technology
The invention relates to a miniature blood detector for rapidly detecting an acute myocardial infarction marker, and a detection method thereof. The miniature blood detector comprises a blood sample inlet (1), a blood separator (2), a serum sample outlet (3), an immunoadsorption reactor (4), a pH-sensitive field effect transistor (5) and a micro-current detection circuit (6); the blood sample is injected from the sample inlet (1), and flows through a blood separation membrane (9), serum enters the immunoadsorption reactor (4), an acute myocardial infarction marker antigen in the serum specifically binds to its antibody, and is cross-linked with an enzyme in order to cause the change of the pH value of a reaction solution in order to change the current signal of the pH-sensitive field effect transistor (5), the current signal is output by the micro-current detection circuit (6), and the concentration of the measured marker is displayed. The concentration of the acute myocardial infarction marker is detected by using whole blood as a biological sample, combining a membrane separation technology with a micro biosensor element, integrating the blood separation membrane with the micropH-sensitive field effect transistor, performing an electronic enzyme-linked immunosorbent reaction and adopting current response as an output in order to quickly and easily achieve clinical detectionof acute myocardial infarction.
Owner:NANJING UNIV OF TECH
Immuno-mass spectrometric detection kit for esophagus cancer
The invention provides an immuno-mass spectrometric detection kit for an esophagus cancer. The detection kit contains a monoclonal antibody secreted by a hybridoma cell strain of which the preservation number is CGMCC No. 5269, and a solid-phase carrier; and the monoclonal antibody is fixed on the solid-phase carrier. The monoclonal antibody related to the detection kit has strong binding specificity aiming at peptide marker antigens. Detection with high flux, high sensitivity and high accuracy of the esophagus cancer can be achieved by the combination of immunization and a mass-spectrometric technique. In addition, the immuno-mass spectrometric detection kit belongs to the technology of diagnosis of molecular level, and is simpler in operation and lower in cost compared with tumor iconography.
Owner:BEIJING C & N INT SCI TECH +1
Synthetic process for alkaline phosphatase marked free thyroid hormone
ActiveCN1683407ASolve hard-to-prepare puzzlesLittle loss of activityHormone peptidesPeptide preparation methodsFree thyroid hormoneLuminescence
The present invention discloses synthesis process of alkaline phosphatase marked free thyroid hormone. The structure of alkaline phosphatase is reformed for coupling with thyroid hormone to prepare marker antigen. The present invention has simple technological process and low cost, and solves the difficult problem of preparing alkaline phosphatase marker as the key component for free thyroid hormone detecting kit in the immunological analysis of chemical luminescence.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Protein capable of specifically detecting mycobacterium tuberculosis infection
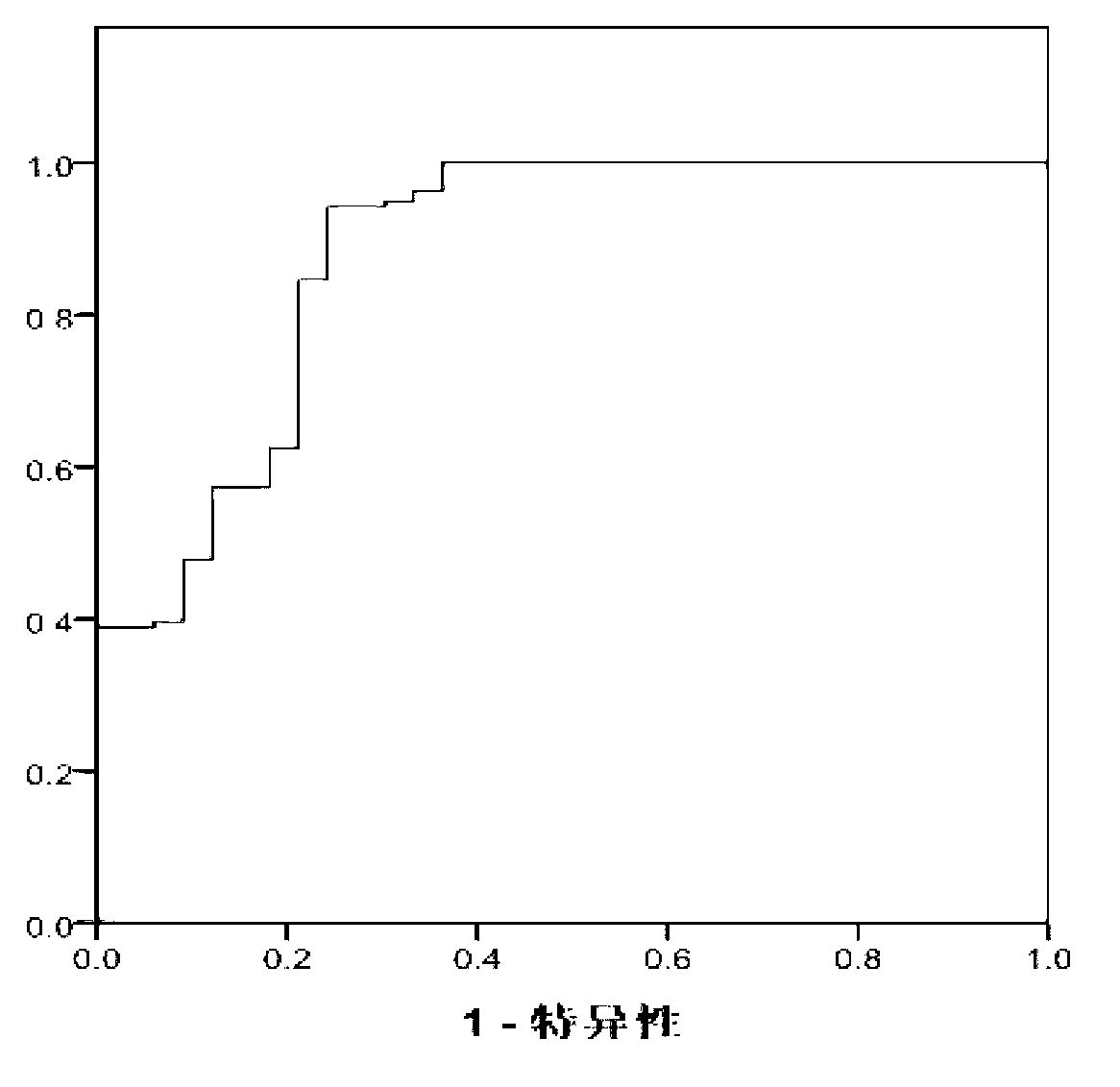
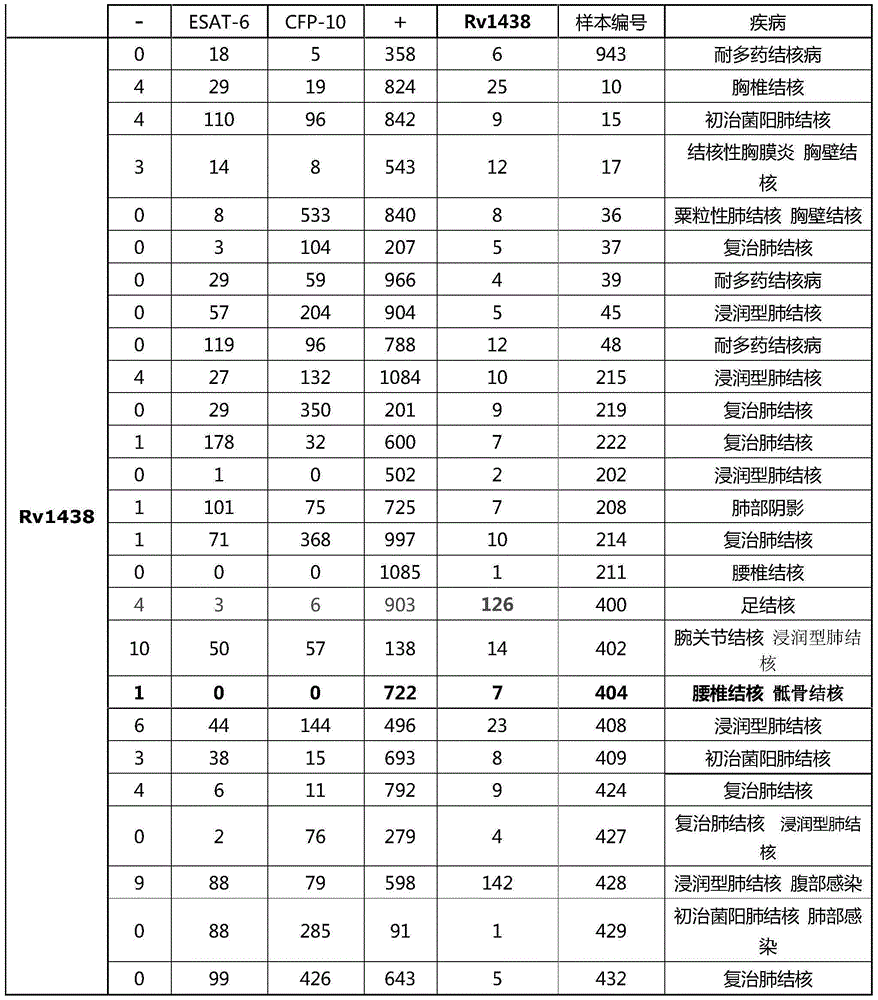
The invention discloses protein capable of specifically detecting mycobacterium tuberculosis infection, and provides application of Rv1438 protein to preparation of products for detecting or assisting in detecting mycobacterium tuberculosis. The experiment of the invention proves that mycobacterium tuberculosis-derived protein segments are screened, a mycobacterium tuberculosis marker antigen RV1438 for detecting tuberculosis is provided, and the antigen is used for in vitro detecting specific T cell immunoreaction to serve as a reference for diagnosing patients suffering from tuberculosis and can be used for diagnosing whether the patients are infected with the mycobacterium tuberculosis. The protein can be used for detecting mycobacterium tuberculosis infection, and the specificity reaches 70% at present.
Owner:TB HEALTHCARE BIOTECHNOLOGY (GUANGDONG) CO LTD
Antibody assay
The present invention relates to a method of detecting liver cancer in a mammalian subject by detecting an antibody in a test sample comprising a bodily fluid from the mammalian subject, wherein the antibody is an autoantibody immunologically specific for a tumour marker protein selected from the group consisting of MMP9, AIF1, EpCAM and CDKN1B, which method comprises contacting the test sample with a tumour marker antigen selected from the group consisting of MMP9, AIF1, EpCAM and CDKN1B and determining the presence or absence of complexes of the tumour marker antigen bound to autoantibodiespresent in the test sample where the presence of said complexes is indicative of the presence of liver cancer. Also included within the invention are corresponding methods of diagnosing and treating liver cancer in a mammalian subject, corresponding methods of predicting response to an anti-liver cancer treatment, a corresponding method of detecting an antibody in a test sample comprising a bodilyfluid from a mammalian subject and kits suitable for performing methods of the invention.
Owner:ONCIMMUNE
Diluent capable of improving stability of acridinium ester antigen-antibody conjugate and reducing background and preparation method thereof
PendingCN111855996AImprove the blocking effectImprove protectionMaterial analysisAntibody conjugateActive agent
The invention provides a diluent capable of improving the stability of an acridinium ester antigen-antibody conjugate and reducing the background. The diluent comprises the components of 5 to 7g of Tris-base, 3.5 to 4.0 mL of concentrated hydrochloric acid, 9 to 11 g of polyethylene glycol-6000, 0.95 mL to 1.05 mL of 10% lauryl sodium sulfate, 9.5 mL to 10.5 mL of Tween, 0.8 to 1.0 g of ethylene diamine tetraacetic acid disodium salt, 9 to 11 g of sodium caseinate, 0.95 mL to 1.05 mL of Proclin 300, 9.5 to 10.5 mL of triton X-100, and 1000mL of purified water for an in-vitro diagnostic reagent. The diluent has the advantages that the components are few; polyethylene glycol 6000, sodium dodecyl sulfate, tween and several active agents are mixed for use, meanwhile, a Tris-base buffer solution system is used, and multiple raw materials play a mutual synergistic effect, so that the diluent has a better protection effect on acridinium ester labeled antigens and antibodies, can be stored for a long time, can provide good blocking property and protectiveness for the antibodies, and reduces non-specific reactions; meanwhile, the material cost is saved, the specificity, stability, repeatability and other properties of the reagent are not influenced, and the reagent is suitable for popularization and application.
Owner:山东康华生物医疗科技股份有限公司
Serological detection method of bovine brucellosis A19-delta VirB12 vaccine
PendingCN111398604ASolve key technologiesSolve productivityDepsipeptidesPeptide preparation methodsAssayImmune adsorption
The invention discloses a serological detection method of a bovine brucellosis A19-delta VirB12 vaccine. An indirect enzyme-linked immunosorbent assay (iELISA) method for identifying and distinguishing a bovine brucellosis immune antibody and a natural infection antibody is established by taking a brucellosis VirB12 protein as a labeled antigen. According to the iELISA method provided by the invention, a problem that bovine brucellosis immune animals and clinical diseased animals are difficult to identify is solved, and the method has practical application value in prevention, control, eradication and purification of bovine brucellosis.
Owner:VETERINARY INST XINJINAG ACADEMY OF ANIMAL SCI CLINIC MEDICAL SCI RES CENT XINJIANG ACADEMY OF ANIMAL HUSBANDRY SCI
Hepatocirrhosis immuno-mass spectrometric detection reagent kit
ActiveCN102507941AEasy to operateStrong specificityMaterial analysis by electric/magnetic meansAntigenHigh flux
The invention relates to a hepatocirrhosis immuno-mass spectrometric detection reagent kit, which comprises a monoclonal antibody and a solid carrier, which are secreted by Hybridoma cell strain with a preserving number of CGMCC No.5269. The monoclonal antibody has strong specificity for polypeptide marker antigens; and by adopting the immunity technology to be combined with the mass spectrum technology, high flux, high sensitivity and high precision on detecting the hepatocirrhosis can be realized, so an early warning effect on the liver cancer can be better played. In addition, the reagent kit belongs to the diagnosis in molecular level, so the early warning capacity is stronger compared with the tumor imaging, and the cost is low.
Owner:BEIJING C & N INT SCI TECH +1
Immune colloidal gold test strip and detection method for karyotype polyhedrosis viruses of bombyx mori
The invention relates to an immune colloidal gold test strip and a detection method for karyotype polyhedrosis viruses of bombyx mori, particularly relates to an immune colloidal gold diagnosis test strip for detecting the karyotype polyhedrosis viruses of the bombyx mori by adopting an immunochromatography technology, and a preparation method of the immune colloidal gold diagnosis test strip, and belongs to the technical field of virus epidemic disease diagnosis. The immune colloidal gold test strip comprises a colloidal gold pad marked with an antigen BmNPV-Lef4 antibody and a coated nitrocellulose membrane, wherein an upper detection line of the nitrocellulose membrane is coated by rabbit-anti BmNPV-Lef4; and a quality control line at the lower part of the nitrocellulose membrane is coated with goat-anti-mouse IgG. The immune colloidal gold test strip is used for detecting the karyotype polyhedrosis viruses of the bombyx mori; compared with a traditional colloidal gold detection method, a double-antibody sandwich method is adopted when the colloidal gold test strip is prepared and a concentration proportion of two virus antibodies is regulated, so that the specificity, the sensitivity and the stability of a detection result can be effectively guaranteed; the detection sensitivity is high and the detection method is simple and convenient and is applied to the detection of the karyotype polyhedrosis viruses of the bombyx mori for the first time.
Owner:JIANGSU UNIV
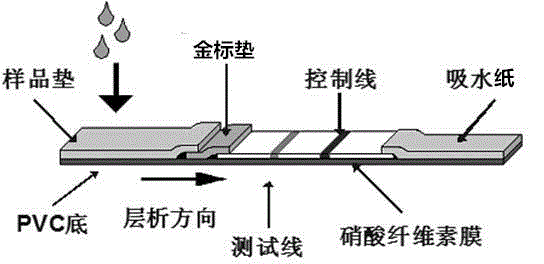
High-sensitivity gradient semi-quantitative immunochromatography detection test strip and detection method
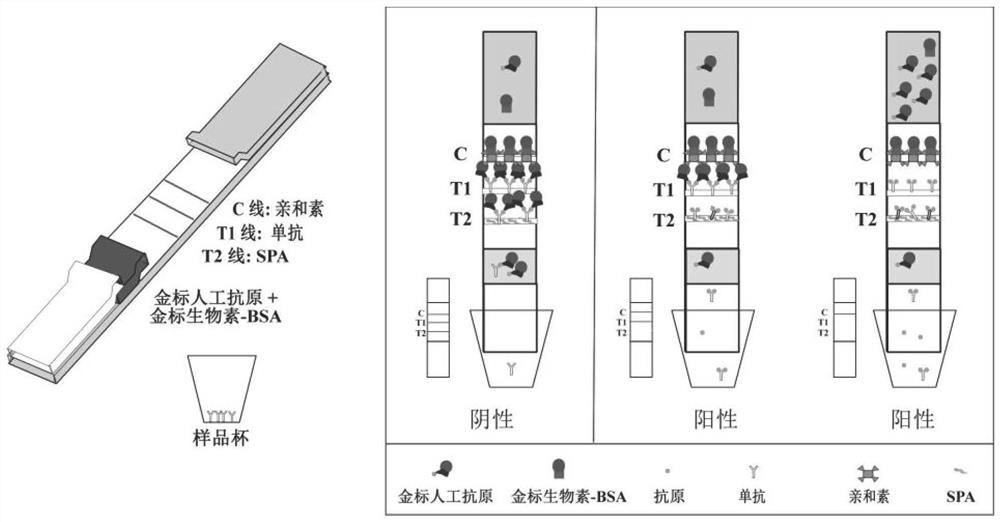
The invention relates to a high-sensitivity gradient semi-quantitative immunochromatography detection test strip. The test strip comprises test paper and a sample cup; the test paper comprises a supporting layer, an adsorption layer and a protective layer; the adsorption layer sequentially comprises a sample pad, a conjugate pad, a cellulose membrane layer and a water absorption material layer; a detection line T1, a detection line T2 and a quality control line are arranged on the cellulose membrane layer; the conjugate pad is made of glass fiber cotton adsorbed with a nano material labeled antigen; the sample cup is a container for diluting a sample, and a target monoclonal antibody diluted by a gold-labeled protein resuspension is pre-fixed in the sample cup. According to the test paper detection method, the two detection lines which do not interfere with each other can be prepared, and gradient semi-quantification is realized; the antibody is independently dried in the sample cup, so that the antibody is prevented from being folded and damaged in the labeling process, and the dosage of the antibody can be accurately controlled; the nano-particle labeled artificial antigen is used as a probe, each antibody intercepted on the detection line T2 can be accurately combined with two labeled antigens, so that signal self-amplification is realized; and compared with a traditional method, the method is higher in sensitivity.
Owner:HENAN ACAD OF AGRI SCI
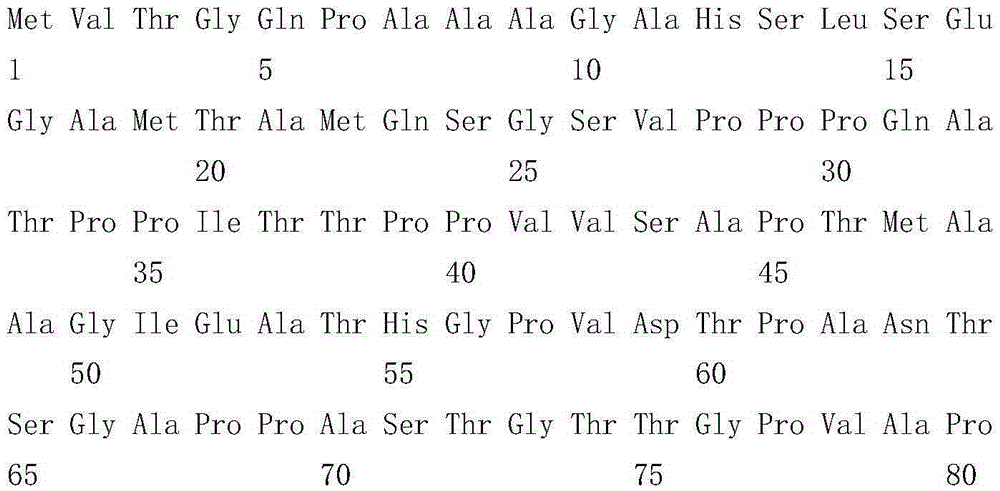
Protein for specifically detecting Mycobacterium tuberculosis infection
ActiveCN106749568AAntibacterial agentsBacterial antigen ingredientsMycobacterium InfectionsPatient diagnosis
The present invention relates to a protein for specifically detecting Mycobacterium tuberculosis infection, wherein the protein is derived from Mycobacterium tuberculosis, has the amino acid sequence represented by SEQ ID NO.1, can be used as the Mycobacterium tuberculosis marker antigen for tuberculosis detection, can be adopted as the reference for tuberculosis patient diagnosis, and is used for diagnosing whether the patient is infected with Mycobacterium tuberculosis.
Owner:TB HEALTHCARE BIOTECHNOLOGY (GUANGDONG) CO LTD
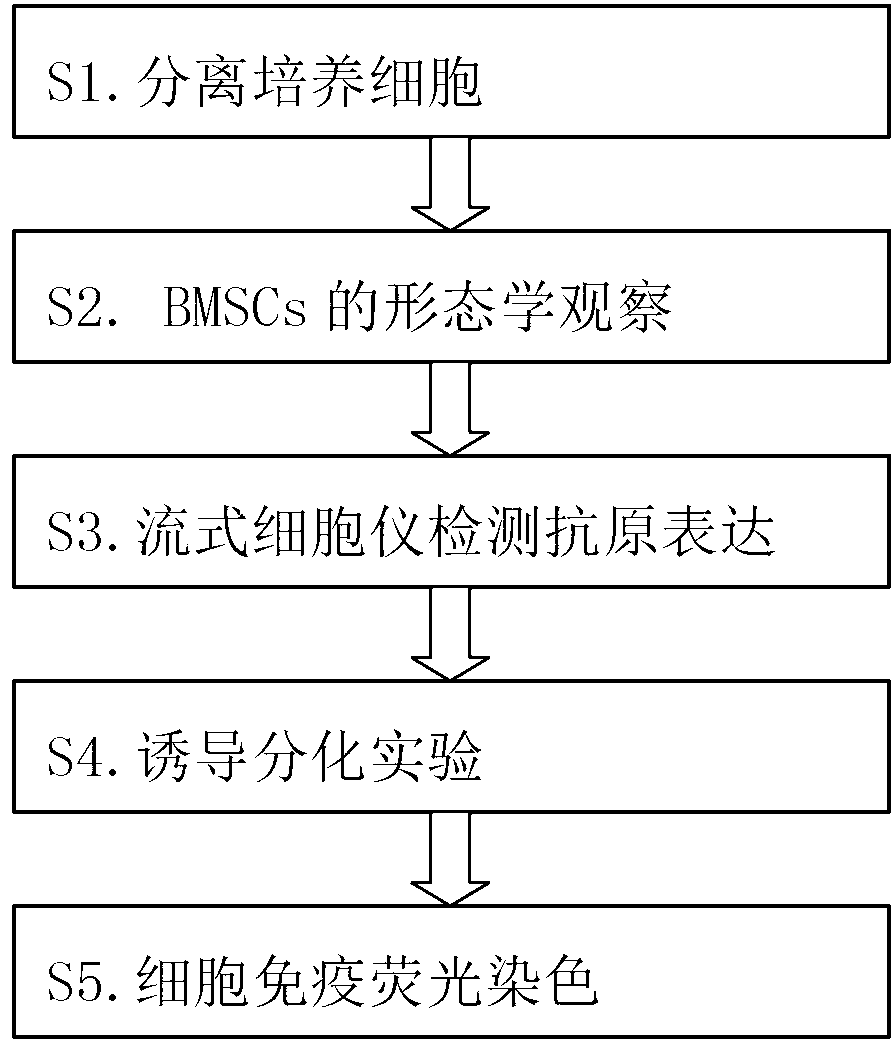
Method for inducing bone marrow mesenchymal stem cells to be differentiated into neuron-like cells
The invention provides a method for inducing bone marrow mesenchymal stem cells to be differentiated into neuron-like cells. The method comprises the steps of cultured cell separation, mesenchymal stem cell morphology observation, flow cytometry antigen presentation detection, differentiation experiment induction and cell immunofluorescence staining. According to the method, the bone marrow mesenchymal stem cells are induced to be differentiated into the neuron-like cells by using all-trans retinoic acids, basic fibroblast growth factors and epidermal growth factors, so that the neuron-like cells not only have the typical morphology of nerve cells, but also express the neuron-specific enolase of neuron marker antigens and the glial fibrillary acidic protein of astrocyte marker antigens; and the bone marrow mesenchymal stem cells maintain the capability that the bone marrow mesenchymal stem cells can be differentiated into non-mesenchymal cells in a germ-layer-crossing manner, and can be differentiated into the neuron-like cells in the germ-layer-crossing manner, so that the neuron-like cells are expected to become seed cells for nerve cell replacement therapy.
Owner:陆华
Micro-fluidic chip for detecting multiple infection markers in peripheral blood through multi-channel ELISA
PendingCN113189349AWithout human interventionDetection repeatability is strongLaboratory glasswaresDisease diagnosisAntigenSerum samples
The invention relates to the technical field of micro-fluidic chip ELISA detection, in particular to a micro-fluidic chip for detecting multiple infection markers in peripheral blood through multi-channel ELISA. The micro-fluidic chip comprises a substrate, a sample adding pool, a capillary channel and a detection area. A serum sample is fed into the sample adding pool through a blood sampling port, driving is carried out through ultrasonic waves, infection marker antigen molecules in a serum sample enter the detection area through the capillary channel, and the infection marker antigen molecules in the serum sample are captured by infection marker antibodies of enzyme-linked immunosorbent assay in the reaction tank; full-automatic chip detection is realized by using an ELISA detection micro-fluidic chip technology, and human interference is not needed; and the detection repeatability of the same sample is high, and the detection result has high stability and reliability.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com